Abstract
OBJECTIVES:
Fluid therapy is a cornerstone of the early treatment of acute pancreatitis (AP), but data are conflicting on whether it affects disease severity. Administering greater fluid volumes (FV) during induction of experimental AP preserves pancreatic perfusion and reduces severity but does not prevent onset of AP. We hypothesized that administering larger FV during endoscopic retrograde cholangiopancreatography (ERCP) associates with less severe post-ERCP pancreatitis (PEP).
METHODS:
In a retrospective cohort study, we identified 6505 patients who underwent 8264 ERCPs between January 1997 and March 2009, 211 of these patients developed PEP (48 mild, 141 moderate, and 22 severe). Data for FVs were available for 173 patients with PEP.
RESULTS:
In univariable analysis of only one of sixteen variables was significantly associated with moderate-severe PEP: larger periprocedural FV was protective (0.94+/−0.3 L vs 0.81+/−0.4 L, P=0.0129). Similarly, multivariable analysis of moderate-severe PEP identified one independent predictor: larger periprocedural FV was protective (OR 0.20, 95% CI 0.05-0.83). Conversely, moderate-severe disease correlated with larger FV administered after PEP diagnosis (reflecting treatment decisions).
CONCLUSIONS:
This hypothesis-generating study suggests that administering larger periprocedural FVs is protective against moderate-severe PEP. Prospective studies on this topic are warranted.
Keywords: acute pancreatitis, severity, fluid volumes, ERCP
INTRODUCTION
Fluid therapy (FT) is an important and accepted component of the early treatment of acute pancreatitis. FT mitigates the hypovolemic shock that commonly accompanies acute pancreatitis, improving pancreatic microvascular perfusion and thereby improving patient outcomes.1-7 Unfortunately, the optimal timing, the volume and the impact of FT in clinical acute pancreatitis are uncertain. A recent systematic review summarized three specific choices pertaining to FT, including fluid type, fluid rate and assessment of adequate resuscitation, but concluded that the evidence for recommending FT “remains paltry and of poor quality.”8 Moreover, data are conflicting as to whether administering higher fluid rates prevents or contributes to clinical outcomes in acute pancreatitis. An important limitation of these studies is that FT commences after but not during initiation of clinical acute pancreatitis such that FT only begins when the “therapeutic window” for FT is closing.
It remains unclear whether patients presenting with acute pancreatitis have an open early therapeutic window for administering FT to achieve better outcomes that would mirror goal-directed FT for managing sepsis and shock.9 Experimental studies partially address this question by reporting that FT begun before/during the inciting trigger for acute pancreatitis maintains systemic/pancreatic perfusion, attenuates without preventing onset of pancreatitis or end organ damage and improves survival.3,5,6 Conversely, maintenance of pancreatic perfusion by FT diminishes after microcirculatory damage has occurred, a process which evolves within 8 hours of onset of acute pancreatitis.5
Based on these experimental data, we hypothesized that administration of greater fluid volumes before completion of ERCP (“periprocedural fluid”) would reduce the severity of post-ERCP pancreatitis (PEP). As a secondary aim we examined whether greater periprocedural fluid volumes reduced the likelihood of PEP of any severity. To test our hypothesis, we collected and added new data for fluid volumes before and after ERCP to an established database with which we previously developed a risk model for PEP.10 We performed univariable and multivariable analyses to quantify the impact of periprocedural fluid volumes and other variables (including established predictors for PEP11-14) on PEP severity and PEP onset.
MATERIALS AND METHODS
Selection of Case and Control Samples
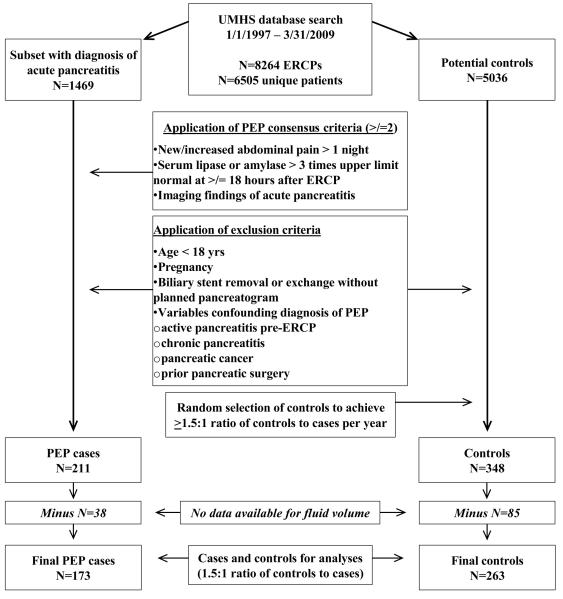
This retrospective cohort study was approved by the University of Michigan Institutional Review Board 2/8/2009. We previously described the methodology used to search our health system’s databases between 1/1/1997 and 3/31/2009 to build a data-set of ERCP patients with and without PEP.10 In short, we identified 6505 unique patients who had 8264 ERCPs. Of these, 1469 patients had an ICD-9-CM code (577.0) for acute pancreatitis and 211 patients met criteria by Cotton et al15 for PEP (Fig 1), and had no exclusion criteria (Fig 1). Based on previously described sample size calculations,10 we used a random number generator to select 1.5 controls (of 5036 potential controls) per case, matching controls to PEP cases by 2-year increments of time. Our existing data set includes patient variables, descriptive data for ERCP indications, and procedural variables,10 including 7 investigational variables hypothesized to influence the risk of PEP (alcohol use and cigarette-smoking status, Charlson comorbidity score, cardiovascular disease, hypertension, aspirin use and body mass),10 6 established variables consistently reported to influence the risk of PEP by multicenter prospective studies (younger age, female, suspected sphincter of Oddi dysfunction (SOD), pancreatic sphincterotomy, ≥2 pancreatic injections, moderate-difficult cannulation),11-14 prophylactic pancreatic stent placement16 and use of any nonsteroidal anti-inflammatory drug (Table 1). We did not include a history of PEP (a validated patient variable) due to concerns of selection bias, which we addressed by extracting data for the first episode of PEP at the University of Michigan. No patient received indomethacin suppositories immediately after ERCP, which may reduce PEP severity.17,18 Because a pancreatitis treatment protocol may reduce PEP severity,19 we emphasize there was no standardized institutional protocol during the study period for fluid administration before, during or after ERCP.
Figure 1.
Methodological Summary
Table 1.
Univariable and Multivariable Analyses of Moderate-Severe PEP
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | Mild (%) N=39 |
Moderate-Severe (%) N=134 |
P-value | OR | 95% CI | P-value |
| Patient Variables | ||||||
| Mean (SD) age*, yrs | 46.4 (13.4) | 47.2 (15.7) | 0.7592 | |||
| Female | 27 (69.2) | 107 (79.8) | 0.1921 | |||
| Suspected SOD | 15 (38.5) | 49 (36.6) | 0.8520 | |||
| Alcohol-use status | 0.1224 | |||||
| Never drinker | 20 (51.3) | 50 (37.3) | Reference | |||
| Current drinker | 10 (25.6) | 58 (43.3) | ‡ 0.0620 | 1.68 | 0.97-2.93 | 0.0653 |
| Former drinker | 9 (23.1) | 26 (19.4) | 0.6525 | |||
| Cigarette-smoking status | 0.5268 | |||||
| Never smoking | 27 (69.2) | 82 (61.2) | Reference | |||
| Current smoking | 5 (12.8) | 16 (11.9) | 1.0000 | |||
| Former smoking | 7 (18.0) | 36 (26.9) | 0.2986 | |||
| Median (IQR) Charlson Score** | 1 (0-2) | 0 (0-2) | 0.3012 | |||
| Cardiovascular disease (any of 8 variables) | 2 (5.1) | 15 (11.2) | 0.3667 | |||
| Cerebral vascular accident | 0 (0) | 0 (0) | 1.0000 | |||
| Transient ischemic attack | 0 (0) | 3 (2.2) | 1.0000 | |||
| Previous myocardial infarction | 2 (5.1) | 10 (7.5) | 1.0000 | |||
| Angina | 1 (2.6) | 0 (0) | 0.2254 | |||
| Congestive heart failure | 0 (0) | 5 (3.7) | 0.5889 | |||
| Claudication | 0 (0) | 2 (1.5) | 1.0000 | |||
| Cardiovascular stent/angioplasty | 1 (2.6) | 1 (0.8) | 0.4011 | |||
| Coronary bypass surgery | 0 (0) | 5 (3.7) | 0.5889 | |||
| Hypertension | 7 (18.0) | 30 (22.4) | 0.6603 | |||
| Mean (SD) body mass (kg)* | 80.6 (21.2) | 76.3 (19.8) | 0.2683 | |||
| Aspirin use | 1 (2.6) | 15 (11.2) | 0.1249 | |||
| NSAIDs | 3 (7.7) | 7 (5.2) | 0.6959 | |||
| Procedural Variables | ||||||
| ≥ 2 pancreatic injections | 0 (0.0) | 10 (7.5) | 0.1192 | |||
| Pancreatic sphincterotomy | 11 (28.2) | 25 (18.7) | 0.2613 | |||
| Moderate/difficult cannulation | 10 (25.6) | 43 (32.1) | 0.5547 | |||
| Pancreatic stent placement | 14 (35.9) | 41 (30.6) | 0.5609 | |||
| Mean (SD) periprocedural fluid volume (L)* | 0.94 (0.27) | 0.81 (0.36) | ‡ 0.0129 | 0.20 | 0.05-0.83 | 0.0272 |
| ERCP duration (per additional minute) | 46.1 (21.3) | 53.0 (23.9) | ‡ 0.0904 | 1.02 | 1.00-1.03 | 0.0962 |
Statistics:
t-test,
Wilcoxon rank sum test, otherwise Fisher’s exact test.
Three variables with P<0.1 were included in the multivariable logistic regression analysis.
CI, confidence interval; IQR, interquartile range; L, liter; NSAID, nonsteroidal anti-inflammatory drug; OR, odds ratio; PEP, post-ERCP pancreatitis; SD, standard deviation; SOD, sphincter of Oddi dysfunction.
Data Collection
For the current study, we collected additional data on 211 cases and 348 controls from paper and/or electronic nursing flow sheets, specifically fluid volumes between arrival time and procedure completion (“periprocedural” time), during recovery, and during 3 additional time periods following diagnosis of PEP: 0-12h, 12-24h and 24-48h. Complete fluid data were not available for 38 patients with PEP (18% of 211) and 85 controls without PEP (24.4% of 348) due to missing flow sheets or lack of documentation, yielding 436 patients for analysis having a similar ratio of 1.5 controls to cases, compared to the entire cohort of 559 cases and controls (Fig 1). To control for rate of fluid administration, we collected data for ERCP duration. Newly collected data were added to our established data set.10 PEP severity was defined as mild or moderate-severe based on criteria by Cotton et al.15 We used previously described definitions for multiple variables,20,10 including cigarette-smoking and alcohol-use, cardiovascular disease (see review by Anderson et al21), suspected SOD, pancreatic sphincterotomy, ≥2 pancreatic injections, moderate-difficult cannulation, pancreatic stent placement, and 19 variables comprising the Charlson Comorbidity Score.22
Outcomes and Statistical Analyses
The primary outcome analyzed was moderate-severe PEP. The secondary outcome was PEP. We used methods identical to our previous studies10,20 to search the entire electronic medical record for data of interest, to reconcile variations in the data recorded in the electronic medical record, and to collect, tabulate and analyze data.10, 20 In brief, data were collected with preprinted data collection forms, tabulated with spreadsheet software (Excel; Microsoft) and analyzed with Pro JMP 10 (SAS Institute, Cary, NC, USA) and Stata 12 (StataCorp, College Station, TX) statistical software. Descriptive statistics were compiled on all variables to evaluate variable distributions prior to modeling. Univariable analyses for moderate-severe PEP and PEP were performed on all variables. We performed two multivariable logistic regression analyses to identify independent predictors of moderate-severe PEP. The first (restrictive) analysis included only those variables with P < 0.1 in the final model.10 The second (more inclusive) analysis also had two markers of health (body mass and Charlson score) and six established predictors of PEP on the basis of our published risk model10 and multicenter prospective studies.11-14 A seventh predictor of PEP (≥ 2 pancreatic injections) was excluded from the model due to insufficient (zero) patients having this variable in the mild PEP group. Categorical and dichotomous variables were assessed by χ2 tests or Fisher’s exact test when necessary. Continuous variables were assessed using either two sample t-tests for normally distributed variables or the Wilcoxon rank sum test for non-normal variables.
RESULTS
Clinical Characteristics and Univariable Analysis of Moderate-Severe PEP
Of the 173 patients in our sample who developed PEP, 22.5% (n=39) developed mild PEP, 66.5% (n=115) moderate PEP and 11.0% (n=19) severe PEP. In univariable analysis the mild and moderate-severe PEP groups had similar patient characteristics and procedural characteristics (Table 1, Left Panel) with the exception that the moderate-severe PEP group had a significantly smaller periprocedural FV. All variables had a rate of missing data < 5%.10
Multivariable Analyses of Moderate-Severe PEP
A multivariable logistic regression analysis identified a single independent predictor of moderate-severe PEP out of 3 variables included in the model (based on having a P value <0.1 in the univariable analysis). Larger periprocedural fluid volume was an independent predictor of protection against moderate-severe PEP (Table 1, Right Panel). Adding additional variables to the multivariate logistic regression, specifically, two markers of health (body mass and Charlson score) and six established predictors of PEP (on the basis of our published risk model10 and multicenter prospective studies11-14), did not change these results (Table 2).
Table 2.
Multivariable Analyses of Moderate-Severe PEP Adjusted for Predictors of PEP
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Investigational variables | |||
| Mean (SD) periprocedural fluid volume (L) | 0.13 | 0.03-0.62 | 0.0107 |
| ERCP duration | 1.02 | 0.99-1.04 | 0.0794 |
| Current drinker | 1.81 | 0.98-3.34 | 0.0563 |
| Markers of health | |||
| Mean body mass (kg) | 0.99 | 0.97-1.01 | 0.4161 |
| Median Charlson Score | 0.89 | 0.69-1.14 | 0.3600 |
| Established predictors of PEP | |||
| Mean (SD) age*, yrs | 1.01 | 0.98-1.04 | 0.5183 |
| Female | 1.42 | 0.85-2.38 | 0.1760 |
| Suspected SOD | 1.25 | 0.79-2.00 | 0.3423 |
| Pancreatic sphincterotomy | 0.63 | 0.33-1.19 | 0.1515 |
| Moderate/difficult cannulation | 0.90 | 0.57-1.42 | 0.6554 |
| Pancreatic stent placement | 1.06 | 0.61-1.84 | 0.8462 |
Statistics: Multivariable logistic regression analysis included 11 variables from the univariate analysis (Table 1): three investigational variables with P <0.1 and eight additional variables with P ≥0.1 but included to adjust for health status (body mass and Charlson score) and six established risk factors for PEP on the basis of our published risk model10 and multicenter prospective studies.12-15 A seventh predictor of PEP (≥ 2 pancreatic injections) was excluded from the model due to insufficient (zero) patients having this variable in the mild PEP group.
CI, confidence interval; IQR, interquartile range; L, liter; OR, odds ratios; PEP, post-ERCP pancreatitis; SD, standard deviation; SOD, sphincter of Oddi dysfunction.
Temporal Relationship Between Fluid Volumes and Moderate-Severe PEP
While fluid volumes before PEP diagnosis were greater in patients with mild PEP compared to those with moderate-severe PEP, fluid volumes after PEP diagnosis were greater in moderate-severe PEP compared to mild PEP, presumably reflecting appropriate treatment decisions related to fluid hydration (Table 3).
Table 3.
| Mild PEP | Moderate-Severe PEP | ||
|---|---|---|---|
| Time Periods | Liters (SD) | Liters (SD) | P-value |
| Periprocedural (arrival to ERCP completion) | 0.94 (0.27) | 0.81 (0.36) | 0.0308 |
| ERCP recovery | 0.94 (0.49) | 0.88 (0.55) | 0.5373 |
| Total fluid before PEP diagnosis | 1.87 (0.69) | 1.66 (0.79) | 0.1786 |
| 0h -12h after PEP diagnosis | 1.11 (0.53) | 1.32 (0.69) | 0.0785 |
| 12h – 24h after PEP diagnosis | 1.02 (0.65) | 1.48 (0.80) | 0.0014 |
| 24h – 48h after PEP diagnosis | 1.16 (1.20) | 2.70 (1.40) | <0.0001 |
| Total fluid after PEP diagnosis | 3.30 (1.91) | 5.50 (2.54) | <0.0001 |
Before PEP diagnosis fluid volumes were greater in patients with mild PEP compared to those with moderate-severe PEP, particularly during the periprocedural period. In contrast, fluid volumes after PEP diagnosis were smaller in mild PEP compared to moderate-severe PEP, presumably reflecting appropriate treatment decisions related to fluid hydration.
Statistics: *t-test.
CI, confidence interval; L, liter; OR, odds ratios; PEP, post-ERCP pancreatitis; SD, standard deviation; SOD, sphincter of Oddi dysfunction.
Clinical Characteristics and Periprocedural Fluid Volumes of PEP Cases and Controls
We previously described patient characteristics and procedural characteristics of the PEP case sample and controls.10 We report similar frequencies in the subgroups of cases and controls, limited to those having periprocedural fluid volume data, except that the frequency of current smoking was no longer significantly different (P=0.1377; data not shown). Regarding periprocedural fluid volumes, mean (SD) values were similar in the PEP and control groups (0.84 +/−0.3 vs 0.84+/−0.4, P=0.9894).
DISCUSSION
In multivariable analysis we found that larger periprocedural fluid volume is independently protective against moderate-severe PEP. The larger periprocedural fluid volume in the mild versus moderate-severe PEP groups is due to a difference of 0.13 L in mean fluid volume (0.94 L vs 0.81 L, P=0.0129). The clinical relevance of this difference is difficult to judge on the basis of published clinical studies but is plausible on the basis of experimental pancreatitis studies.
A recent systematic review, entitled, “Fluid therapy in acute pancreatitis: anybody’s guess”,8 underscores the degree of uncertainty regarding FT in clinical acute pancreatitis. There is insufficient clinical data to determine whether FT has an impact on major outcomes8 other than preventing early death from hypovolemic shock in acute pancreatitis.1-3,5,6 A second major limitation is that clinical studies focus on administering FT after patients present with established acute pancreatitis, which may have little relevance to FT administered before/during the inciting event, before closure of the therapeutic window.
Experimental pancreatitis studies support the paradigm from sepsis management that the benefit of FT is likely limited to an early therapeutic window,9 prior to onset of microcirculatory damage, which heralds a markedly diminished impact of FT on maintaining pancreatic microvascular perfusion.5 Administering FT during induction of acute pancreatitis, however, preserves pancreatic microvascular perfusion, 23,24 associates with lower mortality in mice (31% vs 67%)25 and dogs (9% vs 50%),24 but does not affect onset of acute pancreatitis.24,25 How much and what type of fluid to give is unclear. It is known that administering crystalloid sufficient to maintain cardiac index in dogs better preserves pancreatic microvascular perfusion,23,24 and improves survival.24 These data are confusing, however, because the maximal fluid rates differ by 10-fold in the two dog studies23,24 and because other experimental studies illustrate a biphasic relationship between FT and outcomes.
For example, two experimental pancreatitis studies25,26 support the clinical premise that too little or too much FT may adversely impact outcomes.8In pigs,26 FT that was “goal-directed” (using functional hemodynamic parameters) rather than “less restrictive” (using central venous pressure and mean arterial pressure parameters) resulted in a significantly greater 7-day survival (29.4% vs 11.8%). Mean FT administered during the peri-induction period of pancreatitis was 1.0 vs 2.0 L/hour (normalized to 70 kg body mass), respectively.26In mice,25 the impact of administering crystalloid FT differed by the route of delivery, presumably due to the slower vs rapid absorption of fluid into the circulation from subcutaneous and intraperitoneal locations, respectively. Subcutaneous FT (0, 1.3, 2 and 2.6 ml tid) dose dependently increased survival but had no effect on biochemical or morphological parameters of acute pancreatitis. In contrast, intraperitoneal FT (0, 1.3, 2 and 2.6 ml tid) dose dependently triggered early deaths associated with pulmonary edema. Survival increased with FT of 2.0 ml and 2.6 ml tid but not 1.2 ml tid, corresponding to mean hourly FT of 1.2 L, 1.6 L and 0.8 L (normalized to 70 kg body mass), assuming a constant rate of fluid absorption. Collectively, these data indicate that during induction of acute pancreatitis, outcomes are better with mean hourly FT ranging 1.0-1.6 L (normalized to a 70 kg person) and worse with mean hourly FT ≤ 0.8 L or ≥ 2.0 L. These data25,26 support the plausibility that seemingly small differences in mean periprocedural fluid volumes can differentially impact severity of pancreatitis due to ERCP, particularly when the fluid is administered during an early therapeutic window.
The pattern of FT in our patient population supports but does not prove the concept of an early therapeutic window by illustrating a biphasic relationship between timing of FT and severity of PEP. Specifically there appears to be a different temporal pattern of cumulative fluid volume administered in the mild compared to moderate-severe PEP groups: greater in the ERCP periprocedural period, no significant difference during recovery or 0-12h after diagnosis of PEP, and significantly less 12-24h and 24-48h after diagnosis of PEP. We interpret this data as indicating that small difference in FT may influence severity early in the disease course and that larger cumulative fluid volumes beginning 12-24h after diagnosis of PEP associate with moderate-severe PEP. Although evidence is lacking, we attribute the latter observation to sick patients receiving more FT rather than greater FT contributing to disease severity.
Periprocedural FT was almost exclusively crystalloid in our study. Whether specific fluid types provide superior FT in acute pancreatitis is controversial.3,5,6 Compared to standard crystalloid solutions (e.g. 0.9% normal saline or Ringer’s lactate solution), however, experimental studies generally report less severe or less frequent end organ damage and improved survival with use of hypertonic saline27 and several different colloids, including albumin,1,2 high molecular weight dextrans,28-35 purified bovine hemoglobin36,37 and fresh frozen plasma.38 Although few controlled, non-PEP, clinical studies address this issue,39-41 studies in critically ill or septic patients report a survival benefit for albumin42 but not hydroxyethyl starch43 compared to crystalloid.
In patients undergoing ERCP, better definitions are required to determine which patients would benefit from prophylactic measures. As we previously reviewed,10 nonstandardized application of a variety of variables have been used to identify “high-risk” groups.17,44 To this end we recently generated a risk model for PEP10 by examining investigational variables and those for which there is some consensus by 2 of 4 large multicenter prospective studies.11-14 Options for reducing risk of PEP or severity of PEP have similarities and differences, as previously reviewed.10 Placement of a prophylactic pancreatic duct stent16 or administering indomethacin suppositories immediately after ERCP17,18 in “high-risk” groups may reduce the risk of PEP. Prophylaxis against more severe PEP also may be possible,16,17,18 but protection from pancreatic duct stenting may be limited to those with moderate but not severe PEP according to a systematic review.16 Following ERCP, use of a pancreatitis treatment protocol, initiated after patients present with PEP, may also reduce severity.19
In summary, we report that a larger periprocedural fluid volume is an independent protective factor against moderate-severe PEP. This hypothesis-generating observation reaffirms the concept from experimental pancreatitis (and sepsis) studies that FT administered during the early therapeutic window reduces severity of pancreatitis. Moreover, the observation has clinical implications for preventing moderate-severe PEP because fluid administration is a simple, low cost, and easily modifiable factor. Ultimately, prospective studies are required to validate our findings10 (to address limitations of the retrospective study design), and to determine whether administering greater fluid volumes in the periprocedural period reduces PEP severity independently of other prophylactic measures.
Acknowledgments
Financial support: SDS receives research support from the VA HSR&D CDA-2 Career Development Award. MJD receives research support from the National Institutes of Health (R21 AA017271).
Abbreviations
- CI
confidence interval
- ERCP
endoscopic retrograde cholangiopancreatography
- FT
fluid therapy
- FV
fluid volume
- IDX
information data exchange
- ICD-9
international classification of diseases 9th edition
- NSAIDs
non-steroidal anti-inflammatory drugs
- OR
odds ratio
- PEP
post-ERCP pancreatitis
- SOD
Sphincter of Oddi
Footnotes
Disclosures: MJD received the drug Pioglitazone from Takeda Pharmaceuticals North America for use in an NIH sponsored clinical research trial (2008). MJD received honoraria from Springer (New York, NY, USA) for an article published in Current Gastroenterology Reports and the British Medical Journal (BMJ) Publishing Group Limited for articles published in BMJ Point of Care.
Conflicts of interest None of the authors declare competing interests (MJD, EJW, JR, MAR, JPS, DDB, BJE, SDS).
REFERENCES
- 1.Elliott DW. Treatment of acute pancreatitis with albumin and whole blood. AMA Arch Surg. 1957;75:573–579. doi: 10.1001/archsurg.1957.01280160083010. [DOI] [PubMed] [Google Scholar]
- 2.Elliott DW, Zollinger RM, Moore R, et al. The use of human serum albumin in the management of acute pancreatitis; experimental and clinical observations. Gastroenterology. 1955;28:563–587. [PubMed] [Google Scholar]
- 3.Klar E, Messmer K, Warshaw AL, et al. Pancreatic ischaemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg. 1990;77:1205–1210. doi: 10.1002/bjs.1800771104. [DOI] [PubMed] [Google Scholar]
- 4.DiMagno MJ, Williams JA, Hao Y, et al. Endothelial nitric oxide synthase is protective in the initiation of caerulein-induced acute pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2004;287:G80–87. doi: 10.1152/ajpgi.00525.2003. [DOI] [PubMed] [Google Scholar]
- 5.Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518–530. doi: 10.1002/bjs.5316. [DOI] [PubMed] [Google Scholar]
- 6.Gardner TB, Vege SS, Pearson RK, et al. Fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol. 2008;6:1070–1076. doi: 10.1016/j.cgh.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 7.DiMagno MJ, Wamsteker EJ, Debenedet AT. Adances in managing acute pancreatitis. F1000 Medicine Reports. 2009;1:59. doi: 10.3410/M1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haydock MD, Mittal A, Wilms HR, et al. Fluid therapy in acute pancreatitis: anybody's guess. Ann Surg. 2013;257:182–188. doi: 10.1097/SLA.0b013e31827773ff. [DOI] [PubMed] [Google Scholar]
- 9.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 10.DiMagno MJ, Spaete JP, Ballard DD, et al. Risk models for post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP). Smoking and chronic liver disease are predictors of protection against PEP. Pancreas. 2013;42:996–1003. doi: 10.1097/MPA.0b013e31827e95e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 12.Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139–147. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 13.Williams EJ, Taylor S, Fairclough P, et al. Risk factors for complication following ERCP; results of a large-scale, prospective multicenter study. Endoscopy. 2007;39:793–801. doi: 10.1055/s-2007-966723. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Li ZS, Liu F, et al. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104:31–40. doi: 10.1038/ajg.2008.5. [DOI] [PubMed] [Google Scholar]
- 15.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 16.Choudhary A, Bechtold ML, Arif M, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc. 2011;73:275–282. doi: 10.1016/j.gie.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmunzer BJ, Waljee AK, Elta GH, et al. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut. 2008;57:1262–1267. doi: 10.1136/gut.2007.140756. [DOI] [PubMed] [Google Scholar]
- 19.Reddy N, Wilcox CM, Tamhane A, et al. Protocol-based medical management of post-ERCP pancreatitis. J Gastroenterol Hepatol. 2008;23:385–392. doi: 10.1111/j.1440-1746.2007.05180.x. [DOI] [PubMed] [Google Scholar]
- 20.Debenedet AT, Raghunathan TE, Wing JJ, et al. Alcohol use and cigarette smoking as risk factors for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2009;7:353–358. doi: 10.1016/j.cgh.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson KM, Wilson PW, Odell PM, et al. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Knol JA, Inman MG, Strodel WE, et al. Pancreatic response to crystalloid resuscitation in experimental pancreatitis. J Surg Res. 1987;43:387–392. doi: 10.1016/0022-4804(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 24.Horton JW, Burnweit CA. Hemodynamic function in acute pancreatitis. Surg. 1988;103:538–546. [PubMed] [Google Scholar]
- 25.Niederau C, Crass RA, Silver G, et al. Therapeutic regimens in acute experimental hemorrhagic pancreatitis. Effects of hydration, oxygenation, peritoneal lavage, and a potent protease inhibitor. Gastroenterology. 1988;95:1648–1657. doi: 10.1016/s0016-5085(88)80091-x. [DOI] [PubMed] [Google Scholar]
- 26.Trepte CJ, Bachmann KA, Stork JH, et al. The impact of early goal-directed fluid management on survival in an experimental model of severe acute pancreatitis. Intens Care Med. 2013;39:717–726. doi: 10.1007/s00134-012-2775-x. [DOI] [PubMed] [Google Scholar]
- 27.Shields CJ, Winter DC, Sookhai S, et al. Hypertonic saline attenuates end-organ damage in an experimental model of acute pancreatitis. Brit J Surg. 2000;87:1336–1340. doi: 10.1046/j.1365-2168.2000.01626.x. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MC, Lewis MB. Low-molecular-weight dextran therapy in experimental pancreatitis. JAMA. 1965;192:398–400. doi: 10.1001/jama.1965.03080180056014. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson LA, Williams RW, Schenk WG., Jr Experimental pancreatitis: Effect of plasma and dextran on pancreatic blood flow. Surgery. 1978;84:313–321. [PubMed] [Google Scholar]
- 30.Donaldson LA, Schenk WG., Jr Experimental acute pancreatitis: the changes in pancreatic oxygen consumption and the effect of Dextran 40. Ann Surg. 1979;190:728–731. doi: 10.1097/00000658-197912000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klar E, Herfarth C, Messmer K. Therapeutic effect of isovolemic hemodilution with dextran 60 on the impairment of pancreatic microcirculation in acute biliary pancreatitis. Ann Surg. 1990;211:346–353. doi: 10.1097/00000658-199003000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt J, Fernandez-del Castillo C, Rattner DW, et al. Hyperoncotic ultrahigh molecular weight dextran solutions reduce trypsinogen activation, prevent acinar necrosis, and lower mortality in rodent pancreatitis. Am J Surg. 1993;165:40–44. doi: 10.1016/s0002-9610(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt J, Huch K, Mithofer K, et al. Benefits of various dextrans after delayed therapy in necrotizing pancreatitis of the rat. Intens Care Med. 1996;22:1207–1213. doi: 10.1007/BF01709338. [DOI] [PubMed] [Google Scholar]
- 34.Goodhead B. Acute pancreatitis and pancreatic blood flow. Surg Gynecol Obstet. 1969;129:331–340. [PubMed] [Google Scholar]
- 35.Knol JA, Edgcomb LP, Inman MG, et al. Low molecular weight dextran in experimental pancreatitis: effects on pancreatic microcirculation. J Surgical Res. 1983;35:73–82. doi: 10.1016/0022-4804(83)90128-2. [DOI] [PubMed] [Google Scholar]
- 36.Strate T, Mann O, Kleinhans H, et al. Microcirculatory function and tissue damage is improved after therapeutic injection of bovine hemoglobin in severe acute rodent pancreatitis. Pancreas. 2005;30:254–259. doi: 10.1097/01.mpa.0000157481.22155.2d. [DOI] [PubMed] [Google Scholar]
- 37.Strate T, Mann O, Kleinhans H, et al. Systemic intravenous infusion of bovine hemoglobin significantly reduces microcirculatory dysfunction in experimentally induced pancreatitis in the rat. Ann Surg. 2003;238:765–771. doi: 10.1097/01.sla.0000094442.12395.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leese T, West KP, Morton DB, et al. Fresh frozen plasma therapy in acute pancreatitis: an experimental study. Int J Pancreatol. 1988;3:437–447. doi: 10.1007/BF02788202. [DOI] [PubMed] [Google Scholar]
- 39.Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710–717. doi: 10.1016/j.cgh.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Du XJ, Hu WM, Xia Q, et al. Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas. 2011;40:1220–1225. doi: 10.1097/MPA.0b013e3182217f17. [DOI] [PubMed] [Google Scholar]
- 41.Leese T, Holliday M, Watkins M, et al. A multicentre controlled clinical trial of high-volume fresh frozen plasma therapy in prognostically severe acute pancreatitis. Ann Roy Coll Surg. 1991;73:207–214. [PMC free article] [PubMed] [Google Scholar]
- 42.Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 43.Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 44.Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1354–1365. doi: 10.1016/j.cgh.2007.09.007. [DOI] [PubMed] [Google Scholar]