Abstract
Double strand breaks pose unique problems for DNA repair, especially when broken ends possess complex structures that interfere with standard DNA transactions. Nonhomologous end joining can use multiple strategies to solve these problems. It further uses sophisticated means to ensure the strategy chosen provides the ideal balance of flexibility and accuracy.
Keywords: double strand break repair, nonhomologous end joining, DNA damage
I. Introduction
Assaults on the genome can result in various forms of DNA damage with potentially catastrophic consequences*. A particularly serious class of damage is that of the double strand break (DSB). DSBs arise during programmed recombination events such as those in V(D)J recombination, but also in response to damaging agents like ionizing radiation (IR) and reactive oxygen species (ROS). DSBs that are misrepaired or left unrepaired cause genomic instability. This in turn has devastating effects at both cellular and organismal levels, including cytotoxicity, accelerated aging, cancer predisposition.
Mammalian cells rely on two major repair pathways to resolve DSBs –homologous recombination (HR) and nonhomologous end joining (NHEJ). Considered the most accurate of DSB repair pathways, HR faithfully replaces lost or damaged sequence via extensive DNA synthesis, using an intact sister chromatid as a template. The need for a sister chromatid and an abundant nucleotide pool restricts HR to the S- and G2-phase of the cell cycle [reviewed in e.g. [1, 2]]
In contrast, NHEJ directly rejoins chromosome ends, and is thus not subject to the same requirement for both homology (to use as template) and extensive synthesis, relative to HR. This makes NHEJ available for repair throughout the cell cycle [2]. On the other hand, there are disadvantages to using NHEJ. All other pathways take advantage of redundancy to replace excised damage (a sister chromatid for HR; the intact complementary strand for base excision repair, nucleotide excision repair, and mismatch repair)[3]. The inability to similarly employ sequence redundancy to replace excised damage means the standard DNA transactions – synthesis and ligation – must be made more flexible, and products of repair typically involve deletion. NHEJ thus might not be considered “DNA repair” in the strictest sense.
Compounding the difficulty for NHEJ is the mélange of possible damage at DSBs that could interfere with ligation, and, which must often be excised. DSBs generated by damaging agents are associated with a wide array of different damaged nucleotides, including oxidized nucleotides and nucleotide fragments [4–6]. Intermediates in V(D)J recombination possess hairpin-terminated breaks [7], and alignment of pairs of ends regardless of source often result in mismatches, gaps and flaps. Proteins may also occlude DSB ends, like the covalently-adducted products of aborted topoisomerase activity [8], or non-covalently associated chromatin proteins.
Thus, damage at DSBs presents a particularly onerous challenge to NHEJ. To resolve this challenge NHEJ employs a series of core factors that work to sense a DSB, align ends, and act as a scaffold for a host of processing factors that facilitate removal (and sometimes even replacement) of ligation-blocking damage (discussed in section II) (Table 1). We suggest that together this complex acts as a multi-protein machine, and is capable of employing an amalgam of the of strategies used by other repair pathways, including 1) damage tolerance, 2) targeted, short patch excision, and 3) long patch excision (discussed in section III). Having these multiple strategies at its disposal increases NHEJ’s flexibility, allowing it to resolve DSBs with diverse end structures. However, determining which strategy is used is critical, as it both impacts the fidelity and efficiency of DSB repair.
Table 1.
NHEJ associated proteins
| CORE FACTORS | |
|---|---|
| Factor* | Activity |
| XRCC5, XRCC6 (Ku) | Senses DSB break [152] then recruits proteins to break |
| PRKDC (DNA PKcs) | Tethers broken DNA ends together [21, 22] and has kinase activity [18, 153] |
| LIG4 (DNA ligase IV) | Ligase [154] |
| XRCC4 | Obligate ligase subunit [28] |
| NHEJ1 (XLF) | Promotes end-bridging [49] |
| PROCESSING FACTORS | |
|---|---|
| Factor* | Activity |
| APLF | Histone chaperone [69] 3′-5′ exonuclease, endonuclease [63, 64] |
| PNKP | Removes 3′ phosphates and phosphorylates 5′ hydroxyls [56] |
| APTX | Removes 5′-adenylate adducts [60] |
| TDP1 | Removes Top I adducts [71], 3′ deoxyribose fragments [75, 77, 155] |
| TDP2 | Removes Top II adducts [72] |
| POLM (Pol μ) | Fills in gaps when ends align with no complementarity [81] |
| POLL (Pol λ) | Fills in gaps when ends are partly complementary [81, 82] |
| DCLRE1C (Artemis) | Endonuclease, 5′-3′ exonuclease [101] |
| SETMAR (Metnase) | Endonuclease/exonuclease, histone methylase [116] |
| MRE11/RAD50/NBN (MRN) | 3′-5′ exonuclease, endonuclease [120, 121] |
| WRN | 3′-5′ exonuclease [156, 157] and 3′-5′ helicase [158] |
HUGO gene nomenclature: XRCC5, XRCC6 (Ku80, Ku70), X-ray repair complementing defective repair in Chinese hamster cells 5/6; PRKDC, protein kinase, DNA-activated, catalytic polypeptide; LIG4, ligase IV, DNA, ATP-dependent; XRCC4, X-ray repair complementing defective repair in Chinese hamster cells 4; NHEJ1 (XLF), nonhomologous end-joining factor 1; APLF, aprataxin and PNKP like factor; PNKP, polynucleotide kinase 3′-phosphatase; APTX, aprataxin; TDP1, tyrosyl-DNA phosphodiesterase 1; TDP2, tyrosyl-DNA phosphodiesterase 2; POLM, polymerase mu; POLL, polymerase lambda; DCLRE1C (Artemis), DNA cross-link repair 1C; SETMAR (Metnase), SET domain and mariner transposase fusion gene; MRE11/RAD50/NBN, meiotic recombination 11 homolog/RAD50 homolog/nibrin (Nbs1); WRN, Werner syndrome.
II. NHEJ is a multi-protein machine
The ability to employ several strategies for repair implies an organized, multi-protein machine capable of dynamic control of DNA end metabolism. This machine starts with the assembly of a core complex, capable of recognizing broken ends and - most importantly - aligning ends together such that strand break termini are presented appropriately to the factors that must act on them. Notably, the core-assembly includes a pathway-specific ligase complex, which aside from its essential role it catalyzing the final step in NHEJ also provides an important non-catalytic scaffolding function.
The core assembly then recruits processing factors (Table 1). As argued above, diversity in end structure implies a need for a wide variety of end processing factors, and the list of such factors implicated in NHEJ is still growing. The list includes, not surprisingly, an array of end-cleaning enzymes shared with base excision repair/single strand break repair (BER/SSBR). Several nucleases and polymerases have also been implicated in NHEJ. However, unlike the end cleaning enzymes, nucleases and polymerases are more often uniquely suited to act in the context of a DSB and thus, are less often shared with BER/SSBR.
We discuss below the contribution of factors within the NHEJ machine as well as some gross structural features (a more detailed review of NHEJ factor structures can be found elsewhere in this issue). We emphasize how factors interact with each other and DNA substrate, as these interactions will determine how the machine is assembled as well as how the machine controls access to ends.
II.i Core factors
The core machinery for NHEJ is typically considered to include the Ku heterodimer (Ku 80/70), the DNA dependent protein kinase catalytic subunit (DNA-PKcs), DNA Ligase IV, XRCC4, and the XRCC4-like factor (XLF, or Cernunnos).
Ku
Ku is a heterodimer of 70 and 83 kD subunits (Ku70, Ku80). The subunits have a similar domain organization [9, 10] (Fig. 1A, upper panel), but N- and C-terminal domains especially have diverged in function. An extended C-terminal domain in Ku80 possesses the primary interface for promoting interaction with DNA-PKcs [9, 11, 12], while Ku70 has a C-terminal SAP domain with DNA binding activity [13]. The N-terminal domain (vWA domain) of Ku70 has acquired enzymatic activity, (see below), while Ku80’s vWA domain interacts with APLF [14]. Finally, the central heterodimerization domains of both Ku subunits promote interaction with XLF [15].
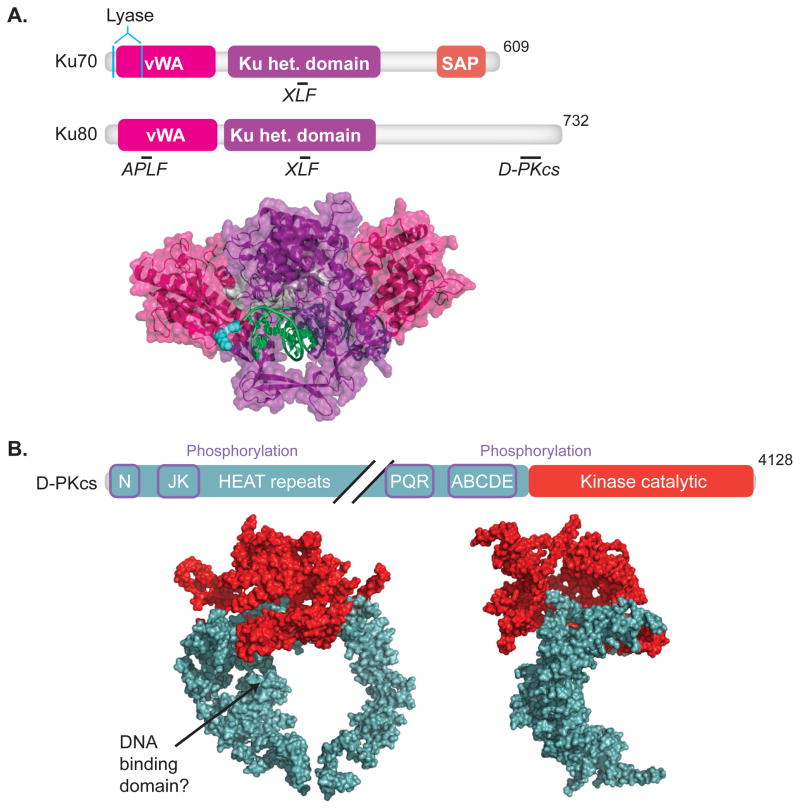
Fig.1. DNA-PK.
Domain maps indicating sites of protein-protein interactions, underlined, interacting proteins, italics, (upper panel) and crystal structures (lower panel) are shown for (A) Ku70 and Ku80 (1JEY)[16] and (B) DNA-PKcs, front and side view (3KGV)[21]. Colors in crystal structures correspond to features in domain maps. (A)Domains of Ku70 include the vWA (von Wilebrand associated) domain, pink, the heterodimerization domain (het.), purple, and SAP (C-terminal SAF-A/B, Acinus and PIAS) domain, orange. Residues important for lyase activity are located in Ku70, cyan [55, 56]. Domains of Ku80 include the vWA domain, and het. domain. Ku 70/80 interactions with APLF [14], DNA-PKcs [11], and XLF [15] are shown. (B) Domains of DNA-PKcs include an N-terminal domain, a kinase catalytic domain, red. Diagonal black lines denote a break in the domain map. Sites of phosphorylation, purple boxes, and HEAT (Huntington elongation factor 2, A subunit of protein phosphatase 2A and TOR1) repeats are indicated.
Ku loads on ends by threading them through a central channel formed by the heterodimer [16] (Fig. 1A, lower panel). This binding mechanism explains its high specificity for ends and, once bound, both its ability to remain stably bound [17] and translocate internally on the DNA fragment [18].
DNA-PKcs
DNA-PKcs is a 460 kD kinase specifically recruited to, and its kinase activity dependent on, Ku-bound DNA ends [19]. It is related to two other kinases also implicated in the DNA damage response - Ataxia Telangiectasia mutated (ATM) and Ataxia Telangiectasia Related (ATR) [20]. DNA-PKcs forms a ~150 angstrom wide C clamp-like shape (Fig. 1B, lower panel) composed largely of HEAT repeats, with the kinase domain and Ku interacting region (Fig. 1B, upper panel) located as a “crown” opposite the clamp’s gap [21] (Fig. 1B, lower panel). DNA-PKcs and Ku together form a very stable complex that remains tethered to the end [22, 23] and blocks access of other factors - nucleases, polymerases, and even ligase IV - in the absence of kinase activity [23, 24]. As discussed below (section III.4) phosphorylation of DNA-PKcs, either autophosphorylation [25] or by ATM [26], plays a key role in regulating access to ends during NHEJ.
Ligase IV, XRCC4, and XLF
Ku and DNA-PKcs also recruit a pre-formed complex of ligase IV and XRCC4 [27–30]. Ligase IV includes a tri-partite N-terminal fragment (1–620) that is required for catalysis [31] (Fig. 2A). This catalytic fragment is related to the other two mammalian ligases (Ligase I and Ligase III) and likely forms a similar complex with DNA substrate, where the three domains of the catalytic fragment wrap around double stranded DNA (dsDNA) (reviewed in [32, 33]). The central portion of the catalytic fragment contains the catalytically critical lysine (K273), which is adenylated in the first step of ligation. In the second step the adenyl group is transferred to the strand break 5′ phosphate terminus, and ligation is completed when this adenylated 5′ phosphate is attacked by the juxtaposed 3′OH terminus of the strand break (reviewed in [32]).
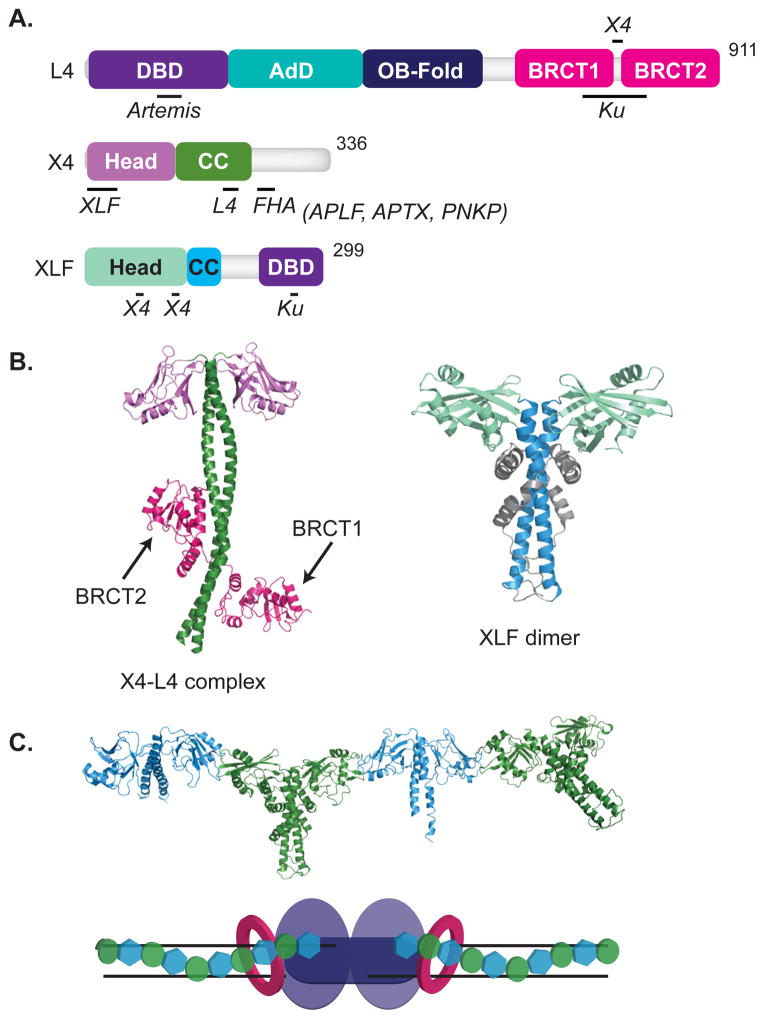
Fig. 2. Ligase Complex.
Features of domain maps are as indicated in Figure 1. (A, B) Domains of DNA Ligase IV include a DNA binding domain (DBD), purple, adenylation domain (AdD), teal, oligonucleotide/oligosaccharide binding domain (OB-fold), dark blue, and breast cancer carboxy terminal (BRCT) domains, pink. Ligase IV interacts with Artemis [109], Ku [151], and XRCC4 [34]. Domains of XRCC4 include the Head domain, lavender, and the coiled-coiled domain (CC), green. XRCC4 interacts with XLF [43], ligase IV [36], APLF [41, 64], APTX [40], and PNKP [39, 152]. XLF/Cernunnos consists of a Head domain, light green, CC domain, cyan, and a DBD, purple. XLF associates with Ku [15] as well as XRCC4 [43]. (B) A crystal structure of XRCC4, green and lavender, in complex with the BRCT domains, pink, of Ligase IV is shown (3II6) [37]. A crystal structure of a XLF dimer is shown (2R9A) [43]. (C) Crystal structure of the filament formed by XLF, cyan, and XRCC4, green, is shown (3RWR) [45]. (D) Representation of the NHEJ core machinery (Ku, pink rings, DNA-PKcs, light purple, Ligase IV, dark purple) in complex with an XRCC4/XLF filament.
Ligase IV is most easily identified as pathway-specific by the addition, relative to other mammalian ligases, of two tandem BRCA1 C-terminal (BRCT) domains and a short region between these two domains that interacts with XRCC4 [34–37] (Fig. 2A). XRCC4 in turn consists of a dimer with N-terminal globular heads and a coiled-coil stalk and interacts with ligase IV through a portion of the coiled-coil stalk (Fig. 2A and B, left panel) [38]. XRCC4’s C-terminus, like the SSBR scaffold XRCC1, possesses a motif phosphorylated by casein kinase II that mediates interactions with related FHA domains in polynucleotide kinase/phosphatase (PNKP) [39], aprataxin [40], and aprataxin and PNKP-like factor (APLF) [41, 42] (Fig. 2A).
The XLF structure is similar to XRCC4, though the coiled-coil region is much shorter [43, 44] (Fig. 2B), and the two interact with each other through symmetrically positioned interfaces on each side of XRCC4 and XLF dimers (Fig. 2A, and C). Together with their ability to interact with DNA, they can form an extended filament (Fig. 2C) that wraps around DNA [43, 45–50] (Fig. 2D). Thus, while Ku and DNA-PKcs alone can promote the alignment of a pair of DSB ends [22], the additional recruitment of XRCC4-ligase IV and XLF and their ability to form a filament are required to further stabilize this alignment [49, 51–53].
II.ii End cleaning enzymes
End cleaning enzymes are specifically targeted to the types of ligation blocking damage that are frequently found at strand break termini. Accordingly, some of them – PNKP, aprataxin, and tyrosine phosphodiesterase 1 (Tdp1) – are employed by BER/SSBR and NHEJ both (reviewed in [54]), while others are specifically suited for DSB associated damage e.g., Ku, tyrosine phosphodiesterase 2 (Tdp2). After removing damage, these enzymes leave behind the “clean” 5′ P and 3′OH termini required for the ligation step.
Ku
Ku has 5′dRP (5′deoxyribose-phosphate)/AP (apurinic/apyrimidinic) lyase activity [55] in addition to a role in recognizing ends and acting as a scaffold for other factors. 5′dRP/AP lyase activity is required for efficient joining of substrates with near-terminal abasic sites both in vitro and in cells [55, 56]. Its activity is highly restricted to abasic sites near 5′ DSB termini, and its active site is ideally located for this function [56] (Fig. 1A, upper panel). It cleaves 3′ of “standard” abasic sites (those with aldehydic isomers, i.e. products of hydrolysis or glycosylase) and generates a downstream 5′ phosphate terminus and a 3′ residue [55]. The 3′ terminal residue is typically released from ends, as Ku is active primarily near 5′ termini [55, 56]. In general, only abasic sites that would otherwise block ligation are excised and only when excision is sufficient to generate clean, ligatable termini [56]. These substrate restrictions reduce unnecessary and probably detrimental processing (e.g. by re-introducing a break after ligation).
Polynucleotide kinase/phosphatase (PNKP)
PNKP [39] like aprataxin [40] and APLF [41, 42], interacts with XRCC4 through its N-terminal forkhead-associated domains (FHA) (Fig. 3A and B).
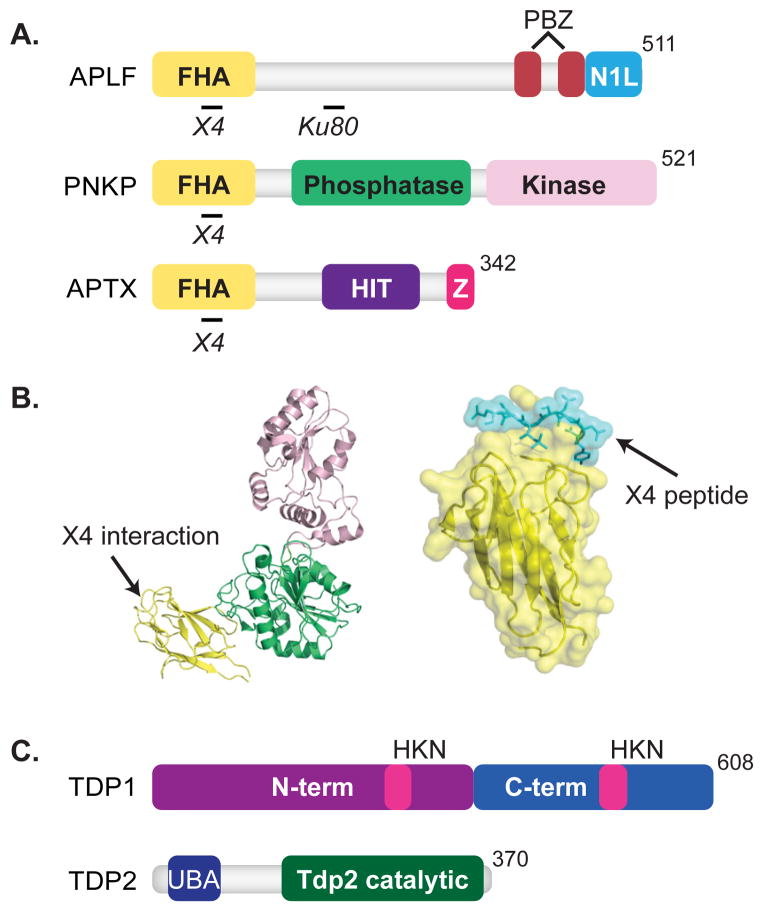
Fig. 3. End Cleaning Enzymes.
Features of domain maps are as indicated in Figure 1. (A) Forkhead-associated (FHA) domains, yellow, are shown for PNKP, APTX, and APLF. APLF also includes two poly(ADP-ribose)-binding zinc finger (PBZ) domains, red, and a nucleosome assembly protein 1-like (NAP1L) motif, blue. APLF associates with XRCC4 [41, 64] and Ku80 [14, 66]. PNKP also contains a phosphatase domain, green, and kinase domain, light pink. PNKP interacts with XRCC4 [39, 152]. APTX contains a histidine triad (HIT) motif, purple, and a zinc-finger motif, pink. APTX interacts with XRCC4 [40]. (B) Crystal structure of PNKP (1YJ5) and the FHA domain of PNKP, yellow, in complex with an XRCC4 peptide, cyan, are shown (1YJM) [58]. (C) Domain maps of Tdp1 and Tdp2 are shown. Tdp1 consists of an N-terminal domain, purple, C-terminal domain, blue, and the catalytic HKN motifs, pink. Tdp2 consists of an ubiquitin-associated (UBA) domain, blue, and a Tdp2 catalytic domain, green.
PNKP has both 5′ kinase and 3′ phosphatase activities [57]. The kinase domain of PNKP (Fig. 3A) has a broad substrate-binding pocket that preferentially recognizes double-stranded substrates with recessed 5′ termini [58, 59]. The 3′ phosphatase domain recognizes its substrate using a deep channel, where the 3′ phosphate-containing terminus must be made single stranded and then buried [58, 60]. Of note, radiation [4, 5] and topoisomerase I can generate strand breaks with both 3′ phosphate and 5′OH termini, suggesting there are advantages to coupling both activities together on the same protein.
Aprataxin
As noted above, ligation proceeds through an intermediate where the 5′ phosphate terminus of the strand break is adenylated. Ligation can abort after adenylation, especially when termini are not readily juxtaposed, e.g., when associated with flanking DNA damage (reviewed in [32]). If the ligase releases after the aborted ligation attempt, the now adenylated 5′ phosphate is no longer a substrate for another ligation attempt. Aprataxin removes the adenylate, thus “resetting” the 5′ phosphate terminus [61, 62]. Biochemical and structural analysis indicates aprataxin is active at DSBs; and considering both its ability to physically interact with XRCC4 [40, 63] (Fig. 3A) and the probable difficulties in aligning some strand termini, it seems likely aprataxin has a significant role in NHEJ.
Aprataxin and PNKP-like factor (APLF) (also termed PALF, Xip1, and C2ORF13)
APLF has been associated with a range of nuclease activity [64, 65], including 3′ exonuclease, dsDNA endonuclease, single stranded DNA (ssDNA) 3′endonuclease activities, and AP endonuclease activity.
However, APLF’s primary role may be to act as a dynamic regulator of the composition and stability of the NHEJ complex, rather than an end processing factor. As noted below (III.iv), APLF may also foster substrate or factor channeling between NHEJ and BER. APLF is capable of direct protein-protein interactions with phosphorylated XRCC4 through APLF’s N-terminal FHA domain [41, 42], as well as Ku through a more central region in APLF [14, 66] (Fig. 3A). These protein-protein interactions alone promote more stable retention of the ligase complex at ends, as well as ligation activity. However, APLF also promotes assembly of higher order NHEJ complexes indirectly, through its ability to bind to Poly (ADP ribose) chains (PAR) [67]. PAR is added to chromatin and repair factors near the site of strand breaks by Poly (ADP ribose) polymerases (PARPs) (reviewed in [68]), and this promotes recruitment of APLF through a pair of tandemly repeated PAR binding zinc finger (PBZ) motifs in its C-terminus [69]. Finally, APLF has a C-terminal nucleosome assembly protein-1 (NAP1)-like motif that is sufficient for partial nucleosome assembly and disassembly in vitro [70]. This latter function may help promote local chromatin remodeling either before or after repair.
Tyrosyl DNA phosphodiesterases
DNA topoisomerases facilitate changes in DNA topology required for transcription, replication, and chromosome segregation. Changes in topology are mediated by cycles of strand-breakage and re-ligation, with the strand-break intermediate in each cycle possessing a covalent bond between a tyrosine in the topoisomerase active site and the strand break terminus (reviewed in [71]). This covalent protein-DNA intermediate can accumulate in cells when the topoisomerase cleaves within abnormal DNA structures. The causes of these structural abnormalities include damage, embedded RNA, and topoisomerase poisons (reviewed in [8]).
Topoisomerases in mammals can be distinguished by whether they cleave one strand, and form either 3′ or 5′ phosphotyrosine intermediates (Type I), or if they cleave both strands, through formation of 5′ phosphotyrosine intermediates on opposing strands (Type II). The adducted products of aborted topoisomerase activity are thus distinct; accordingly, there are two enzymes, each distinctly suited to resolve them. Tyrosine phosphodiesterase (Tdp1) releases the aborted Type I topoisomerase adduct from 3′ strand break termini [72] (Fig. 3C), while Tdp2 activity releases the aborted topoisomerase adducts from 5′ strand break termini [73].
Tdp2 can be clearly linked to NHEJ [74], consistent with the obligate association of aborted topoisomerase II activity and a DSB. By comparison, aborted topoisomerase I activity results in a single strand break (SSB). Nevertheless, Tdp1 has been implicated in helping resolve DSBs associated with type I topoisomerase adducts, e.g. as generated after replication. Tdp1 has also been linked to NHEJ through its ability to process other types of damage at DSB ends [75]. Especially relevant is the ability of Tdp1 to excise the most frequent damaged terminus found at IR-induced DSBs, the 3′ phosphoglycolate [76–78].
It is not clear how the first step in NHEJ, loading of Ku, is possible given the bulkiness of the adducted topoisomerase. Loading of Ku may thus be downstream of both ubiquitin-dependent partial proteolysis of the topoisomerase adduct [79] and, possibly, end cleaning by the tyrosine phosphodiesterase as well. Consistent with this idea, Tdp2 possesses an N-terminal ubiquitin associated (UBA) domain (Fig. 3C) that may help couple end cleaning, and eventually NHEJ, to the strand-break products of aborted topoisomerase activity [80].
II.iii Polymerases and nucleases
For the most part, each of the end cleaning enzymes implicated in strand break repair is targeted to a specific type of damage, and these enzymes target the most frequent types of damage expected given the context. However, they cannot account for all of the types of damage that can be encountered at DSBs. Moreover, end-cleaning enzymes may be unable to recognize their cognate substrate when the density or diversity of damage at an end is too great. End-cleaning enzymes are also unable to generally alter end structure, for example if ends have gaps or mispairs remaining after end-cleaning factors have finished. Therefore, NHEJ also employs several polymerases and nucleases that can more dramatically remodel end structure.
Polymerases
There are four mammalian family X polymerases, and all can function during NHEJ when overexpressed in S. cerevisiae [81]. Their activities are readily distinguished in vitro: in order, pol β>Pol λ>pol μ>TdT, they possess decreasing requirement for complementary sequence opposite the primer terminus and incoming nucleotide triphosphate (Fig. 4B) [82]. The latter three can all be specifically implicated in mammalian NHEJ through specific association with Ku and XRCC4-ligase IV, as mediated by their N-terminal BRCT domains (Fig. 4A). This physical association is essential for polymerase activity at DSB ends with 3′ overhangs [82–85]. Differences in altered size and composition in “loop1”, which may act as a template strand surrogate, further helps distinguish substrate specificities when comparing pol λ, pol μ, and TdT [82, 86–88] (Fig. 4C).
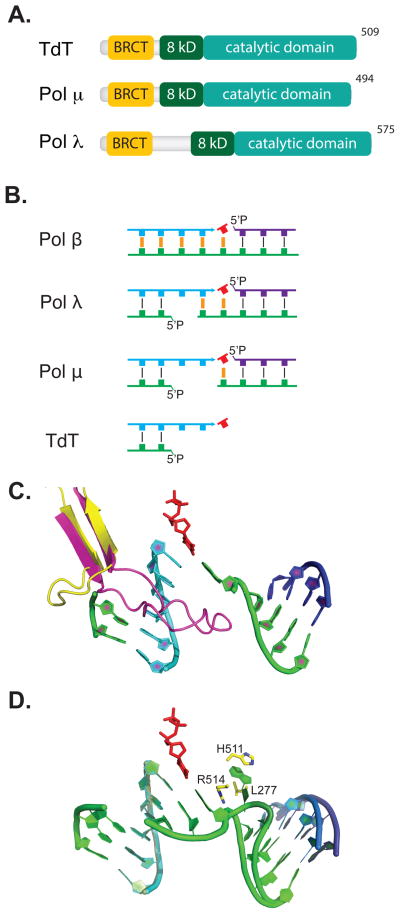
Fig. 4. Polymerases.
(A) Members of X family polymerases implicated in NHEJ possess a BRCA1 C-terminal (BRCT) domain, yellow, an 8 kilodalton (8kD), dark green, and a catalytic domain, teal. (B) The four mammalian Family X polymerases have activity with decreasing requirement for complementary sequence, orange, opposite primer, cyan, and incoming nucleotide triphosphate, red. Template strand, green, and downstream strand, purple, are also shown. (C) The loop1 motif in Pol λ, yellow, is shown from a structure of Pol λ bound to a 1-nucleotide gap with primer, cyan (1XSN) [153], downstream strand, purple, incoming nucleotide, red, and template, green. Four nucleotides of template opposite the primer were omitted. In place of these nucleotides we show loop1 from a structure of TdT, magenta (1JMS) [86], aligned with 1XSN. (D) Aligned substrates from structures of Pol λ with a 1 nt (1XSN) [153] versus a 2 nt gap (3HWT) [92]; primer, cyan, and incoming nucleotide, red, are super-imposable, as is most of template, green, and down stream strands, dark blue & purple. The extra template nucleotide is buried (“scrunched”) in a pocket formed by L277, H511, and R514.
Pol λ
Pol λ is the most orthodox of polymerases specifically implicated in NHEJ, and is indeed also employed as a “backup” to pol β in BER/SSBR [89–91]. Nevertheless, Pol λ’s ability to form a specific complex with NHEJ core factors is essential if it is to remain active in gap filling when ends are only partly complementary [82–84]. Pol λ also uniquely pinches-out (or scrunches) downstream template on gaps longer than 1 nucleotide, thereby increasing its processivity on longer gaps [92] (Fig. 4D). The latter characteristic may be particularly important in the context of NHEJ, where the end alignment needed to generate a gapped substrate is transient.
Pol μ
Pol μ activity involves synthesis using one end as primer, but in contrast to TdT it typically uses a second end as template to select a complementary incoming nucleotide triphosphate (Fig. 4B) [82, 93]. Despite this latter restriction, pol μ has no requirement for pre-existing complementarity sequence in the overhangs. Pol μ activity can thus be distinguished from both pol λ (activity requires the primer be base-paired) and TdT (activity is independent of base pairing, both opposite primer and the site of synthesis) (Figure 4B).
The roles of these polymerases in NHEJ are influenced by both differing expression patterns and partly overlapping substrate specificities. TdT is expressed only in cells active in assembling antigen receptor genes by V(D)J recombination, and its ability to diversify NHEJ repair products is consequently limited to this developmental stage [94, 95]. By comparison, Pol μ is more widely expressed than is TdT, but only at low levels [96, 97]. Like TdT, pol μ is upregulated in a cell type undergoing V(D)J recombination, but it is upregulated at a stage when TdT is not expressed - during recombination of immunoglobulin light chain (IgL) loci [98]. Accordingly, pol μ is essential for mitigating deletion during NHEJ of IgL recombination intermediates [98]. Analysis of NHEJ in pol μ-deficient mice [99] and cultured fibroblasts [100] also confirms an important role for pol μ in promoting NHEJ in non-lymphoid cell types, though it has been suggested this role is independent of pol μ’s synthesis activity [100]. Pol λ appears to have a mild impact on NHEJ, especially in non-lymphoid cell types [95], possibly because its repertoire of substrates may be a subset of Pol μ’s [82].
Nucleases
A variety of nucleases have been implicated in NHEJ; notably, most are bifunctional. Artemis and Metnase are both structure specific endonucleases, while Metnase also possesses histone methyl-transferase activity. The Mre11/Rad50/Nbs1 (MRN) complex possesses structure specific endonuclease activity and exonuclease activity. Finally, the factor mutated in Werner’s syndrome has both exonuclease and helicase activity.
Artemis
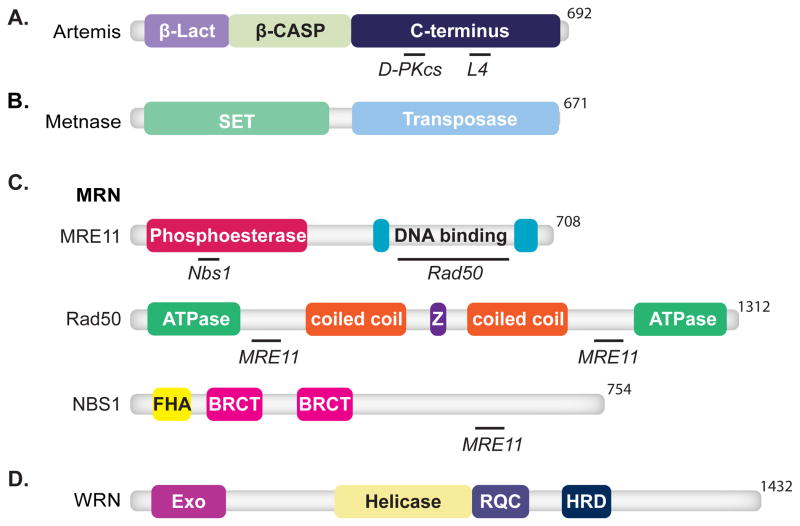
Artemis is the nuclease most clearly linked to NHEJ, through its role in both V(D)J recombination and radioresistance [101, 102]. Nuclease activity resides in an N-terminal domain that combines a metallo-β-lactamase fold with an appended subdomain conserved in other nucleic acid metabolizing enzymes (β-CASP domain) [101, 103–105] (Fig. 5A). A C-terminal domain contains sites for interacting with both DNA-PKcs [106–109] and the DNA binding domain of ligase IV [109, 110] and is hyperphosphorylated in cells by both DNA-PKcs and ATM after damage [106, 111, 112].
Fig. 5. Nucleases.
Features of domain maps are as indicated in Figure 1. (A) Artemis consists of an N-terminal metallo-β-lactamase (β-Lact) domain, purple, and a metallo-β-lactamase-associated CPSF Artemis SNM1 PSO2 (β-CASP) domain, light green, and a C-terminal domain, dark blue. Artemis interacts with DNA-PKcs (D-PKcs) [106, 107] and ligase IV (L4) [109, 110]. (B) Metnase is composed of a N-terminal Su(var) Ez and Trithorax (SET) domain, light green, and a transposase domain, light blue. (C) MRN is composed of MRE11, Rad50, and NBS1. Mre11 contains phosphoesterase, red, and DBDs, cyan. Mre11 interacts with Nbs1 and Rad50 [154]. Domains of Rad50 include ATPase domains, light green, CC domains, orange, and zinc finger domain (Z), purple. Rad50 associates with Mre11 [154]. NBS1 consists of an FHA domain, yellow, and BRCT domains, pink. NBS1 also associates with Mre11 [154]. (D) WRN is composed of a N-terminal exonuclease domain, fuchsia, a helicase domain, yellow, the RecQ carboxy-terminal (RQC) domain, purple, and a helicase and RNase D C-terminal (HRDC) domain, dark blue.
Artemis in complex with phosphorylated DNA-PKcs cleaves generally at ssDNA-dsDNA transitions, including hairpins, overhangs, bubbles, flaps and gapped substrates[113]. Cleavage in ssDNA can also be stimulated by damaged nucleotides [114].
Metnase
Metnase is uniquely found in primates, and possesses both nuclease and histone-methyl transferase activity (Fig. 5B). Both activities have been implicated in NHEJ [115, 116]. Like Artemis, Metnase cleaves within ssDNA and at ssDNA/dsDNA transitions, though there are significant differences in substrate specificity (Metnase requires a strand terminus to engage substrate) [115]. Also like Artemis, Metnase physically interacts with ligase IV [117].
Mre11/Rad50/Nbs1 (MRN) complex
MRN (Fig. 5C) performs many functions central to the DNA damage response (reviewed in [118]). Relevant to NHEJ, it can sense and bridge broken ends [119] and possesses both 3′>5′ exonuclease as well as structure-specific endonuclease activities [120, 121]. However, as discussed in greater detail below, processing of DSB ends by MRN has been implicated in more than one DSB repair pathway.
WRN
WRN helicase and exonuclease (Fig. 5D) interacts with Ku, and this interaction both generally stimulates WRN exonuclease activity [122–125] and allows it to proceed through damaged nucleotides (e.g. 8-oxoguanine) [124]. Like MRN, WRN probably contributes to DSB end processing in multiple DSB repair pathways (discussed below).
III Strategies for resolving ends
The lack of a continuous template to guide repair factor activity distinguishes NHEJ from other repair pathways. This distinction hinders normal function of many repair factors, most obviously polymerases and ligases. NHEJ thus employs a core assembly that aligns ends together, essentially substituting for continuous template with a network of protein-protein and protein-DNA interactions. However, this is only a partial solution, and NHEJ must flexibly employ a number of strategies for repair.
III.i Tolerance at the ligation step
The only essential step in NHEJ is the ligation of at least one strand of the DSB. Thus, one strategy presumably employed by NHEJ is the direct joining of imperfectly aligned ends via low fidelity ligation (Fig. 6A). This is conceptually similar to DNA damage tolerance during translesion DNA synthesis (TLS), which is used to bypass replication blocks caused by sites of DNA damage without the need for repair (reviewed in [126]).
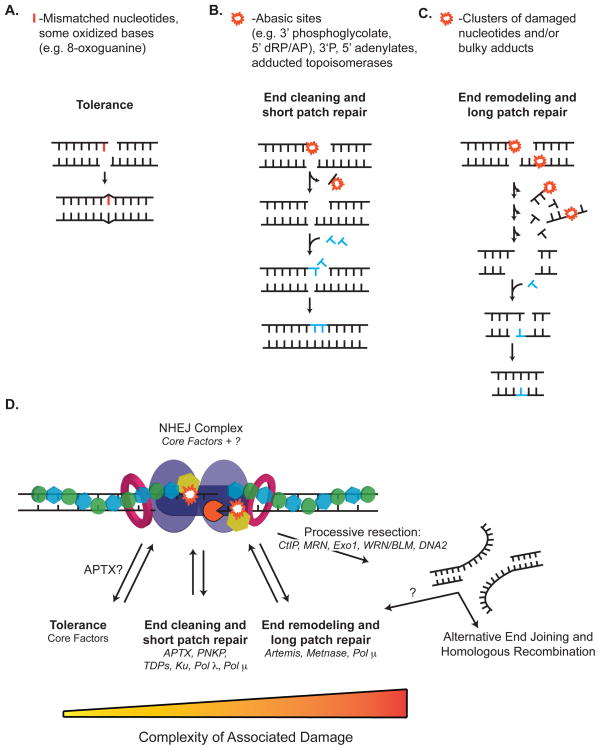
Fig. 6. Strategies for Damage Processing by NHEJ.
NHEJ employs three strategies for resolving damage, red line and starbursts, at ends. After assembly of core factors and alignment of broken ends, the core complex can (A) attempt to tolerate the break by direct ligation, or rearrange the complex and recruit (B) end-cleaning enzymes for short patch excision, or (C) end-remodeling enzymes for more extensive damage processing. Newly synthesized and incoming nucleotides are shown, blue. (D) NHEJ may employ these strategies hierarchically. The choice and transition from one strategy to the next is likely determined by the complexity of damage at the ends. The NHEJ core complex is shown as described in Figure 2D.
There is evidence that NHEJ, at least in vitro, is uniquely tolerant of mismatches at the ligation step [50, 53]. Structural studies suggests this may be attributable in part to differences in how ligase IV interacts with its substrate [31], relative to other ligases. As discussed above (Fig. 2D, section II.i), though, it is probable that the incorporation of the ligase within an extended DNA-protein filament – essentially a “protein splint” to align termini together – is essential. The filament may promote ligation by simply stabilizing end synapsis, thereby increasing the time available for completing catalysis. An attractive idea is that the filament also promotes the active sampling of different alignment geometries (e.g., kinks and rotations in terminal nucleotides) and allows the ligase to take advantage of an alignment geometry that represents the lowest barrier to ligation.
However, low fidelity ligation is prone to aborting after adenylating the 5′ phosphate terminus [32]. An already adenylated ligase cannot engage this substrate; this would occur if the “spent” ligase’s active site is re-adenylated in situ, or if the spent ligase is exchanged for another, already adenylated ligase. The former problem might be mitigated somewhat by the general resistance of ligase IV to re-adenylation, relative to other mammalian ligases [127]. Possibly more significantly, aprataxin can remove the adenylate on the substrate regardless of the reason why the ligase is also adenylated; the NHEJ complex is thus reset for another ligation attempt [61, 62].
As with translesion synthesis, a key feature of tolerance is that the junction retains damage or mismatches after NHEJ is finished (Fig. 6A). Therefore, other DNA repair pathways are required to completely restore the integrity of the DNA. Inasmuch as the retained damage may be bi-stranded, it must be carefully processing by other repair pathways (e.g. BER) to avoid re-introducing a DSB (aborted BER).
III.ii End cleaning and short patch NHEJ
DSB termini may be damaged, mispaired, or adducted such that ligation –even with reduced fidelity - is blocked. Such ends can be resolved by NHEJ using steps equivalent to those used in BER/SSBR, where repair is tailored to the specific class of damage, and excision consequently can be limited to this damage (Fig. 6B). As described above, the two pathways share some of the same enzymes (PNKP, aprataxin, Tdp1, and pol λ).
An example of “short patch repair” in NHEJ involves repair of ends with 3′ phosphoglycolates, probably the most frequent class of damage associated with IR-induced DSBs [128]. They can be resolved in cell extracts using steps borrowed from SSBR – in this case, the nucleotide residue can be excised by Tdp1, leaving a 3′ phosphate substrate for PNKP [76–78]. The resulting one-nucleotide gap can then be filled in by polymerases μ or λ [82, 129].
The enzymes shared between NHEJ and BER/SSBR appear restricted to those involved in the late end cleaning steps expected to be common to both pathways (e.g. PNKP, Tdps). Glycosylases are required for the first step in BER (removal of damaged bases) but have yet to be linked to NHEJ and, when tested, were found to be much less active at DSB termini [130]. Base damage (e.g. oxidized bases) in some contexts can be sufficiently subtle enough to be tolerated at the ligation step (e.g. [130, 131]), thus excision may be delayed until NHEJ is complete and the junction a substrate for “true” BER.
In contrast to nucleotides with damaged bases, abasic sites cannot be tolerated at the ligation step, and the enzymes that excise abasic sites in BER (APE1, pol β) are less active at DSB ends, relative to abasic sites embedded in duplex DNA [55, 130]. NHEJ instead employs Ku [55], at least when the abasic site is near 5′ termini. Ku has a substrate specificity that is appropriately reciprocal to BER enzymes involved in abasic site removal with regard to DSB proximity [56]. Ku’s abasic site substrates can be directly generated in the same damaging event responsible for strand breakage. However, the principle source of this class of abasic site may be DSB products of an “aborted” BER reaction, where BER substrates present in opposite strands can be incised by APE1 to generate 5′dRP-terminated DSBs [132]. This mechanism is probably the primary means for generating the DSB intermediate in immunoglobulin class switch recombination [133].
As long as excision of damaged nucleotides is sufficiently limited, it may be possible to still align ends with remaining partly complementary sequence. Gap-fill-in by a polymerase could then fully reconstitute the sequence of the broken region (Fig. 6B), and this reaction has been reconstituted using cell extracts [134]. Given the parallels between this reaction and BER, the best candidate polymerase is Pol λ (Pol λ is shared with BER). As noted above, both pol μ and pol λ are active in this context (partly complementary ends that align to generate a short gap) in vitro, and it isn’t yet obvious if there are advantages to using one or the other.
III.iii End remodeling and Long-patch NHEJ
Targeted excision of ligation-blocking damage may not be possible, either because there is no cognate enzyme, or because the density of damage blocks the ability of an enzyme to recognize its cognate lesion. Such ends can still be resolved by resecting the damaged strand with endonucleases like Artemis and Metnase. This method of targeting damage is more flexible but less specific than short patch NHEJ (Fig. 6C cf. 6B). For example, Artemis and Metnase can excise the same 3′phosphoglycolate terminus as Tdp1/PNKP, except when nucleases are used more flanking DNA is lost and the products of excision are heterogeneous [78, 114, 135]. Since damage is removed as part of a patch (i.e. including some flanking DNA) this strategy might be considered analogous to long patch BER, or possibly nucleotide excision repair.
A long patch solution creates unique problems for NHEJ. End structures will typically be sufficiently altered or remodeled such that alignment-directed synthesis cannot restore original information, and products have deletions even if they are a substrate for direct ligation (Fig. 6B). More importantly, the odds of generating a ligation substrate after one round of nuclease activity are low. Consequently, ends often need to be subjected to cycles of repeated processing steps (iterative model) [136], whether the step involves a nuclease again or some other end remodeling factor. At the same time, the products of each processing cycle should be interrogated as a possible ligation substrate so as to avoid futile cycles and excessive sequence loss. Accordingly, Artemis interacts directly with both ligase IV and DNA-PKcs (Fig. 5A), and kinase activity of the latter is required for Artemis to be active [101, 108, 109]. These interactions suggest that DNA-PKcs may limit the activity of Artemis to an end within the context of an aligned end-pair, and physical association with ligase IV may promote a trial-ligation of the ends after each cycle of processing.
Polymerase μ probably plays an especially important role in long patch NHEJ. In pol μ’s absence, excision cycles must rely on the chance generation of an overhang with sufficient complementary sequence that ligation can proceed. This can be seen as microhomology dependence, where deletion continues until identical sequence is revealed on opposite strands. Pol μ’s unique ability to convert non-complementary ends to complementary ends solves this problem, as is apparent by the increased frequency of microhomology-mediated junctions observed in Pol μ’s absence [82].
Like Artemis, the MRN complex also promotes a “long patch solution” to ligation-blocking damage. For example, MRN directs the release of a protein adducted to strand termini by promoting endonucleolytic cleavage of DNA a short distance (10–16 nt) away from the adducted end [137]. This is a stark contrast to the short patch solution, wherein a tyrosine phosphodiesterase cleanly removes these adducts [73].
Notably, the MRN complex may solve the problem intrinsic to long patch excision differently than suggested for Artemis. MRN at least in vitro can generates compatible ends using a “microhomology search” mechanism, rather than through cycles of processing and trial ligation. MRN can interact with a pair of ends in a synaptic complex in a manner that couples degradation of 3′ termini with the alignment of now extruded 5′ tails [138]. This presumably gives MRN the ability to sense when 5′ tails possess complementary sequence, as the exonuclease activity can be observed to pause in vitro as soon as complementary sequence has been revealed [139, 140]. The resulting “sticky” 5′ termini can now participate in end joining. The alternative, where each end is processed independently of the other, would be inefficient, slow, and likely require excessive deletion before a chance microhomology is revealed. In mammals, MRN performs microhomology search most efficiently with ligase III, suggesting it’s role might more accurately be considered part of the alternative end joining (Alt-EJ) pathway [140].
It also seems possible that, when limited processing fails, ends are directed towards processive resection. CtIP, MRN, Exo1, and a RecQ helicase (including WRN) promote the release of Ku from ends [141] and generate long (100s of nucleotides) 3′ ssDNA tails (reviewed in [142]). These tails are typically intended to serve as the precursor to repair by HR and can also be resolved by Alt-EJ (Fig. 6D). However, the tails could also be clipped off, perhaps by Artemis, and then resolved by canonical NHEJ. Coupling processive resection with subsequent resolution by canonical NHEJ might be aided by physical interaction between the WRN RecQ helicase and Ku [122–125], as well as the ability of DNA-PKcs to regulate WRN activity [143, 144].
III.iv Regulation of NHEJ strategy
We have proposed that NHEJ employs multiple strategies for repair, with these strategies distinguishable by both the extent and specificity of the end processing steps employed prior to ligation. How does NHEJ regulate which strategy and which processing factor within a strategy should be used?
The type(s) of damage present at the ends might rigidly determine the strategy by which NHEJ processes ends. NHEJ would thus be able to identify the specific type of damage and proceed accordingly. For example, upon recognition of an end with an adducted topoisomerase, the core assembly would specifically recruit a tyrosine phosphodiesterase. However, the complexity of damage at ends (e.g. [6]) and the number of different processing factors involved would probably rapidly overwhelm such a mechanism.
A more reasonable model is to organize processing hierarchically according to the extent of processing required. The core assembly would thus attempt to first ligate an end directly (tolerance) and proceed as needed to short patch excision, then to long patch excision (Fig. 6D). A hierarchical mechanism has the benefit of allowing for the retention of as much sequence as possible, while still eliminating the need for the NHEJ machinery to specifically identify and appropriately process all of the possible types of damage.
Transitions in processing strategies may simply occur spontaneously, as the aligned complex continues to engage a pair of ends unproductively. Processing factor recruitment and access to ends may then be somewhat stochastically determined, perhaps influenced by differing abundance of the processing factors and their affinity for substrate.
Regulation by phosphorylation
However, it is clear that changes in processing are also facilitated by dynamic reorganization of the complex as signaled by post-translational modifications. An interesting notion is that the signaling modification is not limited to the proteins involved; specifically, the 5′ adenylate product of aborted ligation or recognition of the adenylated product by aprataxin could serve as a signal to trigger a change in how the end is processed.
DNA-PKcs has already been implicated in control of processing. Phosphorylation of DNA-PKcs itself, either by itself (autophosphorylation) or by ATM, promotes first the rearrangement of DNA-PKcs at ends as well as end accessibility, then eventually DNA-PKcs dissociation (reviewed in [145]). However, there are over 30 different phosphorylation sites in DNA-PKcs [146] (Fig. 2B). Mutation of different sites has different effects on processing, indicating that there are qualitatively distinct states in accessibility (reviewed in [145]). The molecular basis for these states is not known and will require high resolution mapping of autophosphorylation sites and DNA interactions on the structure of DNA-PKcs.
DNA-PKcs also directly modifies the function of factors involved in NHEJ by phosphorylating them. DNA-PKcs (and in some instances, ATM as well) phosphorylate Artemis [106, 108, 109, 111, 112], PNKP [147], and WRN [143, 144], which in turn typically promotes recruitment and/or activity of these factors. DNA-PKcs also phosphorylates the ligase complex – most prominently XRCC4 [148] and XLF [149]. The latter phosphorylations serve to reduce DNA binding and progressively disrupt the XRCC4-XLF-ligase IV filament [150], suggesting possible intermediate states where the filament is more flexible or allows greater access for processing factors.
Regulation by ribosylation
Post-translational modification by Poly (ADP ribose) polymerases (PARPs) also affects NHEJ complex organization. PARPs are activated by strand breaks and modify factors with poly(ADP ribose) (PAR) chains [68], which directly affect the function of the factor modified and also recruit other factors that possess PAR binding modules. APLF is one such factor and, as noted above, has been shown to stabilize the assembly of NHEJ core factors at breaks directly through multiple protein-protein interactions [14]. However, APLF could also promote dynamic changes in the stability and composition of complexes present at strand breaks; its PBZ domains can interact with uncharacterized factors already present at the strand break after they are modified by PAR [67, 69]. APLF may also interact simultaneously with XRCC1 or XRCC4 [41, 42], Ku, [14, 66] and PAR [69], thus acting as a physical and inducible link between multiple strand break repair pathways (e.g. NHEJ, SSBR, Alt-EJ). This could provide a means for seamlessly re-directing repair to a different pathway. For example, APLF may promote resolution by NHEJ of aborted products of BER, or repair by BER of joined products of NHEJ that still retain damage in flanking DNA.
IV. Making the best of a bad situation
There is a wide spectrum of different end structures that can be encountered. For the fraction of these ends with simple end structures – for example, restriction enzyme-generated breaks - “simple ligation” is sufficient. NHEJ might promote fast joining of these breaks, thus preventing less savory outcomes, including the triggering of a cellular response or destruction of the initially simple end structure by a nuclease.
However, it is clear that NHEJ’s most important role is in resolving those end structures that are not already an easy substrate for ligation, and NHEJ can employ multiple strategies to still join them together (Fig. 6). We’ve suggested these strategies can be likened to those used by other pathways. The strategies include tolerance (similar to TLS), targeted excision (similar to short patch BER and SSBR), and finally a broad excision and remodeling of the initial end structure (with similarities to long patch BER, or NER). They can be ordered as above according to increasing invasiveness and consequent error, but also according to increasing flexibility, and thus capacity for more complex damage (Fig. 6D). We’ve argued therefore that NHEJ would be both most accurate and efficient when these strategies are employed hierarchically – one shouldn’t use a sledgehammer to crack a nut.
NHEJ is often considered error-prone. Such error could presumably be attributed to NHEJ’s use of the wrong strategy; we suggest it is more often due to a failure to engage the broken end soon enough.
Highlights.
We review here how diverse ends at chromosome breaks are resolved by NHEJ
We summarize structures, interactions, and activities of factors implicated in NHEJ
NHEJ employs an amalgam of strategies used by other repair pathways
This gives NHEJ flexibility, but implies strategy choice must be regulated
We speculate on why such regulation might be important
Acknowledgments
Work by the Ramsden Lab is supported by NIH grants CA84442 and CA097096. The authors would like to thank Christina Strom.
Footnotes
Abbreviations : Alt-EJ, alternative end joining; APLF, aprataxin and polynucleotide kinase/phosphatase-like factor; ATM, ataxia-telangiectasia mutated; ATR, ATM-, Rad3-related; APTX, aprataxin; β-Casp, metallo-β-lactamase-associated CPSF Artemis SNM1 PSO2; BER, base excision repair; BRCT, BRCA1 C-terminal; DNA-PK, DNA-dependent protein kinase; DNA-PKcs, DNA protein kinase catalytic subunit; DSB, double-strand break; dRP/AP, deoxyribose-phosphate/apurinic/apyrimidinic; DSB, double strand break; dsDNA, double-stranded DNA; FHA, forkhead-associated; HEAT, Huntington elongation factor 2, A subunit of protein phosphatase 2A and TOR1; HR, homologous recombination; HRDC, helicase and RNase D C-terminal; IR, ionizing radiation; Ku, Ku70/80 heterodimer; MRN, Mre11/Rad50/Nbs1; NAP1, nucleosome assembly protein-1; NHEJ, non-homologous end-joining; PIKK, phosphoinositide 3-kinase-like family of protein kinases; PNKP, polynucleotide kinase/phosphatase; PAR, poly(adenyl-ribose); PARP, PAR polymerase; PBZ, PAR-binding zinc finger; ROS, reactive oxygen species; RQC, RecQ carboxy-terminal; SAP domain, SAF-A/B, Acinus and PIAS domain; SCID, severe combined immunodeficiency; SET, Su(var) Ez and Trithorax; SSBR, single-strand break repair; Tdp1, tyrosyl-DNA phosphodiesterase 1; Tdp2, tyrosyl-DNA phosphodiesterase 2; TdT, terminal deoxyribonucleotidyltransferase; vWa, von Wilebrand; WRN, Werner’s Syndrome helicase; XRCC4, X ray-complementing Chinese hamster gene 4; X4–L4 complex, XRCC4–DNA ligase IV complex; XLF, XRCC4-like factor
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Crystal Ann Waters, Email: cawaters@email.unc.edu.
Natasha Tiffany Strande, Email: strande@email.unc.edu.
David William Wyatt, Email: dwwyatt@email.unc.edu.
John Michael Pryor, Email: pryorjm@email.unc.edu.
Dale A. Ramsden, Email: dale_ramsden@med.unc.edu.
Works Cited
- 1.Heyer WD, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet. 2010;44:113–139. doi: 10.1146/annurev-genet-051710-150955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman JR, Taylor MR, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 4.Goodhead DT. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- 5.Ward JF. The complexity of DNA damage: relevance to biological consequences. Int J Radiat Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 6.Breen AP, Murphy JA. Reactions of oxyl radicals with DNA. Free radical biology & medicine. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- 7.Roth DB, Menetski JP, Nakajima PB, Bosma MJ, Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992;70:983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- 8.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gell D, Jackson SP. Mapping of protein-protein interactions within the DNA-dependent protein kinase complex. Nucleic Acids Res. 1999;27:3494–3502. doi: 10.1093/nar/27.17.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dynan WS, Yoo S. Interaction of Ku protein and DNA-dependent protein kinase catalytic subunit with nucleic acids. Nucleic Acids Res. 1998;26:1551–1559. doi: 10.1093/nar/26.7.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 12.Singleton BK, Torres-Arzayus MI, Rottinghaus ST, Taccioli GE, Jeggo PA. The C terminus of Ku80 activates the DNA-dependent protein kinase catalytic subunit. Mol Cell Biol. 1999;19:3267–3277. doi: 10.1128/mcb.19.5.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aravind L, Koonin EV. SAP - a putative DNA-binding motif involved in chromosomal organization. Trends Biochem Sci. 2000;25:112–114. doi: 10.1016/s0968-0004(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 14.Grundy GJ, Rulten SL, Zeng Z, Arribas-Bosacoma R, Iles N, Manley K, Oliver A, Caldecott KW. APLF promotes the assembly and activity of non-homologous end joining protein complexes. EMBO J. 2013;32:112–125. doi: 10.1038/emboj.2012.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yano K, Morotomi-Yano K, Lee KJ, Chen DJ. Functional significance of the interaction with Ku in DNA double-strand break recognition of XLF. FEBS Lett. 2011;585:841–846. doi: 10.1016/j.febslet.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 17.Blier PR, Griffith AJ, Craft J, Hardin JA. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem. 1993;268:7594–7601. [PubMed] [Google Scholar]
- 18.Paillard S, Strauss F. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 1991;19:5619–5624. doi: 10.1093/nar/19.20.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 20.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 21.Sibanda BL, Chirgadze DY, Blundell TL. Crystal structure of DNA-PKcs reveals a large open-ring cradle comprised of HEAT repeats. Nature. 2010;463:118–121. doi: 10.1038/nature08648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFazio LG, Stansel RM, Griffith JD, Chu G. Synapsis of DNA ends by DNA-dependent protein kinase. EMBO J. 2002;21:3192–3200. doi: 10.1093/emboj/cdf299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weterings E, Verkaik NS, Bruggenwirth HT, Hoeijmakers JH, van Gent DC. The role of DNA dependent protein kinase in synapsis of DNA ends. Nucleic Acids Res. 2003;31:7238–7246. doi: 10.1093/nar/gkg889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy YV, Ding Q, Lees-Miller SP, Meek K, Ramsden DA. Non-homologous end joining requires that the DNA-PK complex undergo an autophosphorylation-dependent rearrangement at DNA ends. J Biol Chem. 2004;279:39408–39413. doi: 10.1074/jbc.M406432200. [DOI] [PubMed] [Google Scholar]
- 25.Meek K, Douglas P, Cui X, Ding Q, Lees-Miller SP. trans Autophosphorylation at DNA-dependent protein kinase’s two major autophosphorylation site clusters facilitates end processing but not end joining. Mol Cell Biol. 2007;27:3881–3890. doi: 10.1128/MCB.02366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen BP, Uematsu N, Kobayashi J, Lerenthal Y, Krempler A, Yajima H, Lobrich M, Shiloh Y, Chen DJ. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J Biol Chem. 2007;282:6582–6587. doi: 10.1074/jbc.M611605200. [DOI] [PubMed] [Google Scholar]
- 27.Calsou P, Delteil C, Frit P, Drouet J, Salles B. Coordinated assembly of Ku and p460 subunits of the DNA-dependent protein kinase on DNA ends is necessary for XRCC4-ligase IV recruitment. J Mol Biol. 2003;326:93–103. doi: 10.1016/s0022-2836(02)01328-1. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Trujillo K, Sung P, Tomkinson AE. Interactions of the DNA ligase IV-XRCC4 complex with DNA ends and the DNA-dependent protein kinase. J Biol Chem. 2000;275:26196–26205. doi: 10.1074/jbc.M000491200. [DOI] [PubMed] [Google Scholar]
- 29.Grawunder U, Wilm M, Wu X, Kulesza P, Wilson TE, Mann M, Lieber MR. Activity of DNA ligase IV stimulated by complex formation with XRCC4 protein in mammalian cells. Nature. 1997;388:492–495. doi: 10.1038/41358. [DOI] [PubMed] [Google Scholar]
- 30.Nick McElhinny SA, Snowden CM, McCarville J, Ramsden DA. Ku recruits the XRCC4-ligase IV complex to DNA ends. Mol Cell Biol. 2000;20:2996–3003. doi: 10.1128/mcb.20.9.2996-3003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochi T, Gu X, Blundell TL. Structure of the catalytic region of DNA ligase IV in complex with an artemis fragment sheds light on double-strand break repair. Structure. 2013;21:672–679. doi: 10.1016/j.str.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellenberger T, Tomkinson AE. Eukaryotic DNA ligases: structural and functional insights. Annu Rev Biochem. 2008;77:313–338. doi: 10.1146/annurev.biochem.77.061306.123941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomkinson AE, Mackey ZB. Structure and function of mammalian DNA ligases. Mutat Res. 1998;407:1–9. doi: 10.1016/s0921-8777(97)00050-5. [DOI] [PubMed] [Google Scholar]
- 34.Grawunder U, Zimmer D, Leiber MR. DNA ligase IV binds to XRCC4 via a motif located between rather than within its BRCT domains. Curr Biol. 1998;8:873–876. doi: 10.1016/s0960-9822(07)00349-1. [DOI] [PubMed] [Google Scholar]
- 35.Modesti M, Junop MS, Ghirlando R, van de Rakt M, Gellert M, Yang W, Kanaar R. Tetramerization and DNA ligase IV interaction of the DNA double-strand break repair protein XRCC4 are mutually exclusive. J Mol Biol. 2003;334:215–228. doi: 10.1016/j.jmb.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Sibanda BL, Critchlow SE, Begun J, Pei XY, Jackson SP, Blundell TL, Pellegrini L. Crystal structure of an Xrcc4-DNA ligase IV complex. Nat Struct Biol. 2001;8:1015–1019. doi: 10.1038/nsb725. [DOI] [PubMed] [Google Scholar]
- 37.Wu PY, Frit P, Meesala S, Dauvillier S, Modesti M, Andres SN, Huang Y, Sekiguchi J, Calsou P, Salles B, Junop MS. Structural and functional interaction between the human DNA repair proteins DNA ligase IV and XRCC4. Mol Cell Biol. 2009;29:3163–3172. doi: 10.1128/MCB.01895-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junop MS, Modesti M, Guarne A, Ghirlando R, Gellert M, Yang W. Crystal structure of the Xrcc4 DNA repair protein and implications for end joining. EMBO J. 2000;19:5962–5970. doi: 10.1093/emboj/19.22.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch CA, Agyei R, Galicia S, Metalnikov P, O’Donnell P, Starostine A, Weinfeld M, Durocher D. Xrcc4 physically links DNA end processing by polynucleotide kinase to DNA ligation by DNA ligase IV. EMBO J. 2004;23:3874–3885. doi: 10.1038/sj.emboj.7600375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clements PM, Breslin C, Deeks ED, Byrd PJ, Ju L, Bieganowski P, Brenner C, Moreira MC, Taylor AM, Caldecott KW. The ataxia-oculomotor apraxia 1 gene product has a role distinct from ATM and interacts with the DNA strand break repair proteins XRCC1 and XRCC4. DNA Repair (Amst) 2004;3:1493–1502. doi: 10.1016/j.dnarep.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Iles N, Rulten S, El-Khamisy SF, Caldecott KW. APLF (C2orf13) is a novel human protein involved in the cellular response to chromosomal DNA strand breaks. Mol Cell Biol. 2007;27:3793–3803. doi: 10.1128/MCB.02269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macrae CJ, McCulloch RD, Ylanko J, Durocher D, Koch CA. APLF (C2orf13) facilitates nonhomologous end-joining and undergoes ATM-dependent hyperphosphorylation following ionizing radiation. DNA Repair (Amst) 2008;7:292–302. doi: 10.1016/j.dnarep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Andres SN, Modesti M, Tsai CJ, Chu G, Junop MS. Crystal structure of human XLF: a twist in nonhomologous DNA end-joining. Mol Cell. 2007;28:1093–1101. doi: 10.1016/j.molcel.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Chirgadze DY, Bolanos-Garcia VM, Sibanda BL, Davies OR, Ahnesorg P, Jackson SP, Blundell TL. Crystal structure of human XLF/Cernunnos reveals unexpected differences from XRCC4 with implications for NHEJ. EMBO J. 2008;27:290–300. doi: 10.1038/sj.emboj.7601942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andres SN, Vergnes A, Ristic D, Wyman C, Modesti M, Junop M. A human XRCC4-XLF complex bridges DNA. Nucleic Acids Res. 2012;40:1868–1878. doi: 10.1093/nar/gks022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammel M, Yu Y, Fang S, Lees-Miller SP, Tainer JA. XLF regulates filament architecture of the XRCC4.ligase IV complex. Structure. 2010;18:1431–1442. doi: 10.1016/j.str.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malivert L, Ropars V, Nunez M, Drevet P, Miron S, Faure G, Guerois R, Mornon JP, Revy P, Charbonnier JB, Callebaut I, de Villartay JP. Delineation of the Xrcc4-interacting region in the globular head domain of cernunnos/XLF. J Biol Chem. 2010;285:26475–26483. doi: 10.1074/jbc.M110.138156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ropars V, Drevet P, Legrand P, Baconnais S, Amram J, Faure G, Marquez JA, Pietrement O, Guerois R, Callebaut I, Le Cam E, Revy P, de Villartay JP, Charbonnier JB. Structural characterization of filaments formed by human Xrcc4-Cernunnos/XLF complex involved in nonhomologous DNA end-joining. Proc Natl Acad Sci U S A. 2011;108:12663–12668. doi: 10.1073/pnas.1100758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai CJ, Chu G. Cooperative assembly of a protein-DNA filament for nonhomologous end joining. J Biol Chem. 2013;288:18110–18120. doi: 10.1074/jbc.M113.464115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai CJ, Kim SA, Chu G. Cernunnos/XLF promotes the ligation of mismatched and noncohesive DNA ends. Proc Natl Acad Sci U S A. 2007;104:7851–7856. doi: 10.1073/pnas.0702620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahnesorg P, Smith P, Jackson SP. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 2006;124:301–313. doi: 10.1016/j.cell.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 52.Cottarel J, Frit P, Bombarde O, Salles B, Negrel A, Bernard S, Jeggo PA, Lieber MR, Modesti M, Calsou P. A noncatalytic function of the ligation complex during nonhomologous end joining. The Journal of cell biology. 2013;200:173–186. doi: 10.1083/jcb.201203128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu J, Lu H, Tsai AG, Schwarz K, Lieber MR. Single-stranded DNA ligation and XLF-stimulated incompatible DNA end ligation by the XRCC4-DNA ligase IV complex: influence of terminal DNA sequence. Nucleic Acids Res. 2007;35:5755–5762. doi: 10.1093/nar/gkm579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 55.Roberts SA, Strande N, Burkhalter MD, Strom C, Havener JM, Hasty P, Ramsden DA. Ku is a 5′-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strande N, Roberts SA, Oh S, Hendrickson EA, Ramsden DA. Specificity of the dRP/AP lyase of Ku promotes nonhomologous end joining (NHEJ) fidelity at damaged ends. J Biol Chem. 2012;287:13686–13693. doi: 10.1074/jbc.M111.329730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jilani A, Ramotar D, Slack C, Ong C, Yang XM, Scherer SW, Lasko DD. Molecular cloning of the human gene, PNKP, encoding a polynucleotide kinase 3′-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. J Biol Chem. 1999;274:24176–24186. doi: 10.1074/jbc.274.34.24176. [DOI] [PubMed] [Google Scholar]
- 58.Bernstein NK, Williams RS, Rakovszky ML, Cui D, Green R, Karimi-Busheri F, Mani RS, Galicia S, Koch CA, Cass CE, Durocher D, Weinfeld M, Glover JN. The molecular architecture of the mammalian DNA repair enzyme, polynucleotide kinase. Mol Cell. 2005;17:657–670. doi: 10.1016/j.molcel.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Bernstein NK, Hammel M, Mani RS, Weinfeld M, Pelikan M, Tainer JA, Glover JN. Mechanism of DNA substrate recognition by the mammalian DNA repair enzyme, Polynucleotide Kinase. Nucleic Acids Res. 2009;37:6161–6173. doi: 10.1093/nar/gkp597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coquelle N, Havali-Shahriari Z, Bernstein N, Green R, Glover JN. Structural basis for the phosphatase activity of polynucleotide kinase/phosphatase on single- and double-stranded DNA substrates. Proc Natl Acad Sci U S A. 2011;108:21022–21027. doi: 10.1073/pnas.1112036108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature. 2006;443:713–716. doi: 10.1038/nature05164. [DOI] [PubMed] [Google Scholar]
- 62.Rass U, Ahel I, West SC. Molecular mechanism of DNA deadenylation by the neurological disease protein aprataxin. J Biol Chem. 2008;283:33994–34001. doi: 10.1074/jbc.M807124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tumbale P, Appel CD, Kraehenbuehl R, Robertson PD, Williams JS, Krahn J, Ahel I, Williams RS. Structure of an aprataxin-DNA complex with insights into AOA1 neurodegenerative disease. Nat Struct Mol Biol. 2011;18:1189–1195. doi: 10.1038/nsmb.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanno S, Kuzuoka H, Sasao S, Hong Z, Lan L, Nakajima S, Yasui A. A novel human AP endonuclease with conserved zinc-finger-like motifs involved in DNA strand break responses. EMBO J. 2007;26:2094–2103. doi: 10.1038/sj.emboj.7601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S, Kanno S, Watanabe R, Ogiwara H, Kohno T, Watanabe G, Yasui A, Lieber MR. Polynucleotide kinase and aprataxin-like forkhead-associated protein (PALF) acts as both a single-stranded DNA endonuclease and a single-stranded DNA 3′ exonuclease and can participate in DNA end joining in a biochemical system. J Biol Chem. 2011;286:36368–36377. doi: 10.1074/jbc.M111.287797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shirodkar P, Fenton AL, Meng L, Koch CA. Identification and functional characterization of a Ku-binding motif in aprataxin polynucleotide kinase/phosphatase-like factor (APLF) J Biol Chem. 2013;288:19604–19613. doi: 10.1074/jbc.M112.440388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rulten SL, Fisher AE, Robert I, Zuma MC, Rouleau M, Ju L, Poirier G, Reina-San-Martin B, Caldecott KW. PARP-3 and APLF function together to accelerate nonhomologous end-joining. Mol Cell. 2011;41:33–45. doi: 10.1016/j.molcel.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 68.Langelier MF, Pascal JM. PARP-1 mechanism for coupling DNA damage detection to poly(ADP-ribose) synthesis. Current opinion in structural biology. 2013;23:134–143. doi: 10.1016/j.sbi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rulten SL, Cortes-Ledesma F, Guo L, Iles NJ, Caldecott KW. APLF (C2orf13) is a novel component of poly(ADP-ribose) signaling in mammalian cells. Mol Cell Biol. 2008;28:4620–4628. doi: 10.1128/MCB.02243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehrotra PV, Ahel D, Ryan DP, Weston R, Wiechens N, Kraehenbuehl R, Owen-Hughes T, Ahel I. DNA repair factor APLF is a histone chaperone. Mol Cell. 2011;41:46–55. doi: 10.1016/j.molcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vos SM, Tretter EM, Schmidt BH, Berger JM. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol. 2011;12:827–841. doi: 10.1038/nrm3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang SW, Burgin AB, Jr, Huizenga BN, Robertson CA, Yao KC, Nash HA. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc Natl Acad Sci U S A. 1996;93:11534–11539. doi: 10.1073/pnas.93.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 74.Gomez-Herreros F, Romero-Granados R, Zeng Z, Alvarez-Quilon A, Quintero C, Ju L, Umans L, Vermeire L, Huylebroeck D, Caldecott KW, Cortes-Ledesma F. TDP2-dependent non-homologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 2013;9:e1003226. doi: 10.1371/journal.pgen.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pouliot JJ, Robertson CA, Nash HA. Pathways for repair of topoisomerase I covalent complexes in Saccharomyces cerevisiae. Genes Cells. 2001;6:677–687. doi: 10.1046/j.1365-2443.2001.00452.x. [DOI] [PubMed] [Google Scholar]
- 76.Inamdar KV, Pouliot JJ, Zhou T, Lees-Miller SP, Rasouli-Nia A, Povirk LF. Conversion of phosphoglycolate to phosphate termini on 3′ overhangs of DNA double strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. J Biol Chem. 2002;277:27162–27168. doi: 10.1074/jbc.M204688200. [DOI] [PubMed] [Google Scholar]
- 77.Zhou T, Lee JW, Tatavarthi H, Lupski JR, Valerie K, Povirk LF. Deficiency in 3′-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1) Nucleic Acids Res. 2005;33:289–297. doi: 10.1093/nar/gki170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou T, Akopiants K, Mohapatra S, Lin PS, Valerie K, Ramsden DA, Lees-Miller SP, Povirk LF. Tyrosyl-DNA phosphodiesterase and the repair of 3′-phosphoglycolate-terminated DNA double-strand breaks. DNA Repair (Amst) 2009;8:901–911. doi: 10.1016/j.dnarep.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao Y, Desai SD, Ting CY, Hwang J, Liu LF. 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J Biol Chem. 2001;276:40652–40658. doi: 10.1074/jbc.M104009200. [DOI] [PubMed] [Google Scholar]
- 80.Caldecott KW. Tyrosyl DNA phosphodiesterase 2, an enzyme fit for purpose. Nat Struct Mol Biol. 2012;19:1212–1213. doi: 10.1038/nsmb.2455. [DOI] [PubMed] [Google Scholar]
- 81.Daley JM, Laan RL, Suresh A, Wilson TE. DNA joint dependence of pol X family polymerase action in nonhomologous end joining. J Biol Chem. 2005;280:29030–29037. doi: 10.1074/jbc.M505277200. [DOI] [PubMed] [Google Scholar]
- 82.Nick McElhinny SA, Havener JM, Garcia-Diaz M, Juarez R, Bebenek K, Kee BL, Blanco L, Kunkel TA, Ramsden DA. A gradient of template dependence defines distinct biological roles for family X polymerases in nonhomologous end joining. Mol Cell. 2005;19:357–366. doi: 10.1016/j.molcel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 83.Lee JW, Blanco L, Zhou T, Garcia-Diaz M, Bebenek K, Kunkel TA, Wang Z, Povirk LF. Implication of DNA polymerase lambda in alignment-based gap filling for nonhomologous DNA end joining in human nuclear extracts. J Biol Chem. 2004;279:805–811. doi: 10.1074/jbc.M307913200. [DOI] [PubMed] [Google Scholar]
- 84.Ma Y, Lu H, Tippin B, Goodman MF, Shimazaki N, Koiwai O, Hsieh CL, Schwarz K, Lieber MR. A biochemically defined system for mammalian nonhomologous DNA end joining. Mol Cell. 2004;16:701–713. doi: 10.1016/j.molcel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 85.Mahajan KN, Nick McElhinny SA, Mitchell BS, Ramsden DA. Association of DNA polymerase mu (pol mu) with Ku and ligase IV: role for pol mu in end-joining double-strand break repair. Mol Cell Biol. 2002;22:5194–5202. doi: 10.1128/MCB.22.14.5194-5202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Delarue M, Boule JB, Lescar J, Expert-Bezancon N, Jourdan N, Sukumar N, Rougeon F, Papanicolaou C. Crystal structures of a template-independent DNA polymerase: murine terminal deoxynucleotidyltransferase. EMBO J. 2002;21:427–439. doi: 10.1093/emboj/21.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Juarez R, Ruiz JF, Nick McElhinny SA, Ramsden D, Blanco L. A specific loop in human DNA polymerase mu allows switching between creative and DNA-instructed synthesis. Nucleic Acids Res. 2006;34:4572–4582. doi: 10.1093/nar/gkl457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romain F, Barbosa I, Gouge J, Rougeon F, Delarue M. Conferring a template-dependent polymerase activity to terminal deoxynucleotidyltransferase by mutations in the Loop1 region. Nucleic Acids Res. 2009;37:4642–4656. doi: 10.1093/nar/gkp460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braithwaite EK, Prasad R, Shock DD, Hou EW, Beard WA, Wilson SH. DNA polymerase lambda mediates a back-up base excision repair activity in extracts of mouse embryonic fibroblasts. J Biol Chem. 2005;280:18469–18475. doi: 10.1074/jbc.M411864200. [DOI] [PubMed] [Google Scholar]
- 90.Tano K, Nakamura J, Asagoshi K, Arakawa H, Sonoda E, Braithwaite EK, Prasad R, Buerstedde JM, Takeda S, Watanabe M, Wilson SH. Interplay between DNA polymerases beta and lambda in repair of oxidation DNA damage in chicken DT40 cells. DNA Repair (Amst) 2007;6:869–875. doi: 10.1016/j.dnarep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Braithwaite EK, Kedar PS, Stumpo DJ, Bertocci B, Freedman JH, Samson LD, Wilson SH. DNA polymerases beta and lambda mediate overlapping and independent roles in base excision repair in mouse embryonic fibroblasts. PLoS One. 2010;5:e12229. doi: 10.1371/journal.pone.0012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia-Diaz M, Bebenek K, Larrea AA, Havener JM, Perera L, Krahn JM, Pedersen LC, Ramsden DA, Kunkel TA. Template strand scrunching during DNA gap repair synthesis by human polymerase lambda. Nat Struct Mol Biol. 2009;16:967–972. doi: 10.1038/nsmb.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis BJ, Havener JM, Ramsden DA. End-bridging is required for pol mu to efficiently promote repair of noncomplementary ends by nonhomologous end joining. Nucleic Acids Res. 2008;36:3085–3094. doi: 10.1093/nar/gkn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benedict CL, Gilfillan S, Thai TH, Kearney JF. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev. 2000;175:150–157. [PubMed] [Google Scholar]
- 95.Bertocci B, De Smet A, Weill JC, Reynaud CA. Nonoverlapping functions of DNA polymerases mu, lambda, and terminal deoxynucleotidyltransferase during immunoglobulin V(D)J recombination in vivo. Immunity. 2006;25:31–41. doi: 10.1016/j.immuni.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 96.Dominguez O, Ruiz JF, Lain de Lera T, Garcia-Diaz M, Gonzalez MA, Kirchhoff T, Martinez AC, Bernad A, Blanco L. DNA polymerase mu (Pol mu), homologous to TdT, could act as a DNA mutator in eukaryotic cells. EMBO J. 2000;19:1731–1742. doi: 10.1093/emboj/19.7.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aoufouchi S, Flatter E, Dahan A, Faili A, Bertocci B, Storck S, Delbos F, Cocea L, Gupta N, Weill JC, Reynaud CA. Two novel human and mouse DNA polymerases of the polX family. Nucleic Acids Res. 2000;28:3684–3693. doi: 10.1093/nar/28.18.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bertocci B, De Smet A, Berek C, Weill JC, Reynaud CA. Immunoglobulin kappa light chain gene rearrangement is impaired in mice deficient for DNA polymerase mu. Immunity. 2003;19:203–211. doi: 10.1016/s1074-7613(03)00203-6. [DOI] [PubMed] [Google Scholar]
- 99.Lucas D, Escudero B, Ligos JM, Segovia JC, Estrada JC, Terrados G, Blanco L, Samper E, Bernad A. Altered hematopoiesis in mice lacking DNA polymerase mu is due to inefficient double-strand break repair. PLoS Genet. 2009;5:e1000389. doi: 10.1371/journal.pgen.1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chayot R, Montagne B, Ricchetti M. DNA polymerase mu is a global player in the repair of non-homologous end-joining substrates. DNA Repair (Amst) 2012;11:22–34. doi: 10.1016/j.dnarep.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 101.Ma Y, Pannicke U, Schwarz K, Lieber MR. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108:781–794. doi: 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 102.Moshous D, Callebaut I, de Chasseval R, Corneo B, Cavazzana-Calvo M, Le Deist F, Tezcan I, Sanal O, Bertrand Y, Philippe N, Fischer A, de Villartay JP. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105:177–186. doi: 10.1016/s0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 103.Callebaut I, Moshous D, Mornon JP, de Villartay JP. Metallo-beta-lactamase fold within nucleic acids processing enzymes: the beta-CASP family. Nucleic Acids Res. 2002;30:3592–3601. doi: 10.1093/nar/gkf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pannicke U, Ma Y, Hopfner KP, Niewolik D, Lieber MR, Schwarz K. Functional and biochemical dissection of the structure-specific nuclease ARTEMIS. EMBO J. 2004;23:1987–1997. doi: 10.1038/sj.emboj.7600206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poinsignon C, Moshous D, Callebaut I, de Chasseval R, Villey I, de Villartay JP. The metallo-beta-lactamase/beta-CASP domain of Artemis constitutes the catalytic core for V(D)J recombination. J Exp Med. 2004;199:315–321. doi: 10.1084/jem.20031142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soubeyrand S, Pope L, De Chasseval R, Gosselin D, Dong F, de Villartay JP, Hache RJ. Artemis phosphorylated by DNA-dependent protein kinase associates preferentially with discrete regions of chromatin. J Mol Biol. 2006;358:1200–1211. doi: 10.1016/j.jmb.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 107.Niewolik D, Pannicke U, Lu H, Ma Y, Wang LC, Kulesza P, Zandi E, Lieber MR, Schwarz K. DNA-PKcs dependence of Artemis endonucleolytic activity, differences between hairpins and 5′ or 3′ overhangs. J Biol Chem. 2006;281:33900–33909. doi: 10.1074/jbc.M606023200. [DOI] [PubMed] [Google Scholar]
- 108.Goodarzi AA, Yu Y, Riballo E, Douglas P, Walker SA, Ye R, Harer C, Marchetti C, Morrice N, Jeggo PA, Lees-Miller SP. DNA-PK autophosphorylation facilitates Artemis endonuclease activity. EMBO J. 2006;25:3880–3889. doi: 10.1038/sj.emboj.7601255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malu S, De Ioannes P, Kozlov M, Greene M, Francis D, Hanna M, Pena J, Escalante CR, Kurosawa A, Erdjument-Bromage H, Tempst P, Adachi N, Vezzoni P, Villa A, Aggarwal AK, Cortes P. Artemis C-terminal region facilitates V(D)J recombination through its interactions with DNA Ligase IV and DNA-PKcs. J Exp Med. 2012;209:955–963. doi: 10.1084/jem.20111437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Ioannes P, Malu S, Cortes P, Aggarwal AK. Structural basis of DNA ligase IV-Artemis interaction in nonhomologous end-joining. Cell Rep. 2012;2:1505–1512. doi: 10.1016/j.celrep.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poinsignon C, de Chasseval R, Soubeyrand S, Moshous D, Fischer A, Hache RJ, de Villartay JP. Phosphorylation of Artemis following irradiation-induced DNA damage. Eur J Immunol. 2004;34:3146–3155. doi: 10.1002/eji.200425455. [DOI] [PubMed] [Google Scholar]
- 112.Riballo E, Kuhne M, Rief N, Doherty A, Smith GC, Recio MJ, Reis C, Dahm K, Fricke A, Krempler A, Parker AR, Jackson SP, Gennery A, Jeggo PA, Lobrich M. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 113.Ma Y, Schwarz K, Lieber MR. The Artemis:DNA-PKcs endonuclease cleaves DNA loops, flaps, and gaps. DNA Repair (Amst) 2005;4:845–851. doi: 10.1016/j.dnarep.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 114.Povirk LF, Zhou T, Zhou R, Cowan MJ, Yannone SM. Processing of 3′-phosphoglycolate-terminated DNA double strand breaks by Artemis nuclease. J Biol Chem. 2007;282:3547–3558. doi: 10.1074/jbc.M607745200. [DOI] [PubMed] [Google Scholar]
- 115.Beck BD, Lee SS, Williamson E, Hromas RA, Lee SH. Biochemical characterization of metnase’s endonuclease activity and its role in NHEJ repair. Biochemistry. 2011;50:4360–4370. doi: 10.1021/bi200333k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fnu S, Williamson EA, De Haro LP, Brenneman M, Wray J, Shaheen M, Radhakrishnan K, Lee SH, Nickoloff JA, Hromas R. Methylation of histone H3 lysine 36 enhances DNA repair by nonhomologous end-joining. Proc Natl Acad Sci U S A. 2011;108:540–545. doi: 10.1073/pnas.1013571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hromas R, Wray J, Lee SH, Martinez L, Farrington J, Corwin LK, Ramsey H, Nickoloff JA, Williamson EA. The human set and transposase domain protein Metnase interacts with DNA Ligase IV and enhances the efficiency and accuracy of non-homologous end-joining. DNA Repair (Amst) 2008;7:1927–1937. doi: 10.1016/j.dnarep.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen L, Trujillo K, Ramos W, Sung P, Tomkinson AE. Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol Cell. 2001;8:1105–1115. doi: 10.1016/s1097-2765(01)00388-4. [DOI] [PubMed] [Google Scholar]
- 120.Paull TT, Gellert M. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- 121.Trujillo KM, Yuan SS, Lee EY, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J Biol Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- 122.Cooper MP, Machwe A, Orren DK, Brosh RM, Ramsden D, Bohr VA. Ku complex interacts with and stimulates the Werner protein. Genes Dev. 2000;14:907–912. [PMC free article] [PubMed] [Google Scholar]
- 123.Li B, Comai L. Functional interaction between Ku and the werner syndrome protein in DNA end processing. J Biol Chem. 2000;275:39800. doi: 10.1074/jbc.C000289200. [DOI] [PubMed] [Google Scholar]
- 124.Orren DK, Machwe A, Karmakar P, Piotrowski J, Cooper MP, Bohr VA. A functional interaction of Ku with Werner exonuclease facilitates digestion of damaged DNA. Nucleic Acids Res. 2001;29:1926–1934. doi: 10.1093/nar/29.9.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karmakar P, Snowden CM, Ramsden DA, Bohr VA. Ku heterodimer binds to both ends of the Werner protein and functional interaction occurs at the Werner N-terminus. Nucleic Acids Res. 2002;30:3583–3591. doi: 10.1093/nar/gkf482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol. 2012;13:141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen X, Ballin JD, Della-Maria J, Tsai MS, White EJ, Tomkinson AE, Wilson GM. Distinct kinetics of human DNA ligases I, IIIalpha, IIIbeta, and IV reveal direct DNA sensing ability and differential physiological functions in DNA repair. DNA Repair (Amst) 2009;8:961–968. doi: 10.1016/j.dnarep.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Henner WD, Rodriguez LO, Hecht SM, Haseltine WA. gamma Ray induced deoxyribonucleic acid strand breaks. 3′ Glycolate termini. J Biol Chem. 1983;258:711–713. [PubMed] [Google Scholar]
- 129.Akopiants K, Zhou RZ, Mohapatra S, Valerie K, Lees-Miller SP, Lee KJ, Chen DJ, Revy P, de Villartay JP, Povirk LF. Requirement for XLF/Cernunnos in alignment-based gap filling by DNA polymerases lambda and mu for nonhomologous end joining in human whole-cell extracts. Nucleic Acids Res. 2009;37:4055–4062. doi: 10.1093/nar/gkp283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dobbs TA, Palmer P, Maniou Z, Lomax ME, O’Neill P. Interplay of two major repair pathways in the processing of complex double-strand DNA breaks. DNA Repair (Amst) 2008;7:1372–1383. doi: 10.1016/j.dnarep.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 131.Datta K, Purkayastha S, Neumann RD, Pastwa E, Winters TA. Base damage immediately upstream from double-strand break ends is a more severe impediment to nonhomologous end joining than blocked 3′-termini. Radiation research. 2011;175:97–112. doi: 10.1667/RR2332.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Malyarchuk S, Castore R, Harrison L. Apex1 can cleave complex clustered DNA lesions in cells. DNA Repair (Amst) 2009;8:1343–1354. doi: 10.1016/j.dnarep.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Masani S, Han L, Yu K. Apurinic/apyrimidinic endonuclease 1 is the essential nuclease during immunoglobulin class switch recombination. Mol Cell Biol. 2013;33:1468–1473. doi: 10.1128/MCB.00026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen S, Inamdar KV, Pfeiffer P, Feldmann E, Hannah MF, Yu Y, Lee JW, Zhou T, Lees-Miller SP, Povirk LF. Accurate in vitro end joining of a DNA double strand break with partially cohesive 3′-overhangs and 3′-phosphoglycolate termini: effect of Ku on repair fidelity. J Biol Chem. 2001;276:24323–24330. doi: 10.1074/jbc.M010544200. [DOI] [PubMed] [Google Scholar]
- 135.Mohapatra S, Yannone SM, Lee SH, Hromas RA, Akopiants K, Menon V, Ramsden DA, Povirk LF. Trimming of damaged 3′ overhangs of DNA double-strand breaks by the Metnase and Artemis endonucleases. DNA Repair (Amst) 2013;12:422–432. doi: 10.1016/j.dnarep.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma Y, Lu H, Schwarz K, Lieber MR. Repair of double-strand DNA breaks by the human nonhomologous DNA end joining pathway: the iterative processing model. Cell Cycle. 2005;4:1193–1200. doi: 10.4161/cc.4.9.1977. [DOI] [PubMed] [Google Scholar]
- 137.Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436:1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Williams RS, Moncalian G, Williams JS, Yamada Y, Limbo O, Shin DS, Groocock LM, Cahill D, Hitomi C, Guenther G, Moiani D, Carney JP, Russell P, Tainer JA. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell. 2008;135:97–109. doi: 10.1016/j.cell.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paull TT, Gellert M. A mechanistic basis for Mre11-directed DNA joining at microhomologies. Proc Natl Acad Sci U S A. 2000;97:6409–6414. doi: 10.1073/pnas.110144297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Della-Maria J, Zhou Y, Tsai MS, Kuhnlein J, Carney JP, Paull TT, Tomkinson AE. Human Mre11/human Rad50/Nbs1 and DNA ligase IIIalpha/XRCC1 protein complexes act together in an alternative nonhomologous end joining pathway. J Biol Chem. 2011;286:33845–33853. doi: 10.1074/jbc.M111.274159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Langerak P, Mejia-Ramirez E, Limbo O, Russell P. Release of Ku and MRN from DNA ends by Mre11 nuclease activity and Ctp1 is required for homologous recombination repair of double-strand breaks. PLoS Genet. 2011;7:e1002271. doi: 10.1371/journal.pgen.1002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- 143.Karmakar P, Piotrowski J, Brosh RM, Jr, Sommers JA, Miller SP, Cheng WH, Snowden CM, Ramsden DA, Bohr VA. Werner protein is a target of DNA-dependent protein kinase in vivo and in vitro, and its catalytic activities are regulated by phosphorylation. J Biol Chem. 2002;277:18291–18302. doi: 10.1074/jbc.M111523200. [DOI] [PubMed] [Google Scholar]
- 144.Yannone SM, Roy S, Chan DW, Murphy MB, Huang S, Campisi J, Chen DJ. Werner syndrome protein is regulated and phosphorylated by DNA-dependent protein kinase. J Biol Chem. 2001;276:38242–38248. doi: 10.1074/jbc.M101913200. [DOI] [PubMed] [Google Scholar]
- 145.Meek K, Dang V, Lees-Miller SP. DNA-PK: the means to justify the ends? Advances in immunology. 2008;99:33–58. doi: 10.1016/S0065-2776(08)00602-0. [DOI] [PubMed] [Google Scholar]
- 146.Dobbs TA, Tainer JA, Lees-Miller SP. A structural model for regulation of NHEJ by DNA-PKcs autophosphorylation. DNA Repair (Amst) 2010;9:1307–1314. doi: 10.1016/j.dnarep.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zolner AE, Abdou I, Ye R, Mani RS, Fanta M, Yu Y, Douglas P, Tahbaz N, Fang S, Dobbs T, Wang C, Morrice N, Hendzel MJ, Weinfeld M, Lees-Miller SP. Phosphorylation of polynucleotide kinase/phosphatase by DNA-dependent protein kinase and ataxia-telangiectasia mutated regulates its association with sites of DNA damage. Nucleic Acids Res. 2011;39:9224–9237. doi: 10.1093/nar/gkr647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leber R, Wise TW, Mizuta R, Meek K. The XRCC4 gene product is a target for and interacts with the DNA-dependent protein kinase. J Biol Chem. 1998;273:1794–1801. doi: 10.1074/jbc.273.3.1794. [DOI] [PubMed] [Google Scholar]
- 149.Yu Y, Mahaney BL, Yano K, Ye R, Fang S, Douglas P, Chen DJ, Lees-Miller SP. DNA-PK and ATM phosphorylation sites in XLF/Cernunnos are not required for repair of DNA double strand breaks. DNA Repair (Amst) 2008;7:1680–1692. doi: 10.1016/j.dnarep.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roy S, Andres SN, Vergnes A, Neal JA, Xu Y, Yu Y, Lees-Miller SP, Junop M, Modesti M, Meek K. XRCC4’s interaction with XLF is required for coding (but not signal) end joining. Nucleic Acids Res. 2012;40:1684–1694. doi: 10.1093/nar/gkr1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Costantini S, Woodbine L, Andreoli L, Jeggo PA, Vindigni A. Interaction of the Ku heterodimer with the DNA ligase IV/Xrcc4 complex and its regulation by DNA-PK. DNA Repair (Amst) 2007;6:712–722. doi: 10.1016/j.dnarep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 152.Mani RS, Yu Y, Fang S, Lu M, Fanta M, Zolner AE, Tahbaz N, Ramsden DA, Litchfield DW, Lees-Miller SP, Weinfeld M. Dual modes of interaction between XRCC4 and polynucleotide kinase/phosphatase: implications for nonhomologous end joining. J Biol Chem. 2010;285:37619–37629. doi: 10.1074/jbc.M109.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garcia-Diaz M, Bebenek K, Krahn JM, Kunkel TA, Pedersen LC. A closed conformation for the Pol lambda catalytic cycle. Nat Struct Mol Biol. 2005;12:97–98. doi: 10.1038/nsmb876. [DOI] [PubMed] [Google Scholar]
- 154.Palmbos PL, Daley JM, Wilson TE. Mutations of the Yku80 C terminus and Xrs2 FHA domain specifically block yeast nonhomologous end joining. Mol Cell Biol. 2005;25:10782–10790. doi: 10.1128/MCB.25.24.10782-10790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]