Abstract
Identification and manipulation of different GABAergic interneuron classes in the behaving animal are important to understand their role in circuit dynamics and behavior. The combination of optogenetics and large-scale neuronal recordings allows specific interneuron populations to be identified and perturbed for circuit analysis in intact animals. A critical aspect of this approach is coupling electrophysiological recording with spatially and temporally precise light delivery. Focal multisite illumination of neuronal activators and silencers in predetermined temporal configurations or a closed loop manner opens the door to addressing many novel questions. Recent progress demonstrates the utility and power of this novel technique for interneuron research.
Introduction
Computation in neural networks relies on dynamic interactions between excitatory and inhibitory cell types [1-4]. Appropriately timed inhibition targeted to specific somatodendritic domains of principal cells selectively filters synaptic excitation and modulates the gain, timing, tuning and bursting properties of pyramidal cell firing [4, 5]. Inhibitory interneurons also secure the transient autonomy of principal cells by flexibly congregating and segregating neuronal populations (often referred to as cell assemblies) through maintenance of oscillations and synchrony [6, 7]. A large variety of inhibitory interneurons are available for such tasks [2, 3, 5, 8, 9], but the specific contributions of the different subtypes are still unclear. Our understanding of neural network functions could therefore be advanced greatly by studying the activity of specific interneuron subtypes in intact circuits and by perturbing them in a targeted manner [10-19].
Several methods have been proposed to identify specific interneuron classes in extracellular recordings, including physiological classification based on spike waveforms, firing patterns and network-affiliated activity [7, 20-23]. Alternatively, intracellular or juxtacellular recordings, either blind [2, 9, 24, 25] or fluorescence-targeted [12, 26-29], have provided valuable information about the activity of various interneuron classes in vivo, including their relation with sensory processing and brain rhythms [2, 9]. Here we discuss how optogenetic approaches, combined with large-scale extracellular recordings in behaving animals, can be used to identify and manipulate interneurons to facilitate the understanding of their computational roles in neural circuits.
Optogenetic identification of genetically-defined interneuron subtypes
Most of our knowledge about the activity of unambiguously identified interneuron subtypes in vivo has been provided by juxtacellular or intracellular recordings combined with post-hoc morphological reconstruction, immunostainings and/or electron microscopy [2, 9, 24, 25]. The yield of this approach can now be considerably increased by using genetically encoded fluorescent markers to identify the cells to be targeted for intracellular recordings [12, 26-29]. A large panel of mouse lines is now available that enable direct visualization of the vast majority of cortical interneuron sub-types [30]. Although these methods allow unambiguous identification of the recorded interneurons and provide information about intracellular features, they are challenging in freely moving animals [25], leading to the increasing use of head-fixed preparations [14, 16, 27-29]. However, fluorescence-targeted in vivo intracellular recording requires optical access for electrode placement, which is not practical in freely moving animals, particularly for deep brain regions. More importantly, simultaneous intracellular recording of multiple neurons is still not feasible in freely moving animals, rendering this method painstakingly slow and precluding the study of interactions between cells. While imaging techniques address the problem of recording multiple neurons simultaneously, they generally suffer from low temporal resolution and difficulty targeting deep structures [31]. In contrast, extracellular recording enables monitoring of the activity of large numbers of individual neurons simultaneously in freely moving rodents [31-35]. With this method, however, the challenge is to identify which types of neurons are recorded.
Optogenetics [36-38] provides a solution for identifying specific genetically-defined neuronal subtypes in blind extracellular recordings by expressing light-sensitive opsins in a given neuronal population and inferring that light-responsive units correspond to members of that population. Both activation [17, 19, 39, 40] and silencing [41] strategies can be used for this purpose. Various methods for cell-type specific expression of opsins have been developed [42-45] along with techniques to deliver optical stimulation during extracellular electrophysiological recordings [46-49]. While implementing this photostimulation-recording method seems relatively straightforward, a number of technical issues must be addressed to exploit its full potential.
Drawbacks of current photostimulation methods
Most of the technical problems of unit recording/analysis arise from the use of large-diameter optical fibers [50-53] or brain surface illumination [14, 54, 55] and the use of high light power. These large fibers are well suited for experiments in which a large illumination area is desired to generate a behavioral phenotype but detrimental for optogenetic circuit analysis and/or extracellular identification of units.
Commercially available optical fibers used in most current optogenetic experiments typically have large diameters (>100 μm) and should be placed at least 200 μm away from the recording electrodes to avoid damage of the targeted neuronal population [52]. Due to the strong light-absorbing nature of neuronal tissue, high light power (several mW) is therefore required to activate neurons at the distant recording sites [50-52, 53]. Brain surface illumination requires even higher power (>30 mW) to reach deeper cortical layers [54, 55]. Continuous application of light at such high power can cause local heating, leading to neuronal dysfunction and potential cellular damage.
Moreover, high power photostimulation can prevent accurate “spike sorting.” This key process in large-scale extracellular recordings consists of assigning the recorded spikes to the individual neurons that generated them by grouping them in distinct clusters based on similarities in spike waveform features. High stimulation power interferes with this process by inducing photoelectric artifacts [40, 50, 51, 55] that can distort spike waveforms, especially when short light pulses are used. Additionally, in the case of optogenetic activation (e.g. with channelrhodopsin - ChR2), high light intensities frequently cause synchronous firing in multiple cells, leading to superimposed spike waveforms that cannot be sorted accurately [47, 56].
Another problem is that neurons not expressing opsins may be stimulated indirectly via synaptic pathways. This occurs primarily when optogenetic stimulation targets excitatory cells, which can excite post-synaptic neurons upon illumination. This problem is exacerbated with large optical fibers and high light intensities because more neurons are stimulated, increasing the probability that downstream neurons fire. Fortunately, this problem is less serious when ChR2 is expressed in GABAergic inhibitory interneurons, which do not excite postsynaptic cells, although post-stimulus rebound spiking [17, 18] or disinhibition [19] may occur. Nonetheless, since single-unit spikes can be detected and sorted only ∼up to 60 μm laterally from the recording sites [56], most of the neurons photostimulated under these conditions are not recorded, making the disambiguation between direct and network-mediated effects more complex. Moreover, other indirect effects such as visual responses evoked by light striking the retina must also be considered [40].
Proposed improvements for optogenetic identification of interneurons
Several laboratories have offered solutions for optical stimulation of local circuits combined with simultaneous neuronal recordings. Although these “optrodes” have proved useful in many applications [50-53, 57], at least three improvements can enhance the reliability of optogenetic identification of interneurons: local delivery of low-intensity light, application of appropriate stimulus waveforms, and replacement of large benchtop lasers with small head-mounted LEDs or laser diodes.
Many of the problems with spike sorting and indirect stimulation can be reduced, if not eliminated, by etching small-core (≤50 μm) optical fibers to a point (∼10 μm or less) and mounting them very close (∼40 μm) to the recording sites (Fig. 1; [47, 49]). Hybrid devices combining silicon probes or tetrodes with etched optical fibers allow the use of extremely low-power (1-10 μW) stimulation due to the proximity of the recorded neurons to the light source. Such configuration aims at photostimulating only neurons that can be recorded from. Overall, this method eliminates photoelectric artifacts and enables identification of the light-responsive units, while also limiting tissue damage [18, 47, 49].
Figure 1. Diode probes for optogenetic identification of interneurons.
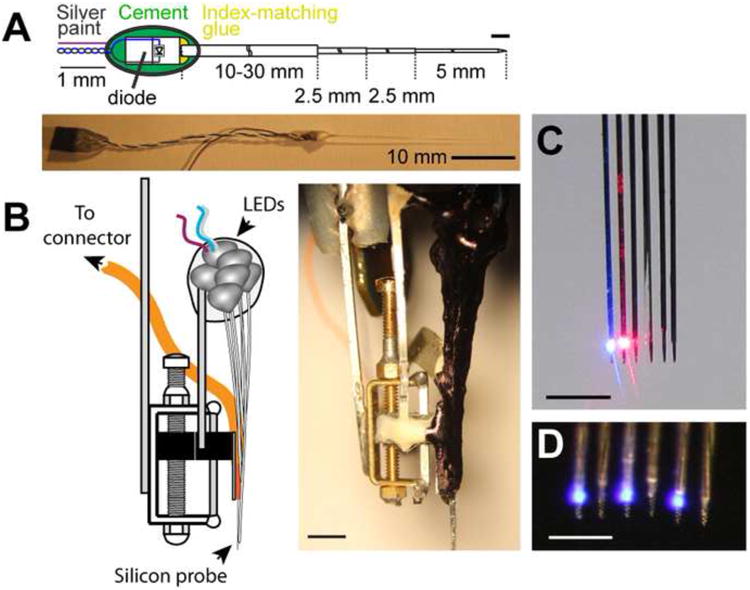
A. Schematic of a single LED-fiber assembly. The LED is coupled to a 50-μm multimode fiber, etched to a point at the distal (brain) end. B. Left: schematic of a drive equipped with a 6-shank diode probe with LED-fibers mounted on each shank. Etched optical fibers are attached ∼40μm above the recordings sites on the silicon probe shanks. Right: picture of the drive depicted on the left. Scale bar: 3 mm. C-D. Local delivery of light. Magnified frontal view of the 6-shank diode probe equipped with diode-coupled optical fibers. C. Two adjacent shanks illuminated with blue and red light. Scale bar: 1mm. D. Four shanks illuminated with blue light. Scale bar: 0.5 mm (A and C) Reproduced from [49].
Overlapping spike waveforms due to synchronous light-evoked action potentials can also be reduced by using structured low-intensity stimulus waveforms such as sinusoids and identifying light-responsive units by their correlation with the stimulus pattern [47, 49]. The rationale behind this approach is that in response to a slowly changing stimulus, targeted neurons do not fire simultaneously because they receive different illumination intensities, express different amounts of opsins, and/or exhibit different resting membrane potentials or thresholds for spike generation. Sinusoidal stimuli have the additional benefit of not inducing photoelectric artifacts that are likely to distort spike waveforms, and they have been used successfully to identify opsin-expressing interneurons in vivo [18, 45].
Optogenetics offers unprecedented opportunities to stimulate and silence neurons at multiple locations and structures, which is extremely useful for studying the role of interneurons in ensemble organization [49]. Fine spatiotemporal control of distributed groups of neurons can be achieved by using multiple benchtop lasers coupled through optical fibers to head fixed animals [47, 48, 53]. However, connecting multiple stiff optic patch cords to a small rodent can seriously restrain its movements in tasks that require free navigation. One solution is to use miniature light emitting diodes (LED) and/or laser diodes that are small enough to be mounted on the head of a freely moving animal. These diodes can be coupled to short, small-diameter (≤50 μm) multimode fibers and attached directly to the shanks of a silicon probe or tetrode (Fig. 1, [49]). The small size and weight of these integrated probes (∼2 g for a 4-shank/4-LED probe) allow fast, multisite and multicolor optogenetic manipulations in freely moving animals with concurrent monitoring of the manipulated neurons.
The currently method of manually attaching fibers to each probe shank is very labor-intensive and may result in inaccurate alignment. However, efforts are underway for automated fabrication of monolithically integrated optical waveguides and LEDs in multi-electrode silicon probes [58, 59], yielding yet smaller and lighter devices.
Optogenetics-supervised, physiology-based classification of interneurons
Over the years, numerous classification schemes based on a variety of physiological criteria were developed to assign extracellular spikes to putative interneurons or pyramidal cells. These include waveform features, firing rate statistics in different brain states, embeddedness in various population activities, firing patterns characterized by their autocorrelograms, and putative monosynaptic connections to other neurons [7, 20-23]. However, it is crucial not only to separate interneurons from pyramidal cells, but also to recognize and correlate activity in different interneuron subtypes with network dynamics and behavior [2, 7, 9]. An important goal of the optogenetic approach is to assist the identification of interneuron classes on the basis of their physiological patterns [7] so that purely physiological criteria could be used in subsequent experiments without the need for optogenetics (Fig. 2) [9]. This would involve iterative refinement of a library of parameters that could be used subsequently for the identification of interneuron subtypes [17-19, 41, 45]. The optogenetically identified neurons would thus provide the necessary “ground truth” for physiology-based cell type identification. Furthermore, physiological classification methods can also serve to distinguish distinct subtypes of interneurons within individual molecularly identified classes [3].
Figure 2. Optogenetic identification of interneurons.
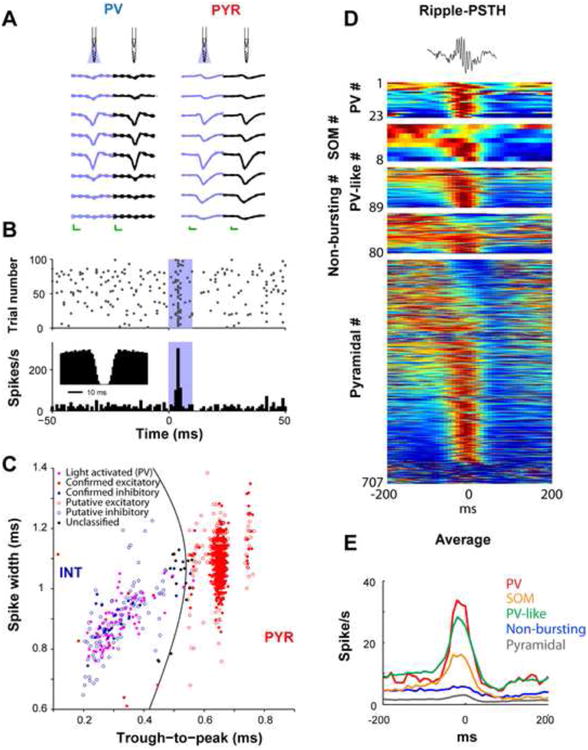
A. Right: unfiltered spontaneous (black) and light-induced (blue) waveforms of a parvalbumin-expressing interneuron (PV) and a pyramidal cell (PYR) at eight recording sites. Note the similarity of the waveforms with and without illumination. Mean and SD; calibration: 0.25 ms, 50 μV. B. Diode probe-induced unit firing in the hippocampal CA1 region (blue shaded area superimposed on the raster plot -top-and the histogram -bottom-; 4 μW at fiber tip). Inset: autocorrelogram shows a shape typical for fast spiking PV interneurons. C. Clustered units are tagged as excitatory or inhibitory based on monosynaptic peaks/troughs in cross-correlation histograms (filled blue and red symbols) and/or response to locally-delivered 50-70 ms light pulses (filled violet symbols) in transgenic mice expressing ChR2 in PV cells. Untagged units (empty symbols) are classified as putative excitatory pyramidal cells (PYR) or inhibitory interneurons (INT) according to waveform morphology; untagged units with low classification confidence are also shown in black (“unclassified”) [18]. D. Optogenetic identification of interneuron classes, including here PV-and somatostatin (SOM)-expressing interneurons, allows studying their relationships to network patterns such as sharp wave ripple events. Top: single ripple. Each row represents the color-coded peri-ripple histogram of the firing rate computed for individual neurons. E. Average firing rate observed for the different cell categories. (B) Reproduced from [49]. (C) Reproduced from [18]. (D and E) Reproduced from [41].
Circuit analysis by interneuron perturbation
Optogenetics not only enables identification of genetically-defined interneuron subtypes but also provides a way to perturb native network patterns locally and identify the causal role of specific interneuron classes in population activity. Experiments combining these perturbation methods with large-scale extracellular recordings or with other techniques (calcium imaging, targeted loose-patch or whole-cell recordings), are becoming increasingly common in the investigation of interneuron function [4].
For instance, the understanding of visual cortex function has benefited recently from the power of optogenetics. Several studies have addressed the role of specific interneuron subtypes in this region by characterizing their specific response properties and manipulating their activity to determine their impact on principal cell responses. Such optogenetic manipulations suggested that PV interneurons principally control the gain of sensory responses, whereas dendrite-targeting, somatostatin-expressing (SOM) neurons sharpen selectivity [12, 15]. However, other interpretations have also been offered [14, 60]. Notably, SOM interneurons have been shown to play a critical role in surround suppression [28].
The location of the visual cortex at the surface of the brain makes it a convenient target for optogenetic manipulations and recordings. Most of the studies mentioned above used fluorescence-targeted in vivo intracellular recordings, which are still extremely challenging in freely moving animals. In order to decipher the respective functions of PV and SOM interneurons in freely moving rodents, several studies have used extracellular recordings combined with optogenetics in other brain regions. For instance, large-scale extracellular recordings in the prefrontal cortex have shown that PV interneurons exert brief and uniform inhibition on their targets while SOM neurons have longer and more variable inhibitory effects [17]. The same study also demonstrated that a subgroup of optogenetically-identified SOM neurons fired preferentially at reward locations, whereas PV neurons responded when the animal was leaving these reward locations [17]. In the CA1 region of the hippocampus, PV and SOM interneurons exerted complementary effects on place cells by suppressing their activity during the rising and decaying parts of the place field, respectively. Inactivation of PV, but not SOM, interneurons was also shown to interfere with the normal phase assignment of spikes to the theta cycle [41]. Moreover, the strong action of SOM interneurons on spike burst generation in principal cells has been demonstrated both in hippocampus and neocortex, an important feature that is not shared with PV cells [16, 41]. Using similar techniques, a recent study also addressed the role of a third population of interneurons, the vasoactive intestinal peptide (VIP) - expressing interneurons. Optogenetic manipulations were used for both identifying VIP interneurons in extracellular recordings and interfering with their activity in the medial prefrontal and auditory cortex of freely moving mice. This study revealed that these cells can release excitatory cells from inhibition by inhibiting other interneurons [19].
Optogenetics combined with extracellular recordings has also advanced the study of interneuron function in oscillatory processes in the neocortex and the hippocampus. In neocortex, for example, strong optogenetic activation of PV-expressing interneurons was shown to amplify gamma oscillations, coordinate the timing of sensory inputs relative to a gamma cycle, and enhance signal transmission [10, 11, 61]. Complementarily, activation of PV interneurons with lower light intensities in both neocortex and hippocampus produced theta resonance and excess spiking in nearby pyramidal cells, demonstrating a specific enhancement of transmission at theta frequency [18]. In the thalamus, repetitive stimulation of the PV neurons of the reticular nucleus switched the thalamocortical firing mode from tonic to bursting, generated state-dependent neocortical spindles [53], and with stronger stimulation evoked generalized spike and wave discharges (Fig. 3) [62]. However, photoactivation of the reticular PV neurons was also found to reduce focal seizures in the neocortex after cortical injury [63]. Similarly, kainic acid-induced seizures could be suppressed by optogenetic activation of PV interneurons in the hippocampus [64]. These recent experiments demonstrate how the power of optogenetics could one day be harnessed for clinical applications, in addition to understanding the role of interneurons in complex cortical functions.
Figure 3. Controlling thalamocortical circuits by optogenetic activation of interneurons.
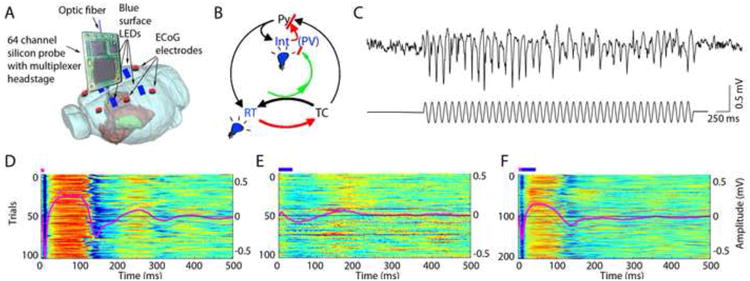
A. Experimental setup. Optical fiber is placed into the thalamic reticular nucleus in a transgenic mouse expressing ChR2 in PV cells to induce spike-wave seizure-like pattern (shown in C). Blue LEDs (squares) are placed epidurally at two positions in each hemisphere. B. Schematic of the reverberation in the thalamocortical loop. Neurons of the thalamus: reticular nucleus cells (RT), thalamocortical projection neurons (TC). Neurons of the cortex: pyramidal cells (Py) and inhibitory interneurons (Int). D. Light stimulation of the parvalbumin RT neurons alone induces spike-wave discharges, whereas light stimulation of cortical parvalbumin interneurons alone induces rebound excitation in cortical pyramidal cells (Py). Combined and phase shifted stimulation of RT and cortex attenuates the induced spike-wave activity. Reprinted from [62].
Outlook
Optogenetics combined with large-scale extracellular recordings has already proven to be effective in studying the functional roles of specific GABAergic interneuron classes in both hippocampus and neocortex, as well as other brain regions [65, 66]. However, optogenetic identification of interneurons does not yet allow one to distinguish between different subpopulations of interneurons that belong to a given molecularly defined class (e.g. the subtypes of PV or SOM cells) that could be distinguished based on other molecular markers, morphology, post-synaptic targets or developmental profiles [2, 3, 5, 67]. Targeting light-sensitive opsin expression to these sub-sub-classes of interneurons will likely require the use of more complex techniques, such as intersectional genetics or induction of recombination at specific gestational time points [68]. Alternatively, a further extension of this approach would be to use immediate early gene expression or photoactivatable fluorescent proteins to label light-activated neurons in vivo [69]. This labeling would subsequently be used to target these cells for in vitro intracellular electrophysiological characterization and/or morphological analysis, providing more detailed information about the cells' identity within each molecular class. Diode probes represent good candidates for this approach because they allow a small number of neurons to be activated selectively and the approximate spatial position of these neurons to be determined based on the silicon probe recording site configuration [33].
Another important extension of current methods is real-time signal processing and closed-loop activation/silencing of interneurons [63, 64]. Illumination could be triggered by spikes of single neurons, combinations of predetermined spike patterns for multiple cells, behavioral parameters, and/or selected features of LFPs [49, 63]. This creates, for instance, the ability to alter timing of action potentials and induce or suppress correlated firing between cells in freely moving animals. Overall, the combination of optogenetic, large-scale recording and single neuron identification methods will pave the way for a better understanding of the complex dynamics of inhibitory interneurons as well as their roles in coordinating the activity in principal cells in local networks and across network modules.
Highlights.
Focal, low-intensity optogenetic stimulation improves interneuron characterization
Diode-probes allow flexible multisite perturbation of interneurons
Methods are needed to study interneuron subtypes within molecularly-defined classes
Acknowledgments
We thank S. Royer, A. Berenyi and R. Eichler for their support and E. Schomburg for his comments on the manuscript. Research was funded by National Institute of Health Grants NS34994, MH54671 and NS074015, the Human Frontier Science Program and the J.D. McDonnell Foundation. LR received also support from the Bettencourt Schueller Foundation. LS is supported by NCATS grant UL1 TR000038.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monyer H, Markram H. Interneuron Diversity series: Molecular and genetic tools to study GABAergic interneuron diversity and function. Trends Neurosci. 2004;27:90–97. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 7.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- 9.Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 10.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Runyan CA, Schummers J, Van Wart A, Kuhlman SJ, Wilson NR, Huang ZJ, Sur M. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron. 2010;67:847–857. doi: 10.1016/j.neuron.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–348. doi: 10.1038/nature11347. This study along with others (refs. 14, 28, 60) demonstrates how different interneurons types can serve specific functions in processing and computation of visual inputs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Uniquefunctional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15:607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- 17**.Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. This article demonstrates the power of optogenetics for the identification of two distinct interneuron subtypes in extracellular recordings, to study their respective activity in relation to behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, Buzsaki G. Inhibition-induced theta resonance in cortical circuits. Neuron. 2013;80:1263–1276. doi: 10.1016/j.neuron.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csicsvari J, Hirase H, Czurko A, Buzsaki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- 21.Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- 22.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sik A, Penttonen M, Ylinen A, Buzsaki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Lapray D, Lasztoczi B, Lagler M, Viney TJ, Katona L, Valenti O, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T. Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci. 2012;15:1265–1271. doi: 10.1038/nn.3176. Juxtacellular recording and labeling of neurons have proven to be a powerful method for to characterize the physiological properties of anatomically characterized interneurons. However, all previous works were carried out in anesthetized animals. This study advance the technique much further by demonstrating that the juxtacellular technique is extendable to behaving animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margrie TW, Meyer AH, Caputi A, Monyer H, Hasan MT, Schaefer AT, Denk W, Brecht M. Targeted whole-cell recordings in the mammalian brain in vivo. Neuron. 2003;39:911–918. doi: 10.1016/j.neuron.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alivisatos AP, Chun M, Church GM, Greenspan RJ, Roukes ML, Yuste R. The brain activity map project and the challenge of functional connectomics. Neuron. 2012;74:970–974. doi: 10.1016/j.neuron.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 33.Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 34.Du J, Blanche TJ, Harrison RR, Lester HA, Masmanidis SC. Multiplexed, high density electrophysiology with nanofabricated neural probes. PloS one. 2011;6:e26204. doi: 10.1371/journal.pone.0026204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berényi A, Somogyvari Z, Nagy A, Roux L, Long J, Fujisawa S, Stark E, Leonardo A, Harris T, Buzsáki G. High-resolution (>500 channels) monitoring of local circuits in behaving rodents. J Neurophysiol. 2013 doi: 10.1152/jn.00785.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 37.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 38.Deisseroth K. Optogenetics. Nature methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PloS one. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kravitz AV, Owen SF, Kreitzer AC. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Res. 2013;1511:21–32. doi: 10.1016/j.brainres.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsaki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. This work demonstrates the complementary roles of parvalbumin and somatostatin-expressing interneurons in controlling burst firing and theta phase distribution of place cell assemblies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nature methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 43.Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ, 3rd, Gu X, Zanella S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light- induced activation and silencing Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. This landmark paper is a demonstration that transgenic methods can rival virus-mediated transduction of neurons. In addition to more even and brain-wide expression of light-responsive opsins, it also offers technical convenience, since only a single surgery is required for physiological experiments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Royer S, Zemelman BV, Barbic M, Losonczy A, Buzsaki G, Magee JC. Multi-array silicon probes with integrated optical fibers: light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur J Neurosci. 2010;31:2279–2291. doi: 10.1111/j.1460-9568.2010.07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci. 2012;15:163–170. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Stark E, Koos T, Buzsaki G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J Neurophysiol. 2012;108:349–363. doi: 10.1152/jn.00153.2012. A demonstration that local delivery of light (see also ref. 47) combined with LED and laser diodes allow multiple-site optogenetic manipulations in freely moving animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nature protocols. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011;14:1118–1120. doi: 10.1038/nn.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore AK, Wehr M. Parvalbumin-expressing inhibitory interneurons in auditory cortex are well-tuned for frequency. J Neurosci. 2013;33:13713–13723. doi: 10.1523/JNEUROSCI.0663-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- 57.Voigts J, Siegle JH, Pritchett DL, Moore CI. The flexDrive: an ultra-light implant for optical control and highly parallel chronic recording of neuronal ensembles in freely moving mice. Frontiers in systems neuroscience. 2013;7:8. doi: 10.3389/fnsys.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Wu F, Stark E, Im M, Cho IJ, Yoon ES, Buzsaki G, Wise KD, Yoon E. An implantable neural probe with monolithically integrated dielectric waveguide and recording electrodes for optogenetics applications. Journal of neural engineering. 2013;10:056012. doi: 10.1088/1741-2560/10/5/056012. A cricital requirement for flexible multi-site, multi-color control of interneurons in behaving animals is the small volume and light weight of devices. Integrating waveguides into silicon substrate (see also ref 59) allows for the production of highly flexible recording-stimulation devices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Optics letters. 2010;35:4133–4135. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Molecular psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berenyi A, Belluscio M, Mao D, Buzsaki G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science. 2012;337:735–737. doi: 10.1126/science.1223154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature communications. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown MT, Tan KR, O'Connor EC, Nikonenko I, Muller D, Luscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- 66.Alonso M, Lepousez G, Sebastien W, Bardy C, Gabellec MM, Torquet N, Lledo PM. Activation of adult-born neurons facilitates learning and memory. Nat Neurosci. 2012;15:897–904. doi: 10.1038/nn.3108. [DOI] [PubMed] [Google Scholar]
- 67.Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31:10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taniguchi H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peter M, Bathellier B, Fontinha B, Pliota P, Haubensak W, Rumpel S. Transgenic mouse models enabling photolabeling of individual neurons in vivo. PloS one. 2013;8:e62132. doi: 10.1371/journal.pone.0062132. [DOI] [PMC free article] [PubMed] [Google Scholar]


