Abstract
The superficial layers of the human vaginal epithelium, which form an interface between host and environment, are comprised of dead flattened cells that have undergone a terminal cell differentiation program called cornification. This entails extrusion of nuclei and intercellular organelles, and the depletion of functional DNA and RNA precluding the synthesis of new proteins. As a consequence, the terminally differentiated cells do not maintain robust intercellular junctions and have a diminished capacity to actively respond to microbial exposure, yet the vaginal stratum corneum (SC) mounts an effective defense against invasive microbial infections. The vaginal SC in reproductive aged women is comprised of loosely connected glycogen-filled cells which are permeable to bacterial and viral microbes as well as molecular and cellular mediators of immune defense. We propose here that the vaginal SC provides a unique microenvironment that maintains vaginal health by fostering endogenous lactobacillii and retaining critical mediators of acquired and innate immunity. A better understanding of the molecular and physicochemical properties of the vaginal SC could promote the design of more effective topical drugs and microbicides.
The human vaginal stratified squamous epithelium has a large surface area (up to 360 cm2) 1,2, and is the first mucosal surface contacted by sexually transmitted pathogens. Yet the healthy vaginal epithelium usually thwarts invasive infections. In contrast, the endocervix, a simple columnar epithelium which presents a much smaller surface area, is a frequent site for infections by a number of sexually transmitted pathogens including Chlamydia trachomatis, Neisseria gonorrhea, Mycoplasma genitalium and oncogenic strains of human papilloma virus 3–6. In this article we review what is known about the distinctive characteristics of the apical layers of the vaginal epithelium, which provide the interface between host and environment and hold the key to its resistance against infection. This layer is comprised of “dead” cornified cells that lack nuclei, intracellular organelles and functional DNA and RNA, and therefore do not express de novo proteins involved in pathogen recognition and defense. A unique feature of the apical layers of the vaginal epithelium is their permeability to microbes as well as cellular and molecular mediators of immune defense. This loosely attached layer of cells creates a microenvironment that plays an important role in fostering endogenous vaginal flora while deterring invasive microbial species. We hypothesize that topically applied microbicides may also infiltrate the vaginal superficial layers to serve a protective function along the perimeter of the vaginal cavity.
Structure of the Vaginal Epithelium
The healthy vaginal mucosa of reproductive aged women is comprised of a multilayered stratified squamous epithelium that rests on a lamina propria. The epithelium undergoes differentiation and contains several distinct layers or “strata”: the mitotically active basal layer (“stratum basale”), the superbasal layer, and a superficial layer of flattened cornified cells (“stratum corneum”) 7,8. Little is known about the differentiation of the vaginal epithelium, but much can be inferred from research on the epidermis (skin), a related stratified squamous epithelium that undergoes a similar differentiation process. The term stratum corneum (SC), from Latin meaning “horny layer”, is often used to describe the superficial layers of the epidermis that consist of keratinized enucleated, dead and flattened cells 9. Cornified epithelia are categorized as “soft” (eg. epidermis) and “hard” (eg. plate of fingernail), depending on the degree and type of keratinization 7. Cornification of skin is a terminal differentiation process that entails a specialized program of cell death; molecular pathways are activated to protect suprabasal keratinocytes from premature apoptosis and necrosis which could trigger the release of damage-associated molecular pattern molecules, inflammation and disturbance of the differentiation process 10. Recent reports suggest that epidermal keratinocytes use NF-kB signaling and alpha catenin to protect suprabasal cells from undergoing apoptosis 11.
During cornification, keratinocytes lose their nuclei and intracellular organelles (mitochondria, endoplasmic reticulii, Golgi) resulting in three hallmarks of keratinocyte terminal differentiation: 1) the molecular machinery that constitutes the cell’s ability to respond to stimuli from the environment is degraded, 2) the production of energy is stopped by the removal of mitochondria, and 3) the nucleus is dismantled and DNA and RNA are destroyed precluding the synthesis of new proteins 11. In keratinizing epithelia, caspase 14 is involved in the processing of filaggrin, a major keratin-binding peptide, and transglutaminases aid in the bundling of keratin intermediate filaments and formation of the cornified envelope 12. Keratins and associated peptides constitute 80–90% of the protein mass of the epidermis 13. Keratinocytes also synthesize lamellar bodies which contain a complex mixture of lipids (ceramides, cholesterol, free fatty acids), antimicrobial peptides (beta-defensins, cathelicidins) and enzymes 14. Terminally differentiated keratinocytes extrude the contents of these lamellar bodies to form a specialized intercellular lipid envelope that strengthens the dead cell layer, provides innate immune defense, and regulates the exchange of moisture. The cells of the epidermal cornified layer do not actively synthesize new proteins and lack tight junctions on their surface; tissue integrity is maintained by modified cell junctions called corneodesmosomes that are cleaved when the apical cells undergo desquamation and are sloughed 11, and the lipid envelope, which has been described as intercellular “mortar” 14.
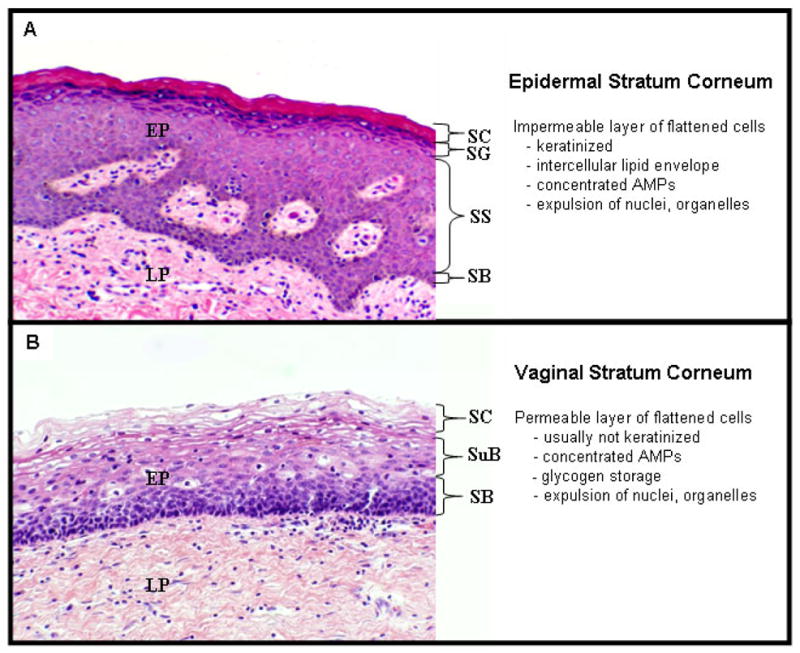
Much less is known about the differentiation of vaginal epithelial cells, but since the vaginal mucosa is also a stratified squamous epithelium that structurally resembles the epidermis in many respects, we infer that vaginal and epidermal differentiation have many features in common. Like the epidermis, the human vaginal epithelium contains basal and suprabasal layers and undergoes terminal differentiation to form a cornified envelope comprised of a flattened layer of specialized cells (Figure 1). As vaginal epithelial cells differentiate, many lose their nuclei and cytoplasmic organelles (Figure 2). Vaginal basal and suprabasal epithelial cells express a number of cytokeratins (K1, 4, 5 and 13), transglutaminases-1 and -3, caspase 14 and filaggrin (A. Islam, personal communication) but do not usually form the prominent keratin bundles that characterize keratinizing epidermal epithelia 15. Instead, the vaginal SC in reproductive-aged women contains large cytoplasmic stores of glycogen (Figure 2).
Figure 1. Comparison of morphology of epidermal vs. vaginal epithelia.
A) Structure of normal human vulvar epidermis. SB = stratum basalis; SS = stratum spinosum, SG = stratum granulosa, SC = stratum corneum.
B) Structure of normal human vaginal epithelium. (SB= stratum basalis, SuB = suprabasal layer; SC= stratum corneum).
Both specimens were collected fresh after surgery, fixed in formalin, mounted in paraffin, sectioned at 6 um and stained with hematoxylin and eosin.
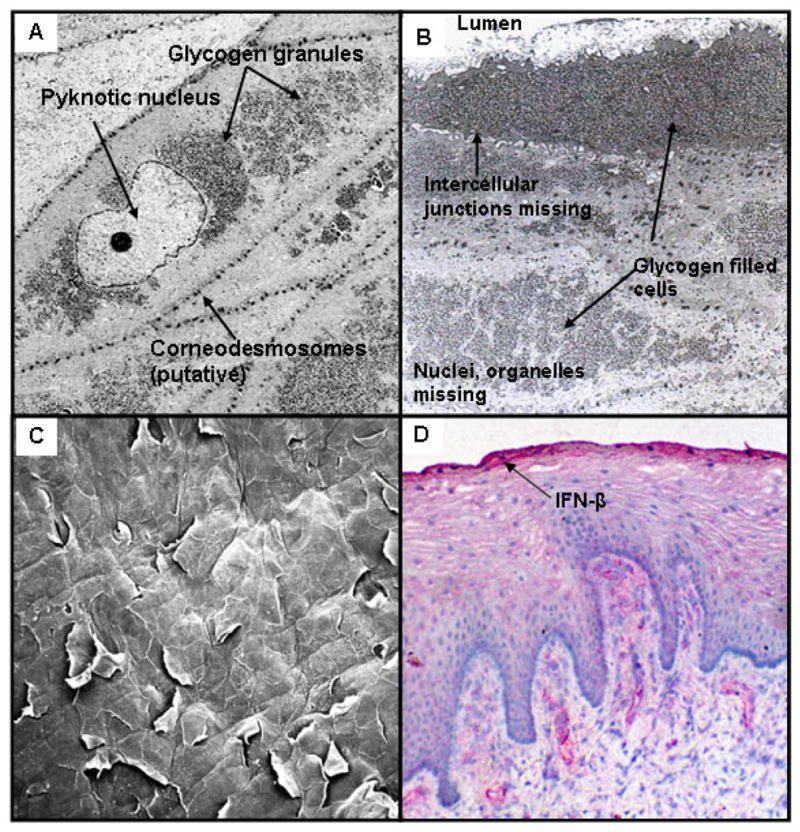
Figure 2. Characteristics of Human Vaginal Stratum Corneum.
A) Transmission electron micrograph showing vaginal epithelial cells transitioning into the stratum corneum. Note pyknotic nucleus, corneodesmosomes and glycogen granules.
B) Transmission electron micrograph showing fully differentiated epithelial cells at the apical surface of the vaginal straum corneum. These cells are devoid of nuclei and cytoplasmic organelles, and are filled with glycogen. The cells are loosely attached with remnants of intracellular junctions.
C) Scanning electron micrograph of the surface of the vaginal epithelium showing exfoliation of flattened corneocytes.
D) Interferon-β is concentrated in the stratum corneum of the human vaginal epithelium, demonstrated by immunohistology.
Like the epidermal SC, cells in the apical layers of the vaginal and ectocervical epithelia are not held together by tight junctions16. Electron micrographs of the human vaginal epithelium reveal intercellular structures resembling corneodesmesomes in the transitional region immediately below the SC, but only remnants of intercellular junctions remain in the SC (Figure 2). Electron microscopy studies have also shown intercellular stacked lipid lamellae in human vaginal mucosa 17, but they do not form an impermeable intercellular lipid envelope as they do in the epidermis. As a consequence, whereas the epidermal SC is relatively impermeable to water, the vaginal SC is permeable to water and soluble proteins 16,18,19. A 3H-thymidine uptake study showed that the minimum transit time of labelled keratinocytes from the basal layer of the human vaginal epithelium to the uppermost layer was 96 hours 20; since this epithelium is approximately 28 cell layers thick 21, this study provides evidence that one cell layer is lost from the vaginal epithelium approximately every 4 hours. Although unknown, the rate of desquamation may be affected by intercourse, use of vaginal products, and hormonal status. Exfoliation is an effective way to eliminate pathogens that have attached to the vaginal surface or that bind to sloughed “decoy” cells. In addition, the exfoliated cells disintegrate and release their contents into the vaginal lumen; a major component is glycogen which serves as a substrate for the resident lactobacilli that produce lactic acid and maintain an acidic pH 22,23.
The structure of the vaginal epithelium changes throughout the lifespan of women, and is also affected by hormonal and environmental conditions. In young girls before puberty the vaginal epithelium is thin and comprised of only basal and parabasal layers 21. During the reproductive years, the vaginal epithelium thickens and develops a distinct cornified layer. Hormonal influences have slight effects on the thickness of the vaginal epithelium 21,25,26, but affect the glycogen stores as the synthesis of glycogen is influenced by estrogen levels 27. After menopause the vaginal epithelium thins, glycogen stores diminish and the SC shows variable degrees of keratinization 17,28,29. Keratinization of the vaginal SC may also occur as a result of trauma, for example in cases of uterine prolapse 29.
The Vaginal Stratum Corneum Microenvironment
As described above, the vaginal SC is a specialized structure with features of the epidermal SC (loosely-connected flattened cells devoid of nuclei and intracellular organelles and with few intercellular adhesions), and specialized features (glycogen deposits, rare keratinization). Furthermore, the vaginal SC does not contain the dense lipid envelope that regulates the flow of moisture across the epidermal epithelium. Because the vaginal SC does not have robust intercellular junctions, and does not usually keratinize or form a complete lipid envelope, it is penetrable by microbes and cellular and molecular mediators of the immune system. For this reason we hypothesize that this region comprises a unique microenvironment that fosters the growth of endogenous vaginal flora while deterring invasive infections through exfoliation and retention of mediators of acquired and innate immune defense.
A variety of immune cells have been described in the human vaginal epithelium, but few have been observed within the vaginal SC layer 30. We and others have observed that leukocytes can penetrate and traverse the SC following placement in the vaginal lumen (apical-to-basal infiltration), suggesting a mechanism for cell-associated HIV transmission via infected cells deposited in semen 31–33. Leukocytes may freely move through this cell layer because the epithelial cells lack e-cadherin 16 and possibly other adhesive molecules that retain and integrate immune cells in mucosal epithelia 34,35. It is currently unknown to what extent leukocytes actively perform immunosurveillance of this cell layer.
It is apparent that a primary role of the vaginal SC is the maintenance of endogenous lactobacilli that are nourished by its glycogen stores. Lactobaccilli in turn make lactic acid that lowers the vaginal pH to create an inhospitable environment for many pathogenic bacterial and viral species 36. The pH within the SC is unknown, but may be very low due to the concentration and activity of lactobacilli at this site. The vaginal SC can also foster biofilm-associated organisms that overgrow in the case of bacterial vaginosis 37. It is probable that the vaginal SC contains mucins and other sialoglycans, known to be enriched on mucosal surfaces, and that these play an important role in the attachment and retention of microbes and molecular mediators at this site 38. Amazingly, little information is available about the specific molecular biology and physicochemical properties of this unique microenvironment, which holds the key to vaginal health.
Most studies on gene expression in vaginal epithelia have been conducted on cell lines that have not undergone differentiation to form a SC. These vaginal epithelial cells can synthesize a number of antimicrobial peptides (AMPs) including lactoferrin, SLPI, elafin, human defensin-5, human beta defensins-1 and -2, and the cathelicidin hCAP-8 39–41, and it is possible that such innate defense molecules are concentrated in the intracellular and/or extracellular matrix of the vaginal SC as they are in the epidermis. Immunohistology studies conducted by our laboratory indicate that the vaginal SC retains antimicrobial peptides (Figure 2D) and immunoglobulins16, which may provide critical immune defense at the vaginal surface. Little is known about the penetration, retention and activity of vaginal microbicides in the vaginal SC; designing vaginal microbicides that penetrate and are retained in active form in the vaginal SC could significantly improve the efficacy of this approach.
Conclusions
HIV-1 and other sexually transmitted pathogens are rarely transmitted across the healthy vaginal epithelium despite their ability to enter the apical cornified layer. This is likely due to a number of protective mechanisms: 1) frequent exfoliation of the superficial SC cell layers, 2) maintenance of a low pH by lactobacilli that are nurtured by glycogen stores in the vaginal SC, 3) interaction with mediators of innate and acquired immunity that are retained in the vaginal SC layer. Much remains to be determined about the microenvironment of the vaginal SC and its role in vaginal health and disease. A better understanding of the molecular and physicochemical properties of the vaginal SC could promote the design of more effective topical drugs and microbicides.
Acknowledgments
This research was supported by the National Institutes of Health (U19 AI096398). The authors thank Drs. Chris Crum, Seyoum Ayehunie and Richard Cone for valuable input.
Bibliography
- 1.Pendergrass PB, Belovicz MW, Reeves CA. Surface Area of the Human Vagina as Measured from Vinyl Polysiloxane Casts. Gynecol Obstet Invest. 2003;55:110–113. doi: 10.1159/000070184. [DOI] [PubMed] [Google Scholar]
- 2.Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA. Acid Production by Vaginal Flora In Vitro Is Consistent with the Rate and Extent of Vaginal Acidification. Infect Immun. 1999;67:5170–5175. doi: 10.1128/iai.67.10.5170-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra M, Ibana JS, Poretta C, Ma L, Myers L, Taylor SN, Greene S, Smith B, Hagensee M, Martin DH, Quayle AJ. A distinct cellular profile is seen in the human endocervix during Chlamydia trachomatis infection. Am J Reprod Immunol 2008. 2008;60:415–425. doi: 10.1111/j.1600-0897.2008.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGowin CL, Anderson-Smits C. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7(5):e1001324. doi: 10.1371/journal.ppat.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. The Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 7.Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–559. doi: 10.1111/j.1469-7580.2009.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asscher AW, DeBoer CH, Turner CJ. Cornification of the human vaginal epithelium. J Anat. 1956;90:547–552. [PMC free article] [PubMed] [Google Scholar]
- 9.Wickett RR, Visscher MO. Structure and function of the epidermal barrier. Am J Infect Control. 2006;34:S98–S110. [Google Scholar]
- 10.Lippens S, Denecker G, Ovaere P, Vandenabeele P, Declercq W. Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ. 2005;12 (Suppl 2):1497–1508. doi: 10.1038/sj.cdd.4401722. [DOI] [PubMed] [Google Scholar]
- 11.Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochim Biophys Acta BBA - Mol Cell Res. 2013;1833:3471–3480. doi: 10.1016/j.bbamcr.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Denecker G, Ovaere P, Vandenabeele P, Declercq W. Caspase-14 reveals its secrets. J Cell Biol. 2008;180:451–458. doi: 10.1083/jcb.200709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, Wolf R. Structure and function of the epidermis related to barrier properties. Clin Dermatol. 2012;30:257–262. doi: 10.1016/j.clindermatol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int J Pharm. 2012;435:3–9. doi: 10.1016/j.ijpharm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85:97–104. doi: 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson IO, van der Bijl P, van Wyk CW, van Eyk AD. A comparative light-microscopic, electron-microscopic and chemical study of human vaginal and buccal epithelium. Arch Oral Biol. 2001;46:1091–1098. doi: 10.1016/s0003-9969(01)00082-6. [DOI] [PubMed] [Google Scholar]
- 18.Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, Hope TJ. Defining the Interaction of HIV-1 with the Mucosal Barriers of the Female Reproductive Tract. J Virol. 2013;87:11388–11400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macht DI. On the Absorption of Drugs and Poisons Through the Vagina. J Pharmacol Exp Ther. 1918;10:509–522. [Google Scholar]
- 20.Averette HE, Weinstein GD, Frost P. Autoradiographic analysis of cell proliferation kinetics in human genital tissues. I. Normal cervix and vagina. Am J Obstet Gynecol. 1970;108:8–17. doi: 10.1016/0002-9378(70)90195-x. [DOI] [PubMed] [Google Scholar]
- 21.Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AE, Eschenbach DA. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol. 2000;183:967–973. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- 22.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high d/l lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16:1809–1813. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 23.Wylie JG, Henderson A. Identity and Glycogen-Fermenting Ability of Lactobacilli Isolated from the Vagina of Pregnant Women. J Med Microbiol. 1969;2:363–366. doi: 10.1099/00222615-2-3-363. [DOI] [PubMed] [Google Scholar]
- 24.Colvin CW, Abdullatif H. Anatomy of female puberty: The clinical relevance of developmental changes in the reproductive system. Clin Anat. 2013;26:115–129. doi: 10.1002/ca.22164. [DOI] [PubMed] [Google Scholar]
- 25.Eschenbach DA, Patton DL, Meier A, Thwin SS, Aura J, Stapleton A, Hooton TM. Effects of oral contraceptive pill use on vaginal flora and vaginal epithelium. Contraception. 2000;62:107–112. doi: 10.1016/s0010-7824(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 26.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96:431–439. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 27.Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet. 2006;273:195–202. doi: 10.1007/s00404-005-0079-x. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson K, Risberg B, Heimer G. The vaginal epithelium in the postmenopause--cytology, histology and pH as methods of assessment. Maturitas. 1995;21:51–56. doi: 10.1016/0378-5122(94)00863-3. [DOI] [PubMed] [Google Scholar]
- 29.Zaino RJ, Nucci M, Kurman RJ. Diseases of the Vagina. In: Tract Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s Pathol Female Genit. Springer US; 2011. pp. 105–154. [Google Scholar]
- 30.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 31.Sallé B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. Infection of Macaques after Vaginal Exposure to Cell-Associated Simian Immunodeficiency Virus. J Infect Dis. 2010;202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 32.Kaizu M, Weiler AM, Weisgrau KL, Vielhuber KA, May G, Piaskowski SM, Furlott J, Maness NJ, Friedrich TC, Loffredo JT, Usborne A, Rakasz EG. Repeated Intravaginal Inoculation with Cell-Associated Simian Immunodeficiency Virus Results in Persistent Infection of Nonhuman Primates. J Infect Dis. 2006;194:912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DJ, Poiltch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting Trojan Horse leukocytes for HIV prevention. AIDS. 2010;24:163–187. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 35.Jenkinson SE, Whawell SA, Swales BM, Corps EM, Kilshaw PJ, Farthing PM. The αE(CD103)β7 integrin interacts with oral and skin keratinocytes in an E-cadherin-independent manner*. Immunology. 2011;132:188–196. doi: 10.1111/j.1365-2567.2010.03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aldunate M, Tyssen D, Johnson A, Zakir T, Sonza S, Moench T, Cone R, Tachedjian G. Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother. 2013;68:2015–2025. doi: 10.1093/jac/dkt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrazzo JM. Vaginal biofilms and bacterial vaginosis: of mice and women. J Infect Dis. 2013;207:1481–1483. doi: 10.1093/infdis/jit050. [DOI] [PubMed] [Google Scholar]
- 38.Lewis AL, Lewis WG. Host sialoglycans and bacterial sialidases: a mucosal perspective. Cell Microbiol. 2012;14:1174–1182. doi: 10.1111/j.1462-5822.2012.01807.x. [DOI] [PubMed] [Google Scholar]
- 40.Patel MV, Fahey JV, Rossoll RM, Wira CR. Innate Immunity in the Vagina (Part I): Estradiol Inhibits HBD2 and Elafin Secretion by Human Vaginal Epithelial Cells. Am J Reprod Immunol. 2013;69:463–474. doi: 10.1111/aji.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole AM, Cole AL. Antimicrobial Polypeptides are Key Anti-HIV-1 Effector Molecules of Cervicovaginal Host Defense. Am J Reprod Immunol. 2008;59:27–34. doi: 10.1111/j.1600-0897.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 42.Anderson DJ. Genitourinary immune defense. In: Holmes K, Sparling P, Stamm W, Piot P, Wasserheit J, Corey L, Cohen M, editors. Sexually transmitted diseases. 4. McGraw Hill Publ; 2008. pp. 271–288. [Google Scholar]


