Abstract
Although cellular therapies hold great promise for the treatment of human disease, results from several initial clinical trials have not shown a level of efficacy required for their use as a first line therapy. Here we discuss how in vivo molecular imaging has helped identify barriers to clinical translation and potential strategies that may contribute to successful transplantation and improved outcomes, with a focus on cardiovascular and neurological diseases. We conclude with a perspective on the future role of molecular imaging in defining safety and efficacy for stem cell clinical implementation.
Introduction: Basics of Molecular Imaging
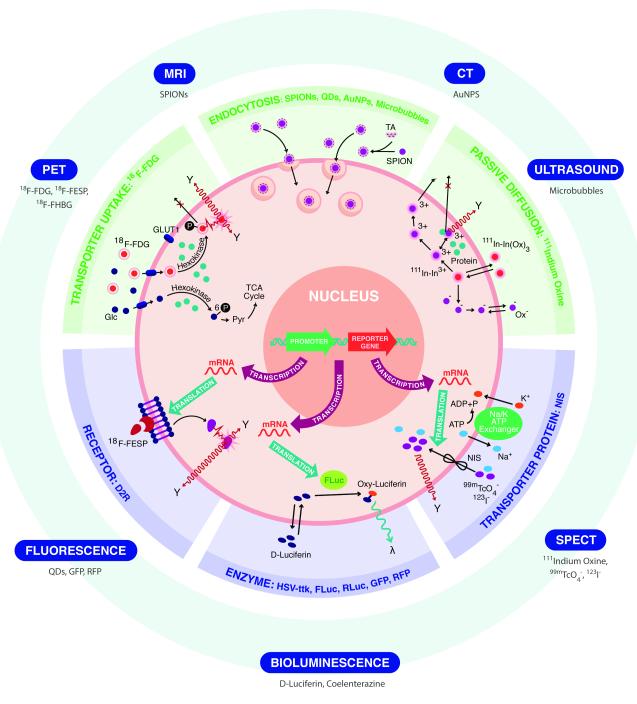
Stem cell therapies provide unique opportunities for treating diseases of the heart and brain, which have limited regenerative capacity. Because somatic stem cells from the heart and brain are rare and difficult to isolate, therapeutic approaches using adult stem cells and differentiated cells derived from pluripotent stem cells provide a promising alternative source for regenerating cardiac and brain tissue (Garbern and Lee, 2013; Yu et al., 2013). Successful implementation of cell therapies will require a better understanding of cell fate after transplantation, which can be achieved by the application of molecular imaging. Molecular imaging enables the longitudinal, non-invasive assessment of cellular behavior in vivo following cell transplantation (Massoud and Gambhir, 2003). Cell tracking can be performed by labeling cells with molecular probes that enter the cell by active/passive transport and are trapped intracellularly (e.g., direct labeling). Alternatively, cells can be labeled by overexpression of specific reporter genes that integrate into the cellular genome via viral or non-viral vectors (e.g., reporter gene labeling) (Figure 1). Once integrated, reporter genes are transcribed into messenger RNA and translated into proteins that interact with a molecular probe for signal generation. Although reporter gene imaging requires genomic manipulation and poses potential safety issues, it is the preferred labeling strategy because signal generation is dependent on cell viability. Signal generated from cells labeled by either technique can then be visualized using imaging systems such as fluorescence imaging (FLI), bioluminescence imaging (BLI), single photon emission computed tomography (SPECT), positron emission tomography (PET), or magnetic resonance imaging (MRI). The advantages and disadvantages of each imaging system are summarized in Table 1 and can be found in other detailed reviews (Chen and Wu, 2011; Nguyen et al., 2011).
Figure 1.
Cell labeling strategies and detectors for stem cell imaging. For direct labeling (in green), cells are incubated with imaging probes that enter the cell via transporter uptake (i.e., 18F FDG, 18F-FESP, and 18F-FHBG), endocytosis (i.e., SPIONs, QDs, Au NPS, and microbubbles), or passive diffusion (i.e., 111In-ox). In reporter gene imaging (in blue), cells are transfected or transduced with the reporter gene construct. Transcription of the reporter gene under the control of a promoter followed by translation of its mRNA, leads to accumulation of different reporter proteins such as receptors (i.e., D2R), enzymes (HSVtk, FLuc, RLuc, GFP, and RFP), and transporter proteins (NIS). Introduction of a reporter gene probe (i.e., 18F-FESP, D-luciferin, coelenterazine) results in signal generation. Labeled cells are detected by imaging systems such as PET, MRI, CT, and ultrasound. Abbreviations: 18F-FDG, 18F-fluorodeoxyglucose; 18F-FESP, 3-(2′-[18F]-fluoroethyl)-spiperone; 18F-FHBG, 9-(4-18Ffluoro-3-[hydroxymethyl]butyl) guanine; SPIONs, superparamagnetic iron oxide nanoparticles; QDs, quantum dots; Au NSPs, gold nanoparticles; 111In-ox, indium oxine; Asp, aspartic acid; Ser, serine; NIS, sodium iodide symporter; I, iodine; 99mTcO −4, technetium pertechnetate; D2R, dopamine 2 receptor; HSV-ttk, herpes simplex virus truncated thymidine kinase; FLuc, firefly luciferase; RLuc, renilla luciferase; GFP, green fluorescent protein; RFP, red fluorescent protein); PET, positron emission tomography; MRI, magnetic resonance imaging; CT, computed tomography; SPECT, single photon emission computed tomography; GLUT1, glucose transport type I.
Table 1.
Comparison of imaging techniques for cell therapies
| Imaging modality | Spatial resolution |
Acquisition time |
Detection limit: cells |
Labeling strategy |
Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Bioluminescence imaging (BLI) (preclinical only) |
5-20 mm depending on depth of signal |
seconds | ~ 103 | Reporter gene | Cheap, simple, high throughput |
Small animals only, low resolution, only 2D images |
| Fluorescence tomography (FMT) (preclinical only) |
2-3 mm | seconds to minutes |
~ 106 | Reporter gene, fluorescent dye |
Cheap, simple | Low resolution, cells need to be close to surface |
| Ultrasound (US) (preclinical and clinical) |
150 μm − 2 mm depending on depth |
seconds to minutes |
not well characterized |
Reporter gene, antibody with microbubble |
Cheap, relatively simple |
Limited 3D capabilities, low signal to noise ratio |
| Single photon emission computed tomography (SPECT) (preclinical and clinical) |
1-2 mm 8-12 mm |
minutes | ~ 105 | Reporter gene, incubation with radiotracer |
3D imaging | Anatomic reference required, radioactive tracer required |
| Positron emission tomography (PET) (preclinical and clinical) |
~1 mm 4-6 mm |
seconds to minutes |
~ 104 | Reporter gene, incubation with radiotracer |
3D imaging, high sensitivity |
Anatomic reference required, radioactive tracer required |
| Magnetic resonance tomography (MRI) (preclinical and clinical) |
25-500 μm 0.5-5 mm |
minutes to hours |
~ 104 | Internalization or surface labeling with nanoparticles or specific ions |
3D imaging, good soft tissue contrast, no radiation, high resolution |
Very expensive, complicated |
| Computed tomography (CT) (preclinical and clinical) |
< 50 μm <1 mm |
seconds to minutes |
not well characterized |
Internalization or surface labeling with nanoparticles |
3D imaging, relatively cheap, high resolutio |
Uses ionizing radiation |
Fields in bold indicate parameters specific to clinical systems if they are different from preclinical systems.
Akin to the use of pharmacokinetics for drug development, the overall goal of molecular imaging in regenerative medicine is to enhance therapeutic efficacy and decrease toxicity. Results from preclinical and clinical studies thus far suggest that cell imaging can and should be incorporated into more studies of cell transplantation in animals and humans. Continued application of molecular imaging for regenerative cell therapies will be critical for its successful implementation. In this review, we will discuss how stem cell imaging has helped identify the hurdles currently limiting the clinical translation of regenerative cell therapies for cardiovascular and neurological diseases, how it can be applied to define strategies to overcome these obstacles, and how it can be incorporated in the clinical implementation of regenerative stem cell therapies.
Defining Hurdles to Clinical Translation: Findings from Preclinical and Clinical Studies
Small and large animal studies have shown that stem cell therapies are effective in treating cardiovascular (van der Spoel et al., 2011) and neurodegenerative disease (Antonic et al., 2013; Lees et al., 2012). Based on these promising results, investigators have launched several Phase I and II studies to evaluate the safety and efficacy of stem cell therapies for the treatment of ischemic heart disease (Bolli et al., 2011; Hare et al., 2012; Heldman et al., 2014; Perin et al., 2012; Traverse et al., 2011; Traverse et al., 2012), peripheral vascular disease (Poole et al., 2013), spinal cord injury (Mothe and Tator, 2012), multiple sclerosis (Uccelli et al., 2011), and stroke (Bang et al., 2005; Kondziolka et al., 2005; Lee et al., 2010). While safety has been clearly demonstrated, efficacy remains more elusive (Clifford et al., 2012; Fadini et al., 2010). Based on these results, one may conclude that cell therapy itself may be inadequate or that better results could be achieved with different cell types. It is also possible that we have yet to apply these novel therapies effectively. Indeed, findings from current trials underscore the need to better understand the fate of transplanted cells and their correlation with structural (i.e., infarct size, left ventricular volume at end diastole) and functional outcome (i.e. left ventricular ejection fraction, neurocognitive and motor function) (Bang et al., 2005; Bolli et al., 2011; Kondziolka et al., 2005; Makkar et al., 2012; Perin et al., 2012; Traverse et al., 2011; Traverse et al., 2012).
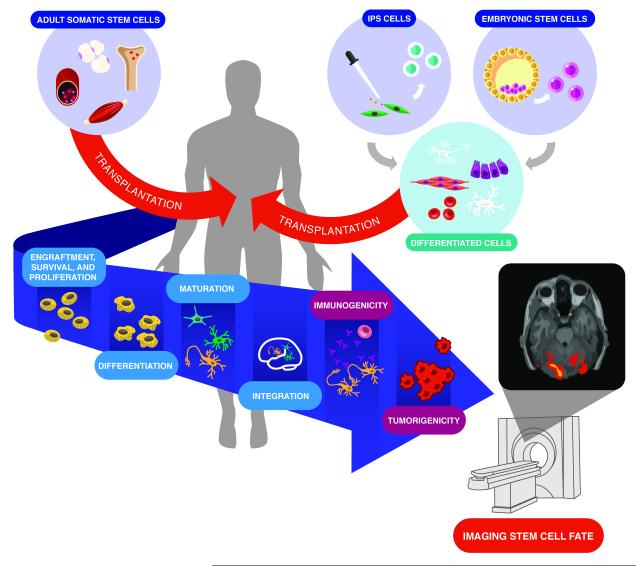
The efficacy of any cell therapy depends on the interaction of many different factors such as disease etiology, cell type, delivery route, cell retention/engraftment, activation of resident cells, or functional integration. In order to optimize cell therapies, we need to improve our understanding of how these factors interact. The inclusion of molecular imaging into preclinical and clinical trials (Table 1) has helped answer some of these important questions, with further discoveries to come. Stem cell imaging studies, for example, have identified the following barriers, among others, to clinical translation in either adult or pluripotent stem cells: 1) limited cell engraftment, survival and proliferation; 2) poor cell differentiation and maturation; 3) immunogenicity with allogeneic transplantation; and 4) potential tumorigenicity with pluripotent stem cell derivatives (Figure 2).
Figure 2.
Barriers to clinical translation. A variety of stem cell types (e.g., adult somatic cells, induced pluripotent stem cells, and embryonic stem cells) are available for transplantation, each with their advantages and disadvantages. Adult somatic stem cells are most commonly transplanted directly into the recipient, whereas embryonic and induced pluripotent stem cells undergo in vitro differentiation prior to transplantation. Regardless of the stem cell type, their successful application in regenerative therapy faces similar clinical hurdles, including: 1) limited engraftment, survival, and proliferation; 2) poor differentiation, maturation and integration; 3) immunogenicity with allogeneic transplantation; and 4) potential tumorigenicity with pluripotent stem cell derivatives. Cell imaging plays a pivotal role in overcoming these hurdles and will help guide the translation of this promising therapy.
Limited Cell Engraftment, Survival and Proliferation
To achieve their maximum clinical benefit, stem cells and their derivatives must engraft, survive, and integrate into the target transplantation tissue. Because the local microenvironment into which cells are delivered will have a substantial impact on retention, survival, and function of these cells, determining the optimal site and timing of delivery is critical. For example, delivering stem cell-derived cardiomyocytes into the normal myocardium next to infarcted tissue might improve their survival, but if these cells are expected to replace dead cardiomyocytes, they will have to migrate into the infarcted tissue. Given that neither situation is ideal, achieving high engraftment and survival rates after transplantation continues to be challenging.
A key factor in determining initial engraftment and retention is the mode of cell delivery. In animal studies of cardiovascular disease using molecular imaging (Huang et al., 2010; Kraitchman et al., 2005; Templin et al., 2012), less than 5% of transplanted stem cells and their derivatives engrafted when delivered intravenously, regardless of cell type (i.e., mesenchymal stem cells (MSCs), induced pluripotent stem derived-cardiomyocytes (iPSC-CMs), embryonic stem cell derived-endothelial cells (ESC-ECs)) and the number of cells implanted. In a direct head-to-head study comparing cell delivery methods, myocardial retention was highest when peripheral blood mononuclear cells (PBMNCs) were injected intramyocardially compared to intracoronary or intravenous delivery (11±3% vs. 2.6±0.1% vs. 3.2±1%) (Hou et al., 2005). Regardless of the delivery route, however, the majority of stem cells and their derivatives (i.e., MSCs and ESC-ECs) were found in the pulmonary vasculature, spleen, liver, and microvasculature (Gyöngösii et al., 2010; Huang et al., 2010; Toma et al., 2009), but not near the area of injury. Because cell size may potentially contribute to significant egress to extra-cardiac tissues, delivering cells as part of a tissue scaffold or graft may improve myocardial retention (Kraehenbuehl et al., 2011; Seif-Naraghi et al., 2013), but has not yet been tested in cell imaging studies.
Some cells, however, do home to areas of injured myocardium, as shown in pre-clinical cell tracking studies using BLI and SPECT discussed below. Sheik et al. demonstrated greater homing of bone marrow mononuclear cells (BMMNCs) expressing firefly luciferase (FLuc) in murine hearts with ischemia/reperfusion compared to sham hearts within one week of delivery (Sheikh et al., 2007). Their findings were confirmed by histology and real-time PCR (for the male SryY gene from donor cells). Similarly, Kraitchman et al. showed a diffuse uptake of indium oxine (111In-oxine) radiolabelled MSCs at 4 to 7 days post-transplantation in infarcted canine hearts (Kraitchman et al., 2005). In a murine model of hindlimb ischemia, Huang et al. also showed that ESC-ECs injected into the femoral vein initially lodged in the pulmonary circulation, but eventually homed in on the ischemic limb (Huang et al., 2010).
More promising results have been found in models of neurovascular disease. In a study directly comparing intra-arterial vs. intravenous injection of neural stem cells (NSCs) in a murine model of hypoxia using BLI, Pendharker et al. found 61% of NSCs were retained in the brain immediately after intra-arterial injection, which declined only slightly to 55% by 7 days (Pendharkar et al., 2010). The BLI signal in the brain was ~12 times higher after intra-arterial injection compared to intravenous injection, where the predominant signal was in the torso. Similar to findings from models of myocardial infarction as discussed previously, homing to the area of injury has been noted in models of cerebral injury. Investigators have tracked ESCs labeled with superparamagnetic iron oxide nanoparticles (SPIONs) and neuroprogenitor cells transfected with FLuc as they move from their injection site in the contralateral hemisphere, across the corpus callosum, and to the injured hemisphere using BLI and MRI, respectively (Hoehn et al., 2002; Kim et al., 2004). Importantly, cells injected into sham mice did not show any evidence of migration.
Reminiscent of results from preclinical studies discussed previously, cell-tracking studies in humans demonstrate low cell retention rates, regardless of delivery method. SPECT and PET are by far the most frequently applied techniques in clinical bio-distribution studies. The majority of studies have been performed in patients for the treatment of myocardial infarction (Hofmann et al., 2005; Kang et al., 2005; Karpov et al., 2005). In one of the initial cell tracking studies, Karpov et al. reported that only 6.8% of autologous BMMNCs labeled with 18F-Fluorodeoxyglucose (18F-FDG) were retained in the heart at 2.5 hours after intracoronary cell infusion (Karpov et al., 2005). Hofmann et al. found higher retention rates with intracoronary delivery (2%) compared to coronary vein delivery (undetectable) at 1.5 hours after infusion of BMMCs (Hofmann et al., 2005). Interestingly, the subset of CD34+ bone marrow cells had a significantly higher retention rate of almost 25%. A modest improvement in retention was also achieved by inflating a balloon to temporarily stop blood flow. Using this modified technique, retention of BMMNCs 24 hours after intracoronary artery or coronary vein delivery increased slightly to 10.3% and 3.1%, respectively (Silva et al., 2009). A more recent study demonstrated that intramyocardial injection of CD34+ cells labeled with 99mTc-hexamethylpropylene amine oxime (99mTc-HMPAO) could significantly improve cell retention to 19% at 18 hours after cell delivery (Vrtovec et al., 2013). Unfortunately, the short half-life of radioactive tracers used in the aforementioned studies (i.e., 1.83 hours for 18F-FDG, 6 hours for 99mTc-HMPAO) did not allow a longer duration of tracking. Using radioactive 111In-oxine, which has a longer half-life of 2.8 days, Schächinger et al. found the number of retained cells dropped from 6.9% at 1 hour to 2% after 3-4 days (Schachinger et al., 2008).
In addition to initial cell engraftment, cell survival may be necessary to achieve maximal therapeutic benefit. Unfortunately, cell tracking studies using BLI in small animal models of myocardial infarction have demonstrated poor survival after transplantation of MSCs (van der Bogt et al., 2008), skeletal myoblasts (van der Bogt et al., 2008), BMMNCs (van der Bogt et al., 2008), adipose stromal cells (van der Bogt et al., 2008), cardiac resident stem cells (Li et al., 2009), ESC-ECs (Li et al., 2008; Li et al., 2007), and ESC-CMs (Cao et al., 2008). Similarly, in a large animal model of myocardial infarction, Gyöngösii et al. failed to detect the presence of porcine MSCs transfected with a PET reporter gene at 7 days after allotransplantation (Gyöngösii et al., 2008). Likewise, in murine models of peripheral vascular disease using BLI, the majority of MSCs (Hoffmann et al., 2010), ESC-ECs (Huang et al., 2010), and iPSC-ECs died shortly after transplantation (Rufaihah et al., 2011).
Interestingly, better long-term survival has been achieved in small animal models of brain injury using human NSCs (Guzman et al., 2007), human ESC-derived NSCs (Daadi et al., 2009), and bone marrow-derived MSCs (Sykova and Jendelova, 2007). In a MRI tracking study, Guzman et al. showed that an average of 51.3% (range: 38.9-74.6%) of human NSCs survived at 5 weeks after stereotactic injection in a rat model of distal middle cerebral artery occlusion, which was verified by histological staining for the human specific marker SC121 (Guzman et al., 2007). However, survival may be overestimated because of the engulfment of dead cells containing SPIONs by macrophages, leading to false-positive signal generation (Li et al., 2008; Terrovitis et al., 2008). To address this question, Daadi et al. labeled human NSCs with both SPIONs and the FLuc reporter gene and demonstrated stable SPIO and BLI signal up to 2 months post-transplantation (Daadi et al., 2009). Importantly, the in vitro cell dose-BLI relationship was maintained in vivo and correlated well with the SPIO dose relationship, suggesting that MRI of SPIONs might be a suitable method for cell tracking in stroke models. Taken together, these findings suggest that the environmental milieu of the central nervous system (CNS) may be less hostile to stem cell engraftment and survival than the cardiovascular system.
Not only does the host environment change depending on the organ transplanted, but it may also vary based on the time after injury. Shortly after myocardial infarction, for example, the host environment may be hostile to cell viability because of the presence of inflammatory cells that are recruited to repair the injured myocardium. As scar tissue forms in the myocardium, the injured area may be devoid of vasculature, which may also impair cell survival. Interestingly, a preliminary study by Swijnenburg et al. showed that timing of delivery actually has little impact on the overall survival of BMMNCs and subsequent change in left ventricular ejection fraction (LVEF) in both acute (<2 hours) and subacute (7 days) models of myocardial infarction (Swijnenburg et al., 2010). Additional imaging studies are needed to determine whether delivery of cells at later times (e.g., 2 to 4 weeks post myocardial infarction when the inflammatory response has subsided) may indeed improve cell engraftment.
Interestingly, timing of delivery has also been shown to affect cell differentiation in stroke models. Rosenblum et al. used the reporter genes FLuc and green fluorescent protein (GFP) to assess in vivo survival and ex vivo phenotypic differentiation after intra-arterial delivery of NSCs, respectively. Improved survival was noted three days after transplantation when levels of vascular adhesion molecules that promote cell homing were highest (Rosenblum et al., 2012). Moreover, early transplantation (i.e., 6 and 24 hours) led to greater differentiation into astrocytes than neurons, which were more predominant with later (7 and 14 days) transplantation times. Transplantation at 3 days post injury resulted in a more even distribution of astrocytes and immature neurons.
Long-term cell tracking in humans presents a greater challenge. The use of radioactive tracers with short half-lives that are more commonly used in clinical stem cell imaging studies is not suitable to assess cell migration due to a short tracking period and limited resolution. The persistence of SPIONs in labeled cells together with the high resolution and superior soft tissue contrast makes long-term clinical cell tracking with MRI feasible. De Vries et al. showed that SPIO-labeled dendritic cells injected into lymph nodes could be readily detected, in addition to their migration to draining lymph nodes two days after injection (de Vries et al., 2005). By co-labeling these cells with 111In-oxine for visualization by SPECT, the investigators also demonstrated that high spatial resolution for MRI is critical for successful transplantation. Because of its lower spatial resolution, SPECT did not accurately locate the lymph nodes in three patients, resulting in injection of dendritic cells into the surrounding fat. Moreover, SPECT provided limited visualization of migration to several draining lymph nodes, which were resolved by MRI. In a second study, Callera et al. used SPIO-labeled CD34+ cells injected into the spine of patients with spinal cord injury to assess their migratory capacity (Callera and de Melo, 2007). MRI at 20 and 35 days after cell delivery was able to detect injected cells and showed their migration towards the side of injury. MRI-based detection of cell migration has also been performed for NSCs injected into patients with brain trauma for up to 3 weeks after cell injection (Zhu et al., 2006). These and other examples demonstrate the ability of MRI for cell tracking (see Table 2).
Table 2.
Selected clinical studies for human cell transplantation
| First Author / Year | # of patients |
Disease | # of cells/ type | Delivery method |
Imaging modality |
Labeling technique |
Cell retention / survival [%] |
Study observations |
|---|---|---|---|---|---|---|---|---|
| Studies based on nuclear medicine techniques (SPECT, PET, GC) | ||||||||
| Hofmann et al (2005) | 9 | STEMI | 2-4.5×109 BMC |
ICA ICV+ICA |
PET |
18F-FDG BMC or 18F-FDG CD34+ fraction of BMC |
2 (ICA 1.5h) 3.8 (ICV+ICA 1.5h) 25 (ICA, CD34+ 1.5h |
Homing only to perfused area of delivery artery, no retention after ICV, higher retention for CD34+ cells |
| Karpov et al (2005) | 44 | Transmural MI |
9×107 BMMNC |
ICA | SPECT | 99mTc-HMPAO | 6.8 (2.5h) 3.2 (24h) |
No differences in cardiac function between control and treatment groups |
| Kang et al (2006) | 20 | STEMI | PBMNC | ICA ICV |
PET | 18F-FDG | 1.5 (ICA 2h) 0 (ICV 2h) |
Cell retention in old and new infarcts are not different |
| Correa et al (2007) | 1 | Ischemic stroke |
3×107 BMMNC |
OTW LCMA |
SPECT | 99mTc-HMPAO | n/a | Substantial amount of delivered cells in brain |
| Schächinger et al7 (2008) | 19 | Acute- chronic MI |
1.5×107 PBMNC |
OTW ICA | GC | 111In-oxine | 6.9 (1h) 2 (3-4 days) |
Reduced retention in chronic compared to acute MI |
| Silva et al (2009) | 30 | STEMI | 1×108 BMMNC |
OTW: ICA or ICV |
SPECT | 99mTc-HMPAO | 10.3 (ICA, 24h) 3.1 (ICV, 24h |
6 month LVEF improvement is correlated with cell retention and higher in ICA group |
| Barbosa da Fonseca et al (2010) | 6 | Ischemic stroke |
1-5×108 BMMNC |
MCA | SPECT WB GC |
99mTc | 1.7 (2h) | Uptake primarily in the hemisphere with the stroke lesion |
| Barbosa da Fonseca et al (2011) | 6 | Chagasic Cardio- myopathy |
1-10×108 BMMNC |
ICA | SPECT WB GC |
99mTc | 5.4 (1h), 4.3 (3h), 2.3 (24h) |
Homing not correlated with number of cells administered Poor uptake in areas with perfusion deficit |
| Vrtovec et al (2013) | 40 | DCM | 1×108 CD34+ from PBMNC |
ICA, IM |
GC | 99mTc-HMPAO | 4.4 (IC, 18h) 19.2 (IM, 18h) |
6 month LVEF improvement is correlated with cell retention and higher in IM group |
| Studies based on magnetic resonance imaging (MRI) | ||||||||
| Zhu et al (2006) | 2 | Brain trauma |
NPC | Stereo- tactical injection |
3T MRI | SPIO | n/a | Migration of cells from injection site to boarder of lesion, signal persistent for 7 weeks |
| Callera et al (2007) | 16 | Spinal cord injury |
0.7×106 CD34+ from PBMNC |
Lumbar puncture |
1T MRI | Antibody - SPIO |
n/a | Cell migration 35 days after injection, cells were not detected in 50% of patients |
STEMI: ST elevation myocardial infarction, BMC: bone marrow cells, ICA: intra-coronary artery infusion, ICV: intra-coronary vein infusion, PET: positron emission tomography, 18F-FDG: 18F-fluorodeoxyglucose, MI: myocardial infarction, BMMNC: bone marrow mononuclear cells, SPECT: single photon emission computed tomography, 99mTc-HMPAO: 99mTc-hexamethylpropyleneamine oxime, PBMNC: peripheral blood mononuclear cells, OTW: over the wire balloon infusion, LCMA: left cerebral middle artery, GC: gamma scintillation camera, LV-EF: left ventricular ejection fraction, MCA: middle cerebral artery, WB GC: whole body gamma scintillation camera, DCM: dilated cardiomyopathy, SPIO: superparamagnetic iron oxide nanoparticle, NPC: neuronal progenitor cells.
The advantages of MRI are high resolution, good contrast, and label persistence. However, it is difficult to estimate biodistribution, initial cell retention, or cell numbers with MRI. In addition, the persistence of SPIONs can also be a disadvantage because resident macrophages can take up the particles after delivered cells have died, leading to false positive signals for cell retention or survival (Li et al., 2008; Terrovitis et al., 2008). These potential confounding factors need to be considered when interpreting cell migration of magnetically labeled cells with MRI, particularly for long-term cell tracking. Interestingly, the ability of macrophages to take up free SPIONs can be used to label them in the human body and track their migration to sites of inflammation (Yilmaz et al., 2013). Combining radioactive labeling or reporter gene imaging with MRI tracking might be the best solution if initial cell retention and spatial localization have to be determined.
Although reporter gene imaging has become very widespread in preclinical studies as discussed previously, only one study has shown a proof-of-principle application for cytolytic T-cell therapy in humans (Yaghoubi et al., 2009). Viability of genetically modified T-cells expressing herpes simplex virus type 1 thymidine kinase (HSV1-tk) could be assessed a few days after cell delivery. This approach could track transplanted cells in the human body as long as they remain alive and would also be suitable to assess proliferative capacity of transplanted cells. Reporter gene imaging, however, requires genetic modification of transplanted cells, which increases the regulatory complexity for their approval and poses additional risks for mutagenesis. Furthermore, the repeated use of radioactive tracers would expose the patient to higher radiation doses. Nevertheless, the potential value of the information that could be gained and improved methods for genetic transformation may justify the use of this technique in the future.
Whether cellular proliferation after implantation can compensate for low levels of retention and survival remains unclear. Signal increase is frequently inferred as proliferation and often occurs early after transplantation of adult progenitor or differentiated cells prior to signal decline as cells die. Progressive signal increase has been reported after transplantation of undifferentiated ESCs and iPSCs (Cao et al., 2009; Cao et al., 2007b; Gutierrez-Aranda et al., 2010; Lee et al., 2009), corresponding to the development of a teratoma. Overreliance on this measure, however, may lead to the underestimation of true proliferation rates because replacement of dying cells is not accounted for. Direct imaging of cell proliferation requires the utilization of radiotracers involved in the thymidine salvage pathway of DNA synthesis (e.g., thymidine analogues), because thymidine contains the only pyridine or purine base that is unique to DNA. Direct imaging of in vivo stem cell proliferation has not yet been performed, but it has been reported in studies tracking tumor progression (Bading and Shields, 2008).
Poor Differentiation, Maturation, and Integration
It is hoped that once cells are delivered and engraftment takes place, they will differentiate, mature, and integrate into the target organ, resulting in tissue regeneration. However, in vivo differentiation of ESCs and iPSCs is hampered by the difficulty of directing differentiation towards the target cell type because the host tissue milieu may lack the complex array of signaling sequences required for directed differentiation and maturation (Cao et al., 2008), and hence resulting in the development of teratomas (Cao et al., 2009; Cao et al., 2007b; Gutierrez-Aranda et al., 2010; Lee et al., 2009). Due to the challenges of in vivo differentiation and the relative ease of in vitro differentiation where reagents can be added under controlled conditions, numerous protocols have emerged for in vitro cardiac (Burridge et al., 2012) and neural differentiation (Kim et al., 2009). Although robust differentiation to cardiac and neural cells is now feasible, generating specific cell subtypes can be more difficult (e.g., atrial vs. nodal vs. ventricular), highlighting the need for better understanding of the signaling pathways associated with cellular differentiation.
Most differentiated stem cell populations exhibit immature features, which may hamper their ability to treat disease. ESC-CMs and iPSC-CMs, for example, exhibit a neonatal form of gap junction distributions, which have slower conduction velocities (Chen et al., 2009). These gap junction connections are randomly distributed at the contact interfaces and do not align properly, making the action potentials across these interfaces less predictable and less homogeneous, which may lead to the development of arrhythmias after cell transplantation.
Because of the slow maturation of certain neuronal subtypes, generating mature functional neurons has also been challenging. Most interneuron precursors derived from human ESCs retain undifferentiated features and even those in more differentiated state still express neural precursor markers within 1 to 2 months after transplantation (Maroof et al., 2013). Development into functional GABAergic interneurons can take up to 7 months in vitro and after transplantation (Nicholas et al., 2013).
In addition to maturation, structural and functional (i.e., electrical and mechanical) integration within the target organ is needed for effective tissue regeneration. Preliminary studies based on ex vivo histopathological data indicate that transplanted stem cells do, at least partially, integrate into the heart and brain (Kehat et al., 2004; Shiba et al., 2012; Wernig et al., 2004). Kehat et al. demonstrated that transplanted human ESC-CMs successfully paced the hearts of swine with complete heart block (Kehat et al., 2004). Although electroanatomical mapping and subsequent histopathological analysis demonstrated that the focus of ventricular activation was the site of cell transplantation, it is possible that pacing resulted from the effects of the ESC-CMs on the host myocardium rather than the cells themselves. Further support for electrical-mechanical coupling was provided by Shiba et al, who showed that guinea pigs grafted with human ESC-CMs had improved mechanical function and lower incidence of arrhythmias (Shiba et al., 2012). By creating human ESC-CMs grafts that stably transfected a fluorescent calcium sensor, the authors showed that calcium fluorescent signal recorded in transplanted grafts synchronized 1:1 with systole (i.e., the phase of left ventricular contraction) on the electrocardiogram (Shiba et al., 2012).
Likewise, Wernig et al. showed that synapses form between the embryonic rat host and donor ESC-derived neuroprecursors in the brain, as supported by the expression of post-synaptic density 95 (PSD-95) of donor cell dendrites on ex vivo histopathological analysis and confirmed by ultra-structural and electrophysiological data (Wernig et al., 2004). However, not all of the transplanted neuronal cells expressed regionally appropriate transcription factors, which are important regulators of the brain’s regional specific activity.
Currently, evaluation of stem cell differentiation, maturation, and integration relies primarily on ex vivo histological examination and, thus, has been limited to animal studies as discussed previously. In vivo monitoring will enable investigators to potentially direct these processes to improve efficacy. Investigators reported an approach to image differentiation using a promoter of a differentiation-specific gene to drive the expression of an established reporter gene, the sodium/iodide symporter (NIS). Kang et al. created a transgenic mouse carrying a reporter gene construct driven by an alpha myosin heavy chain (MHC) promoter in transgenic mice. When exposed to radioactive iodine, the myocardium of transgenic mice showed rapid, increased uptake that was abolished by oral administration of potassium perchlorate, a NIS inhibitor (Kang et al., 2005). Using an alternative strategy, Xie et al. monitored changes in the expression of signal transducers and activators of transcription 3 (STAT3) using a 7-repeat STAT3 reporter construct driving Renilla Luciferase (RLuc) during the in vitro differentiation of mouse ESCs (Xie et al., 2009).
Immunogenicity Associated with Allogeneic Transplantation
As in solid organ transplantations, the immune system remains a formidable barrier to the clinical implementation of stem cell therapy. Poor cell survival after stem cell implantation is partly due to cellular rejection by the immune system (de Almeida et al., 2013; Pearl et al., 2012). Even pluripotent stem cells, which were once considered potentially immunoprivileged due to their lack of major histocompatibility complex (MHC) Class I, MHC Class II, and costimulatory molecules as well as their expression of immune-modulating molecules (Abdullah et al., 2007; Koch et al., 2008; Magliocca et al., 2006), have been found to elicit a donor-specific immune response when transplanted into immune competent mice (Drukker et al., 2006; Swijnenburg et al., 2008a). Although the discovery of iPSCs offered a potential solution to immune rejection (Takahashi et al., 2007; Yu et al., 2007), Zhao et al. reported that murine iPSCs were also rejected after transplantation into syngeneic recipients (Zhao et al., 2011). However, two recent studies by Araki et al. and Guha et al. have disputed that claim; the authors showed that transplantation of tissue grafts derived from iPSC-derived mice (e.g., skin and bone marrow) and terminally differentiated iPSC-derivatives (e.g., endothelial cells, hepatocytes, and neuronal cells), respectively, may be less immunogenic, reigniting hope that iPSCs can still be used to circumvent the immune system (Araki et al., 2013; Guha et al., 2013). Both of the aforementioned studies relied on ex vivo histopathological analysis.
Improvement in survival can be achieved by transplantation into a more immune-tolerant tissue environment. Because the blood-brain barrier separates the CNS from the systemic immune system, the brain is considered more immunoprivileged than other sites of the body, enabling the long-term survival of stem cells as discussed previously (Daadi et al., 2009; Guzman et al., 2007). An alternative strategy is to transplant cells into a more immune tolerant recipient host, which has been shown to be successful in the following small animal cell tracking studies. Using BLI, Swijnenburg et al. found poor survival of cells transplanted into immunocompetent mice compared to immuodeficient mice, with marginal improvement in survival achieved by administration of traditional immunosuppressive regimens (i.e., tacrolimus, sirolimus, and myocophenolate mofetil) (Swijnenburg et al., 2008b). In a follow-up study using BLI, improved engraftment and survival of ESCs, iPSCs, and their differentiated derivatives was achieved by the administration of co-stimulatory receptor blocking agents such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)-Ig, anti-CD40 ligand (anti-CD40L), and anti-lymphocyte function-associated antigen 1 (anti-LFA-1) (Huber et al., 2013; Pearl et al., 2011). More recently, a study by Rong et al. based on ex vivo histopathological data found that constitutive expression of CTL4-Ig and programmed death ligand-1 (PD-L1) in human ESCs were not rejected in humanized mice and showed reduced infiltration of human T-cells (Rong et al., 2014). Furthermore, infiltrating T-cells expressed high levels of immunosuppressive cytokines.
To avoid the potential side effects of immunosuppressants that can increase morbidity and mortality, studies using ex vivo histopathological analysis are currently evaluating encapsulation of MSCs into an artificial semi-permeable membrane as a means for evading the immune system for applications that do not require direct interaction of transplanted cells with the host tissue (Levit et al., 2013). In animal models of myocardial infarction, transplantation of encapsulated human MSCs has been shown to improve cell survival and efficacy, compared to implantation of non-encapsulated counterparts (Levit et al., 2013)
Direct monitoring of transplant cell rejection has not been evaluated, and is currently inferred indirectly by the progressive decline of labeled cells post transplantation. Christen et al. proposed a promising technique to directly image immune cells involved in transplant rejection, namely, macrophages, which release proteases and phagocytose dead or dying cells (Christen et al., 2009). Using a fluorescent protease sensor and a magnetofluorescent phagocytosis marker, the investigators created a 3D functional map showing higher phagocytosis activity during rejection on MRI and higher protease activity on tomographic FLI in mice receiving cardiac allografts than those receiving isografts. Strategies such as this will enhance the discovery of novel tolerance-inducing agents to improve survival of transplanted cells.
Potential Tumorigenicity Associated with Pluripotent Stem Cell Transplantation
The risk for tumor formation is low for adult somatic stem cells and progenitors, whose differentiation is largely limited to the different cell types of their tissue of origin. Given their better safety profile, somatic cells as well as progenitor cells are currently being evaluated in clinical trials. Nevertheless, in vivo cell tracking is still needed to monitor long-term safety after delivery of these cells, as demonstrated by a recent case report of a boy with ataxia telangiectasia who developed a glioneuronal brain tumor 4 years after intracerebellar and intrathecal injection of fetal neural stem cells (Amariglio et al., 2009).
Because they arise from pluripotent stem cells, ESC- and iPSC-derivatives may pose a greater risk for tumor development. These cells can malignantly transform, resulting in the formation of a teratoma, a tumor consisting of cells from all three germ layers. Alternatively, it is possible that a preparation of ESC or iPSC-derivatives could be contaminated with undifferentiated or incompletely differentiated cells that can proliferate and form a teratoma (Cao et al., 2007b; Gutierrez-Aranda et al., 2010; Lee et al., 2009).
In order to prevent tumor development, a pure cell product must be obtained prior to transplantation. Product purity can be achieved by depletion of undifferentiated cells (Ben-David et al., 2013a; Ben-David et al., 2013b; Tang et al., 2011) or enrichment of differentiated cells based on their expression of specific surface markers (Dubois et al., 2011), using either fluorescent activated cell sorting (FACS) or magnetic activated cell sorting (MACS), the latter being more amenable for high-throughput processing. Because 100% purity may be difficult to attain, it will be important to define a minimum threshold of undifferentiated cells that can be tolerated. Based on a previous study using BLI to track the growth of teratomas originating from ESCs transduced with a construct expressing FLuc and GFP in immunodeficient mice, it appears that a “pure” cell product cannot contain more than 104 undifferentiated cells, the threshold required to induce teratoma formation in the heart (Lee et al., 2009). Even lower thresholds may be required based on other cell tracking reports (Cao et al., 2007b).
The removal of potential tumorigenic cells, however, will not preclude the need for tumor surveillance by more traditional imaging techniques to identify abnormal growths (e.g., ultrasound, computed tomography, or MRI) or cellular activity (e.g., PET). Earlier detection may require the use of molecular imaging probes, such as the PET reporter probe 64Cu-radiolabeled cyclic Arg-Gly-ASP (RGD) peptides to image expression of αVβ3 integrins, which play an important role in angiogenesis and metastasis. Cao et al. demonstrated the superiority of this technique compared to more standard 18F-FDG and 18F-Fluorothymidine (18F-FLT) PET imaging for detecting teratoma formation following subcutaneous injection in mice (Cao et al., 2009). Alternatively, the PET reporter gene herpes simplex virus truncated thymidine kinase (HSV-ttk) can serve as both a monitor for tumor development and a suicide gene for ablative therapy (Cao et al., 2007a). Specifically, Cao et al. transplanted mice with ESCs labeled with either a double-fusion (GFP and FLuc) or a triple-fusion construct (GFP, RLuc, and HSV-ttk). BLI detected teratoma formation in both groups after 5 weeks, but only those teratomas expressing HSV-ttk were ablated by the administration of ganciclovir, an inhibitor of herpes viral DNA polymerase.
The Future of Stem Cell Imaging
Although significant insight has been gained from incorporating in vivo imaging in preclinical studies, its application in clinical trials remains underutilized and perhaps undervalued. Molecular imaging provides much needed “pharmacokinetic” information for the safe and effective administration of cellular therapies. For example, when examining a patient’s response to stem cell therapies in clinical trials, individual variation is significant (Alper, 2009; Bang et al., 2005; Bolli et al., 2011; Hare et al., 2012; Heldman et al., 2014; Kondziolka et al., 2005; Perin et al., 2012), with some patients in the therapy group achieving little or no improvement while some have significant functional recovery. Unfortunately, these pivotal trials did not incorporate cell tracking, leaving us with many unanswered questions. It is possible if not likely that individual patients might have different reactions to the therapy administered, and the transplanted cells did not engraft or survive in those that did not respond to therapy. As ESC- and iPSC-derivatives enter clinical trials (Garber, 2013), it will also be important to determine whether inadequate differentiation, maturation, and integration could also lead to poor functional outcome. Although strategies to monitor the latter processes are still in their infant stages and will need to be further explored in animal models, short-term tracking of engraftment and survival of cells directly labeled with imaging probes is currently feasible in humans and could be utilized in future stem cell trials (see Table 2).
At a minimum, molecular imaging should be incorporated into clinical phase II trials to generate a dose response curve to identify the optimal dose and dosing frequency. Depending on the efficacy readout, estimating dose response could be performed purely on the number of cells engrafted. In line with that, both preclinical and clinical studies using molecular imaging have found a considerable variability in the degree of cell retention, which was not correlated with the number of administered cells (Barbosa da Fonseca et al., 2011; Correa et al., 2007; Dedobbeleer et al., 2009; Schachinger et al., 2008). This makes dose response estimates more difficult because of the need for substantial group sizes. Despite the small number of patients enrolled, two recent clinical studies were able to find a positive correlation between early cell retention by cell imaging and late improvement in cardiac function six months after cell delivery (Silva et al., 2009; Vrtovec et al., 2013).
Molecular imaging can play a more extensive role by evaluating strategies to improve the viable cell concentration at the target site. As demonstrated in a growing number of preclinical studies but only a handful of clinical studies, cell imaging can help define the optimal cell type, delivery method (Hofmann et al., 2005; Vrtovec et al., 2013), timing of delivery (Rosenblum et al., 2012; Swijnenburg et al., 2010), and host microenvironment (Pearl et al., 2011; Swijnenburg et al., 2008b). Large-scale clinical studies incorporating molecular imaging are needed to determine which strategies will prove to be the most efficacious.
The other important role that cell imaging provides is to decrease toxicity to patients. The optimal cell dose must not only lead to maximal benefit, but it will also result in minimal risk of tumor formation, which is likely specific to the cell subtype and the injection site (Lee et al., 2009), and perhaps even specific to the patient. Molecular imaging can identify early transformation of cell grafts into tumors by imaging the proliferation and/or expression of tumor specific markers (Cao et al., 2009), which cannot be detected by traditional imaging techniques.
Further optimization of cellular therapy will require implementation of reporter gene imaging in humans, which will enable long-term assessment of survival and proliferation as well as differentiation, maturation, and integration. Adequate tumor surveillance will also require long-term imaging. Although the use of reporter gene imaging for long-term cell tracking in humans is currently restricted by regulatory hurdles, reservations about safety may be addressed by the use of human endogenous versions of reporter genes (e.g., human ferritin reporter for MRI (Campan et al., 2011) and human mitochondrial thymidine-kinase-2 for PET (Ponomarev et al., 2007). To avoid the problem of insertional mutagenesis, safety can be further enhanced by applying newer methods for genomic manipulation such as site-specific integration using phage integrases (Karow et al., 2011; Lan et al., 2012) and safe harbor mediated integration by zinc-finger nuclease (ZFN) (Ochiai et al., 2012; Wang et al., 2012), transcription activator-like effector nuclease (TALEN) (Sommer et al., 2014), or clustered regularly interspaced short palindromic repeats (CRISPR) (Yang et al., 2013).
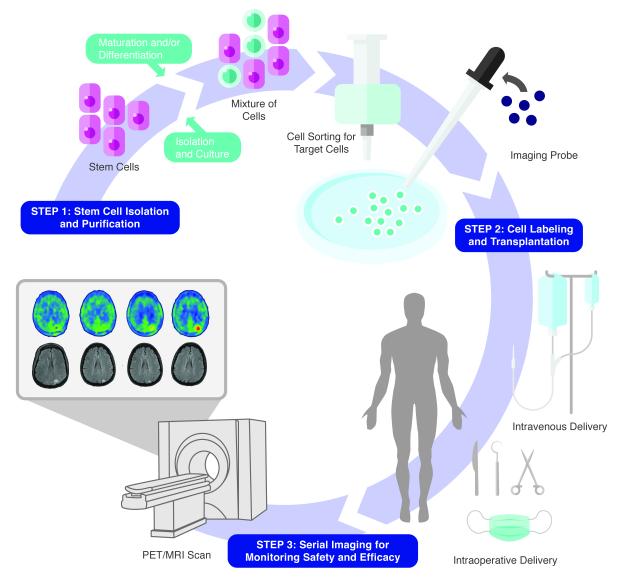
In the future, we predict that molecular imaging will be performed regularly throughout the course of treatment. From the time of delivery, patients will be monitored for safety and efficacy to identify patients at risk for tumor formation or those that may benefit from repeat transplantation, respectively (Figure 3). PET/MRI will likely emerge as the imaging modality of choice, given that it provides exquisite functional and anatomical detail with minimal radiation exposure. Although molecular imaging in humans is restricted to short-term monitoring of engraftment and survival, investigators are encouraged to use the tools at hand to guide the clinical translation of stem cell therapy. With rapid advancements in imaging technology, visualization of such intricate processes as stem cell differentiation and integration will become feasible as well as longer-term tracking, which will enable tracking cell fate beyond engraftment and survival. Finally, it is vital that we conduct more studies to evaluate the relationship between cell fate and functional improvement to confirm the efficacy of cell transplantation.
Figure 3.
A step-by-step approach to successful routine application of stem cell therapy. Step 1: Stem cell isolation and purification. Stem cells must be initially isolated and purified from a tissue source. Because of poor in vivo differentiation, cells are often differentiated in vitro prior to implantation. These techniques, however, are imperfect and can result in a mixture of undifferentiated and differentiated cells. Cell sorting is then required to select for the cell population that can be safely transplanted with maximal therapeutic benefit. Step 2: Cell labeling and transplantation. Prior to transplantation, cells are labeled to track cell fate. Selected cells are labeled in vitro using direct labeling or reporter gene techniques and then implanted at the bedside via intravenous delivery or in the operating room/angiography suite, via intra-lesional or intra-arterial delivery, respectively. Step 3: Serial imaging for safety and efficacy monitoring. After delivery, labeled cells are tracked using PET/MRI, which provides high sensitivity for cell detection as well as excellent spatial localization. Cells can be tracked serially to determine whether they engraft, survive, and proliferate in the target organ. Cells can also be monitored for any potential unwanted effects including tumor formation and migration to other sites. In addition, PET/MRI can provide serial data on the functional and structural recovery of damaged tissues.
Summary
Although significant progress has been made in the field of regenerative medicine, stem cell therapies are still in their infancy. Preclinical studies have helped identifying barriers to clinical translation, but additional studies must be performed to define strategies to overcome these hurdles. Disappointing results from clinical trials thus far suggest that we should perhaps reconsider fundamental strategies while continuing to broaden the application of stem cell imaging for future clinical trials. This could be achieved by refining and expanding the available technologies to enable long-term tracking of cell fate.
Acknowledgements
This work was supported in part by grants from the American Heart Association (PKN), Austrian Science Foundation (JR), NIH HL093172, NIH EB009689, NIH HL095571, CIRM TR3-05556, and CIRM DR2-05394 (JCW). We would like to give a special thanks to Christina Sicoli Design for creating the figures. We also thank Blake Wu and Ian Chen for assistance with manuscript preparation. Due to space limitation, we were unable to include all the important relevant papers in stem cell imaging. We apologize to the investigators who have made significant contributions to this field not discussed in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests None.
References
- Abdullah Z, Saric T, Kashkar H, Baschuk N, Yazdanpanah B, Fleischmann BK, Hescheler J, Kronke M, Utermohlen O. Serpin-6 expression protects embryonic stem cells from lysis by antigen-specific CTL. Journal of Immunology. 2007;178:3390–3399. doi: 10.4049/jimmunol.178.6.3390. [DOI] [PubMed] [Google Scholar]
- Alper J. Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol. 2009;27:213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Medicine. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonic A, Sena ES, Lees JS, Wills TE, Skeers P, Batchelor PE, Macleod MR, Howells DW. Stem cell transplantation in traumatic spinal cord injury: a systematic review and meta-analysis of animal studies. PLoS Biology. 2013;11:e1001738. doi: 10.1371/journal.pbio.1001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. Journal of Nuclear Medicine. 2008;49(Suppl 2):64S–80S. doi: 10.2967/jnumed.107.046391. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Annals of Neurology. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Barbosa da Fonseca LM, Xavier SS, Rosado de Castro PH, Lima RS, Gutfilen B, Goldenberg RC, Maiolino A, Chagas CL, Pedrosa RC, Campos de Carvalho AC. Biodistribution of bone marrow mononuclear cells in chronic chagasic cardiomyopathy after intracoronary injection. Int J Cardiol. 2011;149:310–314. doi: 10.1016/j.ijcard.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013a;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Ben-David U, Nudel N, Benvenisty N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nature Communications. 2013b;4:1992. doi: 10.1038/ncomms2992. [DOI] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem cells and Development. 2007;16:461–466. doi: 10.1089/scd.2007.0083. [DOI] [PubMed] [Google Scholar]
- Campan M, Lionetti V, Aquaro GD, Forini F, Matteucci M, Vannucci L, Chiuppesi F, Di Cristofano C, Faggioni M, Maioli M, et al. Ferritin as a reporter gene for in vivo tracking of stem cells by 1.5-T cardiac MRI in a rat model of myocardial infarction. American Journal of Physiology Heart and Circulatory Physiology. 2011;300:H2238–2250. doi: 10.1152/ajpheart.00935.2010. [DOI] [PubMed] [Google Scholar]
- Cao F, Drukker M, Lin S, Sheikh AY, Xie X, Li Z, Connolly AJ, Weissman IL, Wu JC. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning and Stem Cells. 2007a;9:107–117. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- Cao F, Li Z, Lee A, Liu Z, Chen K, Wang H, Cai W, Chen X, Wu JC. Noninvasive de novo imaging of human embryonic stem cell-derived teratoma formation. Cancer Research. 2009;69:2709–2713. doi: 10.1158/0008-5472.CAN-08-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, van der Bogt KE, Sadrzadeh A, Xie X, Sheikh AY, Wang H, Connolly AJ, Robbins RC, Wu JC. Spatial and temporal kinetics of teratoma formation from murine embryonic stem cell transplantation. Stem Cells and Development. 2007b;16:883–891. doi: 10.1089/scd.2007.0160. [DOI] [PubMed] [Google Scholar]
- Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, et al. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PloS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS, Kim C, Mercola M. Electrophysiological challenges of cell-based myocardial repair. Circulation. 2009;120:2496–2508. doi: 10.1161/CIRCULATIONAHA.107.751412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IY, Wu JC. Cardiovascular molecular imaging: focus on clinical translation. Circulation. 2011;123:425–443. doi: 10.1161/CIRCULATIONAHA.109.916338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen T, Nahrendorf M, Wildgruber M, Swirski FK, Aikawa E, Waterman P, Shimizu K, Weissleder R, Libby P. Molecular imaging of innate immune cell function in transplant rejection. Circulation. 2009;119:1925–1932. doi: 10.1161/CIRCULATIONAHA.108.796888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. The Cochrane database of Systematic Reviews. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- Correa PL, Mesquita CT, Felix RM, Azevedo JC, Barbirato GB, Falcao CH, Gonzalez C, Mendonca ML, Manfrim A, de Freitas G, et al. Assessment of intra-arterial injected autologous bone marrow mononuclear cell distribution by radioactive labeling in acute ischemic stroke. Clin Nucl Med. 2007;32:839–841. doi: 10.1097/RLU.0b013e318156b980. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, Wu JC, Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Molecular Therapy. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida PE, Ransohoff JD, Nahid A, Wu JC. Immunogenicity of pluripotent stem cells and their derivatives. Circulation Research. 2013;112:549–561. doi: 10.1161/CIRCRESAHA.111.249243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- Dedobbeleer C, Blocklet D, Toungouz M, Lambermont M, Unger P, Degaute JP, Goldman S, Berkenboom G. Myocardial homing and coronary endothelial function after autologous blood CD34+ progenitor cells intracoronary injection in the chronic phase of myocardial infarction. J Cardiovasc Pharmacol. 2009;53:480–485. doi: 10.1097/FJC.0b013e3181a7b572. [DOI] [PubMed] [Google Scholar]
- Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- Garber K. Inducing translation. Nat Biotechnol. 2013;31:483–486. doi: 10.1038/nbt.2602. [DOI] [PubMed] [Google Scholar]
- Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12:407–412. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Macia A, Sanchez L, Ligero G, Garcia-Parez JL, Menendez P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D, Capela A, Greve J, Malenka RC, Moseley ME, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyöngösii M, Blanco J, Marian T, Tron L, Petnehazy O, Petrasi Z, Hemetsberger R, Rodriguez J, Font G, Pavo IJ, et al. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circulation Cardiovascular imaging. 2008;1:94–103. doi: 10.1161/CIRCIMAGING.108.797449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyöngösii M, Hemetsberger R, Wolbank S, Kaun C, Posa A, Marian T, Balkay L, Emri M, Galuska L, Mikecz P, et al. Imaging the migration of therapeutically delivered cardiac stem cells. JACC Cardiovascular Imaging. 2010;3:772–775. doi: 10.1016/j.jcmg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Focking M, Arnold H, Hescheler J, Fleischmann BK, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J, Glassford AJ, Doyle TC, Robbins RC, Schrepfer S, Pelletier MP. Angiogenic effects despite limited cell survival of bone marrow-derived mesenchymal stem cells under ischemia. The Thoracic and Cardiovascular Surgeon. 2010;58:136–142. doi: 10.1055/s-0029-1240758. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, et al. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber BC, Ransohoff JD, Ransohoff KJ, Riegler J, Ebert A, Kodo K, Gong Y, Sanchez-Freire V, Dey D, Kooreman NG, et al. Costimulation-adhesion blockade is superior to cyclosporine A and prednisone immunosuppressive therapy for preventing rejection of differentiated human embryonic stem cells following transplantation. Stem Cells. 2013;31:2354–2363. doi: 10.1002/stem.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Lee DS, Paeng JC, Lee JS, Kim YH, Lee YJ, Hwang DW, Jeong JM, Lim SM, Chung JK, et al. Development of a sodium/iodide symporter (NIS)-transgenic mouse for imaging of cardiomyocyte-specific reporter gene expression. Journal of Nuclear Medicine. 2005;46:479–483. [PubMed] [Google Scholar]
- Karow M, Chavez CL, Farruggio AP, Geisinger JM, Keravala A, Jung WE, Lan F, Wu JC, Chen-Tsai Y, Calos MP. Site-specific recombinase strategy to create induced pluripotent stem cells efficiently with plasmid DNA. Stem Cells. 2011;29:1696–1704. doi: 10.1002/stem.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpov RS, Popov SV, Markov VA, Suslova TE, Ryabov VV, Poponina YS, Krylov AL, Sazonova SV. Autologous mononuclear bone marrow cells during reparative regeneratrion after acute myocardial infarction. Bull Exp Biol Med. 2005;140:640–643. doi: 10.1007/s10517-006-0043-1. [DOI] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke. 2004;35:952–957. doi: 10.1161/01.STR.0000120308.21946.5D. [DOI] [PubMed] [Google Scholar]
- Kim JB, Greber B, Arauzo-Bravo MJ, Meyer J, Park KI, Zaehres H, Scholer HR. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–643. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26:89–98. doi: 10.1634/stemcells.2007-0151. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonte R, Lebowitz J, et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. Journal of Neurosurgery. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- Kraehenbuehl TP, Ferreira LS, Hayward AM, Nahrendorf M, van der Vlies AJ, Vasile E, Weissleder R, Langer R, Hubbell JA. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2011;32:1102–1109. doi: 10.1016/j.biomaterials.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Liu J, Narsinh KH, Hu S, Han L, Lee AS, Karow M, Nguyen PK, Nag D, Calos MP, et al. Safe genetic modification of cardiac stem cells using a site-specific integration technique. Circulation. 2012;126:S20–28. doi: 10.1161/CIRCULATIONAHA.111.084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, Connolly AJ, Robbins RC, Wu JC. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, collaborators, S. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- Lees JS, Sena ES, Egan KJ, Antonic A, Koblar SA, Howells DW, Macleod MR. Stem cell-based therapy for experimental stroke: a systematic review and meta-analysis. International Journal of Stroke. 2012;7:582–588. doi: 10.1111/j.1747-4949.2012.00797.x. [DOI] [PubMed] [Google Scholar]
- Levit RD, Landazuri N, Phelps EA, Brown ME, Garcia AJ, Davis ME, Joseph G, Long R, Safley SA, Suever JD, et al. Cellular encapsulation enhances cardiac repair. Journal of the American Heart Association. 2013;2:e000367. doi: 10.1161/JAHA.113.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, et al. Imaging survival and function of transplanted cardiac resident stem cells. Journal of the American College of Cardiology. 2009;53:1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Suzuki Y, Huang M, Cao F, Xie X, Connolly AJ, Yang PC, Wu JC. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26:864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, et al. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliocca JF, Held IK, Odorico JS. Undifferentiated murine embryonic stem cells cannot induce portal tolerance but may possess immune privilege secondary to reduced major histocompatibility complex antigen expression. Stem Cells and Development. 2006;15:707–717. doi: 10.1089/scd.2006.15.707. [DOI] [PubMed] [Google Scholar]
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes & Development. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. The Journal of Clinical Investigation. 2012;122:3824–3834. doi: 10.1172/JCI64124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PK, Lan F, Wang Y, Wu JC. Imaging: guiding the clinical translation of cardiac stem cell therapy. Circulation Research. 2011;109:962–979. doi: 10.1161/CIRCRESAHA.111.242909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H, Sakamoto N, Fujita K, Nishikawa M, Suzuki K, Matsuura S, Miyamoto T, Sakuma T, Shibata T, Yamamoto T. Zinc-finger nuclease-mediated targeted insertion of reporter genes for quantitative imaging of gene expression in sea urchin embryos. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10915–10920. doi: 10.1073/pnas.1202768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl JI, Kean LS, Davis MM, Wu JC. Pluripotent stem cells: immune to the immune system? Science Translational Medicine. 2012;4:164ps125. doi: 10.1126/scitranslmed.3005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl JI, Lee AS, Leveson-Gower DB, Sun N, Ghosh Z, Lan F, Ransohoff J, Negrin RS, Davis MM, Wu JC. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8:309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, De A, Choi R, Chen S, Rutt BK, et al. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke. 2010;41:2064–2070. doi: 10.1161/STROKEAHA.109.575993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev V, Doubrovin M, Shavrin A, Serganova I, Beresten T, Ageyeva L, Cai C, Balatoni J, Alauddin M, Gelovani J. A human-derived reporter gene for noninvasive imaging in humans: mitochondrial thymidine kinase type 2. Journal of Nuclear Medicine. 2007;48:819–826. doi: 10.2967/jnumed.106.036962. [DOI] [PubMed] [Google Scholar]
- Poole J, Mavromatis K, Binongo JN, Khan A, Li Q, Khayata M, Rocco E, Topel M, Zhang X, Brown C, et al. Effect of progenitor cell mobilization with granulocyte-macrophage colony-stimulating factor in patients with peripheral artery disease: a randomized clinical trial. JAMA. 2013;310:2631–2639. doi: 10.1001/jama.2013.282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z, Wang M, Hu Z, Stradner M, Zhu S, Kong H, Yi H, Goldrath A, Yang YG, Xu Y, et al. An Effective Approach to Prevent Immune Rejection of Human ESC-Derived Allografts. Cell Stem Cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum S, Wang N, Smith TN, Pendharkar AV, Chua JY, Birk H, Guzman R. Timing of intra-arterial neural stem cell transplantation after hypoxia-ischemia influences cell engraftment, survival, and differentiation. Stroke. 2012;43:1624–1631. doi: 10.1161/STROKEAHA.111.637884. [DOI] [PubMed] [Google Scholar]
- Rufaihah AJ, Huang NF, Jame S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachinger V, Aicher A, Dobert N, Rover R, Diener J, Fichtlscherer S, Assmus B, Seeger FH, Menzel C, Brenner W, et al. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118:1425–1432. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Science translational medicine. 2013;5:173ra125. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, Chang CP, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SA, Sousa AL, Haddad AF, Azevedo JC, Soares VE, Peixoto CM, Soares AJ, Issa AF, Felipe LR, Branco RV, et al. Autologous bone-marrow mononuclear cell transplantation after acute myocardial infarction: comparison of two delivery techniques. Cell Transplantation. 2009;18:343–352. doi: 10.3727/096368909788534951. [DOI] [PubMed] [Google Scholar]
- Sommer D, Peters A, Wirtz T, Mai M, Ackermann J, Thabet Y, Schmidt J, Weighardt H, Wunderlich FT, Degen J, et al. Efficient genome engineering by targeted homologous recombination in mouse embryos using transcription activator-like effector nucleases. Nature Communications. 2014;5:3045. doi: 10.1038/ncomms4045. [DOI] [PubMed] [Google Scholar]
- Swijnenburg RJ, Govaert JA, van der Bogt KE, Pearl JI, Huang M, Stein W, Hoyt G, Vogel H, Contag CH, Robbins RC, et al. Timing of bone marrow cell delivery has minimal effects on cell viability and cardiac recovery after myocardial infarction. Circulation Cardiovascular Imaging. 2010;3:77–85. doi: 10.1161/CIRCIMAGING.109.872085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swijnenburg RJ, Schrepfer S, Cao F, Pearl JI, Xie X, Connolly AJ, Robbins RC, Wu JC. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells and Development. 2008a;17:1023–1029. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proceedings of the National Academy of Sciences of the United States of America. 2008b;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Progress in Brain Research. 2007;161:367–383. doi: 10.1016/S0079-6123(06)61026-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, Muller E, Kuest SM, Cohrs S, Schibli R, et al. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation. 2012;126:430–439. doi: 10.1161/CIRCULATIONAHA.111.087684. [DOI] [PubMed] [Google Scholar]
- Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schar M, Gerstenblith G, Weiss RG, Marban E, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circulation research. 2009;104:398–402. doi: 10.1161/CIRCRESAHA.108.187724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurology. 2011;10:649–656. doi: 10.1016/S1474-4422(11)70121-1. [DOI] [PubMed] [Google Scholar]
- van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg RJ, Pearl J, Lee A, Fischbein M, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–129. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, van Belle E, Gyongyosi M, Sluijter JP, Cramer MJ, Doevendans PA, Chamuleau SA. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovascular Research. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, Cernelc P, Torre-Amione G, Haddad F, Wu JC. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128:S42–49. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang WY, Hu S, Lan F, Lee AS, Huber B, Lisowski L, Liang P, Huang M, de Almeida PE, et al. Genome editing of human embryonic stem cells and induced pluripotent stem cells with zinc finger nucleases for cellular imaging. Circulation Research. 2012;111:1494–1503. doi: 10.1161/CIRCRESAHA.112.274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Benninger F, Schmandt T, Rade M, Tucker KL, Bussow H, Beck H, Brustle O. Functional integration of embryonic stem cell-derived neurons in vivo. The Journal of Neuroscience. 2004;24:5258–5268. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Chan KS, Cao F, Huang M, Li Z, Lee A, Weissman IL, Wu JC. Imaging of STAT3 signaling pathway during mouse embryonic stem cell differentiation. Stem Cells and Development. 2009;18:205–214. doi: 10.1089/scd.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, Gambhir SS. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Rosch S, Klingel K, Kandolf R, Helluy X, Hiller KH, Jakob PM, Sechtem U. Magnetic resonance imaging (MRI) of inflamed myocardium using iron oxide nanoparticles in patients with acute myocardial infarction - preliminary results. Int J Cardiol. 2013;163:175–182. doi: 10.1016/j.ijcard.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Yu DX, Marchetto MC, Gage FH. Therapeutic translation of iPSCs for treating neurological disease. Cell Stem Cell. 2013;12:678–688. doi: 10.1016/j.stem.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]