Abstract
Introduction
The aim of this study was to provide detailed age-specific (5-year age groups) and histology-specific (histologic subtypes of seminoma and nonseminoma) relative survival estimates of testicular germ cell cancer patients in Germany and the United States (U.S.) for the years 2002–2006 and to compare these estimates between countries.
Methods
We pooled data from 11 cancer registries of Germany and used data from the U.S. (SEER-13 database) including 11 508 and 10 774 newly diagnosed cases (1997–2006) in Germany and the U.S. respectively. We estimated 5-year relative survival (5-year-RS) by histology and age based on period analysis.
Results
5-year-RS for testicular germ cell tumors was 96.7% and 96.3% in Germany and the U.S. respectively. 5-year-RS for spermatocytic seminoma was close to 100% in both countries. 5-year-RS for nonseminoma was lower than for classical seminoma in Germany (93.3% versus 97.6%) and the U.S. (91.0% versus 98.2%). Among nonseminomas, choriocarcinomas provided the lowest 5-year-RS in both countries (Germany 80.1%, U.S. 79.6%). Age-specific 5-year-RS for seminoma showed only little variation by age. 5-year-RS for nonseminomas tended to be lower at higher ages, especially for malignant teratoma.
Discussion
This is the first study that provides up-to-date survival estimates for testicular cancer by histology and age in Germany and the U.S. Survival after a diagnosis of testicular cancer is very comparable between Germany and the U.S. 5-year-RS for spematocytic seminoma was close to 100% and the lowest 5-year-RS occurred among choriocarcinoma. Higher age at diagnosis is associated with a poorer prognosis among nonseminoma patients.
Keywords: Testicular neoplasms, germinoma, seminoma, nonseminomatous germ cell tumor, survival, Germany, United States
Introduction
Information on survival of cancer patients is an important indicator of cancer control, besides the numbers of new cases (incidence) and deaths (mortality). Survival information is needed for estimating how many cancer survivors are alive at any one time in order to plan health services [1]. With modern therapeutic approaches, 5-year survival after the diagnosis of testicular cancer exceed 90% in many countries [2]. Several prognostic factors of testicular cancers have been identified including histologic group, extent of disease, and age at diagnosis. However, the independent prognostic relevance of these factors controlling for confounders has been disputed until recently [3].
A recent large cohort study based on the Surveillance Epidemiology and End Results database (SEER 17) of the U.S. examined for the first time the effect of age on testicular cancer-specific observed mortality while taking into account diseases characteristics (extent of disease: localized or metastasized, i.e. regional or distant metastases), treatment factors (radiotherapy, retroperitoneal lymph node dissection), and sociodemographic variables by use of multivariable regression techniques. An important finding of this study was the adverse effect of age at diagnosis (< 40 years versus 40+ years) on testicular cancer-specific mortality within both subgroups of testicular germ cell tumors, seminoma and nonseminoma. As in many other studies, this study used broad categories of histology (seminoma versus nonseminoma) and age (< 40 years versus 40+ years) [4].
Many previous studies reported relative survival (RS) of testicular cancer. However, many reports did not even distinguish between seminoma and nonseminoma and also included testicular tumors other than germ cell tumors [1,2,5–13]. Some studies distinguished by histology but only in broad terms by providing separate RS estimates for seminoma and nonseminoma [14–19]. Age stratification of RS estimates - if any- either was not reported or was based on broad categories especially in the age range 15–40 years [1,2,6,7,10,12,14,16,18].
The aim of our study was to provide detailed age-specific (10-year age groups) and histology-specific (histologic subtypes of seminoma and nonseminoma) relative survival estimates of testicular germ cell cancer patients in Germany and the United States (U.S.) for the years 2002–2006 and to compare these estimates between countries.
Material & Methods
German Cancer Registry data
A collaborative study of cancer survival in Germany was initiated by the German Cancer Research Center and the Association of Epidemiological Cancer Registries (GEKID) in 2009. Details of the project have been published recently [20]. Briefly, we pooled data from 11 cancer registries of Germany covering a population of 33 million people (Table 1). The estimated completeness of cancer registration was over 80% in all registries and over 90% in most registries in 2004–2006. Follow-up was performed by linkage to death certificates of the respective state and in some registries additional linkage to population registries to get information about deaths or migration to other states. Two of the smallest cancer registries covering urban populations (Hamburg and Bremen) registered out-migrations of cancer patients (any cancer) by record linkage with population registries. Out-migration rates leading to the loss of follow-up were 1.7% in Hamburg and 2.8% in Bremen [20].
Table 1.
Overview of participating cancer registries in the present analysis
| Registry | Population (million) covered in 2006 | Diagnosis period | % DCO (excluded)1) | Testicular Cancer Cases | Median age at diagnosis | Microscopically confirmed cases (%) |
|---|---|---|---|---|---|---|
| Bavaria2) | 8.13 | 2002–2006 | 2.3 | 1 732 | 36 | 100.0 |
| Brandenburg | 2.55 | 1997–2006 | 1.5 | 1 217 | 37 | 99.5 |
| Bremen | 0.66 | 1998–2006 | 3.4 | 228 | 34.5 | 99.6 |
| Hamburg | 1.75 | 1997–2006 | 3.2 | 734 | 35 | 95.6 |
| Mecklenburg-Vorpommern | 1.69 | 1997–2006 | 1.8 | 921 | 36 | 99.5 |
| Lower Saxony | 7.98 | 2001–2006 | 4.4 | 2 175 | 36 | 99.5 |
| North Rhine-Westphalia2) | 2.62 | 1997–2004 | 1.7 | 929 | 35 | 98.9 |
| Rhineland-Palatinate2) | 0.52 | 1998–2006 | 0.9 | 213 | 35 | 100.0 |
| Saarland | 1.04 | 1997–2006 | 0.6 | 488 | 36 | 100.0 |
| Saxony | 4.25 | 1997–2006 | 1.9 | 2 081 | 37 | 99.0 |
| Schleswig-Holstein2) | 1.85 | 1999–2006 | 3.1 | 790 | 36 | 99.9 |
| Total | 33.04 | 2.5 | 11 508 | 36 | 99.2 |
DCO for testicular cancer overall. No autopsy only cases.
Selected administrative districts only
SEER-13 database
In addition, we extracted testicular cancer cases diagnosed in 1997–2006 of the SEER-13 (Surveillance Epidemiology and End Results) database for comparative analysis [21]. Patients with testicular cancer diagnosed in 1997–2006 who were at least 15 years old at the time of diagnosis were included in the analysis. SEER employs resource intensive follow-up to capture the last date of contact for each cancer case, with standards specifying that active follow-up be conducted for 95% of cases [22]. Cases are matched to the National Death Index, Social Security, Medicare and Medicaid data as well as records of contact with physicians, pathology labs and hospital registries. If persons with cancer moved out of a registry area, follow-up was tracked no matter where they moved within the U.S. We excluded cases diagnosed at autopsy and death certificate only cases (DCO).
Coding and grouping of morphology codes
All registries coded cancer topography, morphology and behaviour according to ICD-O-3 (International Classification of Diseases for Oncology) [23]. These codes were converted into ICD-10 (International Classification of Diseases) using the rules of the International Association of Cancer Registries (IACR) [24]. All registries recorded cancer cases according to the rules of the International Agency for Research on Cancer. We used ICD-O morphology codes to classify the tumors. Morphology codes 9060–9062 and 9064 identified classical seminomas. Code 9063 identified spermatocytic seminomas. Nonseminoma without mixed germ cell tumors included codes 9065, 9070–9072, 9080–9084, and 9100–9102 while code 9085 identified mixed germ cell cancers. Specific nonseminoma entities included embryonal carcinoma (9070), yolk sac tumors (9071), malignant teratoma (9080–9084, 9102), and choriocarcinoma (9100–9101). Germ cell tumors coded as 9065 are nonseminomatous germ cell tumors without further specification.
Starting in the late 1980s, a provisional field code for mixed germ cell tumor morphology (MGCT) was introduced into the 1st and 2nd versions of the ICD-O [23]. The MGCT morphology code (9085/3) became a standard morphology code in the ICD-O 3rd version [25]. Incidence trend analyses of testicular cancer in the U.S. and Germany suggest that the ICD-O code “mixed germ cell cancer” is being used for all mixed histologies, whether they are tumours with combinations of nonseminoma histologies or tumours with both seminomatous and nonseminomatous elements [26].
Statistical methods
We estimated 5-year relative survival (5-year-RS) which is the ratio of the observed probability of survival and the probability that would have been expected if the cancer patients had only experienced the normal (background) mortality of the general population in which they live, given the same distribution of factors such as age, sex, geographic area, and calendar period. Expected survival was estimated according to the Ederer II method using life tables stratified by country, calendar year, age, and sex [27]. Conventional survival analysis (cohort analysis) refers to cohorts of individuals diagnosed many years before and not to patients diagnosed more recently. We therefore used period analysis to estimate RS. Unlike cohort analysis, period analysis of RS exclusively takes survival experience of cancer patients in the most recent calendar period into account and closely predicts survival proportions of patients diagnosed in that period [28].
We used cancer patients diagnosed between 1997–2006 for the 5-year-RS estimation of the years 2002–2006. To compare the overall 5-year-RS between countries, we adjusted 5-year-RS estimates to the age distribution of testicular cancer cases (germ cell and non-germ cell) in Germany using the age groups 15–24, 25–29, 30–34, 35–39, 40–44, 45+ years and corresponding weights 0.11, 0,13, 0.19, 0.21, 0.16, and 0.20. Furthermore, we compared 5-year-RS estimates by 10-year age groups (15–24, 25–34, 35–44, 45+ years) (seminoma, nonseminoma). For nonseminoma subtypes, we present only 5-year-RS estimates for three age groups (15–24, 25–34, 35+ years) as the number of cases became too small.
Staging to subdivide testicular cancers into localized, regionally metastasized, or distantly metastasized tumors revealed large proportions of missing data (47%) in the German data set as either information on regional lymph node involvement or on presence of distant metastases was missing. We therefore did not standardize German age-specific RS estimates by stage. For the U.S. data, we standardized age-specific RS estimates by stage (localized, regional lymph node metastases, distant metateses). We used the overall stage distribution of the histologic group for standardizing age-specific RS estimates. All calculations were carried out using SAS software (version 9.2), using macros developed for period analysis [29].
Results
During the period 1997 through 2006, overall 11 508 and 10 774 primary invasive testicular cancers (ICD10: C62) have been registered in Germany and the U.S. (SEER-13). The vast majority of these cancers were germ cell cancers (Germany 96.5%, U.S. 97.9%). Overall 9,628 out of 10,547 germ cell cancer patients in the SEER program were whites. Only about a fifth of all testicular germ cell tumors occurred among men aged 45 years or more (Germany: 19.7%, U.S. 16.4%). The proportion of death certificate only (DCO) and autopsy only cases was small (Germany: 2.5%, U.S. 0.13%) and depended on age.
The estimated age-standardized 5-year-RS for testicular germ cell tumors was 96.7% and 96.3% in Germany and the U.S. respectively. Although based on small numbers, the 5-year-RS for spermatocytic seminoma was close to 100% in both countries. 5-year-RS for nonseminoma was considerably lower than for classical seminoma in Germany (93.3% versus 97.6%) and the U.S. (91.0% versus 98.2%). 5-year-RS estimates for mixed germ cell tumors were between those for classical seminoma and nonseminoma. Among nonseminomas, 5-year-RS was lowest for choriocarcinomas in both countries (Germany 80.1%, U.S. 79.6%). 5-year-RS estimates were very comparable between Germany and the U.S. (Table 2).
Table 2.
Estimated age-standardized 5-year relative survival of testicular tumors by histology in Germany and the U.S. for the years 2002–2006
Nonseminomas: mixed germ cell tumors excluded; estimates are age-standardized using the age distribution in Germany and the age groups as defined in the table (exception: age groups 15–19 and 20–24 years are merged because of small case numbers); for the age standardization of the rate of spermatocytic seminoma in Germany, no cases aged 15–24 years were registered. Therefore, age groups between 15–29 years were therefore collapsed to a single age group for age standardization.
| Germany
|
SEER-13
|
Difference % (U.S. – GER)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Cases | RS (%) | SE (%) | Cases | RS (%) | SE (%) | Estimate | 95%CI | |
| All malignant testicular tumors | 11 508 | 96.3 | 0.3 | 10 774 | 95.7 | 0.3 | −0.6 | −1.4; 0.2 |
| Malignant germ cell tumors | 11 106 | 96.7 | 0.3 | 10 547 | 96.3 | 0.3 | −0.4 | −1.2; 0.4 |
| Classical seminoma | 7 172 | 97.6 | 0.3 | 6 256 | 98.2 | 0.3 | 0.6 | −0.2; 1.4 |
| Spermatocytic seminoma | 62 | 99.2 | 2.0 | 73 | 100.0 | 2.0 | 0.8 | −4.7; 6.3 |
| Mixed germ cell tumors | 1 003 | 94.2 | 1.2 | 2 194 | 93.5 | 1.0 | −0.7 | −3.8; 2.4 |
| Nonseminoma (incl. MGCTs) | 3 871 | 93.6 | 0.7 | 4 218 | 92.5 | 0.8 | −1.1 | −3.2; 1.0 |
| Nonseminomas (excl. MGCTs) | 2 868 | 93.3 | 0.8 | 2 024 | 91.0 | 1.4 | −2.3 | −5.5; 0.9 |
| - Embryonal carcinoma | 1 245 | 95.9 | 1.2 | 933 | 96.5 | 1.5 | 0.6 | −3.2; 4.4 |
| - Malignant teratomas | 1 131 | 93.8 | 1.3 | 555 | 90.7 | 3.1 | −3.1 | −9.7; 3.5 |
| - Choriocarcinoma | 256 | 80.1 | 3.8 | 298 | 79.6 | 4.6 | −0.5 | −12.2; 11.2 |
| - Yolk sak tumor | 195 | 91.3 | 3.4 | 119 | 85.2 | 5.6 | −6.1 | −18.9; 6.7 |
| - Nonseminoma, not other specified* | 40 | 119 | 84.3 | 5.4 | ||||
relative survival estimate for Germany not validly estimable;
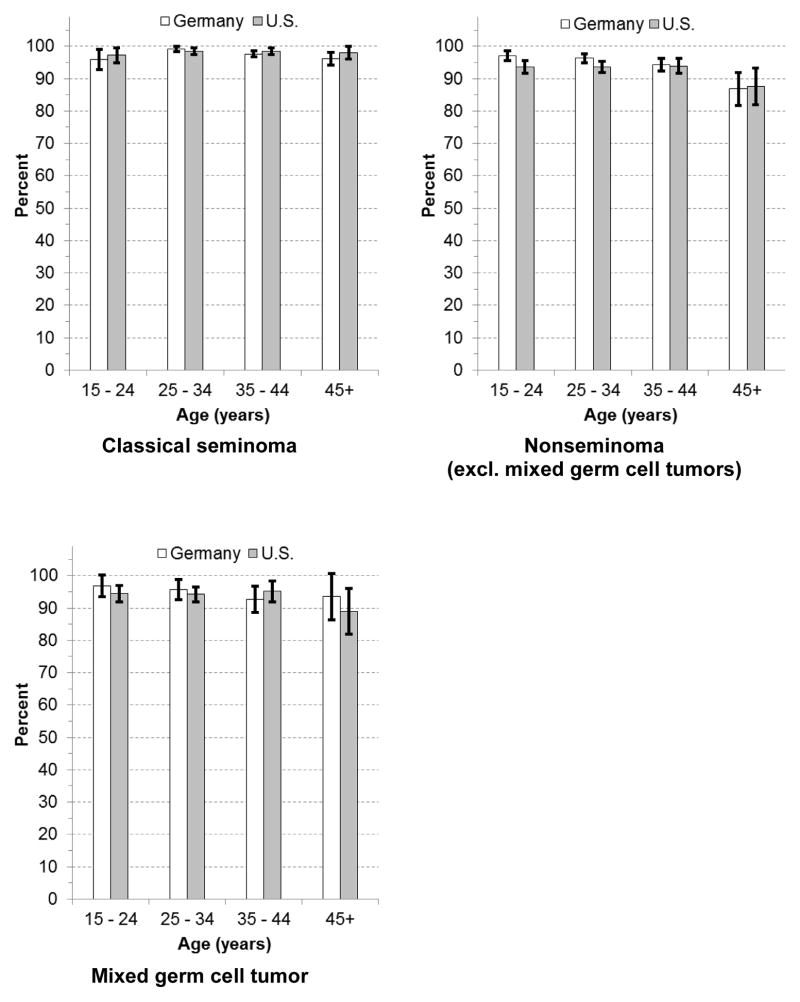
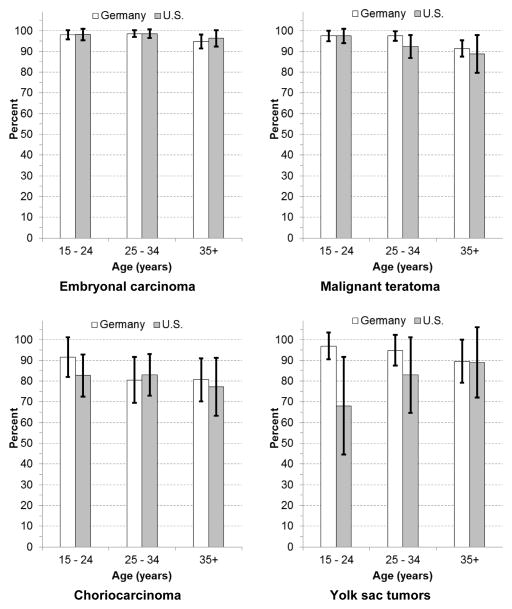
Age-specific 5-year-RS for seminoma showed only little variation by age. 5-year-RS for nonseminoma (mixed GCTs excluded) declined with age in both countries, but lower survival was essentially restricted to the minority of patients aged 45+ years (Germany: 15–24 years 97.1%, 45+ years: 86.8%; U.S.: 15–24 years 93.6%, 45+ years 87.6%). The analysis of 5-year-RS of nonseminoma subtypes revealed that especially 5-year-RS of malignant teratoma and choriocarcinoma declined with increasing age. Age-specific 5-year-RS estimates for yolk sac tumors were based on only small numbers (Figure 1 & Figure 2). Standardization by stage of age-specific 5-year-RS of the U.S. resulted in some attenuation of the age effect. However, higher age remained associated with lower age-specific 5-year-RS of nonseminoma, especially malignant teratoma (Supplementary Table 1).
Figure 1.
Age-specific estimated relative survival of major histologic groups of testicular germ cell tumors in Germany and the U.S
White bars: Germany, black bars: U.S.; whiskers: 95% confidence intervals
Figure 2.
Age-specific estimated relative survival of subgroups of nonseminomatous testicular germ cell tumors in Germany and the U.S.
White bars: Germany, black bars: U.S.; whiskers: 95% confidence intervals
Discussion
The detailed age- and histology-stratified RS estimates of testicular cancer for Germany and the U.S. provide some new insights into the prognosis of testicular cancer. After a diagnosis of spermatocytic seminoma the prognosis is nearly perfect (estimated 5-year-RS of close to 100%) in Germany and the U.S. The WHO classification of testicular tumors considers spermatocytic seminomas as benign tumors that can become a life threatening disease if it progresses to sarcoma (i.e. becomes a new entity) [30]. Furthermore, among subtypes of nonseminoma, estimated 5-year-RS is lowest for choriocarcinoma. Also we provide for the first time RS estimates for MGCTs. Whereas 5-year-RS for seminoma showed only little variation by age, 5-year-RS for nonseminomas tended to be lower at ages 45 years and more. The age dependency of 5-year-RS of nonseminoma occurs especially among malignant teratomas.
The separate 5-year-RS estimation for MGCTs that were earlier treated as nonseminoma showed that 5-year-RS estimates are in between the 5-year-RS estimates of classical seminoma and nonseminoma that exclude MGCTs. Based on previous incidence trend analyses, we believe that the morphology code for MGCTs may include all mixed histologies, whether they are tumors with combinations of nonseminoma histologies or tumors with both seminomatous and nonseminomatous elements [26]. Therefore, MGCTs may reflect a heterogeneous group of histologies. In a population-based study of Luxembourg, 122 of 397 testicular cancer cases (31%) were MGCT. Overall 65 tumors (53%) included a seminomatous component and 47% included only mixed non-seminomatous components [31].
Empirical evidence of the age dependency of the prognosis of testicular cancer goes back at least to 1943 [32]. We were able to corroborate the association between age at diagnosis and prognosis. Standardization by stage across age groups of the U.S. data resulted in some attenuation of survival differences by age. However, two recent studies were able to show the independent effect of age on the prognosis of testicular cancer by adjustment for extent of disease [4,33]. In contrast to detailed analyses by Fossa et al. [4], the association between age and 5-year-RS virtually occurred only among nonseminoma patients in our study. This apparent discrepancy may be explained by the way, Fossa et al. analyzed SEER-17 data. In contrast to our analyses, they adjusted for several variables including extent of disease, treatment, and others and modeled the 10-year testicular cancer-specific mortality rate. Adjustment strengthened the association between age (15–39 years versus 40+ years) and cancer-specific survival among seminoma patients.
Potential explanations for the age dependency of prognosis are reduced treatment intensity combined with increased therapy-related toxicity among older men [4]. The most likely explanation for this age effect is a higher early excess mortality after diagnosis of metastasized testicular cancer. Spermon et al. observed the largest difference in RS between testicular cancer patients younger than 50 years and older ones within the first year after diagnosis [14]. This difference was most prominent among patients with regional or distant metastases at diagnosis. The EUROCARE-4 study revealed that decreasing 5-year-RS with age is mainly due to decreasing 1-year survival with age, while survival conditional on surviving the first year changes only modestly with advancing age [10]. In a Dutch study, RS estimates for patients 50 years or more were only higher after one year of follow-up (< 50 years: 99%, ≥50 years: 93%) [16].
Some limitations to our study need mention. First, when we stratified 5-year-RS estimates by histology, we relied on the morphology codes provided by the cancer registries. Lack of standardization of histological criteria to differentiate between subtypes of germ cell cancers among pathologists and lack of standardization of coding criteria (for example regarding the use of MGCT morphology) can produce some apparent differences between registries. An earlier pathology review study in Australia including 1,009 testicular cancer cases diagnosed between 1950 and 1978 has shown that the diagnostic accuracy can vary across centers and over time [34]. Although all registries that we included in our analyses recorded cancer cases according to the rules of the IACR and coded cancer topography, morphology and behaviour according to ICD-O-3, this does not rule out some heterogeneity of registration and coding. Second, despite of the use of large cancer registry files, some of our 5-year-RS estimates (e.g. choriocarcinoma and yolk sac tumors) were quite imprecise due to the low number of registered cases. Third, we were not able to provide a comparison of stage-adjusted RS estimates due to the substantial proportion of missing data on TNM in the German data set.
In conclusion, we provided detailed up-to-date estimates of histology- and age-specific relative survival of germ cell tumors of the testis in Germany and the U.S. 5-year-RS for spermatocytic seminoma was close to 100% in both countries. Choriocarcinomas provided the lowest 5-year-RS in both countries. Whereas 5-year-RS for seminoma showed only little variation by age, 5-year-RS for nonseminomas tended to be lower in patients aged 45+ years at diagnosis.
Supplementary Material
Acknowledgments
This study was supported in part by a grant from the German Cancer Aid (Deutsche Krebshilfe), no. 108257.
Members of the GEKID Cancer Survival Working Group include Karla Geiss, Martin Meyer (Cancer Registry of Bavaria), Andrea Eberle, Sabine Luttmann (Cancer Registry of Bremen), Roland Stabenow (Cancer Registry of Berlin and the New Federal States), Stefan Hentschel, Alice Nennecke (Cancer Registry of Hamburg); Joachim Kieschke, Eunice Sirri (Cancer Registry of Lower Saxony), Bernd Holleczek (Cancer Registry of Saarland), Katharina Emrich (Cancer Registry of Rhineland-Palatinate), Hiltraud Kajüter, Volkmar Mattauch (Cancer Registry of North Rhine-Westphalia), Alexander Katalinic (Cancer Registry of Schleswig-Holstein), Klaus Kraywinkel (Robert Koch Institute, Berlin), Hermann Brenner, Adam Gondos, Lina Jansen (DKFZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coleman MP, Gatta G, Verdecchia A, Esteve J, Sant M, Storm H, et al. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol. 2003;14 (Suppl 5):v128–v149. doi: 10.1093/annonc/mdg756. [DOI] [PubMed] [Google Scholar]
- 2.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer. 2009;45(6):931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Inci K, Dogan HS, Akdogan B, Ergen A, Tasar C, Ozen H. Does age affect the prognosis of patients with testicular germ cell tumor? Urol Int. 2007;79(2):117–123. doi: 10.1159/000106323. [DOI] [PubMed] [Google Scholar]
- 4.Fossa SD, Cvancarova M, Chen L, Allan AL, Oldenburg J, Peterson DR, et al. Adverse prognostic factors for testicular cancer-specific survival: a population-based study of 27,948 patients. J Clin Oncol. 2011;29(8):963–970. doi: 10.1200/JCO.2010.32.3204. [DOI] [PubMed] [Google Scholar]
- 5.Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44 (10):1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Aareleid T, Sant M, Hedelin G. Improved survival for patients with testicular cancer in Europe since 1978. EUROCARE Working Group. Eur J Cancer. 1998;34(14 Spec):2236–2240. doi: 10.1016/s0959-8049(98)00313-x. [DOI] [PubMed] [Google Scholar]
- 7.Sant M, Capocaccia R, Coleman MP, Berrino F, Gatta G, Micheli A, et al. Cancer survival increases in Europe, but international differences remain wide. Eur J Cancer. 2001;37(13):1659–1667. doi: 10.1016/s0959-8049(01)00206-4. [DOI] [PubMed] [Google Scholar]
- 8.Yu XQ, O’Connell DL, Gibberd RW, Coates AS, Armstrong BK. Trends in survival and excess risk of death after diagnosis of cancer in 1980–1996 in New South Wales, Australia. Int J Cancer. 2006;119(4):894–900. doi: 10.1002/ijc.21909. [DOI] [PubMed] [Google Scholar]
- 9.Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, et al. Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8(9):784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 10.Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8(9):773–783. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 11.Power DA, Brown RS, Brock CS, Payne HA, Majeed A, Babb P. Trends in testicular carcinoma in England and Wales, 1971–99. BJU Int. 2001;87(4):361–365. doi: 10.1046/j.1464-410x.2001.00078.x. [DOI] [PubMed] [Google Scholar]
- 12.Nur U, Rachet B, Mitry E, Cooper N, Coleman MP. Survival from testicular cancer in England and Wales up to 2001. Br J Cancer. 2008;99 (Suppl 1):S80–S82. doi: 10.1038/sj.bjc.6604597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rachet B, Maringe C, Nur U, Quaresma M, Shah A, Woods LM, et al. Population-based cancer survival trends in England and Wales up to 2007: an assessment of the NHS cancer plan for England. Lancet Oncol. 2009;10(4):351–369. doi: 10.1016/S1470-2045(09)70028-2. [DOI] [PubMed] [Google Scholar]
- 14.Spermon JR, Witjes JA, Kiemeney LA. Difference in stage and morphology-adjusted survival between young and elderly patients with a testicular germ cell tumor. Urology. 2002;60(5):889–893. doi: 10.1016/s0090-4295(02)01886-1. [DOI] [PubMed] [Google Scholar]
- 15.Steele GS, Richie JP, Stewart AK, Menck HR. The National Cancer Data Base report on patterns of care for testicular carcinoma, 1985–1996. Cancer. 1999;86 (10):2171–2183. [PubMed] [Google Scholar]
- 16.Verhoeven RH, Coebergh JW, Kiemeney LA, Koldewijn EL, Houterman S. Testicular cancer: trends in mortality are well explained by changes in treatment and survival in the southern Netherlands since 1970. Eur J Cancer. 2007;43(17):2553–2558. doi: 10.1016/j.ejca.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Talback M, Dickman PW. Predicting the survival of cancer patients recently diagnosed in Sweden and an evaluation of predictions published in 2004. Acta Oncol. 2012;51(1):17–27. doi: 10.3109/0284186X.2011.626444. [DOI] [PubMed] [Google Scholar]
- 18.Aareleid T, Gondos A, Brenner H, Pokker H, Leppik K, Magi M. Testicular cancer survival in Estonia: improving but still relatively low. Acta Oncol. 2011;50(1):99–105. doi: 10.3109/0284186X.2010.480981. [DOI] [PubMed] [Google Scholar]
- 19.Talback M, Stenbeck M, Rosen M. Up-to-date long-term survival of cancer patients: an evaluation of period analysis on Swedish Cancer Registry data. Eur J Cancer. 2004;40(9):1361–1372. doi: 10.1016/j.ejca.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Hiripi E, Gondos A, Emrich K, Holleczek B, Katalinic A, Luttmann S, et al. Survival from common and rare cancers in Germany in the early 21st century. Ann Oncol. 2011 doi: 10.1093/annonc/mdr131. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute, DCCPS SRP; Surveillance Systems Branch, editor. Surveillance, Epidemiology, and End Results (SEER) program research data (1973–2009) released April 2012 based on the November 2011 submission. Jan 4, 2012. [Google Scholar]
- 22.SEER Training Modules. National Cancer Institute, editor. Requirements of CoC/Governing Agencies. 2012 http://training.seer.cancer.gov/follow-up/intro/requirements.html. 23-3-2013.
- 23.International Classification of Diseases for Oncology (ICD-O) 2. Geneva: World Health Organization; 1990. [Google Scholar]
- 24.Ferlay J, Burkhard C, Whelan SL, Parkin DM. Check and conversion programs for cancer registries IARC/IACR Tools for Cancer Registries. 42. IARC Technical Report 2005 [Google Scholar]
- 25.International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2002. [Google Scholar]
- 26.Trabert B, Stang A, Cook MB, Rusner C, McGlynn KA. Impact of classification of mixed germ-cell tumours on incidence trends of non-seminoma. Int J Androl. 2011;34 (4 Pt 2):e274–e277. doi: 10.1111/j.1365-2605.2011.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ederer F, Heise H. Methodological note no. 10, End Results Evaluation Section. Bethesda: National Cancer Institute; 1959. Instructions fo IBM 650 programmers in processing survival computations. [Google Scholar]
- 28.Brenner H, Gefeller O, Hakulinen T. Period analysis for ‘up-to-date’ cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer. 2004;40(3):326–335. doi: 10.1016/j.ejca.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H, Gefeller O, Hakulinen T. A computer program for period analysis of cancer patient survival. Eur J Cancer. 2002;38(5):690–695. doi: 10.1016/s0959-8049(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 30.Mikuz G. WHO classification of testicular tumors. Verh Dtsch Ges Pathol. 2002;86:67–75. [PubMed] [Google Scholar]
- 31.Scheiden R, Hein T, Wagener C, Kieffer N, Lamy S, Capesius C. Testicular cancer in Luxembourg: incidence and outcome in relation to the different histo-pathological types (1980–2004) Bull Soc Sci Med Grand Duche Luxemb. 2008;(4):521–539. [PubMed] [Google Scholar]
- 32.Moller H, Friis S, Kjaer SK. Survival of Danish cancer patients 1943–1987. Male genital organs. APMIS Suppl. 1993;33:122–136. [PubMed] [Google Scholar]
- 33.Sant M, Aareleid T, Artioli ME, Berrino F, Coebergh JW, Colonna M, et al. Ten-year survival and risk of relapse for testicular cancer: a EUROCARE high resolution study. Eur J Cancer. 2007;43(3):585–592. doi: 10.1016/j.ejca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Stone JM, Sandeman TF, Ironside P, Cruickshank DG, Matthews JP. Time trends in accuracy of classification of testicular tumours, with clinical and epidemiological implications. Br J Cancer. 1992;66(2):396–401. doi: 10.1038/bjc.1992.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.