Abstract
The function of neocortical interneurons is still unclear, and, as often happens in biology, one may be able to draw functional insights from considering the structure. In this spirit we describe recent structural results and discuss some potential functional implications. In particular, many GABAergic interneurons appear to innervate nearby pyramidal neurons very densely and without any apparent specificity within their immediate vicinity, as if they were extending a “blanket of inhibition”, contacting them often in an overlapping fashion. While it is clear that subtypes of interneurons specifically target subcellular compartments of pyramidal cells, and they also target different layers selectively, they appear to treat all neighboring pyramidal cells the same and innervate them en masse. We explore the functional implications and temporal properties of dense, overlapping inhibition by four interneuron populations.
Introduction
Although functional inhibition was discovered more than half a century ago [1], there is still vigorous debate as to what exactly inhibitory neurons (INs) do. Even for the paradigmatical example of a clearly defined IN population, the chandelier cells, it is still unclear whether they are actually inhibitory [2] or excitatory [3], or whether their function could be a mixed one, depending on the state of the network [4].
To make this problem more complicated, GABAergic interneurons belong to many different subtypes, and their function is unlikely to be homogeneous or simple. However recent data suggest that some INs project densely to nearby principal cells (PCs). To gather information that could constrain hypotheses about IN function we review recent studies on network the connectivity of five IN populations that together encompass ~85% of all neocortical INs: 1) Parvalbumin containing INs (PVs) are virtually always fast spiking cells (FSs), with particularly rapid action potentials. Due to the high overlap between FS and PV groups [5–8], we use only the term PV for simplicity. 2) Chandelier cells (ChCs), also known as axo-axonic cells [9] [10,11]. 3) Neurogliaform cells (NGFCs) [12,13], 4) Somatostatin containing INs (SOMs) [14] and 5) vasoactive intestinal peptide containing INs (VIPs) [15]. Of these five populations PVs, NGFCs, SOMs and VIPs show virtually no overlap with each other [15–17], while some ChCs contain parvalbumin [11]. All studies reviewed here were performed in rats or mice.
Blanket inhibition
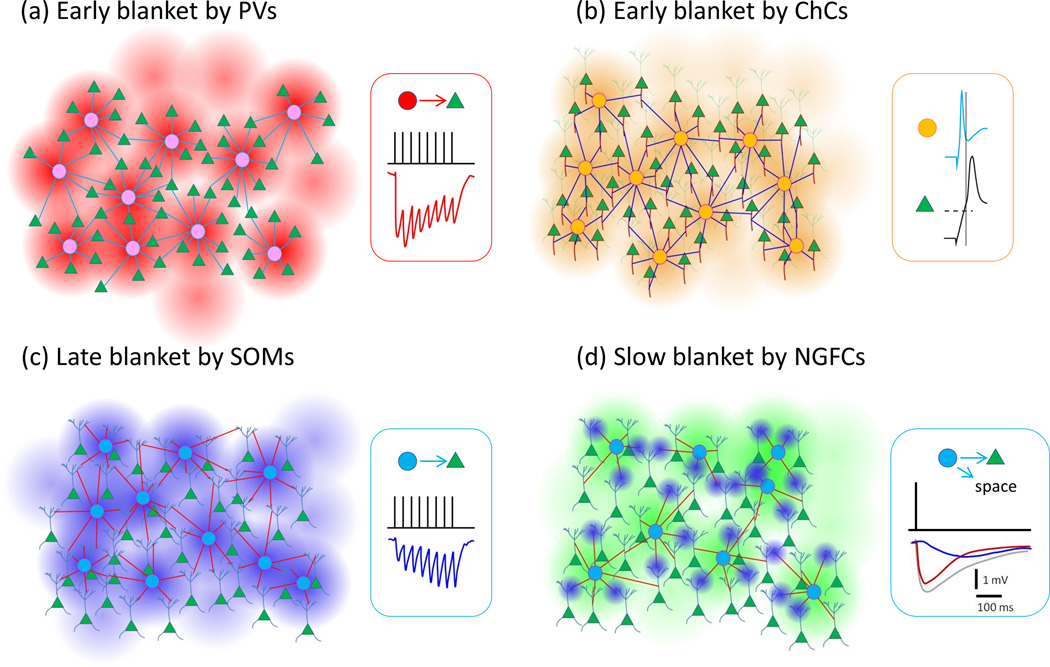
This term describes the dense and unspecific innervation of local PCs by INs, i.e., restricted to immediate intralaminar territories covered by their axons. PVs and SOMs project densely to PCs within an 200 µm radius (Figure 1). This dense innervation pattern was demonstrated in living IN-GFP brain slices across multiple cortical areas and developmental stages using two-photon glutamate uncaging [18,19]. The connection probabilities decayed with distance but at peak, at around 100 µm intersomatic distances, were ~80% for both IN types and in some recordings all INs within 200 µm of a PC were connected to it demonstrating highly overlapping inhibitory connectivity. Given that many axons are cut in slice, we expect these INs project to essentially every PC around them in the intact brain. Since these studies showed that a given PC receives inhibitory input from most PVs and SOMs around it, it stands to reason that any PV or SOM inhibits most PCs around it unspecifically. Prior to these studies, compatible but less comprehensive results had been reported, using paired electrical recordings [20].
Figure 1.
Blanket inhibition by the different subtypes of interneurons. (a) Early blanket inhibition by PVs. (b) Early Blanket inhibition by ChCs. Right panel shows early activation of ChCs compared to PCs after layer 1 stimulation (copied with permission from [4]). (c) Late blanket inhibition by SOMs. (d) Slow blanket inhibition by NGFCs. Inset: Gray trace represents total inhibitory current while blue is a GABAB receptor component and red is the difference. Green triangles represent PCs, and circles in each panel represent INs projecting to PCs. Traces shown in (a), (c), and (d) represent responses of PCs to IN inputs.
The connectivity between INs is less well understood. Some studies report a high degree of connectivity between PVs, from PVs to SOMs and SOMs to PVs [21–23] (but see [5] and [24] for smaller estimates of PV->PV and PV->SOM). Thus the dense inhibitory blankets from PVs and SOMs to PCs might extend to INs too, with the clear exception that SOMs virtually never inhibit each other.
A recent study of ChCs found that within their local axonal cloud they also project densely to local PCs [10]. Nearly 50% of AISs within 200 µm from a ChC soma were apposed by a cartridge. This could be a significant underestimate of the real connectivity because of the technical caveats and stringent analysis methods employed (discussed in detail in [10]). Indeed, some areas within the ChC axonal fields had cartridges apposing nearly every AIS. Consistent with the lack of selectivity, an average of 4 ChCs were estimated to innervate any given AIS, indicating an overlapping pattern of inhibition. Dense innervation of virtually every PC AIS by ChCs in piriform cortex has also been observed [25]. Thus, ChCs appear similar to PVs and SOMs in terms of their local blanket inhibition. It would be important to study ChC connectivity with a similar method used on PVs and SOMs [18,19] to reveal the functional density of this blanket inhibition.
A final case for a “blanket” inhibitory innervation can be made for NGFCs, which mediate a spatially extreme form of blanket inhibition by forming presynaptic boutons that are not directly opposed to postsynaptic densities of other cells and secrete GABA into the neuropil some micrometers away from the functionally postsynaptic cells. This innervation strategy, showering cortical circuits with GABA, presumably accounts for the 87% connection probability observed from NGFCs to nearby neurons within 100 µm [13]. NGFCs additionally modulate synaptic transmission within their axonal field [13] and inhibit cells with more distant somata that have distal dendrites within the NGFC axonal fields [26]. Working through presynaptic GABAB receptors, NGFCs can decrease the effect of repetitive synaptic events [13]. A high degree of connectivity was observed in another recent study on layer 4 NGFCs, although presynaptic modulation of synaptic transmission upon thalamic stimulation was found only on PV-to-PC synapses but not on excitatory synapses formed by thalamocortical afferents which also contain presynaptic GABAB receptors [12]. This discrepancy might be simply due to presence of the whole PV somato-dendritic domain within the NGFC axonal cloud, rather than spatial specificity in the distribution of release sites within the NGFC axonal field.
Early and late blanket inhibition
Subtypes of INs have different temporal properties in their firing and synaptic dynamics and also target separate subcellular compartments of PCs. Due to dynamic changes and variance of synaptic weights [27], blanket inhibition is unlikely to invariantly shut down all PC activity in a region. PCs might rather be varyingly inhibited depending on the timing, synaptic weights and the position of the IN contact (Figure 1).
Two lines of evidence suggest that PVs are specialized for a fast and transient inhibition while SOMs deliver slow-onset, lasting inhibition. Firstly, both the excitatory input synapses and inhibitory output synapses of PVs vs SOMs, have consistently different dynamics [22,28–34]. Synapses on and from PVs are virtually always depressing: postsynaptic potentials peak in the beginning and then decrease dramatically during a high frequency train of action potentials. In contrast, synapses on and, sometimes but not always, from SOMs are facilitating, meaning that they are almost silent in response to a single action potential but subsequent postsynaptic potentials increase by several fold during a high frequency spike train. Dynamics of inhibitory synapses between PVs and SOMs are determined accordingly by the presynaptic cell [31].
Secondly, the amount of monosynaptic feedback inhibition within the populations is dramatically different between subtypes, in terms of synaptic connections between individual cells within the groups [22,23,31] as well as autaptic connections [35]. Individual PVs readily form synapses onto each other and themselves, while SOMs virtually never inhibit each other [22,24,31,36] allowing them to sustain persistent firing [37]. In addition some data suggest that PVs/FSs more often than non-FSs, form reciprocal connections to the local PCs that excite them, forming direct feedback inhibition loops [38–41] (however, see [19,21,42,43]). Moreover, SOMs disinhibit layer 4 through powerful inhibition of PVs [44]. Thus we expect that during lasting high frequency neocortical activity, such as is seen spontaneously and in response to sensory stimuli in vivo [45,46], there is a rapid-onset, transient blanket of inhibition by PVs which is later replaced by a slowly recruited, persistent blanket mediated by SOMs.
ChCs, like other PV cells, appear to have a fast, early action on local PCs [47]. To the best of our knowledge there is currently a lack of information about the detailed dynamics of ChC firing and synapses in the neocortex. However their output synapses might be depressing like PV synapses (see Fig. 4c in [47]) as is the case in hippocampus [48,49].
The spatially extreme NGFC inhibitory blanket is also temporally extreme, in that the slow GABAB receptor-mediated postsynaptic event, elicited by a single action potential in a NGFC, reaches its peak well over 100 ms later [50–52]. Also the GABAA receptor-mediated component of NGFC inhibition is slower and longer lasting than with PVs [50].
Functional relevance of blanket inhibition
In rodents INs form a minority, ~15% [53,54], while PCs constitute the majority (~85%) or neocortical neurons. Despite variance in input strength, every neuron receives both inhibitory and excitatory inputs [55,56] and PCs target only ~10% of the neurons around them [53]. Given these numbers one could infer that blanket inhibition serves to balance excitation and prevent epilepsy. However, more detailed functional roles of inhibition in cortical networks can be divided into five (partially overlapping) hypotheses: sharpening tuning of stimulus response relation through lateral inhibition [57]; generation/pacing of network oscillations through feedback inhibition [58,59]; normalization of input through feed-forward inhibition [56,60], modulation of stimulus-response gain of PCs [61] and computational discrimination of inputs into self-organizing maps [62]. Some of these hypotheses have been recently reviewed elsewhere [29,63], so we will not discuss them in detail but solely focus on some aspects of these hypotheses where the impact of the blanket configuration of inhibition is evident. As a related aside, the blanket configuration might reveal a principle for how cortical circuits are wired up during development and a further impetus to build a disinhibitory network to sculpt specificity into the blanket (Box 1), alongside the PC-specific synaptic weights.
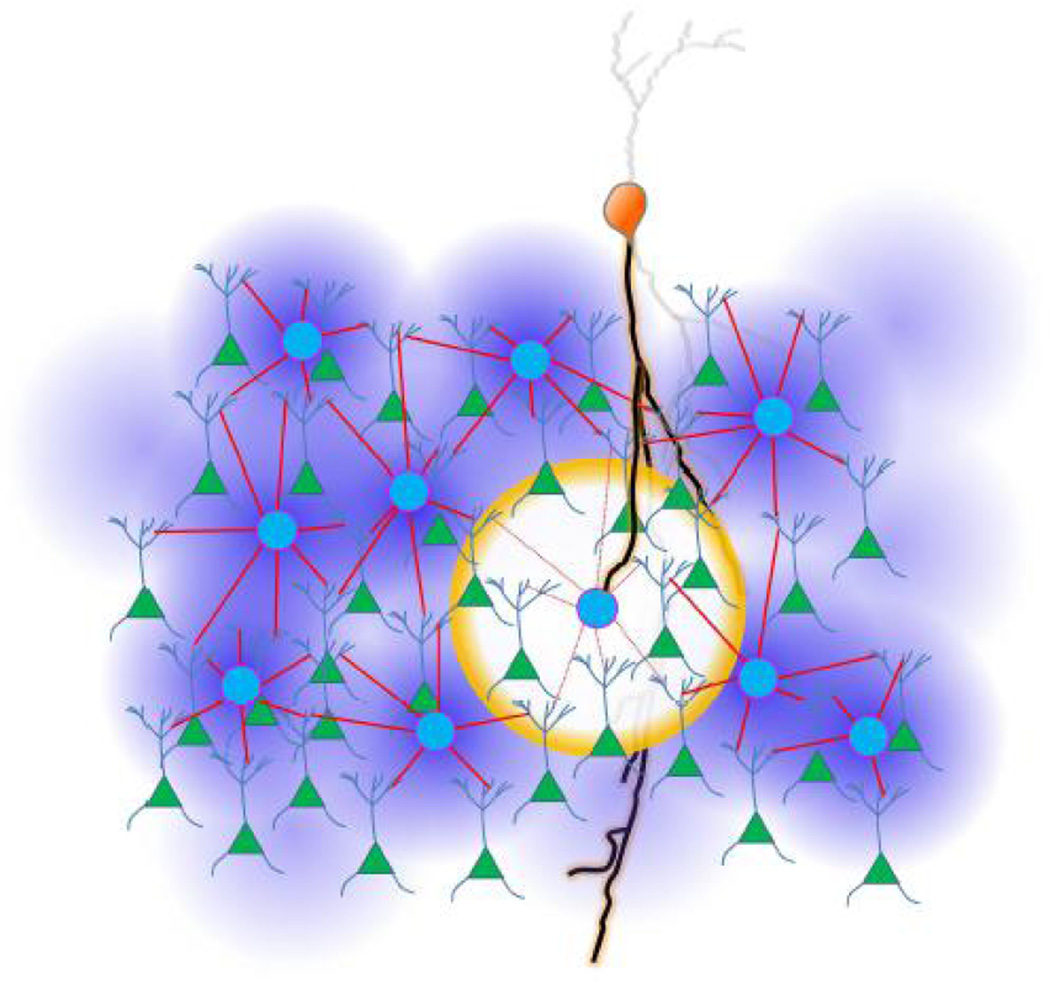
Box 1: “Making holes in the blanket: VIPs as disinhibitory specialists“.
In contrast to the dense blanket inhibition of PCs by INs discussed here so far, VIPs do not connect to most PCs within their reach [24,72,73]. VIPs specifically target SOMs and, to a lesser extent, PVs [24,72,73]. Two recent papers [72,73] show inhibition of SOMs by VIPs during behaviorally triggered excitation of VIPs, by whisking in somatosensory cortex and by aversive feedback stimuli in auditory and prefrontal cortices. This would disinhibit the PCs under SOM blanket inhibition. Given the horizontally restricted extent of VIP axons [72,74,75], activation of a limited number of VIPs could make holes in the blanket of inhibition, selectively disinhibiting PCs in some regions while leaving others under the blanket (Figure 3). These disinhibitory holes might be expected to be the size of the axonal fields of a few SOMs, and to occur at times of behaviorally relevant activation of VIPs [72,73] as well as spontaneously due to the high resting membrane potential and excitability of these neurons. Alternatively, if many or all VIPs can be activated by a stimulus, this would briefly disable the whole SOM blanket, possibly allowing for spread of excitation into an unusual direction (see “Functional relevance of blanket inhibition”). Given that VIPs could be activated by higher cortical feedback [72,73], these holes in the blanket might serve to recall past experiences.
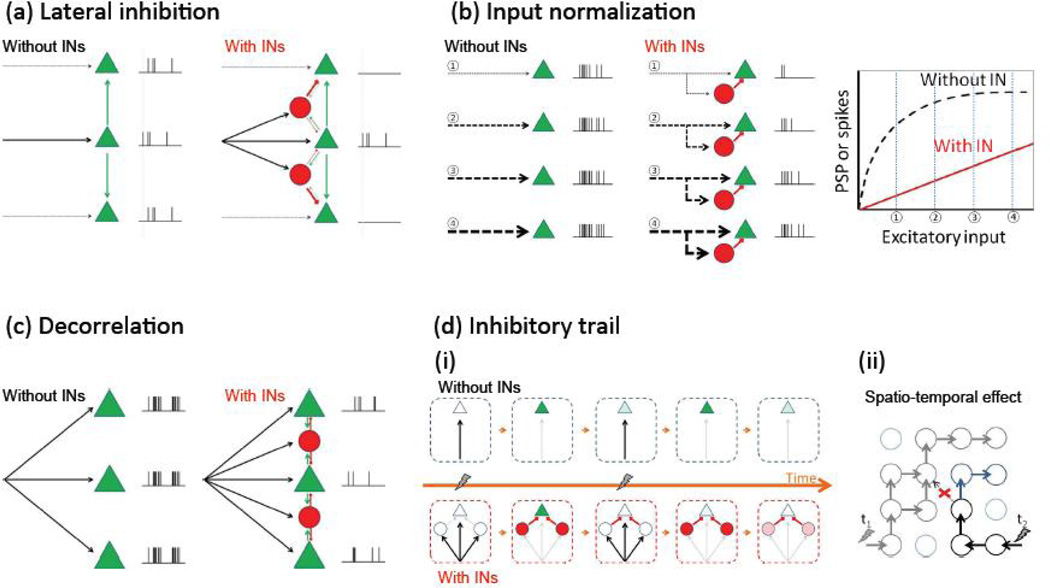
The sharpening of excitatory responses by lateral inhibition is a traditional function of inhibitory circuits and one that seems to be at work throughout the sensory systems of the brain [57]. Any given excitatory input, by firing neighboring inhibitory interneurons, themselves connected to all neighboring excitatory cells, will achieve essentially a “winner take all” strategy, and enable the sole excitation of the desired target (Figure 2A). The design of blanket inhibition goes hand in hand with this functional logic, since one would want a uniform and unspecific inhibitory connectivity. This will assure that all excitatory inputs are subject to a similar degree of local filtering and thus prevent biases in the propagation of signals. An intriguing hypothesis, based on neural network theory, is the possibility that inhibitory circuits serve to separate in a multidimensional vector space similar patterns of excitatory inputs [64]. In fact, a learning rule with a local inhibitory spatial flanks can spontaneously generate self-organizing maps [62], helping to explain the common occurrence of functional maps in most regions of the brain. A local blanket of inhibition is critical for this function, since it will enable a circuit to orthogonalize inputs automatically, and this could be a critical aspect to enable sensory areas of the brain to enhance the discrimination among similar inputs.
Figure 2.
Functional roles of INs. (a) Lateral inhibition. (b) Input normalization. (c) INs decorrelate PC spiking. (d) Inhibitory trail reduces response to a second input. Lightning symbols indicate input along arrows. (ii) Simple temporal effect. Green triangles represent PCs and red circles represent INs. (ii) example of spatio-temporal effect. Here circles represent modules containing both INs and PCs, and arrows the spread of excitation; because of the inhibitory trail stimulation of a pathway at t1, shortly before stimulation of another path at t2 blocks the progress of the latter activity at the red cross and directs it instead to the blue direction.
A blanket inhibitory design is also ideal for feedforward inhibitory circuits to linearize or extend the dynamic range of PCs (Figure 2B). Otherwise some cells could escape this normalization and saturate with increasing excitatory inputs. This would essentially inactivate them from the network, defeating the purpose of this mechanism.
The role of inhibitory circuits in the generation of oscillations and synchronization could be equally well served by a blanket inhibitory design, although the functional implications are more complicated. As a result of the dense blanket inhibition PCs near each other experience synchronous inhibitory postsynaptic events from common presynaptic INs [65], and this could in principle serve to synchronize their spiking [58]. At the same time, electrically or synaptically coupled interneuron groups in slice can synchronize sustained spiking under some experimental manipulations [36,66]. On the other hand, recent work shows that interneuron spiking in neocortical brain slices is largely uncorrelated spontaneously during UP-states or after thalamic stimulation [65]. However, in vivo, the subthreshold membrane potential changes and spiking of some INs tend to be synchronized [67] and sensory evoked responses are apparently similar within cell groups [29]. Moreover, in apparent contradiction to the role of INs in generating oscillations [59], recent work suggests that inhibition can actually decorrelate firing of PCs [65,68,69]. In principle, from an information theoretic aspect both synchrony and decorrelated firing of neurons are useful – the prior for effective transmission and the latter for unambiguous representation of information for example. Perhaps a compromise can be reached by neuronal networks where irregular firing and population level rhythmicity coexist [70]. If this is the case, interneurons could be involved in generating both synchrony and irregular firing, depending on the exact state of the circuit. Future work needs to examine the exact role of INs in synchronizing or decorrelating PCs.
Finally, another potential function of inhibition could be to leave a temporal mark in the circuit, as a refractory trail for the spread of further excitatory patterns (Figure 2C). Inhibition, in particular by the facilitating SOMs and the GABAB receptor-triggering NGFCs, can remain in a given cortical circuit after the excitatory neurons that recruited the INs have ceased firing (see e.g., disynaptic inhibition traces in [30,33,60]). This could result in a transient trail of inhibition left behind by a passing wave of excitation, possibly causing a network level refractory period akin to that associated with the action potential in an axon. Like the latter allows action potential propagation in only one direction along an axon, the inhibitory trail might, to some extent, enforce directionality of spread of activity seen for example across the cortical surface during sensory stimulation [71]. The blanket configuration would be ideal for this function, and infact early and late “blankets” could differentially re-channel excitatory activity into different circuits, enabling a novel type of fast circuit plasticity that does not require the slower Hebbian learning rules.
Since the possible functions of INs are so many, it might be useful to comprehensively study, through simulations and experiments, whether realistic networks can actually perform multiple roles simultaneously.
Conclusions
Blanket inhibition is a general feature of most inhibitory connections to principal cells in the cortex, except for VIP cells which appear to make holes in the blankets. Blanket inhibition exists in different temporal domains and could be critical to implement different functional roles of inhibitory neurons.
Figure 3.
Hole in inhibitory blanket. Orange cell represents a VIP disinhibiting a network through inhibition of a SOM. Green triangles represent PCs, and a light blue circles SOMs.
Highlights.
-
-
Key inhibitory interneurons innervate pyramidal neurons densely and unspecifically.
-
-
Timing of inhibition is different across interneuron populations.
-
-
Dense inhibitory network structure should inform hypotheses of function.
-
-
Disinhibitory interneurons can make holes in the dense inhibitory “blanket”.
Acknowledgements
Our work is supported by the HFSP, the Kavli Institute for Brain Science, the NIH Director Pioneer Award, NEI, NINDS, NIMH, NIDA, Keck Foundation and NARSAD. This material is based upon work supported by, or in part by, the U. S. Army Research Laboratory and the U. S. Army Research Office under contract number W911NF-12-1-0594. MMK thanks Ilkka Kivimäki for useful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coombs JS, Eccles JC, Fatt P. The inhibitory suppression of reflex discharges from motoneurones. J Physiol. 1955;130:396–413. doi: 10.1113/jphysiol.1955.sp005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nature neuroscience. 2009;12(1):21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of gabaergic axoaxonic cells in cortical microcircuits. Science. 2006;311(5758):233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 4.Woodruff AR, McGarry LM, Vogels TP, Inan M, Anderson SA, Yuste R. State-dependent function of neocortical chandelier cells. J Neurosci. 2011;31(49):17872–17886. doi: 10.1523/JNEUROSCI.3894-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking gabaergic interneurons in adult mouse neocortex. Proc Natl Acad Sci U S A. 2002;99(19):12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13(11):4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawaguchi Y, Kubota Y. Gabaergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified gabaergic cells in the rat frontal cortex. Neuroscience. 1998;85(3):677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- 9.Somogyi P. A specific 'axo-axonal' interneuron in the visual cortex of the rat. Brain Res. 1977;136(2):345–350. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- 10. Inan M, Blazquez-Llorca L, Merchan-Perez A, Anderson SA, DeFelipe J, Yuste R. Dense and overlapping innervation of pyramidal neurons by chandelier cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(5):1907–1914. doi: 10.1523/JNEUROSCI.4049-12.2013. * ChCs make distinctive cartridge synapses on AISs of PCs enabling reliable immunolabelling of the postsynaptic targets and presynaptic cartridges. Capitalizing on this and a transgenic mouse where ChCs are labelled with GFP sparsely, these authors revealed the density and overlap of PC inhibition by ChCs.
- 11.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science (New York, N Y ) 2013;339(6115):70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chittajallu R, Pelkey KA, McBain CJ. Neurogliaform cells dynamically regulate somatosensory integration via synapse-specific modulation. Nature neuroscience. 2013;16(1):13–15. doi: 10.1038/nn.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary gaba-mediated volume transmission. Nature. 2009;461(7268):1278–1281. doi: 10.1038/nature08503. ** These authors report a high connection probability when a NGFC is presynaptic. They also make a convincing case that NGFCs secrete GABA into the neuropil at "nonsynaptic" locations and this inhibits nearby neurons.
- 14.McGarry LM, Packer AM, Fino E, Nikolenko V, Sippy T, Yuste R. Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front Neural Circuits. 2010;4:12. doi: 10.3389/fncir.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical gabaergic interneurons expresses ionotropic serotonin receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(50):16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical gabaergic neurons. Dev Neurobiol. 2011;71(1):45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518(3):389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69(6):1188–1203. doi: 10.1016/j.neuron.2011.02.025. ** This paper demonstrates blanket inhibition from SOMs to PCs using two-photon glutamate uncaging to sequentially activate each GFP-tagged SOM while recording in whole cell mode from PCs.
- 19. Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: A canonical microcircuit for inhibition? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(37):13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. ** This paper demonstrates blanket inhibition from PVs to PCs using two-photon glutamate uncaging to sequentially activate each GFP-tagged PV while recording in whole cell mode from PCs.
- 20.Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front Neurosci. 2007;1(1):19–42. doi: 10.3389/neuro.01.1.1.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avermann M, Tomm C, Mateo C, Gerstner W, Petersen CCH. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. Journal of neurophysiology. 2012;107(11):3116–3134. doi: 10.1152/jn.00917.2011. [DOI] [PubMed] [Google Scholar]
- 22.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402(6757):75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 23.Hu H, Ma Y, Agmon A. Submillisecond firing synchrony between different subtypes of cortical interneurons connected chemically but not electrically. J Neurosci. 2011;31(9):3351–3361. doi: 10.1523/JNEUROSCI.4881-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nature neuroscience. 2013;16(8):1068–1076. doi: 10.1038/nn.3446. ** With a technique combining optogenetics in new transgenic mice, this paper sketches out most inhibitory interactions between neurochemically defined IN classes.
- 25.Wang X, Sun Q-Q. Characterization of axo-axonic synapses in the piriform cortex of mus musculus. The Journal of comparative neurology. 2012;520(4):832–847. doi: 10.1002/cne.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nature neuroscience. 2013;16(2):210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of gabaergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 28.Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. Journal of neurophysiology. 2003;90(5):2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- 29.Gentet LJ. Functional diversity of supragranular gabaergic neurons in the barrel cortex. Frontiers in neural circuits. 2012;6:52. doi: 10.3389/fncir.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nature neuroscience. 2007;10(6):743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Hu H, Agmon A. Short-term plasticity of unitary inhibitory-to-inhibitory synapses depends on the presynaptic interneuron subtype. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(3):983–988. doi: 10.1523/JNEUROSCI.5007-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1(4):279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- 33.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by martinotti cells. Neuron. 2007;53(5):735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Tan Z, Hu H, Huang ZJ, Agmon A. Robust but delayed thalamocortical activation of dendritictargeting inhibitory interneurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacci A, Huguenard JR, Prince DA. Functional autaptic neurotransmission in fast-spiking interneurons: A novel form of feedback inhibition in the neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(3):859–866. doi: 10.1523/JNEUROSCI.23-03-00859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu H, Ma Y, Agmon A. Submillisecond firing synchrony between different subtypes of cortical interneurons connected chemically but not electrically. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(9):3351–3361. doi: 10.1523/JNEUROSCI.4881-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanselow EE, Connors BW. The roles of somatostatin-expressing (gin) and fast-spiking inhibitory interneurons in up-down states of mouse neocortex. Journal of neurophysiology. 2010;104(2):596–606. doi: 10.1152/jn.00206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, Zeng H, Lein E, Lesica NA, Mrsic-Flogel TD. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nature neuroscience. 2011;14(8):1045–1052. doi: 10.1038/nn.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy RB, Reyes AD. Spatial profile of excitatory and inhibitory synaptic connectivity in mouse primary auditory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(16):5609–5619. doi: 10.1523/JNEUROSCI.5158-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otsuka T, Kawaguchi Y. Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(34):10533–10540. doi: 10.1523/JNEUROSCI.2219-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nature neuroscience. 2005;8(11):1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- 42.Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. The Journal of physiology. 2003;551(Pt 1):139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaitsev AV, Lewis DA. Functional properties and short-term dynamics of unidirectional and reciprocal synaptic connections between layer 2/3 pyramidal cells and fast-spiking interneurons in juvenile rat prefrontal cortex. Eur J Neurosci. 2013 doi: 10.1111/ejn.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H, Jeong H-Y, Tremblay R, Rudy B. Neocortical somatostatin-expressing gabaergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77(1):155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ch'ng YH, Reid RC. Cellular imaging of visual cortex reveals the spatial and functional organization of spontaneous activity. Frontiers in integrative neuroscience. 2010;4 doi: 10.3389/fnint.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64(3):404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodruff AR, McGarry LM, Vogels TP, Inan M, Anderson SA, Yuste R. State-dependent function of neocortical chandelier cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(49):17872–17886. doi: 10.1523/JNEUROSCI.3894-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganter P, Szucs P, Paulsen O, Somogyi P. Properties of horizontal axo-axonic cells in stratum oriens of the hippocampal ca1 area of rats in vitro. Hippocampus. 2004;14(2):232–243. doi: 10.1002/hipo.10170. [DOI] [PubMed] [Google Scholar]
- 49.Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23(7):2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabadics J, Tamas G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of gabaa,slow and gabaa,fast. Proc Natl Acad Sci U S A. 2007;104(37):14831–14836. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299(5614):1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 52.Wozny C, Williams SR. Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb Cortex. 2011;21(8):1818–1826. doi: 10.1093/cercor/bhq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the c2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61(2):301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 54. Meyer HS, Schwarz D, Wimmer VC, Schmitt AC, Kerr JN, Sakmann B, Helmstaedter M. Inhibitory interneurons in a cortical column form hot zones of inhibition in layers 2 and 5a. Proc Natl Acad Sci U S A. 2011;108(40):16807–16812. doi: 10.1073/pnas.1113648108. * The authors performed a thorough quantitative analysis of the distribution and occurrence of INs in somatosensory cortex.
- 55.Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464(7292):1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci. 2009;12(12):1577–1585. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- 57.Hartline HK, Wagner HG, Ratliff F. Inhibition in the eye of limulus. J Gen Physiol. 1956;39(5):651–673. doi: 10.1085/jgp.39.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual gabaergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 59.Buzsaki G, Wang X-J. Mechanisms of gamma oscillations. Annual review of neuroscience. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murayama M, Pâerez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 2009;457(7233):1137–1141. doi: 10.1038/nature07663. [DOI] [PubMed] [Google Scholar]
- 61.Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488(7411):343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohonen T. Self-organizing neural projections. Neural Networks. 2006;19(6–7):723–733. doi: 10.1016/j.neunet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72(2):231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohonen T, Oja E. Fast adaptive formation of orthogonalizing filters and associative memory in recurrent networks of neuron-like elements. Biol Cybern. 1976;21(2):85–95. doi: 10.1007/BF01259390. [DOI] [PubMed] [Google Scholar]
- 65. Sippy T, Yuste R. Decorrelating action of inhibition in neocortical networks. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(23):9813–9830. doi: 10.1523/JNEUROSCI.4579-12.2013. * This paper shows that although nearby PCs get synchronous inhibitory input from common INs (as expected from blanket inhibition), and while INs are sometimes weakly electrically coupled, nearby INs do not spike synchronously in slice.
- 66.Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(8):2058–2073. doi: 10.1523/JNEUROSCI.2715-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of gabaergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65(3):422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 68. Graupner M, Reyes AD. Synaptic input correlations leading to membrane potential decorrelation of spontaneous activity in cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(38):15075–15085. doi: 10.1523/JNEUROSCI.0347-13.2013. * Like [65], this paper shows that inhibitory postsynaptic events tend to be more synchronous than excitatory ones which is to be expected from blanket inhibition.
- 69.Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD. The asynchronous state in cortical circuits. Science. 2010;327(5965):587–590. doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90(3):1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mohajerani MH, Chan AW, Mohsenvand M, Ledue J, Liu R, McVea DA, Boyd JD, Wang YT, Reimers M, Murphy TH. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nature neuroscience. 2013;16(10):1426–1435. doi: 10.1038/nn.3499. ** This study shows that activation of primary sensory cortical areas by optogenetics results in spread of activity across the cortex in a highly similar pattern as that caused by sensory stimuli that activate the given primary sensory cortex.
- 72. Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16(11):1662–1670. doi: 10.1038/nn.3544. ** These authors demonstrated inhibition of SOMs by VIPs in somatosensory cortex. The activity of VIPs correlated with whisking and was driven by an excitatory projection from motor cortex.
- 73. Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013 doi: 10.1038/nature12676. ** This paper shows inhibition inhibition of SOMs by VIPs in auditory and prefrontal cortices, and the resulting disinhibition of nearby PCs. It was further demonstrated that VIPs are activated by aversive feedback stimuli.
- 74.Bayraktar T, Welker E, Freund TF, Zilles K, Staiger JF. Neurons immunoreactive for vasoactive intestinal polypeptide in the rat primary somatosensory cortex: Morphology and spatial relationship to barrel-related columns. J Comp Neurol. 2000;420(3):291–304. [PubMed] [Google Scholar]
- 75.Tahvildari B, Wolfel M, Duque A, McCormick DA. Selective functional interactions between excitatory and inhibitory cortical neurons and differential contribution to persistent activity of the slow oscillation. J Neurosci. 2012;32(35):12165–12179. doi: 10.1523/JNEUROSCI.1181-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]