Abstract
Significant progress has been made in our understanding of how endogenous cannabinoids (eCBs) signal at excitatory and inhibitory synapses in the central nervous system (CNS). This review discusses how eCBs regulate inhibitory interneurons, their synapses, and the networks in which they are embedded. eCB signaling plays a pivotal role in brain physiology by means of their synaptic signal transduction, spatiotemporal signaling profile, routing of information through inhibitory microcircuits, and experience-dependent plasticity. Understanding the normal processes underlying eCB signaling is beginning to shed light on how their dysregulation contributes to disease.
Introduction
Endocannabinoid (eCB) signaling plays a central role in reward seeking and drug addiction [1,2], anxiety and depression [3], pain [4], learning and memory [5], neurogenesis and development [6,7], and may also serve as a drug target for therapeutic intervention in obesity, autism, epilepsy, and schizophrenia [8–12]. The eCB system, comprising lipid messengers, synthetic and degradative enzymes, carrier proteins/transporters, and receptors [10,13–15], is a neuromodulatory system capable of transiently or persistently suppressing transmitter release from both excitatory and inhibitory synapses throughout the CNS [16–20]. At inhibitory synapses, short-term eCB-mediated plasticity is commonly triggered by postsynaptic depolarization, referred to as depolarization-induced suppression of inhibition (DSI), and long-term plasticity in the form of depression of inhibition, termed iLTD, is a heterosynaptic form of plasticity triggered by repetitive activity of neighboring excitatory synaptic inputs. Classically, eCBs are synthesized by activity within postsynaptic neuronal compartments, retrogradely cross the synapse, occupy presynaptically expressed type-1 cannabinoid receptors (CB1Rs), and depress glutamate and GABA release. While eCBs are prototypical retrograde messengers [21], additional research indicates this canonical interpretation is complicated by their non-retrograde actions on postsynaptic CB1Rs and transient receptor potential type-1 (TRPV1) channels, as well as astrocytic CB1Rs [16]. Here, we emphasize recent experimental advances examining eCB functions at interneurons including their molecular signaling cascades, spatiotemporal signaling profiles, role in microcircuits, and dysregulation in certain pathophysiological conditions. Inhibitory interneurons are a heterogeneous group that support key aspects of brain function including fine-tuning excitatory and inhibitory neuronal networks, controlling membrane excitability and sub-threshold conductances, regulating synaptic and intrinsic input/output transformations, as well as enforcing precise spike-timing and oscillations in downstream targets [22–27]. Continued exploration into the dynamic interplay between eCBs and interneurons is therefore essential for understanding brain function.
Endocannabinoid Signal Transduction and Interneuronal Function
The best defined eCBs are 2-arachidonoyl glycerol (2-AG) and anandamide (AEA) (for comprehensive reviews on eCB signal transduction, see [10,13–20]). 2-AG can be produced postsynaptically in an activity-dependent manner through increased Ca2+ influx; Gq/11 protein coupled receptor (GPCR) activation, commonly group-I metabotropic glutamate receptors (I-mGluRs) or muscarinic acetylcholine receptors (mAChRs); or an associative/synergistic combination thereof [28] (Fig. 1a). Ca2+ and Gq/11 GPCRs signal to phospholipase-C β (PLCβ) activating diacylglycerol lipase-α (DGLα) leading to 2-AG synthesis. Genetic and pharmacological studies strongly support the notion that DGLα is responsible for 2-AG synthesis at inhibitory (and excitatory) synapses [29–33] (see also [34]). The exact role Ca2+ plays in 2-AG synthesis remains unclear. PLCβ is a Ca2+-sensitive enzyme, but PLCβ appears only to regulate synaptically-driven and associative/synergistic eCB release [28]. Recent work on striatal GABAergic medium spiny neurons (MSNs) found that Ca2+/calmodulin-dependent protein kinase-α (CAMKIIα) negatively regulates DGLα activity [35]. In contrast with 2-AG, AEA biosynthesis appears more complex and involves several enzymes [14], most notably N-acyl-phosphatidylethanolamine phospholipase-D (NAPE-PLD). Immunohistochemical studies localized this enzyme to cerebellar Purkinje cells and certain hippocampal interneurons [36,37], and functional evidence indicates postsynaptic AEA release regulates synapse strength onto striatal MSNs [38]. Additional work is needed to ascertain the subcellular expression profile of NAPE-PLD at inhibitory synapses (reviewed in [18]). Given that NAPE-PLD and DGLα can be expressed in the same cell, what determines whether 2-AG or AEA emerges? While the answer could relate to cell-type and/or synapse-specific expression of eCB-synthesizing enzymes, recent in vitro studies provide alternative possibilities. For example, the pattern and/or frequency of synaptic activity [39,40], and the resting membrane potential (e.g. “up” vs. “down” state in striatal MSNs) [41] can preferentially release 2-AG or AEA. In addition, AEA might inhibit 2-AG production [42]. Alternatively, AEA could activate TRPV1 which increases Ca2+ signaling to mobilize 2-AG, or compartmentalized Ca2+ microdomains at inhibitory synapses might selectively generate 2-AG, AEA, or both.
Figure 1. Pre- and postsynaptic mechanisms for associative iLTD.
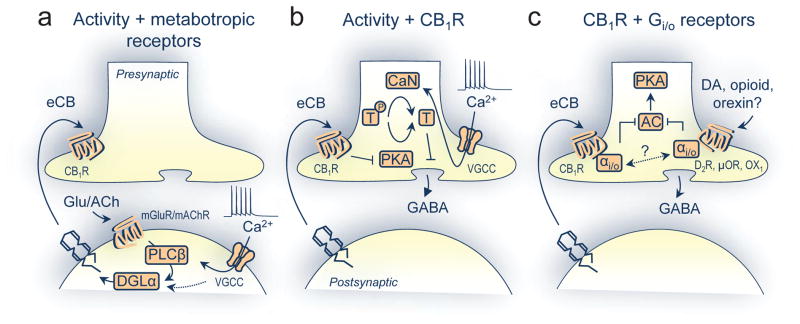
a) Postsynaptic activity (e.g. Ca2+ influx through voltage-gated Ca2+ channels, VGCCs) along with metabotropic receptor activation (e.g. mGluR and mAChR) engages phospholipase-C β (PLCβ) and then diacylglycerol lipase α (DGLα) followed by mobilization of 2-AG. DGLα can also be stimulated via an uncharacterized, Ca2+-dependent mechanism.
b) Presynaptic activity, leading to Ca2+ influx (here, VGCCs), along with CB1R stimulation shifts protein kinase A (PKA)/calcineurin (CaN) activity to favor dephosphorylation of an unknown target (T) essential for eCB-mediated iLTD.
c) Concomitant presynaptic CB1R activation plus dopamine (DA)-like type 2 (D2R), μ opioid (μOR), and/or orexin-1 (OX1) receptors might cooperatively reduce adenylyl cyclase (AC)/PKA signaling [20]. Alternatively, heterodimeric signaling interactions may switch Gi/o signaling to Gs, actually promoting AC/PKA activity (not shown).
Presynaptic CB1Rs are found throughout the brain, whereas CB2Rs and TRPV1 channel expression in the CNS remains controversial. CB1Rs can transduce information during short-term and long-term plasticity via their Gi/o proteins to intracellular effectors including voltage-gated Ca2+ channels, inwardly rectifying K+ channels, and/or PKA [13]. Presynaptic activity [43–45], calcineurin [43] (Fig 1b), the vesicle associated protein Rab3B [46], and Gi/o coupled M2-type mAChRs [47] also seem to be required for iLTD. Interactions between CB1R and other Gi/o GPCRs such as type-2 dopamine-like receptors (D2Rs) [48,49] have been described at inhibitory synapses (Fig 1c), suggesting additional layers of modulatory complexity in presynaptic terminals. Early studies performed in hippocampus and neocortex reported that regular-spiking, but not fast-spiking, interneurons express CB1Rs and are therefore responsive to eCBs [25,50]. New data, however, indicates this dichotomy is not steadfast. At striatal [51], nucleus accumbens [52], visual [45] and somatosensory [53] cortical (but see [54]) fast-spiking interneuron output synapses, CB1Rs were shown to mediate short-term plasticity. In addition, hippocampal fast-spiking interneurons can mobilize eCBs required for long-term plasticity [55]. Beyond retrograde signaling, evidence indicates 2-AG can engage postsynaptic CB1Rs in an autocrine fashion to inhibit cortical interneuron excitability [56,57]. CB2Rs were originally thought to be expressed only in immune cells, but accumulating evidence supports a role for these receptors in regulating inhibitory synaptic transmission [58,59]. Signaling cascades downstream of synaptic CB2Rs remain virtually unknown. While eCBs can also target pre- and postsynaptic TRPV1 as well as astrocytic CB1Rs [16], our understanding of how these receptors modulate GABAergic transmission and inhibitory interneuron physiology is extremely limited. Collectively, several diverse and novel modes of eCB production and detection have been described at inhibitory interneurons, and fully characterizing eCB signaling should help elucidate higher-level circuit functions.
Spatiotemporal Signaling Profile of Endocannabinoids at Interneurons
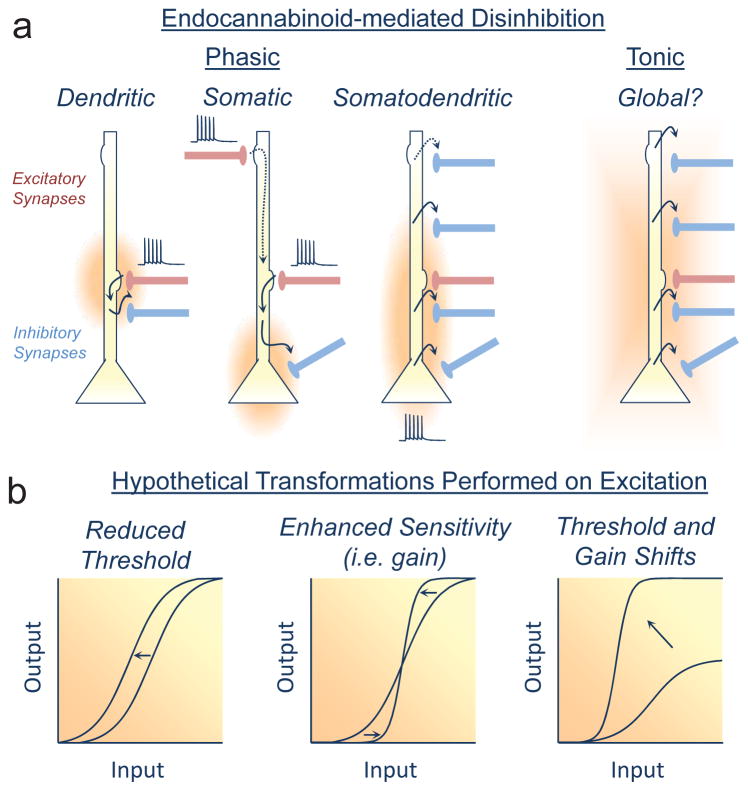
Two eCB signaling modes have been identified at GABAergic synapses: one phasic (i.e. activity-dependent) and one tonic (Fig. 2a) (for review, see [19]). Strong evidence supports “on-demand” phasic eCB release while more indirect findings suggest eCBs arise through preformed reserve pools [33,60] (but see [32,34]). Phasic eCB signaling underlies short- and long-term forms of inhibitory synaptic plasticity such as DSI and iLTD, respectively [17,18]. The type of synaptic plasticity (i.e. short vs. long) may rely on how long the CB1R is occupied by eCBs [61,62]. DSI can be observed at specific inhibitory inputs or distributed more globally across the somatodendritic tree [17,18], and recent research indicates that postsynaptic activity alone (e.g. theta burst-firing that mimics CA1 pyramidal cell firing in vivo) triggers iLTD at both somatic and dendritic compartments [61]. Together, these findings may provide insights into how inhibition shapes somatic spike-timing, Ca2+ dependent plasticity processes, and dendritic integration. Phasic eCB signaling can also be observed by pairing two stimuli that are independently submaximal for eliciting changes in synapse strength. This “associativity” is thought to provide additional computational flexibility to neuronal networks, and new results from several laboratories provided evidence for long-term eCB-mediated associative iLTD [43,47,61,63]. Beyond the role that I-mGluRs and mAChRs play in eCB-mediated associative plasticity, GluK2-containing kainate [64], E2α estrogen [65], neuronal protease-activated type-1 [66], and Trk [67] receptors can also mobilize eCBs at inhibitory synapses. It remains unknown if these receptors participate in long-term associative eCB signaling.
Figure 2. Endocannabinoid-mediated disinhibition and possible functional outcomes.
a) Phasic eCB signaling schematics are based on experimental data obtained in the CA1 area of the hippocampus. Dendritic disinhibition requires afferent activity, glutamate release, I-mGluR activation, and mobilization of eCBs that heterosynaptically depress nearby inhibitory synapses [62]. Somatic disinhibition can be elicited by pairing perforant path and Schaffer Collateral (Sch) inputs [63]. Somatodendritic disinhibition can be triggered with postsynaptic activity alone, which presumably leads to cell-wide increases in Ca2+ signaling (and eCB mobilization) in response to invading back-propagating action potentials [61]. The spatial profile for tonic eCB signaling remains unknown.
b) Hypothetical input (e.g. rate or timing)/output (e.g. firing frequency or probability) transformations inspired by experimentation and modeling [84,132] highlight the potential functional significance of eCB-mediated disinhibition on excitation. These possibilities are not exhaustive nor do we consider here plasticity occurring at excitatory synapses.
Evidence for tonic actions at inhibitory synapses is disparate, but when observed, can be mediated by 2-AG [68] or AEA [69,70]. Several groups have reported tonic eCB signaling [65,71–74], a finding not shared by others [62,75–77]. Tonic eCB signaling can be observed as an increase in GABAergic transmission in the presence of specific CB1R antagonists, all of which exhibit inverse agonism. Inverse agonism effectively decreases the activity of a given receptor below its basal level and can only be observed when GPCRs constitutively signal in the absence of endogenous ligand [78,79]. Refuting the inverse agonist hypothesis of tonic eCB signaling, tonic affects can be blocked by chelating postsynaptic Ca2+ [69,72,80] or inhibiting eCB synthesizing enzymes [65,81]. These manipulations cannot distinguish between intrinsic eCB signaling arising from a specified neuron or tonic endogenous activation of I-mGluRs or mAChRs which can promote eCB mobilization [82,83]. Other potential issues to consider are the concentration of CB1R antagonist/inverse agonist used and where a recording was made, as the health of relatively superficial cells in brain slices is likely compromised. More research is needed to ascertain the magnitude, functional relevance, and the spatial extent of tonic eCB signaling at inhibitory interneurons, as tonic GABAergic signaling can have a significant impact on neuronal input/output transformations [23,84].
Pre- and/or postsynaptic eCB degradative enzymes shape the spatiotemporal profile of eCB signaling at interneurons. Accumulating evidence indicates synaptic 2-AG signaling is primarily regulated by monoacylglycerol lipase (MGL) [68,76,85–87]. Whether changes of MGL expression/activity could be a physiological mechanism for controlling eCB signaling at the synapse is unclear. At hippocampal inhibitory synapses onto CA1 pyramidal cells, 2-AG diffusion between pyramids is more restricted than originally thought [75], even when MGL is pharmacologically inhibited [61]. In the cerebellum, 2-AG diffusion from Purkinje cells is largely unrestricted [88] but seems to be controlled by MGL expression across synapses and glia [85]. At GABAergic synapses onto hypothalamic magnocellular endocrine cells, astrocytes normally impede eCB signaling, and manipulations that lead to astrocytic retraction unmask eCB signaling [70]. These results suggest other degradative enzymes (or lack of) control eCB diffusion. Additional enzymes that terminate eCB signaling are the serine hydrolases αβ-hydrolyzing domain 6 and 12, cyclooxygenase-2, and fatty acid amide hydrolase [10,13,14], but little is known about how these enzymes regulate eCB diffusion at inhibitory synapses.
Endocannabinoid Signaling in Inhibitory Microcircuits
Blueprints for proper mature circuit function are laid down during development, and a myriad of molecular cues, including eCBs, help organize the early CNS by influencing neural specification, migration, target selection, and synaptogenesis [6,7]. Interfering with eCB signaling can disrupt brain patterning which may underlie certain neuropsychiatric conditions like schizophrenia (see below). CB1Rs are enriched in GABAergic axonal growth cones (as well as principal cells), and eCB signaling induces chemo-repulsion and growth cone collapse [89]. Embryonic CB1R deletion leads to reductions in parvalbumin expression, a calcium binding protein found in fast-spiking interneurons, as assessed immunohistochemically in neocortex and striatum [90]. Disrupted eCB signaling thus appears to negatively impact network function.
Perisomatic fast-spiking interneurons help channel information though microcircuits by imparting synchrony to their targets, governing action potential generation, and enforcing precisely timed spikes and oscillations [23,25]. In vitro experiments showed that perisomatic fast-spiking interneurons mediate cholinergically-induced oscillations in hippocampal area CA3 [91]. Furthermore, regular-spiking, perisomatic CB1R-expressing interneurons were found to entrain acetylcholine-induced oscillations in CA1 [92,93]. In vivo, CB1R-bearing CA1 interneurons exhibit homogeneous firing characteristics with respect to extracellular oscillations [94], contrasting sharply with those in CA3 [95]. These findings highlight the need to carefully examine which interneuron cell-types contribute to and participate in neuronal oscillations.
Perisomatic inhibition may also contribute to a network property of hippocampal pyramidal cells known as phase precession. Phase precession manifests as spike-time phase advancement relative to local field potential oscillations and helps encode an animal’s spatial position [96–98]. Activity-dependent eCB release may contribute to the mechanism of phase precession (examined in vitro), which depresses transmitter release from CB1R-bearing GABAergic inputs thereby permitting eCB-insensitive fast-spiking interneurons to dominate and phase-advance spikes [96]. While these results are consistent with the ability of eCBs to shape spike-timing [99], somatic CB1R-expressing inhibitory synapses may exhibit greater eCB sensitivity than dendritic inputs [100] (see also [61]), thus providing an activity-dependent eCB-mediated disinhibitory mechanism for gating somatic output [61,63]. eCB-mediated disinhibition may be a general feature of inhibitory microcircuits as it has been observed in hippocampus [61,63], striatum [41,101], and midbrain periaqueductal gray [102,103]. eCB-mediated disinhibitory states (Fig. 2b) can dynamically route excitation through neuronal networks by increasing the contrast between active and inactive cells still under inhibitory control [25,94]. Validating this hypothesis is challenging because CB1Rs are expressed at glutamatergic, GABAergic, and neuromodulatory synapses as well as astrocytes [16], each of which probably contribute to the complex effects that cannabinoids have on network oscillations in vivo [104,105]. Focused attempts to selectively inactivate each of these cell-types should help disentangle the relative contribution that each makes to local circuit function.
Interneuronal synchrony is facilitated by gap junctions. Cortical fast-spiking interneurons are synchronized via gap junctions [106], and regular-spiking, CB1R-positive forebrain interneurons [107,108] are also electrically coupled. Certain regular-spiking and fast-spiking interneurons express CB1Rs whose activation depresses transmitter release. Thus, eCB-mediated effects at chemical inhibitory synapses, by reducing shunting inhibition, could augment electrical coupling between interneurons. Fast-spiking and regular-spiking interneurons often exhibit different modes of neurotransmitter release (i.e. synchronous vs. asynchronous, respectively) [109,110]. These release modes (and their modifiability by eCBs) may confer additional computational flexibility to oscillating electrical networks such that reduced asynchronous chemical inhibition facilitates downstream spike generation and precision.
Contribution of Endocannabinoid Signaling at Interneurons to Behavior and Disease
Evidence suggests that eCB-mediated inhibitory synaptic plasticity occurs in vivo, and interfering with eCB signaling has aversive effects on learning and memory [5,111]. Mice constitutively lacking CB1R or Rab3B protein exhibit disrupted eCB-mediated iLTD at synapses onto principal amygdala [112] or hippocampal [46] neurons, respectively. These in vitro findings correlated with alterations in the extinction of learned memories, albeit in opposite directions. To circumvent issues inherent in knock-out strategies, an innovative micro-RNA knock-down strategy was recently employed to specifically address the contribution of CB1R-expressing GABAergic neurons to behavior [113]. Baseline learning and memory was unaffected, but mice showed elevated and persistent auditory fear conditioning. To extend these findings, this mouse could be used in conjunction with optogenetic tools to selectively stimulate or inactivate CB1R-expressing interneurons.
Normal experience-dependent maturation of neocortical GABAergic transmission requires eCB-dependent plasticity at inhibitory synapses [45]. In murine visual cortex, inhibition matures relatively slowly until puberty and is thought to sculpt circuits required for sensory processing during the critical period of ocular dominance plasticity. There is evidence that eCB-mediated iLTD likely drives GABAergic transmission into a more mature state [45]. While sequential maturation of GABAergic synaptic transmission has been observed across visual cortical layers, eCB signaling is relevant in L2/3 but not L4 [114]. eCB-mediated iLTD can be reactivated in mature animals re-exposed to dark conditions, indicating critical windows can be reopened by sensory experience [115]. Exactly how this form of plasticity is reinitiated remains to be determined.
Diet and stress can also powerfully influence eCB signaling in vivo [116]. Diet-induced obese mice exhibit enhanced eCB-mediated short-term and long-term hippocampal inhibitory synaptic plasticity [117]. In hypothalamic satiety circuits, food deprivation and acute restraint stress suppresses eCB-mediated iLTD, which favors long-term potentiation by nitric oxide at GABAergic synapses [118]. Several studies found that stress alters eCB signaling [119–123], which is presumably controlled by an elusive membrane-bound Gq/11-coupled glucocorticoid receptor. Altered eCB signaling might be a general feature of stressful events.
Beyond their involvement in inhibitory microcircuits and certain behavioral states, dysregulated eCB signaling is observed in animal models of human neuropsychiatric disorders such as autism, epilepsy, and schizophrenia [3,8–11]. Recent data indicates tonic, not phasic, eCB signaling is dysregulated at CA1 hippocampal inhibitory synapses in which the autism-associated, cell-adhesion molecule neuroligin-3 was disrupted [73]. This finding also suggests neuroligin-3 orchestrates the putative tonic eCB release machinery, but the mechanism for this action is totally unknown. In Fragile X syndrome, the most common single-gene cause of autism, disruption of the mRNA binding Fragile X mental retardation protein (FMRP) causes local dysregulation of I-mGluR signal transduction. In FMRP null mice, where coupling between I-mGluR activation and eCB mobilization is likely altered, eCB signaling was enhanced at inhibitory synapses in the hippocampus [124] and dorsal striatum [125] but impaired at excitatory synapses in the ventral striatum and prefrontal cortex [126]. Dysregulated eCB signaling also contributes to epilepsy [127,128]. GABAergic CB1Rs are generally pro-convulsive whereas glutamatergic CB1Rs exhibit neuroprotection against seizures. CB1R agonists can be pro- or anti-convulsive, the discrepancies possibly resulting from differences in experimental epilepsy models and/or eCB-mediated effects on inhibitory vs. excitatory transmission. In addition, evidence indicates perturbations to GABAergic synaptic transmission, such as reduced CB1R expression, are correlated with schizophrenia [129,130]. Neuregulin-1 is elevated in schizophrenics and prolonged treatment of hippocampal slice cultures with neuregulin-1 enhances MGL expression and curtails 2-AG-mediated forms of inhibitory synaptic plasticity [86]. Together, these findings suggest the role of eCB signaling should be further considered in neuropsychiatric conditions and as therapeutic targets.
Summary and Open Questions
Significant progress has been made in our understanding of how eCBs signal at inhibitory interneurons and their functional consequences in normal and pathophysiological circuits, but several questions remain unanswered. What mechanisms underlie specific pre- and/or postsynaptic activity patterns of 2-AG and/or AEA mobilization, and what function do they serve? Which factor(s) determine whether eCBs affect synapses, intrinsic excitability, or both? How are eCBs transported across the synaptic cleft? What are the relative contributions that various degradative enzymes have on the eCB spatiotemporal signaling profile? What is the precise role of tonic eCB release? How does eCB-mediated disinhibition influence local circuit function in vivo and is runaway disinhibition related to neuropsychiatric conditions such as autism, epilepsy and schizophrenia? While eCB signaling can impact many interneuronal functions, they are only one piece of a larger puzzle. The field should soon have answers to these and other outstanding questions as genetic manipulation of CB1R expressing interneurons in vivo is now possible [113,131].
Highlights.
Endocannabinoids (eCBs) are powerful modulators of inhibitory synaptic transmission
eCBs control inhibitory synapses in an activity-dependent (i.e. phasic) or tonic manner
By regulating inhibitory microcircuits, eCB signaling contributes to behavior
Dysregulated eCB signaling contributes to common neuropsychiatric conditions
Acknowledgments
This work supported by the National Institutes of Health (R01-DA17392 and R01-MH081935) and the Irma T. Hirschl Career Scientist Award to P.E.C. Special thanks to Yuki Hashimotodani and Haleigh R. Smith for comments on the manuscript. We apologize to those authors whose work we did not cite due to space limitations.
Footnotes
There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidhpura N, Parsons LH. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology. 2011;61:1070–1087. doi: 10.1016/j.neuropharm.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillard CJ, Weinlander KM, Stuhr KL. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–229. doi: 10.1016/j.neuroscience.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roques BP, Fournie-Zaluski MC, Wurm M. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat Rev Drug Discov. 2012;11:292–310. doi: 10.1038/nrd3673. [DOI] [PubMed] [Google Scholar]
- 5.Marsicano G, Lafenetre P. Roles of the endocannabinoid system in learning and memory. Curr Top Behav Neurosci. 2009;1:201–230. doi: 10.1007/978-3-540-88955-7_8. [DOI] [PubMed] [Google Scholar]
- 6.Harkany T, Mackie K, Doherty P. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol. 2008;18:338–345. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Alonso J, Guzman M, Galve-Roperh I. Endocannabinoids via CB(1) receptors act as neurogenic niche cues during cortical development. Philos Trans R Soc Lond B Biol Sci. 2012;367:3229–3241. doi: 10.1098/rstb.2011.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ligresti A, Petrosino S, Di Marzo V. From endocannabinoid profiling to ‘endocannabinoid therapeutics’. Curr Opin Chem Biol. 2009;13:321–331. doi: 10.1016/j.cbpa.2009.04.615. [DOI] [PubMed] [Google Scholar]
- 9.Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- 10.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 12.DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35:403–411. doi: 10.1016/j.tins.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2) Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piomelli D. More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 18•.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. An exhaustive review highlighting the state-of-the-art on eCB signaling at synapses. [DOI] [PubMed] [Google Scholar]
- 19.Alger BE. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know. J Physiol. 2012;590:2203–2212. doi: 10.1113/jphysiol.2011.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron. 2012;75:951–962. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 23•.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. This review discusses some of the known roles for inhibition in microcircuit function in vitro and in vivo and raises several important and outstanding issues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–338. doi: 10.1016/j.conb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 28.Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca(2+)-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol. 2007;17:360–365. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, Blat Y, Westphal RS, Zaczek R, Lewis DA, et al. Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol. 2011;589:4857–4884. doi: 10.1113/jphysiol.2011.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimotodani Y, Ohno-Shosaku T, Tanimura A, Kita Y, Sano Y, Shimizu T, Di Marzo V, Kano M. Acute inhibition of diacylglycerol lipase blocks endocannabinoid-mediated retrograde synaptic suppression: evidence for on-demand biosynthesis of 2-arachidonoylglycerol. J Physiol. 2013 doi: 10.1113/jphysiol.2013.254474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Wang M, Bisogno T, Di Marzo V, Alger BE. Endocannabinoids generated by Ca2+ or by metabotropic glutamate receptors appear to arise from different pools of diacylglycerol lipase. PLoS One. 2011;6:e16305. doi: 10.1371/journal.pone.0016305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min R, Testa-Silva G, Heistek TS, Canto CB, Lodder JC, Bisogno T, Di Marzo V, Brussaard AB, Burnashev N, Mansvelder HD. Diacylglycerol lipase is not involved in depolarization-induced suppression of inhibition at unitary inhibitory connections in mouse hippocampus. J Neurosci. 2010;30:2710–2715. doi: 10.1523/JNEUROSCI.BC-3622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shonesy BC, Wang X, Rose KL, Ramikie TS, Cavener VS, Rentz T, Baucum AJ, 2nd, Jalan-Sakrikar N, Mackie K, Winder DG, et al. CaMKII regulates diacylglycerol lipase-alpha and striatal endocannabinoid signaling. Nat Neurosci. 2013;16:456–463. doi: 10.1038/nn.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cristino L, Starowicz K, De Petrocellis L, Morishita J, Ueda N, Guglielmotti V, Di Marzo V. Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience. 2008;151:955–968. doi: 10.1016/j.neuroscience.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 37.Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509:400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- 38.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 39.Lerner TN, Kreitzer AC. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron. 2012;73:347–359. doi: 10.1016/j.neuron.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, Grandes P, Manzoni OJ. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–1547. doi: 10.1038/nn.2974. [DOI] [PubMed] [Google Scholar]
- 41••.Mathur BN, Tanahira C, Tamamaki N, Lovinger DM. Voltage drives diverse endocannabinoid signals to mediate striatal microcircuit-specific plasticity. Nat Neurosci. 2013;16:1275–1283. doi: 10.1038/nn.3478. These authors introduce a new concept: depending on the resting membrane potential of striatal medium spiny neurons, either 2-AG and/or AEA can be preferentially mobilized. This concept may be a general feature of all neurons, and their results provide additional support for eCB-signaling at fast-spiking interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- 43.Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci U S A. 2008;105:10250–10255. doi: 10.1073/pnas.0711880105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J Neurosci. 2007;27:5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Jiang B, Huang S, de Pasquale R, Millman D, Song L, Lee HK, Tsumoto T, Kirkwood A. The maturation of GABAergic transmission in visual cortex requires endocannabinoid-mediated LTD of inhibitory inputs during a critical period. Neuron. 2010;66:248–259. doi: 10.1016/j.neuron.2010.03.021. This research nicely relates experience-dependent modifications at inhibitory synapses to long-lasting forms of eCB-mediated plasticity in vitro, strongly suggesting eCB-mediated changes in synapse strength are critical for shaping cortical processing during development in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsetsenis T, Younts TJ, Chiu CQ, Kaeser PS, Castillo PE, Sudhof TC. Rab3B protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc Natl Acad Sci U S A. 2011;108:14300–14305. doi: 10.1073/pnas.1112237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahumada J, de Sevilla DF, Couve A, Buno W, Fuenzalida M. Long-term depression of inhibitory synaptic transmission induced by spike- timing dependent plasticity requires co-activation of endocannabinoid and muscarinic receptors. Hippocampus. 2013 doi: 10.1002/hipo.22196. [DOI] [PubMed] [Google Scholar]
- 48.Chiu CQ, Puente N, Grandes P, Castillo PE. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci. 2010;30:7236–7248. doi: 10.1523/JNEUROSCI.0736-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 51.Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006;24:2246–2252. doi: 10.1111/j.1460-9568.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- 52.Winters BD, Kruger JM, Huang X, Gallaher ZR, Ishikawa M, Czaja K, Krueger JM, Huang YH, Schluter OM, Dong Y. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc Natl Acad Sci U S A. 2012;109:E2717–2725. doi: 10.1073/pnas.1206303109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De-May CL, Ali AB. Cell type-specific regulation of inhibition via cannabinoid type 1 receptors in rat neocortex. J Neurophysiol. 2013;109:216–224. doi: 10.1152/jn.00272.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galarreta M, Erdelyi F, Szabo G, Hestrin S. Cannabinoid sensitivity and synaptic properties of 2 GABAergic networks in the neocortex. Cereb Cortex. 2008;18:2296–2305. doi: 10.1093/cercor/bhm253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterfi Z, Urban GM, Papp OI, Nemeth B, Monyer H, Szabo G, Erdelyi F, Mackie K, Freund TF, Hajos N, et al. Endocannabinoid-mediated long-term depression of afferent excitatory synapses in hippocampal pyramidal cells and GABAergic interneurons. J Neurosci. 2012;32:14448–14463. doi: 10.1523/JNEUROSCI.1676-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marinelli S, Pacioni S, Bisogno T, Di Marzo V, Prince DA, Huguenard JR, Bacci A. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J Neurosci. 2008;28:13532–13541. doi: 10.1523/JNEUROSCI.0847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- 58.Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–368. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Younts TJ, Chevaleyre V, Castillo PE. CA1 Pyramidal Cell Theta-Burst Firing Triggers Endocannabinoid-Mediated Long-Term Depression at Both Somatic and Dendritic Inhibitory Synapses. J Neurosci. 2013;33:13743–13757. doi: 10.1523/JNEUROSCI.0817-13.2013. This study shows that activity alone is sufficient for eliciting iLTD across the somatodendritic axis of pyramidal cells. This form of plasticity is spatially restricted to single, active neurons and functionally gates excitation by virtue of disinhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 63.Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–1221. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lourenco J, Matias I, Marsicano G, Mulle C. Pharmacological activation of kainate receptors drives endocannabinoid mobilization. J Neurosci. 2011;31:3243–3248. doi: 10.1523/JNEUROSCI.3512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. This article focuses on sex differences related to eCB signaling in the brain, a general concept that might be further considered by other investigators in the future. The authors found that estradiol suppresses inhibitory synaptic transmission in female hippocampus in a manner requiring a not yet fully characterized interaction between estrogen receptor-α and mGluRs that mobilizes AEA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hashimotodani Y, Ohno-Shosaku T, Yamazaki M, Sakimura K, Kano M. Neuronal protease-activated receptor 1 drives synaptic retrograde signaling mediated by the endocannabinoid 2-arachidonoylglycerol. J Neurosci. 2011;31:3104–3109. doi: 10.1523/JNEUROSCI.6000-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J Neurophysiol. 2010;104:1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J, Alger BE. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat Neurosci. 2010;13:592–600. doi: 10.1038/nn.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di S, Popescu IR, Tasker JG. Glial control of endocannabinoid heterosynaptic modulation in hypothalamic magnocellular neuroendocrine cells. J Neurosci. 2013;33:18331–18342. doi: 10.1523/JNEUROSCI.2971-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci U S A. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013;78:498–509. doi: 10.1016/j.neuron.2013.02.036. This is the first report to link tonic eCB signaling and cell-adhesion molecules at inhibitory synapses. While the mechanism for this interaction is unclear, it is open up a potentially new area of research investigating how intercellular proteinaceous bridges contributes to eCB signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu PJ, Lovinger DM. Developmental alteration of endocannabinoid retrograde signaling in the hippocampus. J Neurophysiol. 2010;103:1123–1129. doi: 10.1152/jn.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 76.Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu QS. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Howlett AC, Reggio PH, Childers SR, Hampson RE, Ulloa NM, Deutsch DG. Endocannabinoid tone versus constitutive activity of cannabinoid receptors. British journal of pharmacology. 2011;163:1329–1343. doi: 10.1111/j.1476-5381.2011.01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farkas I, Kallo I, Deli L, Vida B, Hrabovszky E, Fekete C, Moenter SM, Watanabe M, Liposits Z. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Watanabe M, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dvorzhak A, Semtner M, Faber DS, Grantyn R. Tonic mGluR5/CB1-dependent suppression of inhibition as a pathophysiological hallmark in the striatum of mice carrying a mutant form of huntingtin. J Physiol. 2013;591:1145–1166. doi: 10.1113/jphysiol.2012.241018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silver RA. Neuronal arithmetic. Nat Rev Neurosci. 2010;11:474–489. doi: 10.1038/nrn2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85•.Tanimura A, Uchigashima M, Yamazaki M, Uesaka N, Mikuni T, Abe M, Hashimoto K, Watanabe M, Sakimura K, Kano M. Synapse type-independent degradation of the endocannabinoid 2-arachidonoylglycerol after retrograde synaptic suppression. Proc Natl Acad Sci U S A. 2012;109:12195–12200. doi: 10.1073/pnas.1204404109. These investigators found that a subset of nerve terminals and astrocytes express MGL that can actually regulate 2-AG actions at relatively remote sites in the cerebellum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du H, Kwon IK, Kim J. Neuregulin-1 Impairs the Long-term Depression of Hippocampal Inhibitory Synapses by Facilitating the Degradation of Endocannabinoid 2-AG. J Neurosci. 2013;33:15022–15031. doi: 10.1523/JNEUROSCI.5833-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 89.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 90.Fitzgerald ML, Lupica CR, Pickel VM. Decreased parvalbumin immunoreactivity in the cortex and striatum of mice lacking the CB1 receptor. Synapse. 2011;65:827–831. doi: 10.1002/syn.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hajos N, Karlocai MR, Nemeth B, Ulbert I, Monyer H, Szabo G, Erdelyi F, Freund TF, Gulyas AI. Input-output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro. J Neurosci. 2013;33:11677–11691. doi: 10.1523/JNEUROSCI.5729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagode DA, Tang AH, Karson MA, Klugmann M, Alger BE. Optogenetic release of ACh induces rhythmic bursts of perisomatic IPSCs in hippocampus. PLoS One. 2011;6:e27691. doi: 10.1371/journal.pone.0027691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nagode DA, Tang AH, Yang K, Alger BE. Optogenetic Identification of an Intrinsic Cholinergically-Driven Inhibitory Oscillator Sensitive to Cannabinoids and Opioids in Hippocampal Ca1. J Physiol. 2013 doi: 10.1113/jphysiol.2013.257428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lasztoczi B, Tukker JJ, Somogyi P, Klausberger T. Terminal field and firing selectivity of cholecystokinin-expressing interneurons in the hippocampal CA3 area. J Neurosci. 2011;31:18073–18093. doi: 10.1523/JNEUROSCI.3573-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Losonczy A, Zemelman BV, Vaziri A, Magee JC. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2010;13:967–972. doi: 10.1038/nn.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsaki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dubruc F, Dupret D, Caillard O. Self-tuning of inhibition by endocannabinoids shapes spike-time precision in CA1 pyramidal neurons. J Neurophysiol. 2013 doi: 10.1152/jn.00099.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee SH, Foldy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adermark L. Modulation of endocannabinoid-mediated long-lasting disinhibition of striatal output by cholinergic interneurons. Neuropharmacology. 2011;61:1314–1320. doi: 10.1016/j.neuropharm.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 102.Drew GM, Lau BK, Vaughan CW. Substance P drives endocannabinoid-mediated disinhibition in a midbrain descending analgesic pathway. J Neurosci. 2009;29:7220–7229. doi: 10.1523/JNEUROSCI.4362-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liao HT, Lee HJ, Ho YC, Chiou LC. Capsaicin in the periaqueductal gray induces analgesia via metabotropic glutamate receptor-mediated endocannabinoid retrograde disinhibition. Br J Pharmacol. 2011;163:330–345. doi: 10.1111/j.1476-5381.2011.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sales-Carbonell C, Rueda-Orozco PE, Soria-Gomez E, Buzsaki G, Marsicano G, Robbe D. Striatal GABAergic and cortical glutamatergic neurons mediate contrasting effects of cannabinoids on cortical network synchrony. Proc Natl Acad Sci U S A. 2013;110:719–724. doi: 10.1073/pnas.1217144110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- 106.Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 107.Galarreta M, Erdelyi F, Szabo G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci. 2004;24:9770–9778. doi: 10.1523/JNEUROSCI.3027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108••.Iball J, Ali AB. Endocannabinoid Release Modulates Electrical Coupling between CCK Cells Connected via Chemical and Electrical Synapses in CA1. Front Neural Circuits. 2011;5:17. doi: 10.3389/fncir.2011.00017. The implications of this research are far-reaching as they suggest activity-dependent changes in chemical synaptic transmission can profoundly alter electrical coupling, a concept that must be further developed if neuroscience is to more fully understand how information is routed through circuits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 110.Ali AB, Todorova M. Asynchronous release of GABA via tonic cannabinoid receptor activation at identified interneuron synapses in rat CA1. Eur J Neurosci. 2010;31:1196–1207. doi: 10.1111/j.1460-9568.2010.07165.x. [DOI] [PubMed] [Google Scholar]
- 111.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 112.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, et al. The endogenous cannabinoid system controls extinction of aversive meories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 113.Brown JA, Horvath S, Garbett K, Schmidt MJ, Everheart M, Gellert L, Ebert P, Mirnics K. The role of cannabinoid 1 receptor expressing interneurons in behavior. Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang B, Sohya K, Sarihi A, Yanagawa Y, Tsumoto T. Laminar-specific maturation of GABAergic transmission and susceptibility to visual deprivation are related to endocannabinoid sensitivity in mouse visual cortex. J Neurosci. 2010;30:14261–14272. doi: 10.1523/JNEUROSCI.2979-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang S, Gu Y, Quinlan EM, Kirkwood A. A refractory period for rejuvenating GABAergic synaptic transmission and ocular dominance plasticity with dark exposure. J Neurosci. 2010;30:16636–16642. doi: 10.1523/JNEUROSCI.4384-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iremonger KJ, Wamsteeker Cusulin JI, Bains JS. Changing the tune: plasticity and adaptation of retrograde signals. Trends Neurosci. 2013;36:471–479. doi: 10.1016/j.tins.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 117.Massa F, Mancini G, Schmidt H, Steindel F, Mackie K, Angioni C, Oliet SH, Geisslinger G, Lutz B. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci. 2010;30:6273–6281. doi: 10.1523/JNEUROSCI.2648-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crosby KM, Inoue W, Pittman QJ, Bains JS. Endocannabinoids gate state-dependent plasticity of synaptic inhibition in feeding circuits. Neuron. 2011;71:529–541. doi: 10.1016/j.neuron.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci. 2010;30:11188–11196. doi: 10.1523/JNEUROSCI.1046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu W, Zhang M, Czeh B, Zhang W, Flugge G. Chronic restraint stress impairs endocannabinoid mediated suppression of GABAergic signaling in the hippocampus of adult male rats. Brain Res Bull. 2011;85:374–379. doi: 10.1016/j.brainresbull.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 121.Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2- arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sumislawski JJ, Ramikie TS, Patel S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress- induced behavioral adaptation. Neuropsychopharmacology. 2011;36:2750–2761. doi: 10.1038/npp.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol. 2012;26:56–70. doi: 10.1177/0269881111409606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124•.Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. This is the first report examining dysregulated eCB signaling at inhibitory synapses in the hippocampus. The authors report that lack of FMRP enhances eCB-mediated synaptic plasticity, and indicates, along with other studies, that altered eCB signaling should be considered further in the treatment of Fragile X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A, Bernardi G, Bagni C, Centonze D. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology. 2010;35:1500–1509. doi: 10.1038/npp.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alger BE. Endocannabinoids and their implications for epilepsy. Epilepsy Curr. 2004;4:169–173. doi: 10.1111/j.1535-7597.2004.04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004;68:1691–1698. doi: 10.1016/j.bcp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 129.Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cellular and molecular life sciences : CMLS. 2006;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Volk DW, Lewis DA. GABA Targets for the Treatment of Cognitive Dysfunction in Schizophrenia. Current neuropharmacology. 2005;3:45–62. doi: 10.2174/1570159052773396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron. 2009;61:774–785. doi: 10.1016/j.neuron.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]