Abstract
Background
Danger associated molecular patterns (DAMPs) are nuclear or cytoplasmic proteins that are released from the injured tissues and activate the innate immune system. Mitochondrial DNA (mtDNA) is a novel DAMP that is released into the extracellular milieu subsequent to cell death and injury. We hypothesized that cell death within the central nervous system in children with traumatic brain injury (TBI) would lead to release of mtDNA into the cerebrospinal fluid (CSF) and has the potential to predict the outcome after trauma.
Methods
CSF was collected from children with severe TBI that required intracranial pressure monitoring with Glasgow Coma Scale (GCS) scores ≤ 8 via an externalized ventricular drain. Control CSF was obtained in children without TBI or meningoencephalitis that demonstrated no leukocytes in the diagnostic lumbar puncture.
Results
The median age for patients with TBI was 6.3 y and 62% were male. The common mechanisms of injury included motor vehicle collision (35.8%) followed by falls (21.5%) and inflicted TBI (19%); 6 children (14.2%) died during their ICU course. The mean CSF mtDNA concentration was 1.10E +05 ± 2.07E+05 and 1.63E+03 ± 1.80E+03 copies/µL in the pediatric TBI and control population respectively. Furthermore, the mean CSF mtDNA concentration in pediatric patients who later died or had severe disability was significantly higher than that of the survivors (1.63E+ 05 ± 2.77E+05 vs. 5.05E+04 ± 6.21E+04 copies/µL) (p<0.0001). We found a significant correlation between CSF mtDNA and HMGB1, another prototypical DAMP, concentrations (ρ = 0.574, p<0.05), supporting the notion that both DAMPs are increased in the CSF following TBI.
Conclusions
Our data suggest that CSF mtDNA is novel DAMP in TBI, and appears to be a useful biomarker that correlates with neurological outcome after TBI. Further inquiry into the components of mtDNA that modulate the innate immune response will be helpful in understanding the mechanism of local and systemic inflammation after TBI.
Keywords: Mitochondrial DNA, Cerebrospinal fluid, Traumatic Brain Injury
Introduction
Severe traumatic brain injury (TBI) accounts for 25% of all TBI and exacts a considerable societal and economic toll (1, 2). According to the Centers for Disease Control and Prevention, TBI accounts for an estimated 7400 deaths in children 0–19 years of age annually (3). The pro-inflammatory cytokine response after TBI has been fairly well characterized (4–6) and recently the role of damage-associated molecular patterns (DAMPs, also known as alarmins) has also been recognized. The pattern recognition receptors (PRRs) present on the microglia and astrocytes can sense cellular damage and stress in the absence of infection due to the presence of DAMPs. DAMPs are rapidly released into the extracellular milieu following injury and modulate the innate immune system. Some examples of DAMPs include nuclear and cytoplasmic proteins e.g., high mobility group box 1 (HMGB1), heat shock proteins, the S100 family of calcium-binding proteins, histones, and interleukin 1 (IL-1) family members (7, 8).
HMGB1 is a prototypical danger associated molecular patterns (DAMP) molecule that activates the innate immune system in response to tissue injury due to trauma, ischemia/reperfusion or infection (9). HMGB1 exerts pro-inflammatory properties through its effects on Toll-like receptor (TLR) -2, -4 and the receptor for advanced glycation end products (RAGE) (7, 10–13), and it has been identified as a late mediator of LPS-induced mortality in mice (7, 8). Previously, we reported that cerebrospinal fluid (CSF) levels of HMGB1 are markedly increased in children after severe TBI. We also demonstrated that peak HMGB1 levels are inversely and independently associated with Glasgow Outcome Scale (GOS) scores. The traumatic events leading to release of HMGB1 into the CSF can also lead to release of other DAMPs into the CSF with subsequent activation of the innate immune response. Since mitochondria are abundantly distributed along axons, nerve terminals and dendrites, mitochondrial constituents once released into the extracellular space can also act as DAMPs (14). Examples of mitochondrial DAMPs include mitochondrial DNA (mtDNA), N-formyl peptides and mitochondrial transcription factor A (TFAM) (15). MtDNA is a novel DAMP that is released into the extracellular milieu subsequent to cell death and injury and acts via TLR-9, a PRR of the immune system that detects bacterial and viral DNA (16). In addition, as compared to genomic DNA, there are multiple copies of mtDNA per cell. Following trauma, circulating mitochondrial DNA (mtDNA) acts as a DAMP, leading to systemic inflammatory response syndrome (17).
To our knowledge, little is known about CSF mitochondrial DNA concentrations in pediatric TBI. We hypothesized that cell death within the CNS in children with TBI would lead to release of mtDNA into the CSF in TBI and has the potential to predict the outcome after trauma.
Methods
This study was approved by the Institutional Review Board at the University of Pittsburgh Medical Center. As part of other past and ongoing clinical studies, informed consent was obtained for collection of the CSF made available for this study.
A clinical protocol for the treatment of children with severe TBI has been used at our institution and has been previously described (18). Briefly, all children with severe TBI received comprehensive neurocritical care to rapidly stabilize and assess for injuries, prevent secondary insults, and promote neurological recovery, in accordance with published guidelines (19). Intracranial pressure (ICP) monitoring was instituted for all children with post-resuscitation Glasgow Coma Scale (GCS) scores ≤ 8 (although some children had an initial GCS > 8, but later deteriorated to meet the above criteria) via an externalized ventricular drain. CSF drainage was part of standard management throughout this study and CSF was drained continuously at a level of 10 cm above the midbrain. Attempts to mitigate intracranial hypertension (generally defined as ICP ≥ 20 mm Hg) were instituted including head positioning (raising the head of the bed to 30°) and other mechanical maneuvers. Episodic intracranial hypertension (lasting > 5 min) were treated with a step-wise escalation of therapies including analgesia with fentanyl, neuromuscular blockade with vecuronium, PaCO2 34–36 mm Hg, and hyperosmolar therapy (mannitol or 3% NaCl). Failure of these maneuvers led to addition of second-tier therapies including barbiturate administration, hypothermia, or decompressive craniectomy, based on the decisions made by the clinicians caring for the child. No interventions were made for the performance of the procedures within this manuscript.
CSF collection
As previously described (20), CSF was continuously drained by gravity into a sterile buretrol at the bedside at 10 cm above the mid-brain. Once drained, CSF of the study subjects was extracted from this system using sterile technique. This procedure was done daily for up to 7 days after TBI, with samples analyzed at two time intervals (0–24h, 72h after injury). Control CSF was obtained in children without TBI or meningoencephalitis that demonstrated no leukocytes in the diagnostic lumbar puncture. The CSF was immediately centrifuged for 10 min at 2000 rpm to remove cellular debris and frozen at −80°C until analysis. Study samples (from both TBI subjects and controls) were centrifuged (10 min at 2000 rpm) again prior to DNA extraction.
DNA was extracted from 200 µl of CSF using the QIAamp Blood Kit (Qiagen GmbH), and the concentration of mito DNA (cytochrome c oxidase I) was measured by real-time quantitative PCR assay in CSF (qPCR, SYBR® Green chemistry). We chose COX1gene to assess mitochondrial DNA, as plasma levels of mtDNA encoding COX1 has been reported to be elevated in severely injured patients (21). The primer sequences COX1 used were (forward: GCC TCC GTA GAC CTA ACC ATC TTC; reverse: GTA AGT TAC AAT ATG GGA GAT TAT TCC). PCR reaction mixture was prepared using SYBR Green PCR master mix (PE Applied Biosystems, Foster City, CA), using the primers described above. PCR was set up in a reaction volume of 20 µL using 10 µ of 2X SYBR® Green Master Mix (2X), 1 µL Forward primer (1 µM), 1 µL Reverse primer (1 µM), 3 µL of nuclease-free H2O and 5 µL of CSF nuclear extract The following thermocycler conditions were used: 3 minute incubation at 95° C followed by 40 cycles of an initial denaturation step at 95° C for 30 seconds, an annealing step of 54° C for 45 seconds, and an elongation step of 68° C for 1 min.
Standard Curve Preparation
The linearity of the quantitative assay was assessed by using a cloned plasmid DNA, which was serially diluted to prepare a series of calibrators with known concentrations as described by Chiu et al (22). Briefly, using primers described above we amplified bases between 421 and 781 encoding the mitochondrial gene cytochrome c oxidase I (Ref Seq: NC_012920). The PCR amplicons were cloned into a pGEM-T-Easy vector (Promega), and the identity of the cloned insert was confirmed. DNA was extracted, quantified and used as a calibrator. As has been described previously, the mitochondrial DNA copy number of this calibrator was derived by dividing the total DNA concentration by the weight of each plasmid molecule (22). Calibrators were prepared by serial dilution of the stock solution and contained 1 – 109 mitochondrial DNA copies/µL.
Statistical analysis
Clinical data collection included patient age, sex, mechanism of injury, admission GCS score, GOS score determined 6 months after injury, and mortality. GOS was defined as GOS 1=dead, 2=vegetative state, 3=severe disability, 4=moderate disability, and 5=normal. Furthermore, good outcome was defined as a GOS score of 4 or 5, and poor outcome as a GOS score of 1–3. Data are expressed as mean ± standard error of the mean (SEM), or median [range], as appropriate. Comparisons between DNA levels as it relates to clinical variables age, sex, initial GCS score, mechanism of injury, and GOS scores in TBI and control patients were made using the Mann-Whitney rank sum test. Changes over time were analyzed using repeated-measures analysis of variance (RM-ANOVA). To compare the correlation between CSF mtDNA and another prototypical DAMP, HMGB1, we used spearman’s correlation test. Receiver-operating characteristic (ROC) curves were carried out for mtDNA levels as a predictor of outcome at 6 months. Statistical calculations were performed with SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA) and Stata/IC (StataCorp LP, College Station, TX).
Results
There were 42 TBI and 13 control patients. The median age for patients with TBI was 6.3 y and 62% were male (Table 1). The GCS scores on presentation to the hospital were diverse ranging from 3–15, but all the patients had a GCS score <8 prior to their enrollment in the ICP monitoring protocol. The common mechanisms of injury included motor vehicle collision (35.8%) followed by falls (21.5%) and inflicted TBI (iTBI) (19 %) (Table 2); 6 children (14.2%) died during their ICU course.
Table 1.
Demographic Data of the Study Subjects
| Age, years [range] | 6.31 ±0.9 [0.16–16 years] |
| Number of male patients (%) | 26 (61.9%) |
| Initial Glasgow Coma Score | 7 [3–15] |
| Glasgow Outcome scale score at 6 months* | 3 [1–5] |
1=dead, 2=vegetative state, 3=severe disability, 4=moderate disability, 5=normal
Age expressed as mean ± standard error of the mean GCS/GOS – expressed as mean [range]
Table 2.
Mechanism of Injury
| n | Percent | |
|---|---|---|
| Motor vehicle collision | 15 | 35.8 |
| Fall | 9 | 21.5 |
| Inflicted traumatic brain injury | 8 | 19.0 |
| Pedestrian/automobile accident | 4 | 9.5 |
| Bicycle/automobile accident | 2 | 4.7 |
| Other | 4 | 9.5 |
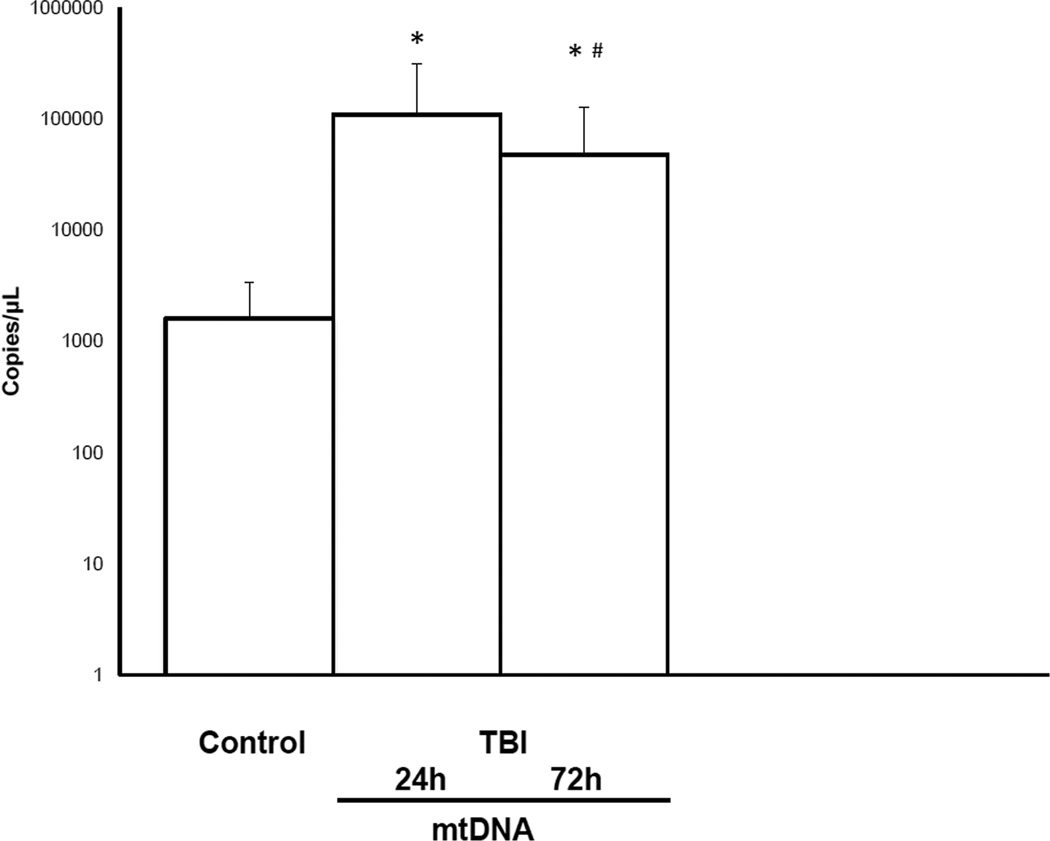
On admission to the ICU, the mean CSF mtDNA was significantly higher in TBI patients as compared to the controls. The mean CSF mtDNA concentration in pediatric TBI patients was 1.10E +05 ± 2.07E+05 copies/µL as compared to 1.63E+03 ± 1.80E+03 copies/µL in the control population (P = 0.003, Mann–Whitney test)(Figure 1). However, neither age nor sex was associated with CSF mtDNA concentrations (data not shown; p=0.877 and p=0.684 respectively). Similarly, there was no association between CSF mtDNA and GCS. The mean concentrations of CSF mtDNA in the mild/moderate TBI (GCS ≥ 8) and the severely injured (GCS ≤ 8) cohort were 5.71 E+04 ± 7.69E+04 and 7.22+04 ± 8.27E+04 copies/µL. Furthermore, there was no significant difference in CSF mtDNA concentrations in children with iTBI (n=8) as compared to children with accidental TBI (n=34) (1.02E+05 ± 1.99E+05 vs. 1.03E +05 ± 1.78E+05 copies/µL) respectively.
Figure 1.
CSF mtDNA levels in children with severe TBI versus controls at 24 and 72 h after injury (*, p<0.05, vs. control and #, p<0.05, vs. mtDNA 24 h).
We measured mtDNA concentrations at 72 h and compared it to the mtDNA levels at 24 h. At 72 h, there was a significant reduction in CSF concentrations of mtDNA as compared to mtDNA at 24 h (4.80E+04 ± 8.07E+04 vs. 1.10E +05 ± 2.07E+05 copies/µL) respectively (Figure 1).
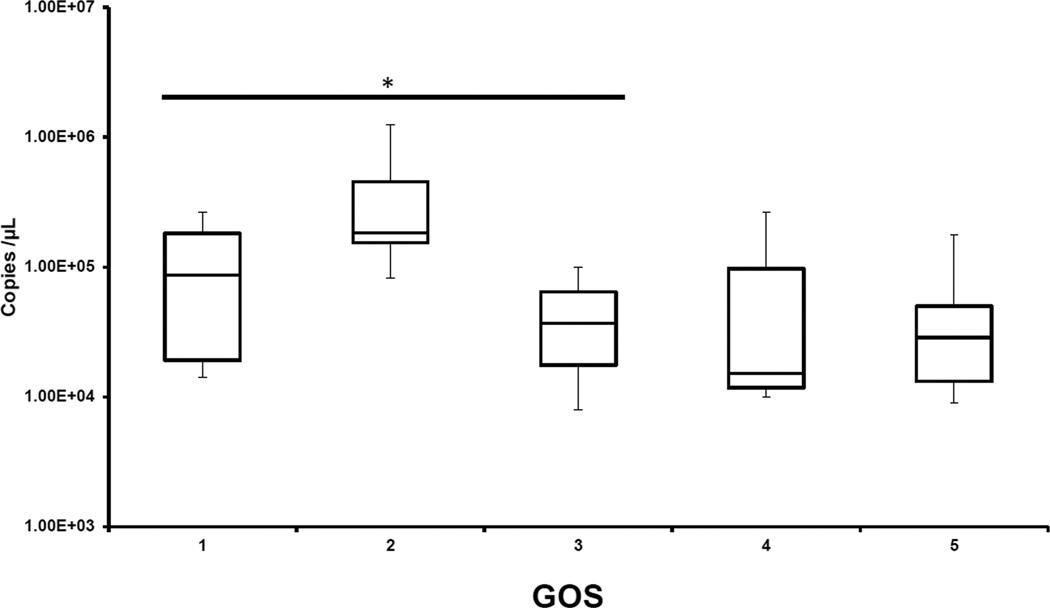
To assess the predictive ability of CSF mtDNA, we examined the correlation of mtDNA with GOS. GOS was dichotomized into unfavorable vs favorable outcome — GOS 1–3 was indicative of severe disability to death as compared to GOS 4–5 that signifies moderate disability to normal outcome. We noted that initial mtDNA concentrations were inversely proportional to the GOS score at 6 months i.e., the mean CSF mtDNA concentration in pediatric patients who later died or had severe disability were significantly higher than that of the patients who were normal or had mild disability (1.63E+ 05 ± 2.77E+05 vs. 5.05E+04 ± 6.21E+04 copies/µL) (p<0.0001), Mann–Whitney test; Figure 2).
Figure 2.
CSF mtDNA concentrations plotted against 6-month Glasgow Outcome Scale (GOS) score. GOS: 1=dead, 2=vegetative state, 3=severe disability, 4=moderate disability, 5=normal; the mean CSF mtDNA concentrations in pediatric patients who later had death - severe disability were significantly higher than that of the survivors (*, p< 0.0001, vs. GOS 4 and 5).
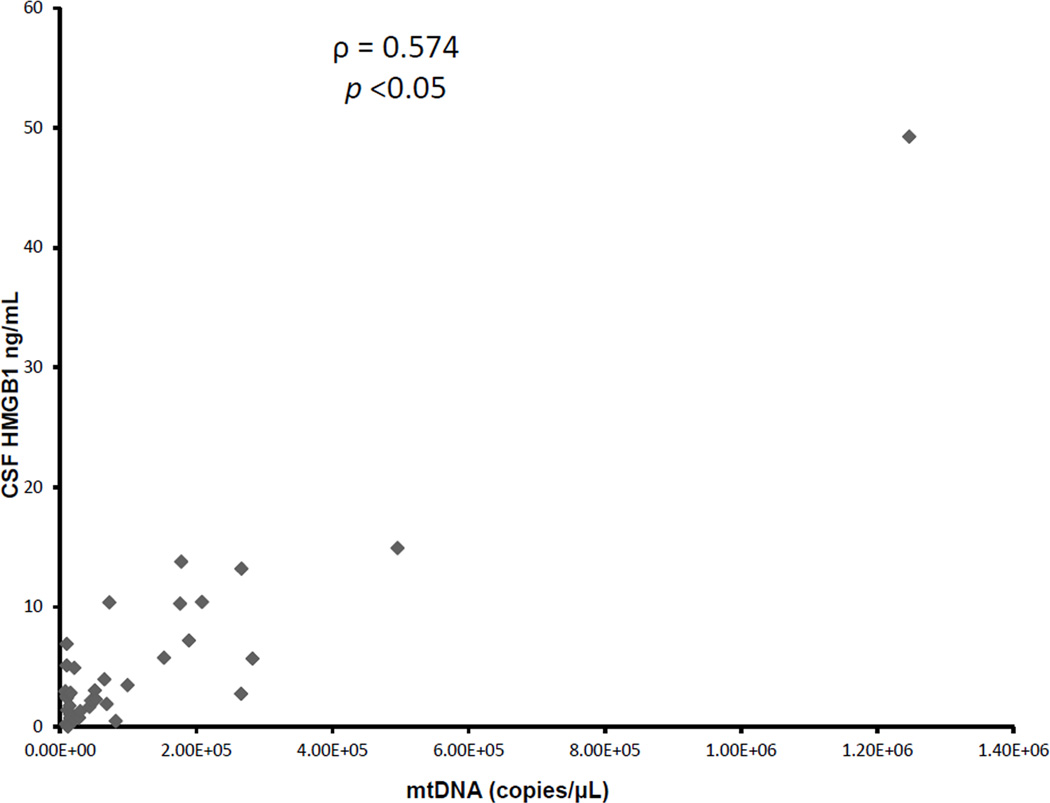
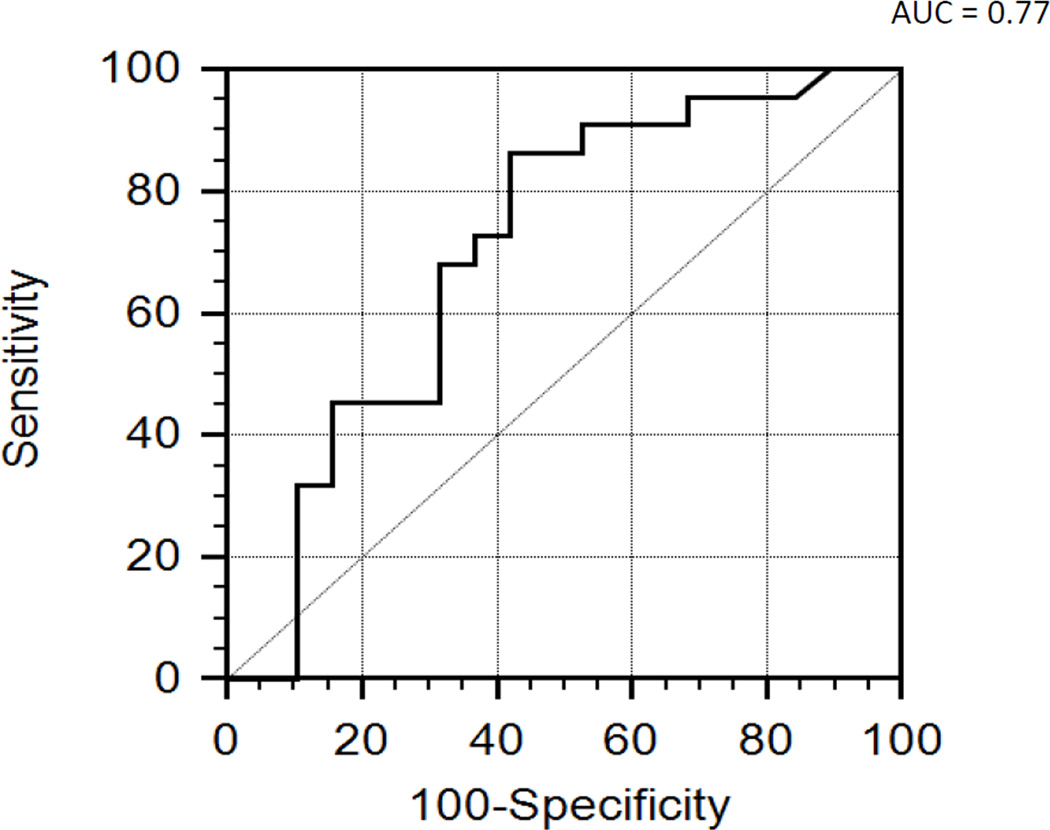
To investigate if changes in CSF mtDNA concentration were parallel to another prototypical DAMP, HMGB1, we compared the CSF mtDNA concentrations to HMGB1 concentrations. Using a commercial ELISA, we had previously measured CSF HMGB1 levels in the same samples (23). We found a significant correlation between CSF mtDNA and HMGB1 concentrations (ρ = 0.574, p<0.05), supporting the notion that both DAMPs are increased in the CSF following TBI (Figure 3). Subgroup analysis demonstrated parallel increase in CSF HMGB1 and mtDNA in both the group of patients with favorable (ρ = 0.511, p=0.021) and unfavorable outcome (ρ = 0.807, p=0.00047). We next performed an ROC analysis to determine the ability to predict outcome at 6 months. Previously, we had reported that ROC analysis of the performance of peak HMGB1 level to predict outcome at 6 months had an area under the curve (AUC) of 0.70 (95% confidence interval [CI] 0.51,0.90 (23). Similarly for CSF mtDNA concentrations, ROC analysis demonstrated an area under the curve (AUC) of 0.77 (95% confidence interval (CI) 0.51, 0.90; Fig 4). CSF mtDNA concentration of 15.50E+03 copies/µL had a sensitivity of 45.45% and specificity of 84.2%. Combining the two biomarkers did not yield any additive effect. A repeat ROC analysis, after exclusion of children with iTBI demonstrated an area under the curve (AUC) of 0.76 (95% confidence interval (CI) 0.57, 0.89).
Figure 3.
Scatterplot of CSF mtDNA versus HMGB1 in patients with severe TBI. There was a significant correlation between CSF mtDNA and HMGB1 concentrations following TBI.
Figure 4.
Receiver-operating characteristic (ROC) analysis using CSF mtDNA as a predictor of outcome at 6 months. ROC analysis demonstrated an AUC of 0.77 (95% CI 0.51, 0.90).
Discussion
The “danger model” theory, as proposed by Polly Matzinger, states that the immune system can discriminate self from non-self, due to DAMPs that are nuclear or cytoplasmic proteins released from the injured tissues (24). These intracellular molecules are derived from different subcellular compartments, including the plasma membrane, nucleus, endoplasmic reticulum and cytosol, and they have the capacity to affect the function of the antigen presenting cells(25), mast cells and neutrophils (17).
Mitochondria have evolved from saprophytic bacteria; therefore unlike the non formylated human genomic proteins, mitochondria possess formyl peptides and circular DNA with non-methylated repeats like bacterial DNA. Human mitochondrial DNA is a double stranded circular molecule of 16569 base pairs and contains 37 genes coding for 2 ribosomal RNAs, 22 transfer RNAs and 13 polypeptides (26, 27). Recent studies have examined the role of extracellular mtDNA in modulation of the innate immune response (15, 17). Circulating mtDNA activates human polymorphonuclear neutrophils (PMNs) through formyl peptide receptor-1 and TLR-9, respectively (17). Similarly, mtDNA from supernatants of fracture reamings from trauma patients was shown to activate polymorphonuclear cells (15).
Since the CSF is in direct contact with the brain parenchyma, examining CSF has always been considered an attractive source for biomarker discovery in the brain and neurological diseases. As mentioned previously, the circular form of mtDNA and the presence of multiple copies of mtDNA per cell make it a potential candidate for release into the CSF. CSF mtDNA has been previously studied in patients with Alzheimer’s disease (28). In asymptomatic at-risk individuals and symptomatic patients with sporadic Alzheimer’s disease, CSF mtDNA levels were significantly lower than in healthy age-matched controls. In contrast, we noticed an increase in CSF mtDNA concentrations, suggesting that mtDNA is released either directly or indirectly from traumatic tissues. Our findings support the novel paradigm that brain injury contributes to the genesis of mitochondrial-derived DAMPs as measured in the CSF. We also report changes in mtDNA concentration within the first 3 days after injury in these patients; mtDNA concentrations in critically ill children with trauma demonstrated a significant decrease at 72 h after trauma as compared to concentrations within the first day after admission. To our knowledge, this is the first study which documents elevated concentration of mtDNA in TBI and its correlation with outcome.
The clinical findings are different in iTBI as compared to accidental TBI. The constellation of findings observed in iTBI include interhemispheric subdural hematoma (SDH), shear injuries, diffuse axonal injury, contusional white matter tears, and retinal hemorrhage as compared to accidental TBI that demonstrates skull fracture, epidural, subdural or subarachnoid hemorrhage, or cortical contusion depending on the impact of the force (29, 30). There is some evidence to suggest that children with iTBI sustain more severe injuries, have a higher mortality rate, and endure worse outcomes than children who experience non-inflicted TBI (31). However, our data did not demonstrate any difference in CSF mtDNA concentrations of children with inflicted as compared to children with accidental TBI. These findings need to be validated in a larger cohort of children with inflicted TBI.
There was no association between CSF mtDNA and GCS. The GCS is difficult to apply precisely to infants and young children—several criteria such as “follows commands” are developmentally inappropriate for the young child, particularly <4 y old. Additional factors such as presence of subclinical seizures, residual neuromuscular blockade or overestimation of injury severity in some children may have contributed to this discordance between clinical and biological measure of brain injury (32).
Cellular disruption by trauma releases DAMPs which signal via the innate immune pathways and subsequently induce local and systemic inflammation. Previously, it has been shown that mtDNA from shock-injured tissues activates neutrophils, produces degranulation and contributes to the systemic inflammatory response syndrome (15); in contrast nuclear DNA did not induce neutrophil activation, thereby affirming that mtDNA is a DAMP that is capable of activating the innate immune system. Furthermore, mechanically disrupted mitochondria when injected into rats led to hepatic inflammation as evidenced by activation of MAPK signaling and cytokine synthesis (15).
We previously demonstrated that CSF concentrations of HMGB1, a prototypical DAMP, are elevated in CSF after trauma, and furthermore, these were independently associated with poor outcome (23). The release of HMGB1 in inflammation supports the notion of HMGB1 as an endogenous danger signal alerting the immune system to the presence of inflammation and necrosis (33, 34). Germane to the current studies, we demonstrated that there was a significant correlation between CSF mtDNA and HMGB1 concentrations at 24 h after trauma. In addition, elevated levels of CSF mtDNA and HMGB1 portended a poor prognosis for children with TBI. We also determined the potential utility of CSF mtDNA as a prognostic biomarker. Surprisingly, ROC analysis after exclusion of children with iTBI, demonstrated an area under the curve (AUC) of 0.76 (95% confidence interval (CI) 0.57, 0.89). Thus, elevated mtDNA concentrations were highly predictive of poor outcome.
All together these observations suggest that DAMPs play a possible role in either inflammation or other aspects of the secondary injury response to pediatric TBI. We have demonstrated that elevated CSF mtDNA concentrations were highly predictive of poor outcome. Although not ready for prime-time, the use of CSF mtDNA concentrations can assist in patient stratification for clinical research, and opens up additional lines of investigation as a potential therapeutic target.
This study was not powered to identify association of mtDNA with demographic parameters i.e., age and sex of the patient. Another limitation of the study was the lack of age matched CSF controls as a majority of children undergoing diagnostic lumbar puncture are infants and toddlers.
In summary, our data suggest that CSF mtDNA is novel DAMP in TBI, and appears to be a useful biomarker that correlates with neurological outcome after TBI. Further inquiry into the components of mtDNA that modulate the innate immune response will be helpful in understanding the mechanism of local and systemic inflammation after TBI.
Bibliography
- 1.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States: 2003. J Head Trauma Rehabil. 2008;23(2):123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- 2.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Faul M, Xu L, Wald MM, C VG. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Atlanta (GA): 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- 4.Hutchinson PJ, O'Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, Timofeev I, Al-Rawi PG, Menon DK, Pickard JD. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24(10):1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- 5.Fink KB, Andrews LJ, Butler WE, Ona VO, Li M, Bogdanov M, Endres M, Khan SQ, Namura S, Stieg PE, Beal MF, Moskowitz MA, Yuan J, Friedlander RM. Reduction of post-traumatic brain injury and free radical production by inhibition of the caspase-1 cascade. Neuroscience. 1999;94(4):1213–1218. doi: 10.1016/s0306-4522(99)00345-0. [DOI] [PubMed] [Google Scholar]
- 6.Bell MJ, Kochanek PM, Doughty LA, Carcillo JA, Adelson PD, Clark RS, Wisniewski SR, Whalen MJ, DeKosky ST. Interleukin-6 and interleukin-10 in cerebrospinal fluid after severe traumatic brain injury in children. J Neurotrauma. 1997;14(7):451–457. doi: 10.1089/neu.1997.14.451. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255(3):320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 10.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and coexpression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270(43):25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L958–L965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 12.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279(9):7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 14.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010;34(1):55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 16.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Exo J, Kochanek PM, Adelson PD, Greene S, Clark RS, Bayir H, Wisniewski SR, Bell MJ. Intracranial pressure-monitoring systems in children with traumatic brain injury: combining therapeutic and diagnostic tools. Pediatr Crit Care Med. 2011;12(5):560–565. doi: 10.1097/PCC.0b013e3181e8b3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MP, Selden NR, Warden CR, Wright DW. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 19. The role of anti-seizure prophylaxis following severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(3 Suppl):S72–S75. [PubMed] [Google Scholar]
- 20.Satchell MA, Lai Y, Kochanek PM, Wisniewski SR, Fink EL, Siedberg NA, Berger RP, DeKosky ST, Adelson PD, Clark RS. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J Cereb Blood Flow Metab. 2005;25(7):919–927. doi: 10.1038/sj.jcbfm.9600088. [DOI] [PubMed] [Google Scholar]
- 21.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258(4):591–596. doi: 10.1097/SLA.0b013e3182a4ea46. discussion 596–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu RW, Chan LY, Lam NY, Tsui NB, Ng EK, Rainer TH, Lo YM. Quantitative analysis of circulating mitochondrial DNA in plasma. Clin Chem. 2003;49(5):719–726. doi: 10.1373/49.5.719. [DOI] [PubMed] [Google Scholar]
- 23.Au AK, Aneja RK, Bell MJ, Bayir H, Feldman K, Adelson PD, Fink EL, Kochanek PM, Clark RS. Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. J Neurotrauma. 2012;29(11):2013–2021. doi: 10.1089/neu.2011.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 25.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805(1):53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Taanman J-W. The mitochondrial genome: structure, transcription, translation and replication. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1999;1410(2):103–123. doi: 10.1016/s0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 27.Andersson SG, Karlberg O, Canback B, Kurland CG. On the origin of mitochondria: a genomics perspective. Philos Trans R Soc Lond B Biol Sci. 2003;358(1429):165–177. doi: 10.1098/rstb.2002.1193. discussion 177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podlesniy P, Figueiro-Silva J, Llado A, Antonell A, Sanchez-Valle R, Alcolea D, Lleo A, Molinuevo JL, Serra N, Trullas R. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Annals of Neurology. 2013 doi: 10.1002/ana.23955. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman RA, Bilaniuk LT, Bruce D, Schut L, Uzzell B, Goldberg HI. Computed tomography of craniocerebral injury in the abused child. Radiology. 1979;130(3):687–690. doi: 10.1148/130.3.687. [DOI] [PubMed] [Google Scholar]
- 30.Chan KH, Yue CP, Mann KS. The risk of intracranial complications in pediatric head injury. Results of multivariate analysis. Childs Nerv Syst. 1990;6(1):27–29. doi: 10.1007/BF00262262. [DOI] [PubMed] [Google Scholar]
- 31.Barlow K, Thompson E, Johnson D, Minns RA. The neurological outcome of non-accidental head injury. Pediatr Rehabil. 2004;7(3):195–203. doi: 10.1080/13638490410001715331. [DOI] [PubMed] [Google Scholar]
- 32.Shore PM, Berger RP, Varma S, Janesko KL, Wisniewski SR, Clark RS, Adelson PD, Thomas NJ, Lai YC, Bayir H, Kochanek PM. Cerebrospinal fluid biomarkers versus glasgow coma scale and glasgow outcome scale in pediatric traumatic brain injury: the role of young age and inflicted injury. J Neurotrauma. 2007;24(1):75–86. doi: 10.1089/neu.2006.0062. [DOI] [PubMed] [Google Scholar]
- 33.Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol. 2013;55(1):76–82. doi: 10.1016/j.molimm.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 34.Manganelli V, Signore M, Pacini I, Misasi R, Tellan G, Garofalo T, Lococo E, Chirletti P, Sorice M, Delogu G. Increased HMGB1 expression and release by mononuclear cells following surgical/anesthesia trauma. Crit Care. 2010;14(6):R197. doi: 10.1186/cc9316. [DOI] [PMC free article] [PubMed] [Google Scholar]