Abstract
Convention holds that ionotropic receptors mediate fast neurotransmission and that ‘slow’ G-protein coupled metabotropic receptors have a secondary, modulatory role in the control of neuronal networks. Here, we discuss recent evidence showing that activation of metabotropic GABAB receptors in cortical layer 1 can powerfully inhibit principal cell activity and that their activation can rapidly halt ongoing network activity. Inputs from both within and outwith the cortex converge upon layer 1 where they target various populations of interneurons, including neurogliaform cells. We argue that neurogliaform cells are the main effector of a powerful inhibitory circuit that, acting through GABAB receptors, can be differentially recruited by long-range connections to serve in roles as diverse as conscious perception and memory consolidation.
Introduction
In undergraduate neuroscience courses, students are taught that ionotropic receptors mediate fast neurotransmission and that ‘slow’ G-protein coupled metabotropic receptors play more of a supporting, modulatory role in cortical circuits [1]. While ionotropic glutamate (AMPA, NMDA and kainate) and GABA (GABAA) receptors are the primary effectors of local synaptic transmission, the role of metabotropic receptors in normal network function is often overlooked. Here, we will review recent evidence suggesting that at least one metabotropic receptor, the GABAB receptor, can exert a fast, powerful inhibitory influence over cortical networks, capable of rapidly silencing ongoing network activity.
GABAB receptors are members of the G-protein coupled receptor (GPCR) superfamily, a group of receptors that include metabotropic glutamate, opioid and olfactory receptors. All GPCRs share a common structure comprising seven transmembrane domains: ligand-binding domains are located in the extracellular region and they activate second messenger systems via interactions with G-proteins in the cytosolic region. Functional GABAB receptors are heterodimers of two GPCR proteins, the GABAB1 and GABAB2 subunits, the presence of both being essential for membrane trafficking. Several isoforms of the GABAB1 subunit exist, but the most abundant are GABAB1a and GABAB1b, which vary in the extracellular domain and are conserved throughout the vertebrate family [2]. The isoform of GABAB1 subunit affects the synaptic location of the GABAB receptor: receptors containing the GABAB1a subunit are predominantly found on presynaptic terminals of both excitatory and inhibitory synapses whilst those containing the GABAB1b subunit predominantly exist in postsynaptic locations [3,4], an observation that holds for all excitatory synapses examined throughout the CNS [5].
Inhibition via GABAB receptors is mediated through the adenylyl cyclase/protein kinase A (PKA) second messenger pathway (via activation of Gαi/o-type G proteins) and, through liberation of Gβγ subunits, by activation of G-protein coupled inward-rectifying K+ (GIRK) channels and inhibition of voltage-gated Ca2+ channels (VGCCs) [2]. Presynaptic GABAB receptors inhibit transmitter release through inhibition of VGCCs and possibly via interactions with vesicular release machinery [5], while postsynaptic GABAB receptors exert their effects through multiple pathways. Recent studies have shown that postsynaptic GABAB receptors inhibit dendritic Ca2+ signals mainly by inhibiting VGCCs, with little contribution from GIRK channels [6*]. While GABAB receptors inhibit dendritic NMDA receptor Ca2+ signals through the PKA/cAMP pathway [7], inhibition of dendritic Ca2+ spikes appears to occur, at least in part, via a direct interaction between the Gβγ-subunit and L-type Ca2+ channels [8].
Traditionally, GABAB receptors are thought to regulate slow changes in neuronal excitability, with their hypofunction being implicated in disorders such as depression, epilepsy and impaired sleep [2]. Despite their wide distribution throughout the hippocampus, it was believed that GABAB receptors were primarily activated during periods of strong network activation, via GABA spill-over [9]. Although GABAB receptors have the potential to affect multiple cellular and network processes through a myriad of mechanisms, including cross-talk with other GPCR systems via the PKA/cAMP pathway, most early studies into their function appeared to preclude a dominant role in controlling fast network processes, perhaps only having relevance in pathological states [10].
‘Slow’ inhibition can rapidly terminate persistent activity
Evidence that GABAB receptors may have a significant role in normal network function comes from the study of the slow oscillation. During sleep, cortical networks participate in the slow oscillation when neurons display periods of synchronised depolarisation and firing (Up states) punctuated by periods of relative hyperpolarisation (Down states). Up states are involved in memory consolidation and Down states may play a role in regulating neuronal homeostasis [for reviews, see e.g. 11,12,13]. A study using an in vitro model of the oscillation in the medial entorhinal cortex (mEC) demonstrated that GABAA and GABAB receptors had different roles in mediating Up states [14]. Up states occurred either spontaneously or in response to electrical stimulation in layer 3, with a mean duration of ~3 seconds. Progressively blocking GABAA receptors through increasing concentrations of antagonist shortened Up states, with complete blockade inducing epileptiform activity [14]. Given that Up states are sustained by a dynamically-regulated balance between inhibitory and excitatory conductances [15], this result was perhaps not too surprising.
Unexpectedly, however, Mann et al. also found that GABAB receptors could control Up state termination [14]. Blockade of GABAB receptors prolonged the duration of Up states by around 50% and, interestingly, electrical stimulation in layer 1 applied 500ms after the onset of the Up state, could immediately evoke a Down state, terminating ongoing activity. Notably, this effect was abolished by application of GABAB receptor antagonists. This important study demonstrated that GABAB receptors could be the major determinant of Up state duration, with GABAB receptors both controlling the spontaneous and afferent-evoked termination of Up states. Given the lack of subtype-specific GABAB receptor antagonists, pharmacology alone cannot determine whether these two phenomena are controlled by presynaptic or postsynaptic GABAB receptors, or a combination of both.
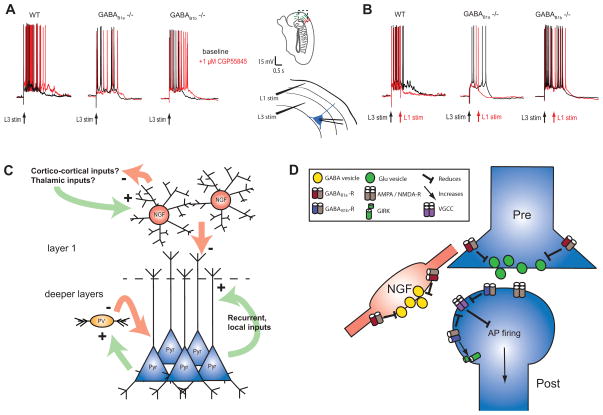
Recently, we further studied the role of GABAB receptors in termination of Up states using mice in which either GABAB1a or GABAB1b subunits had been genetically ablated [16**]. We found that, in mice lacking GABAB1a-containing receptors, electrical stimulation in layer 1 could still terminate Up states, but that blockade of the remaining GABAB1b-containing receptors did not prolong Up states (Fig. 1A). Conversely, we found that in mice lacking GABAB1b-containing receptors, electrical stimulation in layer 1 could not actively terminate Up states, but that they were prolonged by blockade of the remaining GABAB1a-containing receptors (Fig. 1B). These experiments demonstrate that presynaptic GABAB1a-containing receptors alone are important in controlling the duration of spontaneous Up states, perhaps by regulating neurotransmitter release. They also show that postsynaptic GABAB1b-containing receptors are essential for afferent-evoked termination of persistent activity, with electrical stimulation in layer 1 activating a powerful mechanism by which interneurons in layer 1, targeting GABAB receptors, can halt ongoing network activity (see Fig. 1C and D). In addition to the mEC [14,16], prolongation of Up states via GABAB receptor blockade has been demonstrated in visual [17], prefrontal [18] and somatosensory cortices [Craig et al., unpublished], suggesting that this mechanism is conserved across cortical regions.
Figure 1. Presynaptic GABAB receptors contribute to spontaneous termination of the Up state whilst postsynaptic GABAB receptors are necessary for layer 1 stimulus-evoked termination of the Up state.
(A), blockade of GABAB receptors with the selective antagonist CGP55845 prolongs the Up state (evoked in response to layer 3 stimulation in medial entorhinal cortex) in wildtype mice and those lacking the GABAB1b subunit, but not those lacking the GABAB1a subunit, implying that presynaptic GABAB receptors are involved in the spontaneous termination of the UP state. (B), electrical stimulation in layer 1 can terminate an ongoing Up state in wildtype mice and those lacking the GABAB1a subunit, but not in those lacking the GABAB1b subunit, demonstrating that afferent-evoked termination of the UP state is mediated by postsynaptic GABAB receptors. (C) and (D), schematic representation of proposed model: recurrent excitatory and inhibitory (GABAA receptor-mediated) connections sustain the Up state, with presynaptic GABAB receptors regulating the duration of the Up state by inhibiting transmitter release. Inputs to layer 1 (perhaps from the thalamus or other cortical regions) activate neurogliaform (NGF) cells, which target dendritic GABAB receptors to inhibit principal cell firing through reduced calcium entry, causing the network to enter a Down state. Neurogliaform cells may also regulate Up state duration by targeting GABAB receptors on the presynaptic glutamatergic terminals of the inputs arriving in layer 1. (A) and (B) adapted from [16], with permission.
Layer 1: important regulator of network activity?
Electrical stimulation in layer 1 can halt ongoing network activity, presumably by activating interneurons that target postsynaptic GABAB1b-containing receptors. While it is remarkable that a ‘slow’ metabotropic receptor can have a profound effect on the state of a network, one has to ask whether this effect is specific to the slow oscillation or whether this experimental manipulation is tapping into a system capable of inhibiting any activity within the cortical circuit?
Early hints of a GABAB receptor-mediated inhibitory circuit in layer 1 came from a study of GABAB receptor location and synaptic inhibition. The study found that layer 1 stimulation blocked dendritic Ca2+ spike generation in layer 5 pyramidal cells, acting via postsynaptic GABAB1b-containing receptors, whilst presynaptic GABA release from GABAergic terminals, detected by GABAA receptor-mediated IPSPs on principal cells, was inhibited by activation of GABAB1a-containing receptors [3]. This study suggested the presence of a complex circuit in layer 1 that could control the excitability of principal cells, and provides a mechanism by which presynaptic and postsynaptic GABAB receptors have different roles in terminating persistent activity [16], with presynaptic receptors regulating transmitter release and postsynaptic receptors actively halting Up states by inhibiting the firing of principal cells. This begs the question of whether such an inhibitory circuit might regulate the operation of other processes?
Evidence that the layer 1 inhibitory circuit is also active during sensory processing was provided by an elegant in vivo study demonstrating that interhemispheric inhibition is mediated by postsynaptic GABAB receptors [19**]. Using a combination of calcium imaging, optogenetics and transgenic mice, it was shown that, in response to hindpaw stimulation, the firing of layer ipsilateral 5 pyramidal cells was disynaptically inhibited by excitatory afferents arising from the contralateral hemisphere that specifically targeted interneurons in layer 1; this effect was mediated by GABAB1b-containing receptors located on the apical dendrites of the pyramidal cells. Importantly, the GABAB-receptor mediated inhibition resulted in only a small hyperpolarisation at the soma: due to the contribution of dendritic Ca2+ channels to overall pyramidal cell excitability, their blockade provided a form of shunting inhibition apparent only when the cell was spiking.
Palmer et al. also observed that activation of dendritic GABAB receptors could reduce layer 5 pyramidal cell spiking by up to 50%, even in the absence of excitatory dendritic input [19]. In addition to the axon hillock, a second action potential initiation site is present in the apical tufts of principal cells and Ca2+ spikes generated there can actually drive more axonal action potentials than supratheshold input near the soma [reviewed by 20], so inhibition of dendritic Ca2+ channels alone can strongly affect the output of principal cells. Taken together this suggests that in addition to halting ongoing network activity, the layer 1 GABAB receptor-mediated inhibitory circuit can also suppress the activity of principal cells during awake states.
Source(s) of inhibition in layer 1?
All of the studies considered so far point to the existence of a specific circuit in layer 1 that, acting through GABAB receptors, can inhibit the activity of principal cells by inhibiting dendritic Ca2+ channels [3,6–8,19] and terminate persistent activity [14,16]. Although the phenomenon is widely observed throughout many cortical regions, key mechanistic questions remain: is the source of inhibition a single class of interneuron? What are the inputs to this circuit? Whilst these questions remain open, several recent studies have begun to address them.
The class of inhibitory interneurons referred to as neurogliaform cells provide an attractive candidate as the source of inhibition in layer 1, as they inhibit pyramidal cells through a mixed GABAA/GABAB receptor-mediated mechanism [21]. Furthermore, a small number of neurogliaform cells have the potential to evoke far-reaching inhibition by activating GABAB receptors via a seemingly non-discriminatory volume transmission [22,23] although new evidence [24**] suggests that the targets of the neurogliaform cells may be more synapse-specific than previously assumed. Neurogliaform cells can target both postsynaptic GABAB receptors and presynaptic GABAB receptors located on glutamatergic terminals [22]. Many studies have described the presence of these late-spiking neurogliaform cells in layer 1 [e.g. 25] and a recent study carried out using connected pairs of layer 1 interneurons and layer 2/3 pyramidal cells demonstrated that neurogliaform cells can strongly inhibit, but do not receive input from, layer 2/3 pyramidal cells [26*]. Importantly, this study found that neurogliaform cells inhibited layer 2/3 pyramidal cells through both GABAA and GABAB receptors, whereas other interneurons in layer 1 acted through GABAA receptors alone.
In a Herculean display of electrophysiological panache, Jiang et al. demonstrated, through quadruple – octruple patch clamp recordings, that a group of layer 1 neurogliaform cells could strongly inhibit layer 5 pyramidal cells via direct inhibition and through electrical connections with three types of layer 2/3 interneurons; the layer 1 neurogliaform cells described appeared different to their counterparts in deeper layers, with elongated processes that spanned multiple cortical columns [27**]. All neurogliaform cells described in the hippocampus and amygdala stain for neuropeptide Y (NPY), and hippocampal neurogliaform cells can be further parsed into two sub-populations differentiated by the expression of nitric oxide synthase (NOS) [28,29]. However, the situation may be more complex in the cortex. While NPY expression has been reported for all cortical neurogliaform cells including layer 1 [30,31*], a recent study reported that NOS-positive elongated neurogliaform cells in layer 1 (morphologically similar to those reported by Jiang et al. [27]) do not stain for NPY, raising the possibility that they represent a third distinct group [32*], perhaps with different embryonic origins. This is not without precedent: while neocortical neurogliaform cells are believed to share common embryonic origins [33–35], hippocampal neurogliaform cells parse into two groups with distinct embryonic origins but similar morphological and electrophysiological properties [36]. It is possible that two (or perhaps three) classes of neurogliaform-like interneurons reside in layer 1: those expressing NPY with ‘typical’ neurogliaform morphology, and an NPY lacking population with a wider dendritic arbour. Interestingly, direct application of NPY to the distal dendrites of layer 5 pyramidal cells is sufficient to inhibit dendritic Ca2+ transients, without the need for either GABAergic or glutamatergic transmission [37*].
While current evidence strongly implies neurogliaform cells as the effector of the layer 1 GABAB receptor-mediated inhibitory circuit, further work is needed to conclusively test this hypothesis. The origin of the inputs to this circuit also remains unclear. Only 10% of the inputs to layer 1 arise from the local circuit, with long-range connections providing the remainder [20]. Layer 1 receives cortico-cortical inputs from the ipsilateral hemisphere [e.g. 38,39,40] and, as already discussed, interhemispheric inhibition relies on inputs to layer 1 from the contralateral hemisphere [19]. Layer 1 receives additional input from subcortical regions: one in vivo study reported that layer 1 interneurons responded to whisker stimulation with sub-10 millisecond latencies, implying direct thalamic input [41], which was anatomically confirmed by a later study [42]. Through expression of channelrhodopsin in the midline thalamus, a recent study found that layer 1 interneurons, especially late-spiking (presumably neurogliaform) cells, could be strongly activated by thalamic input, even driving feed-forward inhibition of other L1 interneurons and deeper pyramidal cells [43**]. Thus, future studies could try to selectively drive neurogliaform cells to actively terminate Up states.
Functional significance?
While the circuitry of the GABAB receptor-mediated inhibitory system in cortical layer 1 has yet to be fully mapped, it is clear that GABAB receptors can strongly regulate cortical activity in a state-dependent manner. In the awake animal, the system regulates interhemispheric inhibition [19], and feedback inhibition via long-range connections to layer 1 is important in cognition and conscious perception, with regulation of dendritic calcium signalling being proposed as a key cellular mechanism for association [20].
Slow wave sleep is believed to be important for long-term memory consolidation [e.g. 44,45], where hippocampal sharp waves can drive cortical Up states [46], allowing the activity of neuronal ensembles to be temporally coordinated across different brain regions [47]. As the layer 1 inhibitory circuit can rapidly terminate an ongoing Up state [14,16], this could provide the mechanism for mediating the long-range synchrony of Up-to-Down state transitions observed in vivo [48]. Inhibition via GABAB receptors could ensure that only the appropriate ensemble is selected for memory consolidation, and may provide a mechanism for a proposed thalamic driver of the slow oscillation [12].
Concluding remarks
This review has focused primarily on GABAB receptors in layer 1 and in particular, their role in modulation of slow oscillations. However, GABAB receptors also influence faster hippocampal oscillations, such as those in the theta and gamma frequency ranges [reviewed by 49]. The mechanisms by which this is achieved may be numerous: recent work has shown that postsynaptic GABAB receptors in the dentate gyrus enhance the function of extrasynaptic GABAA receptors [50], and that postsynaptic GABAB receptors inhibit perisomatic-targeting but not dendritic-targeting parvalbumin-positive interneurons [51]. Presynaptic GABAB receptors, in addition to contributing to spontaneous termination of the Up state [16], mediate disinhibition of dentate granule cell output [52], and may regulate excitability at the hippocampal mossy fibre terminal [53]. Dysfunction of GABAB receptor-mediated signalling has been implicated in several models of absence epilepsy [54], highlighting the importance of this receptor in normal network function.
Future work should focus on uncovering the nature of the layer 1 inhibitory circuit that is mediated by GABAB receptors, such as identifying which cell type(s) are the main effectors and mapping the inputs to the circuit. While several existing optogenetic tools will be useful for dissecting this circuitry, the creation of mice with floxed GABAB1a and GABAB1b subunits to allow conditional inactivation of pre- or post-synaptic GABAB receptors in a neuron subtype-specific manner would greatly aid this research, especially given the multitude of mice available that express Cre recombinase in different interneuron subtypes [55]. Additionally, the study of a group of recently-identified GABAB receptor auxiliary subunits from the KCTD family [56], which have variable expression throughout the brain and modify receptor properties in a subtype-specific manner [56–59], could provide new insights into how GABAB receptors influence neuronal circuits. Although challenges clearly remain, future advances in understanding how the ‘slow’ GABAB receptor can effect rapid changes in network state could present exciting new insights into the genesis of synchronous neuronal activity, in both healthy and pathological conditions.
Highlights for Craig & McBain.
Metabotropic GABAB receptors can, unexpectedly, mediate rapid termination of persistent network activity in the cortex.
Layer 1 interneurons modulate network activity by targeting GABAB receptors on principal cells.
Dendritic GABAB receptors can inhibit the firing of principal cells by acting on voltage-gated calcium channels.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. 4. New York ; London: McGraw-Hill, Health Professions Division; 2000. [Google Scholar]
- 2.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gassmann M, Bettler B. Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat Rev Neurosci. 2012;13:380–394. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
- *6.Chalifoux JR, Carter AG. GABAB Receptor Modulation of Voltage-Sensitive Calcium Channels in Spines and Dendrites. J Neurosci. 2011;31:4221–4232. doi: 10.1523/JNEUROSCI.4561-10.2011. Using calcium imaging and local agonist application, this study demonstrated that GABAB receptors strongly and locally modulate dendritic Ca2+ signals through activation of multiple types of VGCC. Importantly, they showed that modulation of dendritic Ca2+ signals does not correlate with somatic voltage changes observed using patch-clamp recordings, questioning the utility of using somatic recordings alone to study GABAB receptor function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalifoux JR, Carter AG. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Garci E, Larkum ME, Nevian T. Inhibition of dendritic Ca2+ spikes by GABAB receptors in cortical pyramidal neurons is mediated by a direct Gi/o-beta-subunit interaction with Cav1 channels. J Physiol. 2013;591:1599–1612. doi: 10.1113/jphysiol.2012.245464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scanziani M. GABA Spillover Activates Postsynaptic GABAB Receptors to Control Rhythmic Hippocampal Activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 10.Mott D, Lewis DV. The Pharmacology and Function of Central GABA~ B Receptors. International review of neurobiology. 1994:97–97. doi: 10.1016/s0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 11.Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vyazovskiy VV, Harris KD. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 2013;14:443–451. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29:7513–7518. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- **16.Craig MT, Mayne EW, Bettler B, Paulsen O, McBain CJ. Distinct roles of GABAB1a- and GABAB1b-containing GABA(B) receptors in spontaneous and evoked termination of persistent cortical activity. J Physiol. 2013;591:835–843. doi: 10.1113/jphysiol.2012.248088. Extending the findings of [14], this study demonstrated that the dual effects of GABAB receptors in mediating both spontanoeus and evoked termination of the UP state were carried exclusively by presynaptic and postsynaptic GABAB receptors, respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Vives MV, Mattia M, Compte A, Perez-Zabalza M, Winograd M, Descalzo VF, Reig R. Inhibitory modulation of cortical up states. J Neurophysiol. 2010;104:1314–1324. doi: 10.1152/jn.00178.2010. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Neubauer FB, Luscher HR, Thurley K. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. Eur J Neurosci. 2010;31:1582–1594. doi: 10.1111/j.1460-9568.2010.07191.x. [DOI] [PubMed] [Google Scholar]
- **19.Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science. 2012;335:989–993. doi: 10.1126/science.1217276. This study used a combination of in vivo and in vitro recordings, optogenetics and Ca2+ imaging to show that interhemispheric inhibtion is mediated by principal cells projecting to the contralateral hemisphere where they target layer 1 and cause a form of shunting inhibition in pyramdal cell dendrites, mediated by postsynaptic GABAB1b-containing receptors and can strongly inhibit pyramidal cell output. [DOI] [PubMed] [Google Scholar]
- 20.Larkum M. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 2013;36:141–151. doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 22.Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentet LJ. Functional diversity of supragranular GABAergic neurons in the barrel cortex. Front Neural Circuits. 2012;6:52. doi: 10.3389/fncir.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **24.Chittajallu R, Pelkey KA, McBain CJ. Neurogliaform cells dynamically regulate somatosensory integration via synapse-specific modulation. Nat Neurosci. 2013;16:13–15. doi: 10.1038/nn.3284. This study challenged the assumption that neurogliaform cells act through a non-discriminatory volume transmission by demonstrating that they inhibit fast-spiking interneurons in layer 4 somatosensory cortex to reduce feed-forward inhibition but do not inhibit feed-forward excitation at thalamocortical synapses onto either stellate cells or fast-spking interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hestrin S, Armstrong WE. Morphology and physiology of cortical neurons in layer I. J Neurosci. 1996;16:5290–5300. doi: 10.1523/JNEUROSCI.16-17-05290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Wozny C, Williams SR. Specificity of Synaptic Connectivity between Layer 1 Inhibitory Interneurons and Layer 2/3 Pyramidal Neurons in the Rat Neocortex. Cereb Cortex. 2011;21:1818–1826. doi: 10.1093/cercor/bhq257. By studying the morphology and electrophysiological properties of more than 200 interneurons in layer 1 of rat somatosensory cortex, this study showed that at least 4 types of interneuron reside in layer 1 and, using paired recordings, examined reciprocal connectivity with layer 2/3 pyramdal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013 doi: 10.1038/nn.3305. This study, by making 4 to 8 simultaneous patch-clamp recordings and examining over 14000 synaptic connections, found two new inhibitory circuits driven by layer 1 interneurons with one circuit disinhibiting layer 5 pyramidal cells and the other, acting through neurogliaform cells, inhibiting the initiaion of dendritic spikes in layer 5 pyramidal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R, Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25:6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manko M, Bienvenu TC, Dalezios Y, Capogna M. Neurogliaform cells of amygdala: a source of slow phasic inhibition in the basolateral complex. J Physiol. 2012;590:5611–5627. doi: 10.1113/jphysiol.2012.236745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karagiannis A, Gallopin T, David C, Battaglia D, Geoffroy H, Rossier J, Hillman EM, Staiger JF, Cauli B. Classification of NPY-expressing neocortical interneurons. J Neurosci. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Ma J, Yao XH, Fu Y, Yu YC. Development of Layer 1 Neurons in the Mouse Neocortex. Cereb Cortex. 2013 doi: 10.1093/cercor/bht114. This study looks at the development of layer 1 interneurons from P2 to P4 and reported that most (60 to 70%) late-spiking interneurons expressed NPY. While this may seem to contradict [32], a sizeable proportion of late-spiking cells did not express NPY, and the GAD67-GFP mouse used in this study to target layer 1 interneurons does not report every cell. [DOI] [PubMed] [Google Scholar]
- *32.Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y. Selective Coexpression of Multiple Chemical Markers Defines Discrete Populations of Neocortical GABAergic Neurons. Cerebral Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. This is a comprehensive study of the morphological and neurochemical features of cortical interneurons. Importantly, the authors describe a form of neurogliaform cell in layer 1 that does not express NPY and has a wider dendritic arbor than neurogliaform cells in other layers, suggesting that these elongated neurogliaform cells may be members of a distinct subgroup. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Developmental neurobiology. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis T. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cerebral cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tricoire L, Pelkey KA, Daw MI, Sousa VH, Miyoshi G, Jeffries B, Cauli B, Fishell G, McBain CJ. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30:2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Hamilton TJ, Xapelli S, Michaelson SD, Larkum ME, Colmers WF. Modulation of distal calcium electrogenesis by neuropeptide y1 receptors inhibits neocortical long-term depression. J Neurosci. 2013;33:11184–11193. doi: 10.1523/JNEUROSCI.5595-12.2013. Here, the authors show that direct application of the neuropeptide NPY is sufficient to inhibit Ca2+ influx at the distal dendrites of layer 5 pyramidal cells, and that NPY could inhibit long-term depression at distal dendrites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson JC, Martin KA. Synaptic connection from cortical area V4 to V2 in macaque monkey. J Comp Neurol. 2006;495:709–721. doi: 10.1002/cne.20914. [DOI] [PubMed] [Google Scholar]
- 39.Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron. 2011;72:111–123. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Zhu JJ. Rapid arrival and integration of ascending sensory information in layer 1 nonpyramidal neurons and tuft dendrites of layer 5 pyramidal neurons of the neocortex. J Neurosci. 2004;24:1272–1279. doi: 10.1523/JNEUROSCI.4805-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubio-Garrido P, Perez-de-Manzo F, Porrero C, Galazo MJ, Clasca F. Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb Cortex. 2009;19:2380–2395. doi: 10.1093/cercor/bhn259. [DOI] [PubMed] [Google Scholar]
- **43.Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, Elmaleh M, Connors BW. Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci. 2012;32:17813–17823. doi: 10.1523/JNEUROSCI.3231-12.2012. Through expression of channelrhodpsin in the ventromedial thalamic nucleus, this study demonstrated that thalamic input was sufficient to drive layer 1 interneurons and could evoke feedforward inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7 (Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 45.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 46.Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Born J. Slow-wave sleep and the consolidation of long-term memory. World J Biol Psychiatry. 2010;11 (Suppl 1):16–21. doi: 10.3109/15622971003637637. [DOI] [PubMed] [Google Scholar]
- 48.Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected] J Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohl MM, Paulsen O. The roles of GABAB receptors in cortical network activity. Adv Pharmacol. 2010;58:205–229. doi: 10.1016/S1054-3589(10)58009-8. [DOI] [PubMed] [Google Scholar]
- 50.Tao W, Higgs MH, Spain WJ, Ransom CB. Postsynaptic GABAB receptors enhance extrasynaptic GABAA receptor function in dentate gyrus granule cells. J Neurosci. 2013;33:3738–3743. doi: 10.1523/JNEUROSCI.4829-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Booker SA, Gross A, Althof D, Shigemoto R, Bettler B, Frotscher M, Hearing M, Wickman K, Watanabe M, Kulik A, et al. Differential GABAB-Receptor-Mediated Effects in Perisomatic- and Dendrite-Targeting Parvalbumin Interneurons. J Neurosci. 2013;33:7961–7974. doi: 10.1523/JNEUROSCI.1186-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foster JD, Kitchen I, Bettler B, Chen Y. GABAB receptor subtypes differentially modulate synaptic inhibition in the dentate gyrus to enhance granule cell output. Br J Pharmacol. 2013;168:1808–1819. doi: 10.1111/bph.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabezas C, Irinopoulou T, Gauvain G, Poncer JC. Presynaptic but not postsynaptic GABA signaling at unitary mossy fiber synapses. J Neurosci. 2012;32:11835–11840. doi: 10.1523/JNEUROSCI.5543-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han HA, Cortez MA, Snead OC. GABAB Receptor and Absence Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. 2012. [PubMed] [Google Scholar]
- 55.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, et al. Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature. 2010;465:231–235. doi: 10.1038/nature08964. [DOI] [PubMed] [Google Scholar]
- 57.Metz M, Gassmann M, Fakler B, Schaeren-Wiemers N, Bettler B. Distribution of the auxiliary GABAB receptor subunits KCTD8, 12, 12b, and 16 in the mouse brain. J Comp Neurol. 2011;519:1435–1454. doi: 10.1002/cne.22610. [DOI] [PubMed] [Google Scholar]
- 58.Seddik R, Jungblut SP, Silander OK, Rajalu M, Fritzius T, Besseyrias V, Jacquier V, Fakler B, Gassmann M, Bettler B. Opposite effects of KCTD subunit domains on GABA(B) receptor-mediated desensitization. J Biol Chem. 2012;287:39869–39877. doi: 10.1074/jbc.M112.412767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivankova K, Turecek R, Fritzius T, Seddik R, Prezeau L, Comps-Agrar L, Pin JP, Fakler B, Besseyrias V, Gassmann M, et al. Upregulation of GABAB Receptor Signaling by Constitutive Assembly with the K+ Channel Tetramerization Domain-containing Protein 12 (KCTD12) J Biol Chem. 2013 doi: 10.1074/jbc.M113.476770. [DOI] [PMC free article] [PubMed] [Google Scholar]