Abstract
Background
Initiation criteria and pediatric antiretroviral treatment (ART) regimens have changed over the past few years in South Africa. We reported worse early virological outcomes associated with the use abacavir (ABC)-based regimens at one large site: here we expand this analysis to multiple sites in the IeDEA-Southern Africa collaboration.
Methods
Data for 9543 ART-naïve children <16 years at treatment initiation started on either stavudine/lamivudine (d4T/3TC) or ABC/3TC with efavirenz (EFV) or ritonavir-boosted lopinavir (LPV/r) treated at six clinics in Johannesburg and Cape Town, South Africa, were analysed with Chi-square tests and logistic regression to evaluate viral suppression at six and twelve months.
Results
Prevalence of viral suppression at six months in 2174 children started on a d4T-based LPV/r regimen was greater (70%) than among 438 children started on an ABC-based LPV/r regimen (54%, p<0.0001). Among 3189 children started on a d4T-based EFV regimen a higher proportion (86%) achieved suppression at six months compared to 391 children started on ABC-containing EFV regimens (78%, p<0.0001). Relative benefit of d4T vs. ABC on six month suppression remained in multivariate analysis after adjustment for pre-treatment characteristics, cohort and year of program (LPV/r – OR 0.57 [CI: 0.46–0.72]; EFV – OR 0.46 [CI: 0.32–0.65]).
Conclusion
This expanded analysis is consistent with our previous report of worse virological outcomes after ABC was introduced as part of first-line ART in South Africa. Whether due to the drug itself or coincident with other changes over time, continued monitoring and analyses must clarify causes and prevent suboptimal long term outcomes.
Keywords: HIV, children, Abacavir, first-line ART
South African paediatric antiretroviral treatment (ART) guidelines have been adapted in terms of initiation criteria and recommended regimens in response to changes adopted by the World Health Organization (WHO).1–3 Abacavir (ABC) was incorporated into paediatric ART first-line regimens in April 2010 largely due to concerns around stavudine (d4T) toxicity.4,5 Under current guidelines, ABC is combined with lamivudine (3TC) as a preferred nucleoside reverse transcriptase inhibitor (NRTI) backbone option.3,6 Concurrent with the introduction of ABC, the South African prevention of mother to child transmission (PMTCT) guidelines included a longer duration of antenatal zidovudine administration and extended postnatal nevirapine prophylaxis for HIV-exposed infants.7
We recently reported poor early virological efficacy and durability of the ABC- compared to d4T-based regimens from a large paediatric HIV clinic in Johannesburg, South Africa.8 Here we investigate whether this phenomenon is more widespread by including multiple sites in the International epidemiologic Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) collaboration and whether poor virological performance in recent years may be attributed to the national programmatic switch to ABC-containing first line regimens.
METHODS
Characteristics of the IeDEA-SA collaboration (www.iedea-sa.org) participating sites have been previously described.9 Each site collects and enters prospective data into electronic databases which are centrally collated at annual intervals extracting a standard set of routine data for analysis. Eight South African sites affiliated to this collaboration contributed data including the Rahima Moosa Mother and Child Hospital (RMMCH) which previously reported poor performance of ABC-based regimens.8
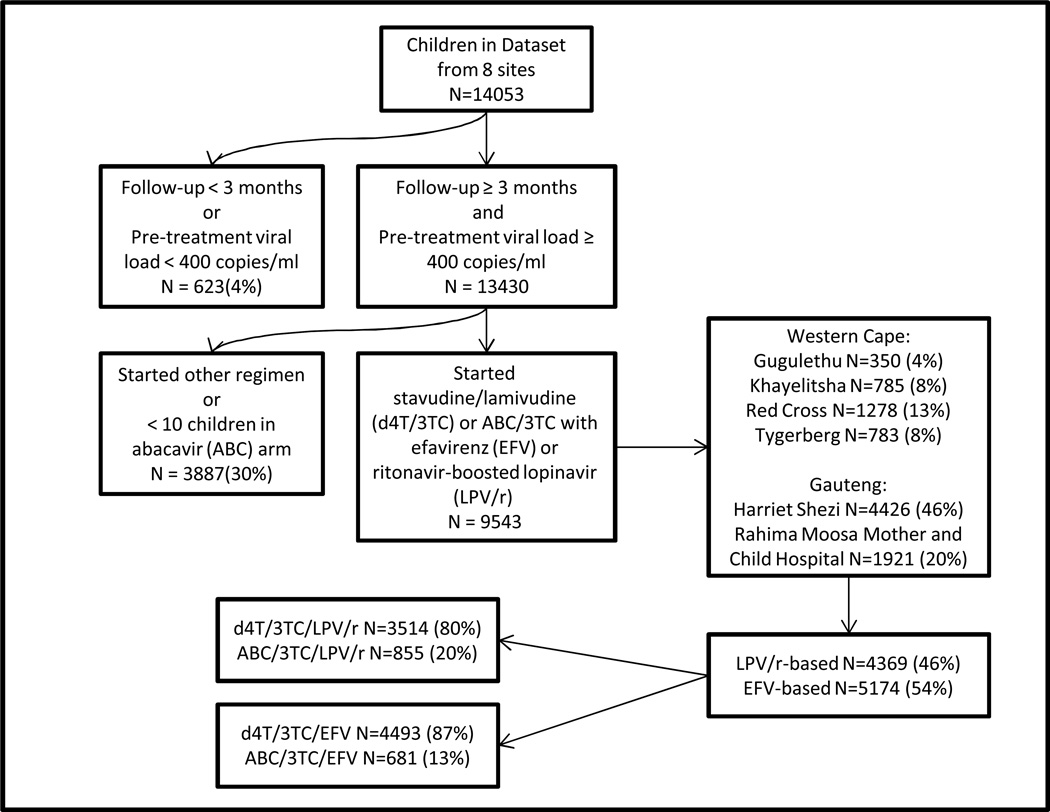
Data of ART-naïve children (<16 years at ART start) initiating d4T/3TC or ABC/3TC with efavirenz (EFV) or ritonavir-boosted lopinavir (LPV/r) who commenced ART three months before the last visit recorded for that cohort were included. Two sites with fewer than 10 children on any one ABC arm were excluded (Figure 1). Data from two Johannesburg and four Cape Town sites were included with a data window from the 17th August 1998 to the 5th of April 2013. All sites were related to academic centres and situated within these two large metropolitan areas. Children with a pre-treatment VL of 0–400 copies/ml were excluded and assumed not to have been ART-naïve. Available pre-treatment characteristics included age at ART initiation, year of ART initiation, weight-for-age z-score (WAZ), height for age z-score (HAZ), CD4 percentage and absolute count and VL (log10 copies/ml) value. Pre-treatment WAZ and HAZ included measurements from one month before to two weeks after, while pre-treatment CD4 and VL values were from six months before to one week after ART initiation, in cases with multiple measurements, the value closest to ART initiation was used.
Figure 1.
Study Population.
Virological outcomes were evaluated at six and twelve months using results of samples collected within a window between three to nine months and nine to fifteen months after treatment initiation respectively. Virological outcomes included time since treatment initiation at VL measurement, actual VL log10 value and suppression to <400 copies/ml and <50 copies/ml at these two time windows compared for children on ABC vs. d4T. If more than one result was available the one nearest the middle of the window was chosen. Chi-square tests were used to compare proportions suppressed while VL log10 values were compared across groups using t-tests if normally-distributed or Wilcoxon tests if not normally-distributed. Year of program was defined as the calendar year ART was initiated and was plotted against suppression rates stratified by regimens for all children and assessed using the Cochran-Armitage test for trend. Associations between first-line regimen (ABC/d4T) and not attaining VL<400 copies/ml at six and twelve months were examined using logistic regression adjusted a priori for gender, age at initiation, pre-treatment WAZ, CD4 percentage, pre-treatment VL (greater or lower than 100,000 copies/ml), year of ART initiation and cohort. Missing data for WAZ, VL log10, CD4 and six month suppression were imputed using multiple imputation.10 Results were combined with Rubin’s rules and are presented as odds ratios with 95% confidence intervals.11 A sensitivity analysis was performed using a restricted two year time window around ABC introduction (1st April 2009 – 31st March 2011) and the interaction between cohort and d4T/ABC was investigated.
Each site has institutional ethical approval to contribute data to IeDEA analyses. Data were analysed using Microsoft Excel, SAS (Version 9.3, SAS Institute Inc., Cary, NC, USA) and STATA 12.0 (College Station, Texas, USA) software.
RESULTS
Figure 1 shows the total of 9543 ART-naïve children <16 years included in the analyses. Two thirds of the final data set was from the two Johannesburg sites, contributing 59% of the data for children on LPV/r regimens but 73% of data for children on EFV regimens.
Table 1 outlines pre-treatment characteristics grouped by ABC/3TC vs. d4T/3TC for children on LPV/r and EFV separately. Differences are noted between the groups, particularly age at initiation; children on ABC/LPV/r were slightly younger than children having started d4T. In contrast, those on EFV were more recently initiated (on ABC) and older. Children started on ABC/3TC, with either EFV and LPV/r had higher pre-treatment WAZ, HAZ, CD4 absolute and percentage values but also marginally higher VL. Sites differed in proportions of children initiated on d4T compared to ABC for those initiating LPV/r (ranging from 78% on d4T at Harriet Shezi Clinic and Red Cross Children’s Hospital to 90% at Gugulethu - p=0.0002) while the distribution between d4T and ABC for children on EFV was more constant ranging from 84% to 90% on d4T. Overall, 20% initiated LPV/r with ABC, while only 13% of children initiated EFV with ABC (p<0.0001).
Table 1.
Pre-treatment characteristics and originating site of the study population stratified by starting regimen.
| Ritonavir-boosted lopinavir (LPV/r) based | Efavirenz (EFV) based | |||||||
|---|---|---|---|---|---|---|---|---|
| ABC/3TC | d4T/3TC | p | ABC/3TC | d4T/3TC | p | |||
| Pre-treatment Characteristics | N | 855 | 3514 | 681 | 4493 | |||
| Age at ART initiation in months | N median (IQR) | 855 7 (4;18) | 3514 10 (5;21) | 0.043 | 681 96 (63;129) | 4493 81 (55;112) | <0.0001 | |
| Male Gender | N (column %) | 427 (50) | 1741 (50) | 0.84 | 337 (49) | 2298 (51) | 0.42* | |
| Pre-treatment CD4 cells/mm3 | N median (IQR) | 574 766 (315;1349) | 2488 662 (319;1158) | 0.0006 | 517 294 (115;529) | 3195 294 (131;522) | 0.44 | |
| Pre-treatment CD4 % | N median (IQR) | 564 18.8 (12.2;27.1) | 2478 16 (10.2;23.0) | <0.0001 | 496 13.8 (6.9;20.2) | 3087 11.5 (6.3;16.3) | <0.0001 | |
| Weight for age Z-score | N median (IQR) | 641 −2.4 (−3.6;−1.0) | 2683 −2.5 (−3.9;−1.3) | 0.030 | 397 −1.3 (−2.1;−0.5) | 2889 −1.5 (−2.4;−0.7) | 0.019 | |
| Height for age Z-score | N median (IQR) | 508 −2.3 (−3.5;−1.2) | 2330 −2.7 (−3.9;−1.5) | 0.0005 | 478 −2.1 (−2.8;−1.3) | 3179 −2.2 (−3.0;−1.4) | 0.039 | |
| Pre-treatment Viral Load (VL) log10 | N median (IQR) | 522 6.0 (5.3;6.5) | 2249 5.8 (5.1;6.3) | 0.0004 | 447 5.1 (4.5;5.5) | 2878 5.0 (4.4;5.5) | 0.062 | |
| <100,000 copies/ml | N (column %) | 96 (18) | 468 (21) | 0.22 | 202 (45) | 1501 (52) | 0.0061* | |
| Site | ||||||||
| Gugulethu | N (row %) | 12 (10) | 109 (90) | 0.0002 | 36 (16) | 193 (84) | 0.32* | |
| Harriet Shezi | N (row %) | 375 (22) | 1360 (78) | 345 (13) | 2346 (87) | |||
| Khayelitsha | N (row %) | 55 (15) | 317 (85) | 55 (13) | 358 (87) | |||
| RMMCH | N (row %) | 141 (17) | 704 (83) | 157 (15) | 919 (85) | |||
| Red Cross | N (row %) | 175 (22) | 627 (78) | 58 (12) | 418 (88) | |||
| Tygerberg | N (row %) | 97 (20) | 397 (80) | 30 (10) | 259 (90) | |||
Abbreviations: Stavudine (d4T), lamivudine (3TC), abacavir (ABC), antiretroviral treatment (ART).
Chi-square tests used to compare proportions. All continuous variable comparisons were done using t-tests if normally-distributed or Wilcoxon tests if not normally-distributed. RMMCH: Rahima Moosa Mother and Child Hospital.
Table 2 shows the virological outcomes in the six and twelve month window for all children and then excluding data from RMMCH. A smaller proportion of children in the ABC groups reached the windows and if they reached the windows, fewer had VLs done compared to children on d4T. Within the group of children on ABC, uptake (i.e. reached window and had VL done) of testing at 6 and 12 months was similar (65% at six and 63% twelve months, p= 0.60 [LPV/r] and 67% and 52%, p=0.13 [EFV]); similarly uptake in children on d4T remained the same for six and twelve month testing (72% at six and 70% twelve months, p= 0.10 [LPV/r]; 75% at six and 74% at twelve months, p=0.31 [EFV]). A comparison in children reaching the six and twelve month follow-up windows was done comparing those who had VLs compared to those who did not have VLs. In both the LPV/r and EFV groups, among children who reached the VL windows, there were no clinically significant differences between children who had or did not have VL measurements.
Table 2.
Virological Outcomes in Children at 6 and 12 months after treatment initiation in children on ritonavir-boosted lopinavir (LPV/r) and efavirenz (EFV) comparing stavudine (d4T)-based to abacavir (ABC)-based treatment for all children (A) and children not from RMMCH (B).
| LPV/r based | EFV based | |||||||
|---|---|---|---|---|---|---|---|---|
| ABC/3TC | d4T/3TC | p | ABC/3TC | d4T/3TC | p | |||
| All Children with available data (A) | N | 855 | 3514 | 681 | 4493 | |||
| Follow-up duration | 9 (4–14) | 31 (12–51) | <0.0001 | 9 (5–14) | 40 (23–64) | <0.0001 | ||
| 3 to 9 month window | ||||||||
| Reached window§ | N (%) | 676 (79) | 3018 (86) | <0.0001 | 586 (84) | 4254 (94) | <0.0001 | |
| Viral load (VL) done if reached window* | N (%) | 438 (65) | 2174 (72) | 0.0002 | 391 (67) | 3189 (75) | <0.0001 | |
| VL log10 value | median (IQR) | 2.6 (1.6;3.8) | 2.1 (1.4;2.9) | <0.0001 | 1.7 (1.4;2.6) | 1.4 (1.4;2.1) | <0.0001 | |
| VL<400 in 6 month window | N (%) | 235 (54) | 1528 (70) | <0.0001 | 304 (78) | 2741 (86) | <0.0001 | |
| VL<50 in 6 month window | N (%) | 136 (31) | 956 (44) | <0.0001 | 208 (53) | 2110 (66) | <0.0001 | |
| 9 to 15 month window | ||||||||
| Reached window§ | N (%) | 405 (47) | 2725 (78) | <0.0001 | 327 (48) | 4057 (90) | <0.0001 | |
| VL done if reached window* | N (%) | 256 (63) | 1909 (70) | 0.0056 | 202 (62) | 3002 (74) | <0.0001 | |
| VL log10 value | median (IQR) | 2.6 (1.6;3.8) | 1.7 (1.4;2.6) | <0.0001 | 1.7 (1.4; 2.6) | 1.4 (1.4; 2.1) | 0.0002 | |
| VL<400 in 12 month window | N (%) | 158 (62) | 1437 (75) | <0.0001 | 155 (77) | 2535 (84) | 0.0038 | |
| VL<50 in 12 month window | N (%) | 104 (41) | 985 (52) | 0.0010 | 115 (57) | 1996 (66) | 0.0055 | |
| All Children excluding RMMCH (B) | N | 714 | 2810 | 524 | 3574 | |||
| 3 to 9 month window | ||||||||
| VL log10 value | median (IQR) | 2.5 (1.6;3.7) | 2.1 (1.4;2.9) | <0.0001 | 1.7 (1.6;2.3) | 1.4 (1.4;2.1) | 0.0051 | |
| VL<400 in 6 month window | N (% of vl done) | 199 (56) | 1196 (70) | <0.0001 | 254 (82) | 2161 (85) | 0.25 | |
| VL<50 in 6 month window | N (% of vl done) | 126 (35) | 726 (42) | 0.016 | 180 (58) | 1654 (65) | 0.022 | |
| 9 to 15 month window | ||||||||
| VL log10 value | median (IQR) | 2.3 (1.4;3.4) | 1.8 (1.4;2.6) | 0.0026 | 1.7 (1.4; 2.6) | 1.4 (1.4; 2.2) | 0.0016 | |
| VL<400 in 12 month window | N (% of vl done) | 144 (65) | 1118 (75) | 0.0014 | 126 (77) | 1990 (83) | 0.046 | |
| VL<50 in 12 month window | N (% of vl done) | 96 (43) | 728 (49) | 0.11 | 92 (56) | 1536 (64) | 0.042 | |
Abbreviation: Lamivudine (3TC), Rahima Moosa Mother and Child Hospital (RMMCH).
Children who did not reach the window were transferred out, lost to follow up or had died before the start of the window.
Measure of uptake of VL testing in each window. VL cut-off values of 400 and 50 are in copies/ml.
The VL log10 values (Table 2) were significantly lower in children on d4T at both the six and twelve month window in both LPV/r and EFV regimens. The proportions suppressed (400 and 50 copies/ml thresholds) were significantly lower in the ABC groups for the six and twelve month windows for both LPV/r and EFV regimens. When data excluding RMMCH are analysed, the VL values were still significantly lower in children on d4T. The proportions suppressed to <400 copies/ml were lower with ABC except for suppression to <400 copies/ml at the six month window for children on EFV-based treatment. Supplemental digital content 1 shows the virological outcomes for each individual site at six and twelve months stratified by regimen. This shows that the differences in six and twelve month virological outcomes between d4T and ABC regimens were strongest at RMMCH. The trend is present at all other sites but significant values are seen in three of the six sites.
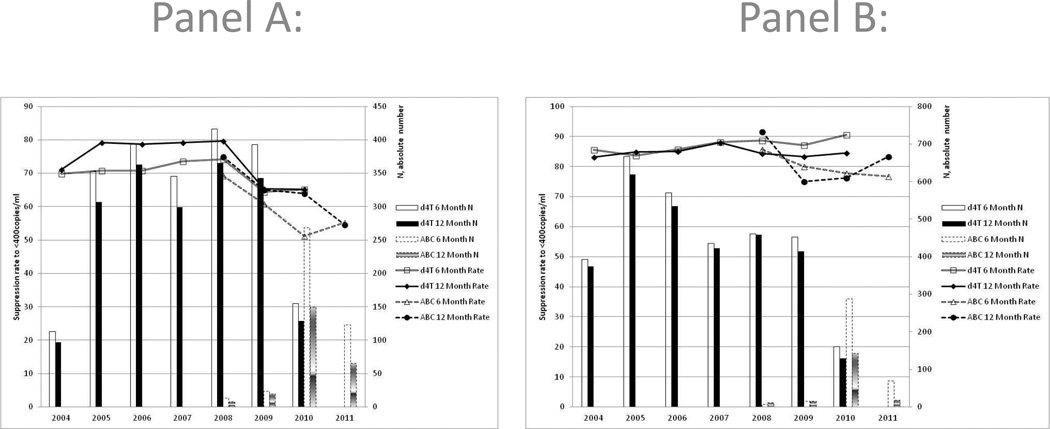
The effect of advancing program year is demonstrated in Figure 2. The LPV/r data (Panel A) show that there is a decline in the six and twelve month suppression rates of children on d4T which seems to be continued as the graph continues into the ABC era from 2010 onwards. The decline of suppression over program year is significant for both the six and twelve month rates (p<0.0001). When examining the six and twelve month rates for all children on EFV (Figure 2, panel B) the trend was not significant.
Figure 2.
Program Year effect on 6 and 12 month viral suppression rates stratified by abacavir (ABC) and stavudine (d4T) for children on ritonavir-boosted lopinavir (LPV/r – panel A) and efavirenz (EFV – panel B) respectively.
Table 3 shows the results of the adjusted logistic regression which indicates in both crude and adjusted analyses that there was a higher risk of failing to suppress associated with ABC-compared to d4T-containing regimens in both the LPV/r and EFV groups at six and twelve months but this is not significant at twelve months in the LPV/r group. Analyses included adjustment for cohort. ART initiation at <6 months of age had an independent beneficial effect on suppression by six months in the LPV/r group (OR: 0.70 [CI: 0.57–0.87], p=0.002). Another significant other effect on both six and twelve months suppression in both the LPV/r and EFV groups was a lower VL (<100,000 copies/ml) at ART initiation. The sensitivity analysis restricted to 2009–2011 shows similar effects; after adjustment the negative effect of ABC on suppression was significant at six months (LPV/r group – OR: 0.50 [CI: 0.34–0.73], p<0.0001; EFV group – OR: 0.38 [CI: 0.23–0.65], p=0.001) and twelve months (LPV/r group – OR: 0.48 [CI: 0.28–0.83], p=0.012; EFV group – OR: 0.48 [CI: 0.25–0.92], p=0.03). When examining for interaction between cohort and d4T/ABC effect, no interaction was found in the LPV/r group (at six months p=0.46 and twelve months p=0.73) while in the EFV group there was a possible interaction between effect of d4T/ABC by cohort (at six months, p=0.046 but not at twelve months, p=0.33).
Table 3.
Odds Ratios (OR) with 95% Wald Confidence Intervals (CI) for adjusted logistic regression of failure to reach a viral load (VL) <400 copies/ml at six and twelve months of treatment in children initiating ritonavir-boosted lopinavir (LPV/r)- or efavirenz (EFV)-based antiretroviral treatment (ART)
| LPV/r-based N=4369 | EFV-based N=5174 | |||||||
|---|---|---|---|---|---|---|---|---|
| Six Months | p | Twelve Months | p | Six Months | p | Twelve Months | p | |
| Unadjusted OR (95% CI): | ||||||||
| d4T-based vs. ABC-based | 0.49 (0.40–0.60) | <0.0001 | 0.52 (0.39–0.69) | 0.001 | 0.56 (0.43; 0.72) | <0.0001 | 0.55 (0.36–0.85) | 0.013 |
| Adjusted OR (95% CI): | ||||||||
| d4T-based vs. ABC-based | 0.57 (0.46–0.72) | <0.0001 | 0.69 (0.46–1.02) | 0.061 | 0.46 (0.32–0.65) | <0.0001 | 0.56 (0.36–0.86) | 0.012 |
| Male | 0.99 (0.86–1.15) | 0.942 | 1.05 (0.85–1.29) | 0.62 | 1.07 (0.90–1.27) | 0.415 | 1.05 (0.83–1.33) | 0.648 |
| Age at Initiation* | 0.70 (0.57–0.87) | 0.002 | 0.86 (0.57–1.30) | 0.411 | 0.99 (0.99–1.00) | 0.001 | 1.00 (0.99–1.00) | 0.631 |
| Pre-Treatment Weight-for-Age Z-score | 0.89 (0.83–0.96) | 0.007 | 0.93 (0.89–0.98) | 0.006 | 0.91 (0.85–0.98) | 0.009 | 0.93 (0.86–1.01) | 0.085 |
| Pre-Treatment CD4% | 0.98 (0.96–1.00) | 0.043 | 0.98 (0.97–0.99) | 0.003 | 0.98 (0.97–1.00) | 0.032 | 0.98 (0.96–0.99) | 0.002 |
| Pre-Treatment VL < 100,000 copies/ml | 0.65 (0.52–0.82) | <0.0001 | 0.69 (0.49–0.96) | 0.032 | 0.72 (0.58–0.88) | 0.002 | 0.79 (0.64–0.99) | 0.04 |
| Year of ART initiation | 1.06 (1.01–1.12) | 0.026 | 1.12 (1.04–1.20) | 0.004 | 0.97 (0.92–1.03) | 0.358 | 1.02 (0.95–1.10) | 0.555 |
Adjusted for cohort (individual cohort results not shown). Odds Ratios < 1 indicate factors improving viral suppression rate at six months.
Age at initiation categorized as < six months (reference) and > six months of age for LPV/r group and as continuous variable (months) for EFV group.
Abbreviations: stavudine (d4T) and abacavir (ABC)
DISCUSSION
Our study showed reduced virological suppression at both six and twelve months in children treated with ABC-based compared to d4T-based regimens with either LPV/r or EFV as the third drug in the regimen. As the change to ABC-based regimens was concurrent with a number of PMTCT and paediatric treatment protocol and program changes, it is difficult to ascribe the lower suppression rates to ABC. Nevertheless, the effect remains despite adjustment for pre-treatment characteristics, calendar time and cohort.
Both the RMMCH and this analysis were based on routinely-collected observational data.8 Careful consideration needs to be given before attributing causality of these trends and associations. Routine data provide useful sentinel surveillance monitoring to inform policy-makers and program managers and alert clinical researchers to potential problems. Although these data do not provide definitive evidence for the superiority of either of the two specific NRTI backbones, urgent attention is warranted to ensure that early paediatric virological outcomes are improved in South Africa. Randomised control trial evidence for the superiority of ABC/3TC as an NRTI backbone is drawn from the Paediatric European Network for the Treatment of AIDS (PENTA-5) trial.6 Children enrolled were older (median 5.4 years) than children on LPV/r in our data, the trial included asymptomatic children on dual therapy and use of nelfinavir rather than LPV/r or EFV as starting regimen. The mean VL at starting ART (5.1 log10 copies/ml) was lower than that reported in our LPV/r group and similar to that reported in our EFV group.
Pre-treatment characteristics have changed since inception of the South African ART program reflecting a trend towards earlier initiation of healthier children. These changes in pre-treatment characteristics are likely to be related to changing initiation guidelines. More favourable pre-treatment characteristics would lead one to expect better treatment outcomes in the ABC groups.
The low and declining uptake (65–75%) of VL testing at six and twelve months is a further concerning finding for the South African program. The proportion of children who had VL testing done differs between those on d4T and those on ABC. This may be related to the more recent introduction of ABC and therefore fewer data for children started on ABC. It may also be a selection bias if testing was done in children who appeared to be doing well although this would have been inconsistent with the VL testing guidelines in place. Nevertheless, there were no clinically significant differences in pre-treatment characteristics between children with and without VL tests done. This analysis demonstrates poorer early virological outcomes, but it is too early to assess the effect of this decline on mortality, clinical events, regimen switches and long term outcomes.
In both children treated with LPV/r and EFV, viral suppression rates were greater with d4T than with ABC. Adult data suggest that higher pre-treatment VL levels may predispose to poor performance of ABC-based therapy.12–15 Achieving suppression was more likely in children with lower pre-treatment VL levels in our cohort. The youngest children (< six months old) starting LPV/r based therapy had better outcomes than those starting older than six months. This may indicate the benefits of early treatment.16 Better growth recovery has also been observed amongst children starting ART before compared to after six months of life.17
The majority of reported data was from Johannesburg where isolated incidents of ABC stock-outs were reported during 2011. In some cases tablet formulations ran out, for example ABC tablets were not available and had to be substituted by syrup. Caregivers may have had to return more frequently than usual as only limited stock could be issued. The available data does not contain details of formulation changes or pharmacy only visits and the continuity of ABC or particular formulation supply can therefore not be included in the analysis but may have contributed to poorer performance or durability of ABC containing regimens.
While PMTCT coverage has increased with lower overall numbers of vertically-infected infants, there may be a reversal of the in-utero vs. intrapartum ratio of infection emerging in HIV-infected infants who were exposed to perinatal antiretroviral prophylaxis.18 A larger proportion of intrauterine-acquired infection may contribute to worse clinical outcomes due to infection occurring in the fetus when the immune system is very immature,19 but the effect on virological suppression is not clear. Improved coverage of PMTCT and ART for adults combined with expanding use of a wider range of antiretrovirals for PMTCT may have led to a larger proportion of infected children with primary antiretroviral drug resistance both selected and transmitted.20 With transmission of M184V there may be reduced activity of ABC.21 This could also contribute to treatment outcomes, but requires further study.
Data suggesting ABC levels are reduced by 32% in the presence of LPV/r are further cause for concern when interpreting our results.22 Children, especially infants, who receive LPV/r based treatment tend to have higher pre-treatment VL values. The change to ABC was a clear switch of protocol but switches to generic versions of d4T, 3TC or EFV cannot be accounted for and cannot be excluded as a cause for the problem if drug quality was inferior. Similarly quality of LPV/r formulations was assumed to have remained constant. Such assumptions may be problematic given the size of the South African epidemic and the quantities of medications that have to be ordered, shipped, redistributed, checked and dispensed on a regular basis and in correct conditions (especially with need for cold-chain for LPV/r syrup). The data available for analysis does not include exact dosing or formulation, neither was there a consistent adherence measure across sites. These potentially confounders were not controlled for. Further pharmacological studies investigating these factors in the large South African program are appropriate.
Limitations of this analysis include shorter follow-up time for children on ABC-based regimens. Some sites still have too few children who started ABC-based regimens for meaningful analysis. Significantly fewer children have results available at both the six and twelve month windows in the ABC group compared to the d4T groups. Details of exact formulations issued and month to month drug supply are not available which would have been useful to quantify whether ABC supply issues were a factor.
This analysis (which includes the site originally reporting a concern about ABC performance) is consistent with the prior observation of worse outcomes in children treated with ABC-containing regimens as recommended in the more recent guidelines. Whether this is due to the ABC-based regimen per se or other factors in more recent time cannot be distinguished with these data. Continued evaluation of the South African program and early paediatric outcomes is required as thousands of children still initiate ART annually. There is enough evidence of possible poorer virologic efficacy of ABC to warrant ongoing careful monitoring, analyses in other settings and pharmacological studies; a randomised control trial in infants and young children in Africa to determine best NRTI options may be required. There are few NRTI options for children in resource limited settings, especially with moves away from d4T and didanosine. Zidovudine may be one option but may complicate matters in malaria endemic areas. Another alternative may be to consider d4T or AZT initially and then switch to ABC after suppression is reached but switch protocols add significant complexity to national protocols. While more recent data are scrutinized, VL monitoring should continue, quality control of drugs should be ensured, strong adherence messaging should continue and if trends persist, guideline review may be required.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the National Institutes of Health (National Institute of Allergy and Infectious Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development - grant number U01AI069924 - PI: Egger and Davies). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All the children whose data were used in this analysis, as well as their caregivers are gratefully acknowledged. We appreciate the efforts of all site and data centre staff who contribute to the improvement of cohort data quality in the collaboration as well as to the South African Department of Health that strives to improve the lives of children affected by HIV.
Footnotes
Conflict of interest:
The authors have no conflict of interest to disclose.
REFERENCES
- 1.National Department of Health. Pretoria: National Department of Health; 2008. Guidelines for the Management of HIV in Children - 2nd Edition 2008. 2008 rev. ed. [Google Scholar]
- 2.National Department of Health. Pretoria: National Department of Health; 2010. Guidelines for the Management of HIV in Children - 2nd Edition 2010. 2010 rev. ed. [Google Scholar]
- 3.World Health Organization. Geneva: World Health Organization; 2010. Antiretroviral therapy for HIV infection in infants and children: towards universal access - 2010 revision. 2010 rev. ed. [PubMed] [Google Scholar]
- 4.Van Dyke RB, Wang L, Williams PL. Toxicities associated with dual nucleoside reverse-transcriptase inhibitor regimens in HIV-infected children. J Infect Dis. 2008;198:1599–1608. doi: 10.1086/593022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Innes S, Cotton MF, Haubrich R, et al. High prevalence of lipoatrophy in pre-pubertal South African children on antiretroviral therapy: a cross-sectional study. BMC Pediatr. 2012;12:183. doi: 10.1186/1471-2431-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green H, Gibb DM, Walker AS, et al. Lamivudine/abacavir maintains virological superiority over zidovudine/lamivudine and zidovudine/abacavir beyond 5 years in children. AIDS. 2007;21:947–955. doi: 10.1097/QAD.0b013e3280e087e7. [DOI] [PubMed] [Google Scholar]
- 7.National Department of Health. Pretoria: National Department of Health; 2008. Policy and Guidelines for the Implementation of the PMTCT Program. 2008 ed. [Google Scholar]
- 8.Technau KG, Lazarus E, Kuhn L, et al. Poor Early Virologic Performance and Durability of Abacavir-based First-line Regimens for HIV-infected Children. Pediatr Infect Dis J. 2013;32:851–855. doi: 10.1097/INF.0b013e31828c3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies MA, Keiser O, Technau K, et al. Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99:730–737. [PMC free article] [PubMed] [Google Scholar]
- 10.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DB. Multiple Imputation after 18+ Years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 12.Staszewski S, Keiser P, Montaner J, et al. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: A randomized equivalence trial. JAMA. 2001;285:1155–1163. doi: 10.1001/jama.285.9.1155. [DOI] [PubMed] [Google Scholar]
- 13.Sax PE, Tierney C, Collier AC, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204:1191–1201. doi: 10.1093/infdis/jir505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar PN, Salvato P, Lamarca A, et al. A randomized, controlled trial of initial anti-retroviral therapy with abacavir/lamivudine/zidovudine twice-daily compared to atazanavir once-daily with lamivudine/zidovudine twice-daily in HIV-infected patients over 48 weeks (ESS100327, the ACTION Study) AIDS Res Ther. 2009;6:3. doi: 10.1186/1742-6405-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill A, Sawyer W. Effects of nucleoside reverse transcriptase inhibitor backbone on the efficacy of first-line boosted highly active antiretroviral therapy based on protease inhibitors: meta-regression analysis of 12 clinical trials in 5168 patients. HIV Med. 2009;10:527–535. doi: 10.1111/j.1468-1293.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- 16.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiau S, Arpadi S, Strehlau R, et al. Initiation of Antiretroviral Therapy Before 6 Months of Age is Associated with Faster Growth Recovery in South African Children Perinatally Infected with Human Immunodeficiency Virus. J Pediatr. 2013;162:1138–1145. doi: 10.1016/j.jpeds.2012.11.025. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilian RR, Kalk E, Bhowan K, et al. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J Clin Microbiol. 2012;50:2373–2377. doi: 10.1128/JCM.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mphatswe W, Blanckenberg N, Tudor-Williams G, et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS. 2007;21:1253–1261. doi: 10.1097/QAD.0b013e3281a3bec2. [DOI] [PubMed] [Google Scholar]
- 20.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med. 2011;8:e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson VA, Calvez V, Gunthard HF, et al. 2011 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2011;19:156–164. [PMC free article] [PubMed] [Google Scholar]
- 22.Waters LJ, Moyle G, Bonora S, et al. Abacavir plasma pharmacokinetics in the absence and presence of atazanavir/ritonavir or lopinavir/ritonavir and vice versa in HIV-infected patients. Antivir Ther. 2007;12:825–830. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.