Summary
Recent advances in gene editing technology have introduced the potential for application of mutagenesis approaches in non-human primates to model human development and disease. Here we report successful TALEN-mediated mutagenesis of an X-linked, Rett Syndrome (RTT) gene, the methyl-CpG binding protein 2 (MECP2), in both rhesus and cynomolgus monkeys. Microinjection of MECP2-targeting TALEN plasmids into rhesus and cynomolgus zygotes leads to effective gene editing of MECP2 with no detected off-target mutagenesis. Male rhesus (2) and cynomolgous (1) fetuses carrying MECP2 mutations in various tissues including testes were miscarried during mid-gestation, consistent with RTT-linked male embryonic lethality in humans. One live delivery of a female cynomolgus monkey occurred after 162 days of gestation, with abundant MECP2 mutations in peripheral tissues. We conclude that TALEN-mediated mutagenesis can be an effective tool for genetic modeling of human disease in non-human primates.
Non-human primates (NHPs) provide powerful experimental models to study human development and disease because of their genetic and physiological similarities to humans. Monkeys, such as rhesus and cynomolgus macaques, are particularly appropriate for studying cognitive functions and neurological disorders as well as complex behaviors (Niu et al., 2010; Chen et al., 2012a). To date, there have been only a few reported successes in producing transgenic monkeys, all of which involved virus-mediated gene transfer (Niu et al., 2010; Chan et al., 2001; Chen et al., 2012b; Wolfgang et al., 2001; Yang et al., 2008; Sasaki et al., 2009). As germline-competent ES cells and somatic cell nuclear transfer (SCNT) are not broadly available for NHPs, recently-developed gene editing technologies involving ZFNs, TALENs and CRISPR/Cas9 offer new opportunities for disease modeling (Mali el al., 2013; Moscou et al., 2009; Cho et al., 2013; Cong et al., 2013; Wood et al., 2011; Deng et al., 2012; Takada et al, 2013).
Here, we applied TALENs to generate MECP2 mutant rhesus and cynomolgus macaques from one-cell embryos. MECP2 is an X-linked, monoallelically expressed gene encoding a methyl-CpG binding protein (Nan et al., 1998; Jones et al., 1998; Skene et al., 2010). Loss-of-function mutations in MECP2 generally lead to a neurodevelopmental disorder on the autism spectrum known as Rett syndrome (RTT). In humans, hemizygous loss-of-function mutations of MECP2 in males leads to embryonic lethality (Hagberg, 1985; Hagberg et al., 1983; Amir et al., 1999), but in females random X-chromosome inactivation leads to graded chimerism of mutant and wild-type allele-expressing cells and a wide range of severities in RTT phenotype. In this study, we used TALEN-based mutagenesis to generate a total of four rhesus and cynomolgous monkeys carrying MECP2 mutations with chimerism. All three male fetuses died during mid-gestation, consistent with the human RTT male phenotype. To our knowledge, this is first report of targeted mutagenesis in monkeys with TALEN technology for modeling of human disease.
The monkey MECP2 gene contains 4 exons, similar to that of humans and mice (Figure 1A), with the methyl-CpG binding domain (MBD) encoded by both exon 3 and exon 4 (Nan et al., 1998). We decided to target exon 3 with three pairs of TALEN constructs (Figure 1A) because exon 3 is the 5’ most common exon for the A and B form MECP2 transcripts, and our aim was to disrupt the gene. These sites are conserved in humans, so we initially used a luciferase reporter assay in 293T cells to evaluate the efficacy of our TALEN-mediated mutagenesis approach (Figure S1A-C). In addition, when these TALEN pairs were transfected individually into Macaca fascicularis mesenchymal stem cells (MSCs) as well as human MSCs, MECP2 mutagenesis occurred (Figure S1D-E).
Figure 1. MECP2 mutant monkey fetuses generated by TALEN (see also Figure S1 in the supplement).
(A) Schematic of TALEN-targeting sites within monkey MECP2 locus exon 3. The first nucleotides of exon 3 is numbered as 1, the last nucleotides of exon 3 is number 351. TALEN-targeting sequences are labeled as T1, T2, T3 (L, R). (B) Summary of embryo transfer (ET) after TALEN injection in both rhesus and cynomolgous monkeys (#, embryos transferred between 2-cell and blastocyst stage; a, including 1 singleton and 1 twin; b, miscarriage happened on the 30th and 63rd/64th days after ET; c, one female cynomolgus was born alive after 162 days of gestation; d, one male cynomolus fetus was miscarried on the 92nd day of gestation). (C) Images and gender identification of the two aborted Rhesus monkey fetuses. (D) Paternal and maternal DNAs were subjected to Sanger sequencing and T7EN1 cleavage assays. Since the paternal allele is homogenous, T7EN1 is incapable to cut. The maternal alleles are heterogeneous (with one 217C allele and one 217T allele), thus in T7En1 cleavage assay, positive digestion was observed (two arrows point at cleaved products). (E) Positive T7EN1 cleavage results from the miscarried monkey fetuses’ tail, brain, and testis tissues, indicative of presence of mutations at the targeted exon (cleaved products are indicated by the bracket with a star). (F) All detected point mutations were plotted based on its position and mutation rate in the entire 351bp exon. Mutation distribution plots were displayed for tail, brain and testes of the two miscarried fetuses. (G) Detrimental mutations (leading to early truncations or in-dels) detected in brain and tails of the fetuses. (H) Mutation rates calculated from Sanger sequencing of MECP2 exon 3. From total clones sequenced, we counted perfectly matched sequences with reference and sequences with at least one mismatch. When the percentage of perfectly matched sequences were calculated, we simultaneously acquired the mutation rate in each tissues sequenced (see also Table S1 and Figure S2 in the supplement).
As TALEN targeting efficiency could have cell-type specificity, we decided to inject all three TALEN pairs of targeting plasmids either individually or in combination, together with RAD51, into one-cell monkey zygotes. RAD51 was included to enhance DNA repair following TALEN activity. We found that compound targeting with all three pairs was the most effective approach for creating MECP2 mutations. Comparing mRNA and DNA versions (supercoiled/circular plasmids) of each TALEN targeting constructs, at a dose of 5 pl of 2 ng/μl per injected zygote, the DNA version appeared to be more efficacious in target editing. Thus circular plasmids were used for future injections. Cytoplasmic injection of circular plasmid DNA in one-cell embryos results in prolonged episomal-form of plasmid-carried gene expression during early embryonic stages (Iqbal et al., 2009), without genomic integration. If plasmid integrations did occur, they can be easily detected by TALEN-plasmid-specific PCR amplification from monkey genomic DNA, especially from later stage fetuses, where free plasmids would have been diluted by cell divisions. In our study, we did not detect any plasmid integration (Figure S2A). With this approach, the mutation rate of the manipulated embryos at the MECP2 locus was within the range of 40-50% when assayed beyond 8-cell stage (Figure S1F-H). Our approach, with prolonged TALEN expression and action, is anticipated to create a large repertoire of targeted MECP2 mutations, thus maximizing the disruption of the target gene and increasing the chance for generating mutations in the germ-line. If presented with a replacement DNA target template carrying specific mutations, this approach could also enhance homologous recombination to generate knock-ins. Although this type of approach is not classically used to generate mutant animals, it could be particularly advantageous when animal resources are limited, as in the case of NHPs.
As the TALEN-mediated mutation rate in early embryos was reasonably high (40-50%), we moved on to transferring manipulated embryos into surrogate female monkeys. The surrogate pregnancy rates were 28.6% (2/7) and 31.6% (6/19) for rhesus and cynomolgus, respectively (Figure 1B). From 7 surrogate rhesus mothers that were transferred with 3 embryos each, two became pregnant. One went through miscarriage at day 30 of gestation. The aborted embryo was not collected. The second pregnant monkey with a twin pregnancy went through miscarriage at day 63 of gestation with the first fetus (A) followed by the second fetus (B) 24hours later (Figure 1C). Both fetuses were collected and analyzed. Using the Y chromosome-associated SRY gene as a male genetic marker, we confirmed that both were males.
To determine whether the two miscarried male rhesus fetuses contained MECP2 mutations, we used a T7EN1 cleavage assay (Shen et al, 2013). To establish the validity of this assay, we PCR amplified the entire MECP2 exon 3 from paternal and maternal genomic DNAs. There is a single nucleotide polymorphism (SNP) found at position 217 with either a T or a C. The maternal parent is a heterozygote, with one allele being 217C and the other, 217T, while the paternal parent is 217C (Figure 1D). T7EN1, which cuts single strand (unpaired) DNA, was unable to cleave the denatured and re-natured PCR products from the father's genomic DNA because they are all derived from the same MECP2 allele (Figure 1D). However the maternal DNA has both 217T and 217C alleles, so when the genomic DNA PCR product was denatured and re-natured, mismatch at 217 could occur, generating a single strand bubble that could then be cleaved by T7EN1, generating two fragments (Figure 1D). We then examined several tissues from the two miscarried fetuses using this assay, and detected multiple cleavage products, indicating the existence of mutations (Figure 1E). As both animals are male with one X chromosome inherited from their mother, if no mutation is present the T7EN1 cleavage result should be negative, as for the paternal DNA. However, our T7EN1 cleavage assay on A and B fetus tail, brain, and testis DNAs clearly indicated that they harbored de novo MECP2 mutations (Figure 1E). All three tissues from both animals appeared to have many different kinds of MECP2 mutations because the cleaved products formed multiple seemingly smeary bands (Figure 1E). Through Sanger sequencing, we confirmed that many alleles contained mutations, with some insertions and deletions (in-dels), but mostly point mutations (Figure 1F-G). We used maternal DNA as the reference (because male fetuses acquire their X chromosomes from their mother), and confirmed that point mutations detected were indeed de novo mutations. From more than 50 DNA clones sequenced from the brains of both fetuses (A and B), we found that 35% and 22% of the clones in A and B, respectively, contained mutations (Figure 1H). Using the same method, we sequenced both maternal and paternal DNA from more than 50 clones each, and detected no mutations, demonstrating the validity of our approach for mutation analysis. We detected mutations in a range of different tissues using sequencing, T7EN1 cleavage, or both (Figure S2B-C), including in the testes of both fetuses (Figure 1E-H), making germ-line transmission a formal possibility. We plotted the mutations that we detected along the exon in each tissue and found that they were spread across the entire length of exon 3 (Figure 1F). This pattern differs from that seen in mutagenesis with single TALEN pairs, suggesting that the use of three TALEN pairs simultaneously targeting the same exon influences, the types and positions of mutations that are elicited, potentially because of interference or interaction between different TALENs targeting simultaneously.
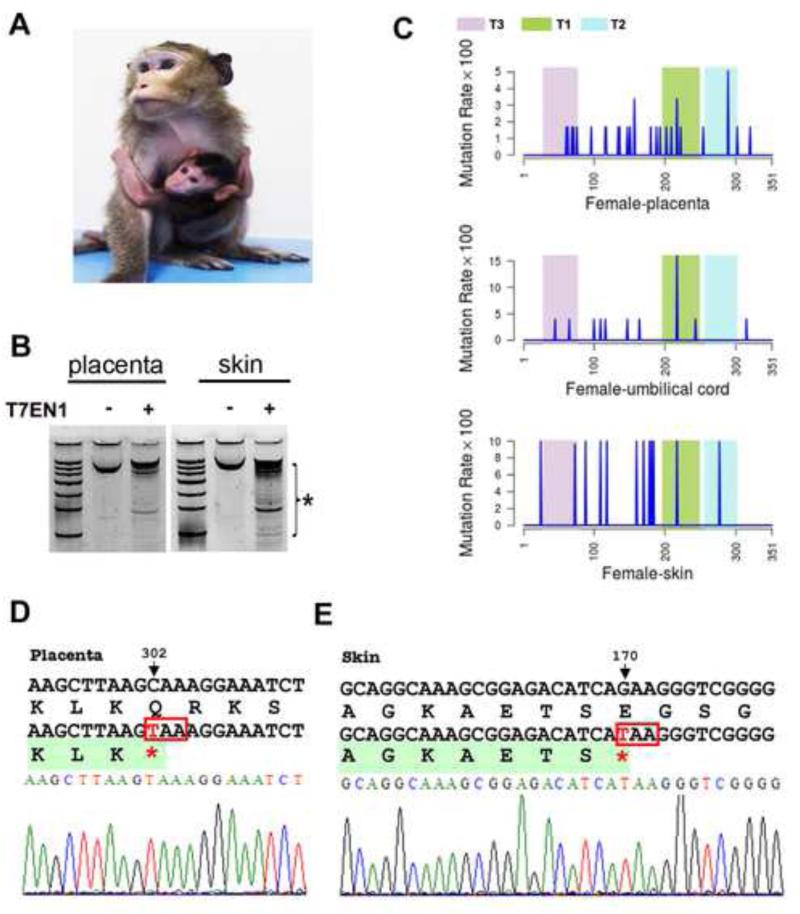
We have also applied our TALEN-targeting strategy in cynomolgus monkeys, which have an advantage over rhesus monkeys in that they breed all year round rather than seasonally. From a total of 54 embryos transferred into 19 cynomolgus surrogates, we obtained 6 successful pregnancies, and subsequently one live birth of a female cynomolgus baby, which is currently about four months old (Figure 1B). From the placenta, the umbilical cord, and skin biopsies, we detected a significant degree of MECP2 mutagenesis as measured by T7EN1 cleavage assays and Sanger sequencing (Figure 2A-E). As MeCP2 is located on the X chromosome, manifestation of genetic mutations in MECP2 depends on which allele is expressed. So far we have not observed any behavioral deficits in this female monkey. However, as RTT patients usually start to develop symptoms at 6-18 months after birth depending on the severity of the disease, which is related to the pattern of X-chromosome inactivation, it is possible that a longer time will be needed for detection of phenotype. Recently, we saw miscarriage of another cynomolgus fetus, which was male and contained MECP2 mutations (Figure S2D). So far, therefore, all three of the mid-gestation stage lethality events have been for male fetuses carrying MECP2 mutations. The high incidence and similarity to human phenotypes suggests that this embryonic lethality may be an RTT-related phenotype.
Figure 2. Generation of TALEN-targeted live cynomolgus female monkey.
(A) Images of three-month-old MECP2-mutant cynomolgus with her mother. (B) T7EN1 cleavage analysis of the placenta and skin tissue of the female cynomolgus baby born alive, indicative of presence of mutations by cleaved products (bracket with a star). (C) Mutation distributions along the targeted exon 3 based on Sanger sequencing in placenta, skin, and umbilical cord. (D-E) Sequencing traits of nonsense mutations detected in placenta and skins of the cynomolgus female baby.
As we used one-cell embryo cytoplasmic injection of circular plasmid TALEN DNAs to induce mutagenesis, our TALEN constructs likely had prolonged expression in the form of episomal vectors during early embryonic development (Iqbal et al., 2009). Continuous TALEN action and thus, repetitive mutagenesis in the targeted region (in this case exon 3 of the MECP2 gene), would undoubtedly create a high degree of mosaicism in the resulting embryo. We also used three pairs of TALENs to target the same MECP2 exon 3, which apparently resulted in many point mutations across the entire targeted exon, sometime even slightly outside the TALEN arms. The advantage of this approach is that it greatly increases the chance of disrupting the targeted gene, including in the germ line, but it also leaves open a legitimate concern about whether mutations occur elsewhere in the genome via off-target mutagenesis. Compared to CRISPR/Cas9, TALENa are considered to have much lower off-target activity (Ding et al., 2013; Ousterout et al.; 2013). There are only a few reported cases of TALEN off-target actions (Mussolino et al., 2011; Hockeymeyer et al., 2011; and Osborn et al., 2013). A new computational prediction tool called TALENoffer successfully predicted 5 out of 6 reported off-target events in the three aforementioned studies (Garu et al., 2013). We therefore tested our 3 pairs of TALEN and all possible cross-pair combinations to TALENoffer. A total of 41 exonic loci (with TALENoffer score > -1.8), 68 intronic loci (with TALENoffer score > -1.6) and 5 intergenic loci (with TALENoffer score > -1.4) were identified as potential off-target sites (Table S1). We then PCR amplified these “off-target” loci from genomic DNA of the paternal and maternal donors, the surrogate mother, and the testes, and brains of the two miscarried fetuses, and examined them using Sanger sequencing. A total of 798 samples were sequenced and no mutations were detected (Table S1). In addition, we genotyped the 41 exonic loci from 24 additional tissue samples of miscarried fetuses as well as the surviving female cynomolgus and her parents. An additional 984 PCR products were sequenced and again no mutations with confidence were detected (Table S1). In sum, a total of 1882 sequencings suggested no “off-target” effects beyond the targeted exon with our approach. In addition, as mentioned above, using TALEN plasmid-specific and Fok1-specific PCR of genomic DNAs from miscarried fetuses, we confirmed that no TALEN plasmid genomic integrations exist in our mutant monkeys (Figure S2A).
Overall, therefore, our study describes the successful application of TALEN-mediated mutagenesis to produce MECP2 mutant monkey, and illustrates an approach that could open up great opportunities in the future for the use of NHP models to study and development treatments for human diseases.
Supplementary Material
Highlights.
Human and primate MECP2 genes are targeted effectively by 3 TALEN pairs
Plasmid-based targeting in primate zygotes leads to mutagenesis
Extensive analysis detects no evidence of off-target activity
Male fetuses show mid-gestation lethality but a female was live born
Acknowledgements
The authors would like to acknowledge the Division of Animal Resources at the Kunming Biomed International for providing expertise and services that contributed to this project. This work was supported by the Major State Basic Research Development Program of China (973 Program) (No. 2012CBA01300, 2012CB966900, 2010CB945202, 2011CB965103), the National High Technology Research and Development Program of China (863 Program) (No. 2012AA020701), Yunnan Innovation Talents of Science and Technology (No. 2012HA013), the National Natural Science Foundation of China (31271599, U1302227, 31301058, 90919057, 31271371, 91319309), Key program for the basic research of the science and technology commission of Shanghai (13JC1407102), “Western Light” Talents Training Program of CAS and Yunnan Academic Leaders and Reserve Personnel, Young Talents Training Program of Tongji University. We also acknowledge grants from NIH (P01 GM081621-01A1, 1R01MH082068-01A2) and CIRM (RB3-02129) to Y.E.S. and the Transcriptome and Epigenetics Core of Center for Study of Opioid Receptors and Drugs of Abuse (CSORDA center grant: NIH-P50DA005010) and Intellectual and Developmental Disabilities Research Center (IDDRC center grant: NIH-P30HD004612) at UCLA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Chan AW, Chong KY, Martinovich C, Simerly C, Schatten G. Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science. 2001;291:309–312. doi: 10.1126/science.291.5502.309. [DOI] [PubMed] [Google Scholar]
- Chen Y, Niu Y, Ji W. Transgenic nonhuman primate models for human diseases: approaches and contributing factors. J. Genet. Genomics. 2012a;39:247–251. doi: 10.1016/j.jgg.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Niu Y, Yang S, He X, Ji S, Si W, Tang X, Xie Y, Wang H, Lu Y, Zhou Q, Ji W. The available time window for embryo transfer in the rhesus monkey (Macaca mulatta). Am. J. Primatol. 2012b;74:165–173. doi: 10.1002/ajp.21017. [DOI] [PubMed] [Google Scholar]
- Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013;31:230–232. doi: 10.1038/nbt.2507. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT, et al. A TALEN Genome-Editing System for Generating Human Stem Cell-Based Disease Models. Cell Stem Cell. 2013;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng D, Yan C, Pan X, Mahfouz M, Wang J, Zhu JK, Shi Y, Yan N. Structural basis for sequence-specific recognition of DNA by TAL effectors. Science. 2012;335:720–723. doi: 10.1126/science.1215670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau J, Boch J, Posch S. TALENoffer: genome-wide TALEN off-target prediction. Bioinformatics. 2013;29(22):2931–2932. doi: 10.1093/bioinformatics/btt501. [DOI] [PubMed] [Google Scholar]
- Hagberg B. Rett's syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr. Scand. 1985;74:405–408. doi: 10.1111/j.1651-2227.1985.tb10993.x. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann. Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 2011;29:731–734. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Barg-Kues B, Broll S, Bode J, Niemann H, Kues W. Cytoplasmic injection of circular plasmids allows targeted expression in mammalian embryos. Biotechniques. 2009;47:959–968. doi: 10.2144/000113270. [DOI] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- Mussolino C, Morbitzer R, Lütge F, Dannemann N, Lahaye T, Cathomen T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011;39:9283–9293. doi: 10.1093/nar/gkr597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Niu Y, Yu Y, Bernat A, Yang S, He X, Guo X, Chen D, Chen Y, Ji S, Si W, Lv Y, Tan T, Wei Q, Wang H, Shi L, Guan J, Zhu X, Afanassieff M, Savatier P, Zhang K, Zhou Q, Ji W. Transgenic rhesus monkeys produced by gene transfer into early-cleavage-stage embryos using a simian immunodeficiency virus-based vector. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17663–17667. doi: 10.1073/pnas.1006563107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn MJ, Starker CG, McElroy AN, Webber BR, Riddle MJ, Xia L, DeFeo AP, Gabriel R, Schmidt M, von Kalle C, et al. TALEN-based gene correction for epidermolysis bullosa. Mol. Ther. 2013;21:1151–1159. doi: 10.1038/mt.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousterout DG, Perez-Pinera P, Thakore PI, Kabadi AM, Brown MT, Qin X, Fedrigo O, Mouly V, Tremblay JP, Gersbach CA. Reading frame correction by targeted genome editing restores dystrophin expression in cells from Duchenne muscular dystrophy patients. Mol. Ther. 2013;21:1718–1726. doi: 10.1038/mt.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda SK, Wefers B, Ortiz O, Floss T, Schmid B, Haass C, Wurst W, Kühn R. Highly Efficient Targeted Mutagenesis in Mice Using TALENs. Genetics. 2013;195:703–713. doi: 10.1534/genetics.113.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z, Zhang X, Zhang P, Huang X. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 2013;23:720–723. doi: 10.1038/cr.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Baek IJ, Kim DH, Jeon J, Lee J, Lee K, Jeong D, Kim JS, Lee HW. Knockout mice created by TALEN-mediated gene targeting. Nat. Biotechnol. 2013;31:23–24. doi: 10.1038/nbt.2477. [DOI] [PubMed] [Google Scholar]
- Takada S, Sato T, Ito Y, Yamashita S, Kato T, Kawasumi M, Kanai-Azuma M, Igarashi A, Kato T, Tamano M, Asahara H. Targeted Gene Deletion of miRNAs in Mice by TALEN System. PLoS. One. 2013;8:e76004. doi: 10.1371/journal.pone.0076004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang MJ, Eisele SG, Browne MA, Schotzko ML, Garthwaite MA, Durning M, Ramezani A, Hawley RG, Thomson JA, Golos TG. Rhesus monkey placental transgene expression after lentiviral gene transfer into preimplantation embryos. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10728–10732. doi: 10.1073/pnas.181336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AJ, Lo TW, Zeitler B, Pickle CS, Ralston EJ, Lee AH, Amora R, Miller JC, Leung E, Meng X, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Meyer BJ. Targeted genome editing across species using ZFNs and TALENs. Science. 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, Snyder B, Larkin K, Liu J, Orkin J, Fang ZH, Smith Y, Bachevalier J, Zola SM, Li SH, Li XJ, Chan AW. Towards a transgenic model of Huntington's disease in a non-human primate. Nature. 2008;453:921–924. doi: 10.1038/nature06975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.