Abstract
The myeloid C-type lectin receptor Dectin-2 directs the generation of Th2 and Th17 immune responses to the house dust mite Dermatophagoides farinae (Df) through the generation of cysteinyl leukotrienes (cys-LTs) and pro-inflammatory cytokines, respectively, but a role for Dectin-2 in effector phase responses has not been described. Here, we demonstrate that administration of the Dectin-2 mAb solely at the time of Df challenge abrogated eosinophilic and neutrophilic inflammation in the bronchoalveolar lavage (BAL) fluid and Th1, Th2, and Th17 inflammation in the lung of previously sensitized mice. Furthermore, Dectin-2 null mice (Clec4n−/−) sensitized with the adoptive transfer of Df-pulsed wild-type (WT) bone marrow-derived DCs (BMDCs) also had less Df-elicited pulmonary inflammation, supporting an effector function for Dectin-2. The protection from pulmonary inflammation seen with the Dectin-2 mAb or in Clec4n−/− mice was associated with little or no reduction in lung-draining lymph node cells or their cytokine production, and with no reduction in serum IgE. WT and Clec4n−/− mice recipients, sensitized with Df-pulsed WT BMDCs, had comparable levels of Df-elicited IL-6, IL-23, TNF-α, and cys-LTs in the lung. By contrast, Df-elicited CCL4 and CCL8 production from pulmonary CD11c+CD11b+Ly6C+ and CD11c+CD11b+Ly6C−CD64+ monocyte-derived DCs was reduced in Clec4n−/− recipients. Addition of CCL8 at the time of Df challenge abrogated the protection from eosinophilic, neutrophilic, and Th2 pulmonary inflammation seen in Clec4n−/− recipients. Taken together, these results reveal that Dectin-2 regulates monocyte-derived DC function in the pulmonary microenvironment at Df challenge to promote the local inflammatory response.
Keywords: Knockout mice, Dendritic Cells, Lipid mediators, Allergy, Inflammation
Introduction
House dust mite (HDM) is the most common allergen worldwide, and sensitization to HDM is present in almost half of U.S. asthmatics (1). While HDM exposure increases the risk of developing asthma in a dose-dependent fashion (2, 3), suggesting a causal role in asthma pathogenesis, the mechanism(s) by which HDM is so immunogenic is only recently being uncovered. In mouse models, HDM can promote Th2-dependent pulmonary inflammation through the specific activation of pattern recognition receptors (4-8). The HDM species Dermatophagoides pteronyssinus (Dp) can activate lung stromal cell TLR4 signaling to drive LPS-dependent pulmonary eosinophilia and serum IgE (4). This activation is, in part, due to structural homology between the Dp-derived protein, Der p 2, and MD-2, the LPS-binding portion of the TLR signaling complex that is critical to facilitate signaling in epithelial cells (5). TLR5, a receptor for the bacterial protein flagellin, also promotes eosinophilic pulmonary inflammation and impairment of airflow to HDM in mice, with contributions from both the hematopoietic and structural compartments (7).
Carbohydrate structures in HDM can also trigger dendritic cell- (DC)-mediated Th2 immune responses through interactions with members of the C-type lectin receptor (CLR) family. The mannose receptor binds to the immunodominant proteins Der p 1 and Der p 2 and inhibition of its signaling by siRNA in human monocyte-derived DCs (hMDDCs) reduces allergen uptake and inhibits Th2 cell development in DC-T cell cocultures (9). Moreover, mannose receptor binding and endocytosis of Der p is enhanced in allergic individuals, suggesting a role in disease pathogenesis (10). Der p 1 and Der p 2 bind to Dendritic Cell-specific Intercellular Adhesion Molecule-3-grabbing Non-integrin (DC-SIGN), but inhibition of DC-SIGN in hMDDCs has variably led to enhancement or inhibition of Th2 cell development (11, 12), indicating that HDM-dependent Th2 immunity might be finely regulated by multiple carbohydrate receptors.
The CLR Dectin-2 recognizes high-mannose ligands found in diverse microbes, including Candida albicans (13), Malassesia furfur (14), and Schistosoma mansoni (15), and we have previously demonstrated that Dectin-2 also recognizes HDM (16). HDM-induced activation of Dectin-2 in DCs triggers the generation of cysteinyl leukotrienes (cys-LTs), proinflammatory lipid mediators, through an FcRγ-, Syk-, and leukotriene C4 synthase-dependent pathway (16). Activation of Dectin-2 by HDM and by other ligands additionally triggers Syk- and CARD9-dependent activation of NFκB and production of pro-inflammatory cytokines IL-1β, IL-6, IL-23, and TNF-α (13, 17-19). Dectin-2 signaling in DCs instructs Th2 and Th17 immunity. Dectin-2 null mice (Clec4n−/−) or mice treated with a Dectin-2 blocking antibody have impaired Th17 immunity to C. albicans (13, 17), and knockdown of Dectin-2 in human DCs similarly impairs in vitro Th17 development in response to C. albicans (20). Dectin-2 knockdown in murine bone marrow-derived dendritic cells (BMDCs) attenuates their capacity to sensitize wild-type (WT) recipients for the generation of Th2 pulmonary inflammation and lymph node expansion after Df challenge (6). Moreover, administration of the Dectin-2 blocking antibody solely during sensitization, similarly attenuates eosinophilic pulmonary inflammation and airways hyperresponsiveness induced by HDM (21). Although Dectin-2 has an established role in sensitization, the influence of this receptor in regulating systemic immunity and tissue inflammation in effector phase responses is unknown. As Dectin-2 mediates DC production of many pro-inflammatory mediators, we sought to determine whether Dectin-2 signaling could promote inflammation in the effector phase of HDM-elicited pulmonary immune responses, in addition to its established role in sensitization.
Here we show that antibody-mediated blockade of Dectin-2 in the challenge phase reduced Df –elicited bronchoalveolar lavage (BAL) inflammation and T cell recruitment to the lungs of actively sensitized mice. Furthermore, Df-elicited BAL inflammation and T cell recruitment to the lung was similarly reduced in Clec4n−/− mice sensitized by the adoptive transfer of WT Df-pulsed DCs. The attenuated pulmonary inflammation in Clec4n−/− mice was associated with a reduction in monocyte-derived DC (MDDC) generation of CCL4 and CCL8 in the lung and was reversed with addition of CCL8 to the airway at the time of Df challenge. In contrast, cell numbers and Th2 cell cytokine production from the lung-draining lymph nodes showed little or no reduction, and the serum levels of IgE and specific IgG1 were unchanged in Clec4n−/− mice. These findings indicate that Dectin-2 exerts a dominant effect on pulmonary MDDCs at challenge which is independent from its role in sensitization. Furthermore they suggest that innate inflammatory pathways induced simultaneously with classical Th2 immunity can contribute significantly to allergic pulmonary inflammation elicited by native allergens.
Materals and Methods
Clec4n−/− mice
Clec4n−/− mice (13) were backcrossed to C57BL/6 for 8 generations, and to the C57BL/6 substrain (Charles River Laboratories) for an additional three generations. Clec4n−/−, WT C57BL/6, and WT Balb/c mice were used at 12-16 weeks age. All animal studies were approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute.
Df sensitization and challenge protocols
Df extract (Greer Laboratories, Lenoir, NC) contained 0.2 mg Der f1 and 6,136 EU endotoxin per mg protein weight. For direct sensitization and challenge experiments, WT Balb/c mice received saline or 1 μg of Df on days 0 and 1, and 1 μg Df on days 14 and 15 by intranasal injection. Two days after the last injection, mice were killed by i.p. injection of pentobarbital. For blocking experiments, 200 μg Dectin-2 antibody (clone D2.11E4, Thermo Scientific Pierce, Rockford, IL) or a rat IgG2a control (BioLegend, San Diego, CA) was injected intraperitoneally on day 13 and day 15, 12 h and 6 h prior to each Df challenge, respectively. For sensitization by adoptive transfer of Df-pulsed BMDCs into naïve mice, the protocol was carried out as described (6). In brief, bone marrow cells from C57BL/6 mice were harvested and cultured in RPMI medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 50 μM 2-ME, and recombinant mouse GM-CSF, as described (22). Day 7 BMDCs were pulsed with either PBS or 50 μg/ml of Df at a concentration of 1 × 106 cells/ml in a 35-mm culture dish (Sumilon Celltight X, Sumitomo Bakelite, Japan) for 24 h. The next day, the BMDCs were washed twice with PBS and resuspended in PBS. 1 × 104 cells in 20 μl were transferred intranasally to C57BL/6 Clec4n−/− and WT littermate recipients. The cells were routinely greater than 85% CD11c+. At days 12 and 14 after DC transfer, recipient mice were challenged with 1 μg Df intranasally. 2 d after the last challenge, mice were killed by i.p. injection of pentobarbital. For CCL8 reconstitution experiments, 5 μg CCL8 (Biolegend) was given by intranasal injection on day 12 and 14, 30 min prior to Df.
BAL fluid cell analysis
2 d after the last intranasal injection, the trachea was cannulated and BAL fluid was obtained by three repeated lavages with 0.75 ml of Ca2+- and Mg2+-free PBS with 1 mM EDTA. The BAL fluid was centrifuged at 500 × g for 5 min. Cells were resuspended in 0.2 ml of PBS with 1% FCS, and the total cells were counted manually with a hemocytometer. For the differential cell counts of macrophages, neutrophils, eosinophils, and lymphocytes, the cells were cytospun onto a glass slide and stained with Diff-Quik, and cell types in a total of 200 cells were identified by morphologic criteria.
Intracellular cytokine staining of pulmonary mononuclear cells
For pulmonary mononuclear cell assessment, the lungs were homogenized with sterile scalpels, and digested for 30 min with 500 U/ml type IV collagenase (Worthington, Lakewood, NJ) and 20 μg/ml DNase I at 37°C. Mononculear cells were separated on a Nycoprep gradient (Cosmo Bio USA, Carlsbad, CA) at 600 × g for 20 min at 4°C; resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM non-essential amino acids, 43 μM 2-ME, 2 mM l-glutamine, 25 mM Hepes buffer, and 1 mM sodium pyruvate; plated at 8 × 106 cells/ml in 24-well plate; and stimulated with 50 ng/ml PMA and 1 μM ionomycin for 2 h at 37°C. 2.5 μM monensin was added and the cells were incubated for an additional 4 h. Cells were then treated with DNase I at 60 μg/ml for 15 min at 37°C; fixed and permeabilized according to the manufacturer's protocol (eBioscience, San Diego, CA); blocked with 1% mouse IgG (Sigma) and 1% anti-mouse CD16/CD32 (BD Biosciences, San Jose, CA) for 20 min; and then stained with anti-mouse CD4-APC (clone RM4-5, BD Biosciences), anti-mouse CD8α-APC-Cy7(clone 53-6.7, BD Biosciences), anti-mouse IL-4-PE (clone 11B11, BD Biosciences), anti-mouse IL-5-PE (clone TRFK5, BD Biosciences), anti-mouse IL-17A-PE (clone TC11-18H10, BD Bioscienes), anti-mouse IFN-γ-PE (clone XMG1.2, eBioscience), or rat IgG1-PE isotype control Ab (BD Biosciences) at a concentration of 0.2 μg/0.1 ml/1 × 106 cells. Analyses were performed on a FACSCanto flow cytometer, and data were analyzed with the FlowJo 7.5.
Cytokine production by peribronchial lymph node cells after ex vivo Df restimulation
2 d after the last intranasal injection, three peribronchial lymph node cells were excised from each mouse and homogenized. The cell suspensions were filtered through a 70-μm cell strainer, centrifuged at 300 × g for 5 min at room temperature, and resuspended in RPMI1640 medium containing heat-inactivated 10% FCS. After the total number of cells was counted for each mouse, cells were cultured at 4 × 106 cells/ml in the presence of 5 μg/ml Df (Balb/c) or 20 μg/ml Df (C57BL/6) for 72 h (23-25). The concentrations of IL-4, IL-5, IL-13, IL-17A, and IFN-γ in the supernatants were measured with ELISA kits (eBioscience).
Measurement of total IgE and Df-specific IgG1
Sera were collected by cardiac puncture 2 d after the last intranasal injection. Total IgE was determined with an ELISA kit (BD Biosciences). Df-specific IgG1 was measured as described (26). Briefly, 96-well plates were coated with a 5 μg/ml solution of Df and incubated with diluted serum followed by alkaline phosphatase-conjugated anti-mouse IgG1 (SouthernBiotech, Birmingham, AL) and p-nitrophenyl phosphate substrate (Sigma-Aldrich, St. Louis, MO).
Histology
The lung tissues were excised, fixed, and stained as described previously (27). For general morphology, tissue sections were stained by chloroacetate esterase (CAE) reaction and counterstained with hematoxylin. Congo red staining was used to identify eosinophils, and periodic acid-Schiff (PAS) staining was used to assess mucus and goblet cells. The slides were analyzed with a Leica DM LB2 microscope (Leica Microsystems, Germany). The pictures were taken by a Nikon digital camera DXM 1200 with Nikon ACT-1 (version 2.70) image acquisition software.
Flow Cytometry, Sorting, and Culture of Dectin-2-expressing populations
To assess Dectin-2 expression on lung cells, the lungs were perfused with PBS + 1 mM EDTA, homogenized with sterile scalpels, and digested in a shaker for 40 min with 650 U/ml type IV collagenase and 0.02 mg/ml DNase I at 37°C. Single cell suspensions were blocked with 10% donkey serum (Jackson Immunoresearch, West Grove, PA), and serially stained with rat anti-Dectin-2 (AbCAM, Cambridge, MA) or rat IgG2a isotype control Ab (BD Biosciences) at 1 μg/0.2 ml/106 cells, donkey anti-rat IgG-APC (Jackson Immunoresearch) at 1:100 dilution, and then a tertiary antibody. Combinations of CD11c-Ultraviolet (clone N418), CD11b-PECy7 (clone M1-70), CD103-PE (clone 2E7), Ly6C-APCCy7 (clone HK1.4), and CD64-PE (clone X54-5/7.1) were used (Biolegend). Propidium iodide or Fixable Viability Dye eFluor780 (eBioscience) was used to exclude dead cells. Analyses were performed on a FACSCanto II flow cytometer (BD Biosciences), and data were analyzed with FlowJo 7.5. Lung cells were sorted on a FACS Aria IIu (BD Biosciences) and cultured for 24 h with 5 ng/ml GM-CSF. CCL-4 and CCL-8 in culture supernatants were measured by ELISA (R&D, Minneapolis, MN).
Measurement of cytokine mRNA and protein expression in the lung
Total RNA was isolated from the right lungs with TRIzol reagent and cDNA was generated using the first-strand kit (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. Quantities of IL-6, IL-10, IL-23, TNF-α, CCL2, CCL4, CCL8, CCL12, CCL17, CCL22, CXCL1 and CXCL2 were measured relative to GAPDH using the Mx3005P Real-Time PCR System (Agilent Technologies, Santa Clara, CA) with gene-specific primers (SA Biosciences, Valencia, CA). Protein levels of chemokines were assessed by ELISA (R&D) on lung homogenates.
BAL fluid cys-LT measurement
24 h after the last intranasal injection, the trachea was cannulated and BAL fluid was obtained by three repeated lavages with 0.75 ml of Ca2+- and Mg2+-free PBS with 1% BSA. The BAL fluid was centrifuged at 500 × g for 5 min and the supernatant was mixed with 5 volumes methanol on ice for 30 min, and spun at 3,000 × g. The methanolic supernatant was dried on a rotovac, resuspended in a 2:1 mixture of prostaglandin B2 (PGB2)/methanol (400 ng/mL) and HEPES buffer (50mM) and reverse phase-HPLC for cys-LT separation was performed as described (28). Briefly, samples were applied to a C18 Ultrasphere reverse phase column (Restek, Bellefonte, PA) equilibrated with a solvent of methanol/acetonitrile/water/acetic acid (10:15:100:0.2, v/v), pH 5.6 (Solvent A). After injection of the sample, the column was eluted at a flow rate of 1 ml/min with a programmed concave gradient to 55% of the equilibrated Solvent A and 45% of Solvent B (100% methanol) over 2.5 min. After 5 min, Solvent B was increased linearly to 75% over 15 min. The retention times for PGB2, LTC4, LTD4, and LTE4 were 20.8, 21.7, 23.5, and 24.2 min, respectively. Individual HPLC fractions containing LTC4, LTD4, and LTE4 were collected at 54, 162, and 204 seconds after the peak of PGB2, respectively, dried, and reconstituted in assay buffer. Cys-LT quantification was performed by ELISA, per the manufacturer's protocol (GE Healthcare Life Sciences, Pittsburgh, PA).
Statistical analysis
Results were expressed as means ± SEM. Student's unpaired, two-tailed t test was used for the statistical analysis. A value of p < 0.05 was considered significant.
Results
Dectin-2 Antibody Inhibits Df-elicited Pulmonary Inflammation in Sensitized Mice
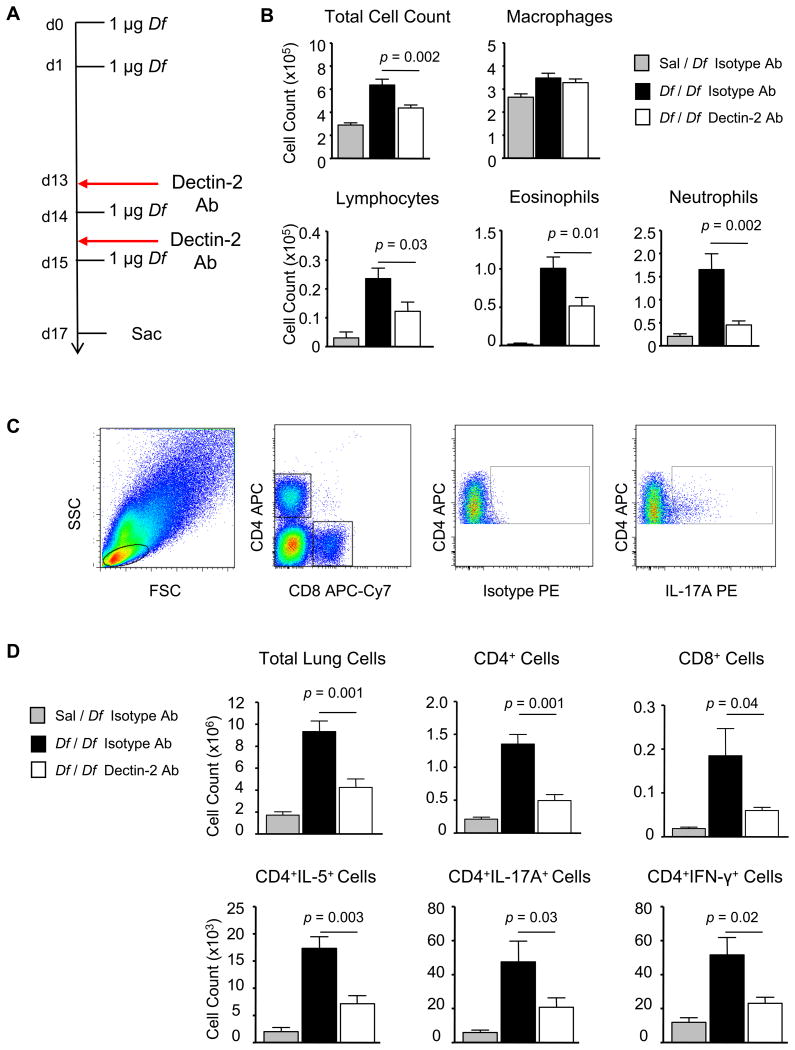
To assess the role of Dectin-2 in the effector phase of allergic pulmonary inflammation, we administered Dectin-2 mAb or rat IgG2a isotype control i.p. preceding each Df challenge of previously sensitized mice (Fig. 1A). Df sensitized and challenged mice treated with isotype control (Df / Df / Iso Ab) developed a significant influx of BAL fluid inflammatory cells, as compared with Df challenged mice sensitized with saline and treated with isotype control (Sal / Df / Iso Ab) (Fig. 1B). This influx included recruitment of lymphocytes, eosinophils, and neutrophils to the BAL fluid. Df sensitized and challenged WT mice treated with the Dectin-2 mAb (Df / Df / Dec2 Ab) had significant reductions in BAL fluid total cells (31.3%), lymphocytes (48.0%), eosinophils (48.5%), and neutrophils (72.9%), as compared to Df / Df / Iso Ab-treated mice.
Figure 1. Dectin-2 Blocking Antibody Inhibits Df-elicited Pulmonary Inflammation in Sensitized WT Mice.
WT mice were sensitized with 1 μg intranasal Df or saline control on days 0 and 1, challenged with 1 μg intranasal Df on days 14, and 15, treated with 200 μg Dectin-2 antibody or a rat IgG2a control i.p. on days 13 and 15, and killed on day 17. A. Experimental diagram. B. Cells from the BAL fluid were counted, cytospin preparations were stained, and 200 cells/slide were counted for specific cell types. Results are means ± SEM (n = 10-12 mice per group) combined from 3 independent experiments. C and D. Pulmonary mononuclear cells were isolated, stimulated with 50 ng/ml PMA and 1 μM ionomycin for 6 h in the presence of 2.5 μM monensin, stained for cell surface expression of CD4 and CD8, and for intracellular expression of IL-4, IL-5, IL-17A, and IFN-γ, and analyzed by flow cytometry. C. Representative dot plots gating on cell size, CD4 and CD8 expression, and IL-17A are shown. D. The total numbers of cells and subsets recruited to the lung are shown. Data are means ± SEM (n = 6-8 mice per group) combined from 2 independent experiments.
To determine whether the reductions in BAL fluid cells were associated with reductions in T cell infiltration in the lung, pulmonary mononuclear cells were isolated from sensitized and challenged mice, stimulated with PMA and ionomycin and assessed for the numbers of CD4+ and CD8+ cells, and for IL-5, IL-17A, and IFN-γ positive subsets. Fig. 1C shows representative staining, including the lymphocyte gate (far left), CD4 and CD8 staining (left), and intracellular cytokine staining on CD4+ lymphocytes using anti-IL-17A PE (far right) or isotype control (right). As compared to saline controls, Df / Df / Iso Ab-treated mice had dramatic increases in pulmonary mononuclear cells, including CD4+ cells, CD8+ cells, and CD4+IL-5+, CD4+IL-17A+, and CD4+IFN-γ+ subpopulations (Fig. 1D). In contrast, Df / Df / Dec2-mAb treated mice had significant reductions in total mononuclear cells (54.6%) and in each subpopulation at 67.6% (CD4+), 63.4% (CD8+), 58.9% (CD4+IL-5+), 56.2% (CD4+IL-17A+), and 55.0% (CD4+IFN-γ+), as compared to Df / Df / Iso Ab-treated controls. The number of CD4+IL-5+, CD4+IL-17A+, and CD4+ IFN-γ+ cells from Df / Df / Dec2 Ab-treated mice was 2 fold above that of Sal / Df / IsoAb-treated controls and paralleled the finding that the total CD4+ T cell count remained 2 fold above baseline.
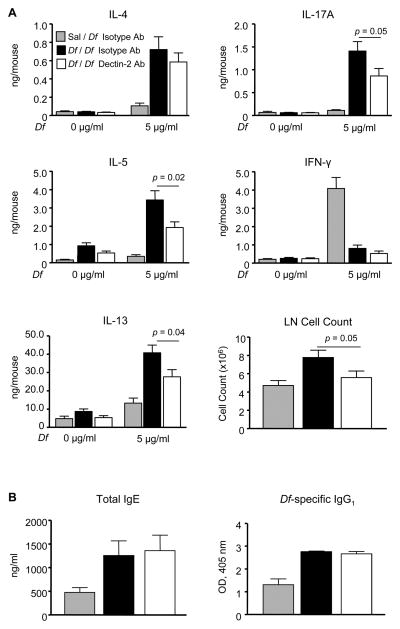
We next sought to determine whether the effects of systemic Dectin-2 blockade extended to the lung-draining lymph nodes. Upon ex vivo restimulation with 5 μg/ml Df, lymph node cultures from Sal / Df / IsoAb-treated mice produced little IL-4, IL-5, or IL-17A (Fig. 2A). IFN-γ was produced in these cultures, reflecting an innate response to Df. A small amount of IL-13 was also produced that was not statistically significant. In cultures from Df / Df / Iso Ab-treated mice, Df-restimulation generated IL-4, IL-5, IL-17A, and IL-13. There were modest but statistically significant reductions in IL-5 (43.9%), IL-13 (32.1%), and IL-17A (38.6%) in cultures from Df / Df / Dec2 Ab-treated mice, as compared to Df / Df / Iso Ab-treated controls, which resulted from a 28.4% reduction in total lymph node cell count, and not from significant differences in cytokine generation on a per cell basis. The levels of IL-5 and IL-17A in cultures from Df / Df / Dec2 Ab-treated mice remained 4-5 fold above that of Sal / Df / IsoAb-treated controls.
Figure 2. Dectin-2 Blocking Antibody Reduces Lymph Node Cytokine Production in Sensitized WT mice and Has No Effect on Serum Immunoglobulins.
WT mice were sensitized, challenged, and treated with antibody as in Fig. 1. A. Peribronchial lymph node cells were isolated, counted, and restimulated for 72 h with 0 or 5 μg/ml Df, and cytokines in the supernatant were measured by ELISA. Data are means ± SEM (n = 10-12 mice per group) combined from 3 independent experiments. B. Serum IgE and serum Df-specific IgG1 were measured by ELISA. Data are means ± SEM (n = 10-12 mice per group) combined from 3 independent experiments.
Total serum IgE and Df-specific IgG1 were also measured in each treatment group (Fig. 2B). After sensitization and challenge, Df / Df / Iso Ab-treated mice had significant increases in serum total IgE and Df-specific IgG1, as compared to Sal / Df / IsoAb-treated controls. However, serum IgE and Df-specific IgG1 titers were not reduced in Df / Df / Dec2 Ab-treated mice, suggesting a dominant role for Dectin-2 in the pulmonary microenvironment during the effector phase.
Dectin-2-Deficient Recipients Sensitized With WT DCs Have Impaired Df-Elicited Pulmonary Inflammation
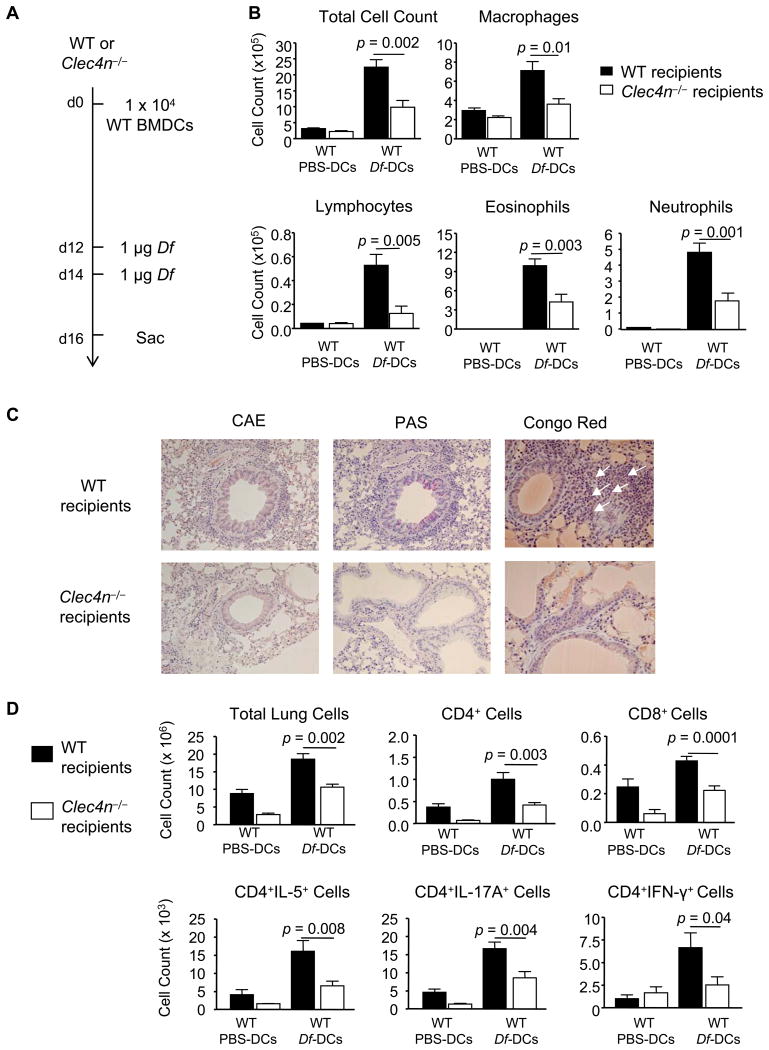
To assess Dectin-2 function in the effector phase by an alternate approach, we generated WT C57BL/6 BMDCs, pulsed them with PBS or Df, and adoptively transferred 1 × 104 BMDCs intranasally to sensitize WT or Clec4n−/− (Dectin-2 null) recipients (13) (Fig. 3A). WT mice sensitized with Df-pulsed BMDCs and challenged with Df had a robust recruitment of inflammatory cells to the BAL fluid including macrophages, lymphocytes, eosinophils, and neutrophils, as compared to WT mice sensitized with PBS-pulsed BMDCs and challenged with Df (Fig. 3B). In contrast, Clec4n−/− mice sensitized with WT Df-pulsed BMDCs had a significantly lower number of Df-elicited total BAL fluid cells (56%), as compared to WT recipients. This included significant reductions of macrophages (49.3%), lymphocytes (76.3%), eosinophils (57.3%), and neutrophils (63.2%). Histologic analysis of the lung showed a cellular infiltrate around the bronchovascular bundles of WT recipients that was reduced in Clec4n−/− recipients (Fig. 3C, left). Goblet cell metaplasia and mucous production, as determined by periodic acid-Schiff staining, was also present in WT and largely absent in Clec4n−/− recipients (Fig. 3C, middle). Congo Red staining identified abundant tissue eosinophilia in WT that was reduced in Clec4n−/− recipients (Fig. 3C, right).
Figure 3. Sensitized Clec4n−/− Mice Have Impaired Df-elicited Pulmonary Inflammation.
WT or Clec4n−/− mice were sensitized with 1 × 104 WT BMDC pulsed with PBS (PBS-DC) or with Df (Df-DC) and challenged with 1 μg Df on days 12 and 14. A. Experimental diagram. B. On day 16, cytospins from BAL fluid were stained and counted. Data are means ± SEM (n = 8-11/Df-DC; n = 3/PBS-DC) combined from 3 independent experiments. C. Histologic analyses of the lung. Lung tissues were fixed with paraformaldehyde and stained with the chloroacetate esterase (CAE) reaction and hematoxylin, periodic acid-Schiff (PAS), or Congo red. Eosinophils are indicated by white arrows in Congo red staining, and mucus is stained in purple in PAS. Images were acquired at 20× (CAE and PAS) and 40× (Congo red) magnifications. D. On day 16, pulmonary mononuclear cells were isolated, stimulated, fixed, and stained for CD4, CD8, and intracellular cytokines, and analyzed by flow cytometry, as in Fig. 1 C&D. Data are means ± SEM (n = 13-15/Df-DC; n = 5/PBS-DC) combined from 3 independent experiments.
Pulmonary mononuclear cells from each treatment group were isolated, stimulated with PMA and ionomycin, and assessed for the numbers of CD4+ and CD8+ cells, and for IL-5, IL-17A, and IFN-γ positive subsets (Fig. 3D). WT mice sensitized with Df-pulsed BMDCs and challenged with Df recruited more CD4+ cells, CD8+ cells, and CD4+IL-5+, CD4+IL-17+, and CD4+IFN-γ+ subpopulations, as compared to mice sensitized with PBS-pulsed BMDCs. Clec4n−/− recipients had a significant reduction in total mononuclear cells (42.7%) and in each subpopulation at 58.3% (CD4+), 47.9% (CD8+), 59.8% (CD4+IL-5+), 48.6% (CD4+IL-17A), and 62% (CD4+IFN-γ+), as compared to WT recipients.
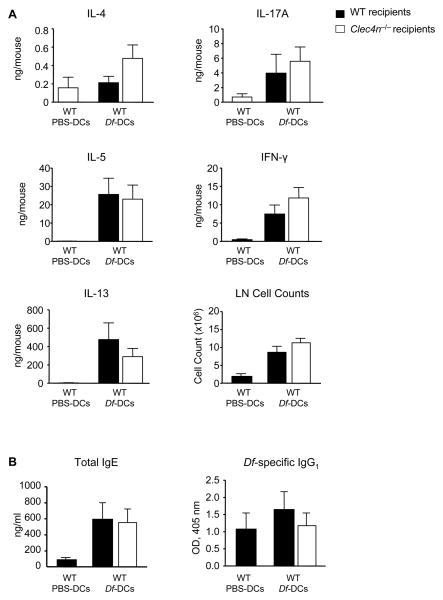
Df-induced cytokine generation from the lung-draining lymph nodes of each treatment group was also assessed (Fig. 4A). Upon ex vivo restimulation with 20 μg/ml Df, lymph node cultures from WT mice sensitized with Df-pulsed BMDCs and challenged with Df produced greater quantities of IL-5, IL-13, IL-17A, and IFN-γ than WT mice sensitized with PBS-pulsed BMDCs. Lymph node cultures from Clec4n−/− recipients sensitized with Df-pulsed BMDCs and challenged with Df had similar numbers of lung-draining lymph node cells and similar quantities of IL-5, IL-13, IL-17A, and IFN-γ detected, as compared to WT recipients. There was a trend to increased IL-4 in cultures from Clec4n−/− recipients that was not statistically significant. Thus, the immunologic responses measured in the lung-draining lymph nodes of Clec4n−/− recipients were not impaired, in sharp contrast to the reductions in airway inflammation and T cell recruitment to the lung.
Figure 4. Sensitized Clec4n−/− Mice Have Intact Df-Elicited Serum Immunoglobulins and Lymph Node Cytokine Production.
WT or Clec4n−/− mice were sensitized and challenged as in Fig. 3. A. On day 16, peribronchial lymph node cells were isolated, counted, and restimulated for 72 h with 20 μg/ml Df, and cytokines in the supernatant were measured by ELISA. Data are means ± SEM (n = 9-11/Df-DC; n = 4/PBS-DC) combined from 3 independent experiments. B. Serum IgE and serum Df-specific IgG1 were measured by ELISA. Data are means ± SEM (n = 9-10/Df-DC; n = 4/PBS-DC) combined from 3 independent experiments.
After sensitization with WT Df-pulsed BMDCs and challenge with Df, WT mice had a significant increase in serum total IgE, as compared to PBS-pulsed BMDC controls (Fig 4B). There was no increase in Df-specific IgG1. Serum IgE and Df-specific IgG1 titers were not reduced in Clec4n−/− mice sensitized with WT Df-pulsed BMDCs and challenged with Df. The intact serum antibody production and lung-draining lymph node responses seen in Clec4n−/− recipients contrast with their impaired airway and pulmonary inflammation, indicating a specific role for Dectin-2 in the pulmonary microenvironment.
Dectin-2 is Specifically Expressed on CD11c+ Cells in the Lung of Naive and Df-Sensitized and Challenged Mice
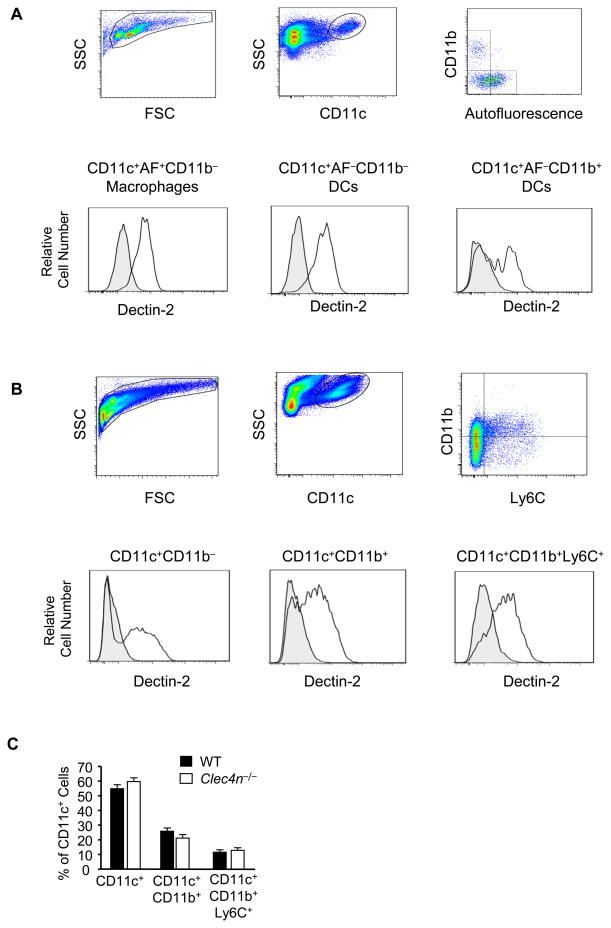
To identify the cell types expressing Dectin-2 that might regulate pulmonary inflammation at Df challenge, we characterized Dectin-2 expression in naive and in sensitized and challenged lung from C57BL/6 WT and Clec4n−/− mice. Single cell suspensions from lungs were stained with antibody for Dectin-2, and with antibodies to discriminate lymphocytes, granulocytes, monocytes, and macrophage/DC populations. In naive lung, Dectin-2 was expressed on autofluorescent CD11c+CD11b− macrophages and on non-autofluorescent CD11c+CD11b− and CD11c+CD11b+ DCs (Fig. 5A). Dectin-2 was not expressed on non-autofluorescent CD11c+CD103+ DCs (data not shown). Nearly 100% of Dectin-2+ cells were CD11c+, as the small numbers of CD11c− cells stained with the Dectin-2 antibody were detected both in WT and Clec4n−/− mice. In Df sensitized and challenged WT lung, Dectin-2 was expressed on CD11c+CD11b− and CD11c+CD11b+ cells, and additionally on CD11c+CD11b+Ly6C+ MDDCs (Fig. 5B), which accounted for 35%, 40%, and 20% of Dectin-2+CD11c+ cells, respectively. While CD11c+CD11b+Ly6C+ cells were newly recruited to Df-challenged lung, Dectin-2+CD11c+CD11b− cells and Dectin-2+CD11c+CD11b+ cells also increased by 2 and 10 fold over that of naive lung. Again nearly 100% of Dectin-2+ cells were CD11c+. CD11c−CD115+Ly6C+ monocytes did not express Dectin-2. CD3+ T cells, CD19+ B cells, NK1.1+ natural killer cells, and SSChiLy6GhiCCR3− neutrophils and SSChiLy6GhiCCR3+ eosinophils were also negative for Dectin-2 (data not shown). All CD11c+ subsets identified in sensitized and challenged WT mice were present in comparable numbers in treated Clec4n−/− mice (Fig. 5C), suggesting that Dectin-2 has no role in the development or recruitment of any specific subpopulation of CD11c+ cells to the lung.
Figure 5. Dectin-2 is Specifically Expressed on CD11c+ Cells in the Lung of Naive and Df-Sensitized and Challenged Mice.
Single cell suspensions from the lung of WT C57BL/6 mice were generated and stained with the indicated antibodies or isotype controls. A. Top row shows gating of viable cells from naïve mice using FSC/SSC, staining for CD11c, and autofluorescence (AF)/staining for CD11b. Bottom row shows Dectin-2 expression (black lines) and isotype control (shaded gray) on CD11c+AF+CD11b− macrophages (left), CD11c+AF−CD11b− DCs (middle) and CD11c+AF−CD11b+ DCs (right). B. Top row shows gating of viable cells from WT C57BL/6 mice sensitized to 1 μg Df on day 0 and 1, challenged to 1 μg Df on days 14 and 15, and killed on day 17. FSC/SSC and stainings for CD11c, and CD11b/Ly6C are shown. Bottom row shows Dectin-2 expression (black lines) and isotype control (shaded gray) on CD11c+CD11b− (left), CD11c+CD11b+ (middle), and CD11c+CD11b+Ly6C+ (right) populations. C. CD11c+ subsets from WT and Clec4n−/− lung after sensitization and challenge as in B. Data are means ± SEM (n = 4-7 mice/group) combined from 2 independent experiments.
Dectin-2 Regulates Pulmonary Chemokine Generation from MDDCs
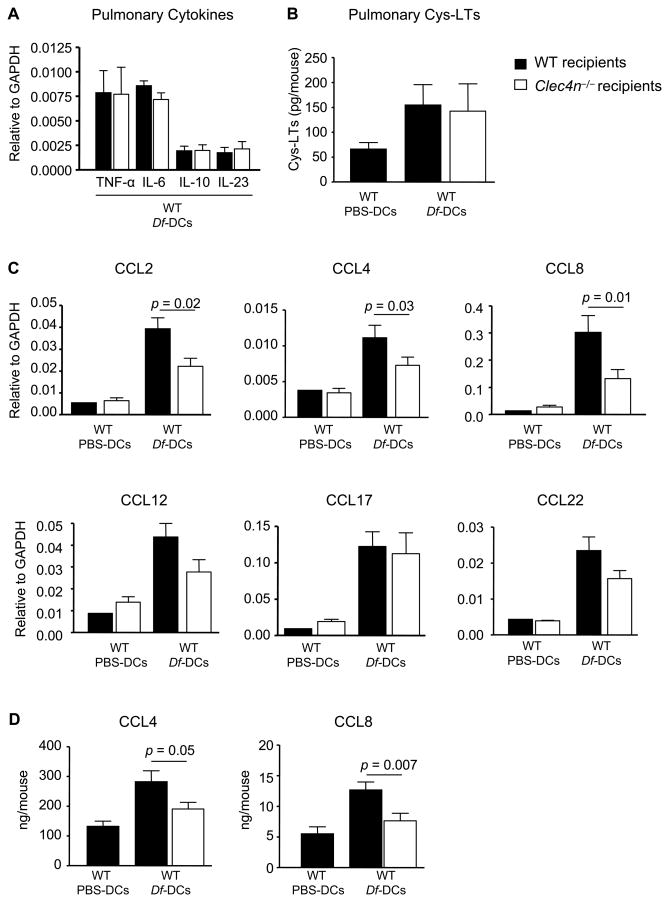
We next sought to determine whether the absence of Dectin-2 on CD11c+ cell populations influenced Df-induced generation of inflammatory mediators in the lung at challenge. We first assessed the lung transcript levels of inflammatory cytokines known to be expressed by BMDCs and induced by Dectin-2 signaling in vitro (6, 13, 17). WT and Clec4n−/− mice sensitized with Df-pulsed BMDCs and challenged with Df had comparable levels of TNF-α, IL-6, IL-10, and IL-23 transcripts (Fig. 6A). To examine whether cys-LT production in the lung may be altered in Clec4n−/− mice, BAL fluid was obtained 24 h after the last challenge. After methanol extraction and HPLC separation, LTC4, LTD4, and LTE4 from BAL fluid were quantified by ELISA. Clec4n−/− mice sensitized with WT Df-pulsed BMDCs and challenged with Df had BAL fluid cys-LT levels (combined contents of LTC4, LTD4, and LTE4) comparable to WT recipients (Fig. 6B). As CD11c+ cells are major sources of pulmonary chemokines in allergic inflammation (29), we reasoned that Dectin-2 may regulate the generation of these mediators and assessed transcript levels of several chemokines from the lungs of each treatment group (Fig. 6C). WT recipients sensitized with Df-pulsed BMDCs and challenged with Df, had increased transcript levels of CCL2, CCL4, CCL8, CCL12, CCL17, and CCL22 in the lung, as compared to PBS controls. Clec4n−/− recipients sensitized with Df-pulsed BMDCs and challenged with Df had reduced levels of CCL2, CCL4, and CCL8, but similar levels of CCL12, CCL17, CCL22, CXCL1, and CXCL2 in the lung, as compared to WT recipients (Fig. 6C and data not shown). ELISA on lung homogenates (Fig. 6D) confirmed a significant reduction of CCL4 (32%) and of CCL8 (40%) protein in Clec4n−/− recipients, as compared to WT controls.
Figure 6. Sensitized Clec4n−/− Mice Have Impaired Df-Elicited Pulmonary Chemokines.
WT or Clec4n−/− mice were sensitized with WT BMDCs and challenged with Df, as in Figs. 3 and 4. A. Cytokine transcripts in lung 48 h after the last Df challenge. B. Cys-LTs from BAL 24 h after the last Df challenge. C. Chemokine transcripts from WT and Clec4n−/− recipients 48 h after the last Df challenge. Data are means ± SEM (n = 9-11/Df-DCs; n = 3/PBS-DCs) combined from 3 independent experiments. D. CCL4 and CCL8 from lung homogenates were measured by ELISA. Data are means ± SEM (n = 13-15/ Df-DCs; n = 4/PBS-DCs) combined from 4 independent experiments.
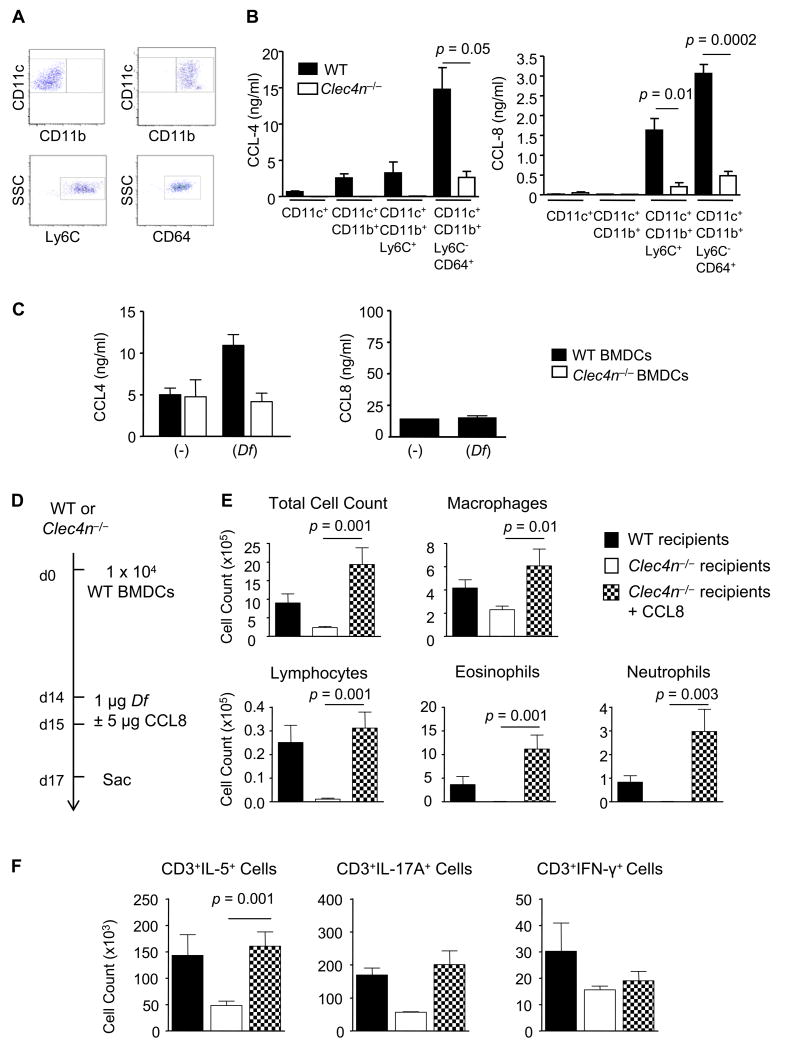
To understand which cell produced CCL4 and CCL8, each Dectin-2-expressing pulmonary CD11c+ population was isolated by flow cytometric sorting from WT and Clec4n−/− recipients that had been sensitized with Df-pulsed BMDCs and challenged with Df. Figure 7A demonstrates a representative dot plot of each sorted population: CD11c+CD11b− (upper left), CD11c+CD11b+ (upper right), CD11c+CD11b+Ly6C+ MDDCs (lower left), and an additional subpopulation of MDDCs which are CD11c+CD11b+Ly6C−CD64+ (lower right) (30). After 24 h culture, neither CCL4 nor CCL8 was detected in the supernatants of CD11c+CD11b− cells from WT recipients (Fig. 7B), despite their capacity to make TNF-α with zymosan stimulation (data not shown). WT CD11c+CD11b+ cells produced CCL4, but no detectable CCL8. While WT CD11c+CD11b+Ly6C+ MDDCs produced nanogram quantities of CCL4 and CCL8, CD11c+CD11b+Ly6C−CD64+ MDDCs produced the largest quantity of each chemokine. Production of CCL4 and CCL8 was significantly reduced in MDDC cultures from Clec4n−/− recipients, suggesting that Dectin-2 regulates CC chemokine production from recruited MDDCs. Df –induced CCL4 generation was also demonstrated in WT BMDCs but absent from Clec4n−/− cultured BMDCs (Fig. 7C), suggesting that Dectin-2 can directly regulate Df-induced chemokine generation. There was no generation of CCL8 from BMDCs.
Figure 7. Sensitized Clec4n−/− Mice Have Impaired MDDC Chemokine Production and Restoration of Airway CCL8 Reconstitutes Allergic Airway Inflammation.
A and B. WT or Clec4n−/− mice were sensitized with WT BMDCs and challenged with Df, as in Figs. 3 and 4. Single cell suspensions from the lung were generated, stained with the indicated antibodies or isotype controls, and sorted. A. Top row shows staining from sorted CD11c+CD11b− cells (left) and CD11c+CD11b+ cells (right). Bottom row shows staining from sorted CD11c+CD11b+ Ly6C+ cells (left) and from CD11c+CD11b+ Ly6C−CD64+ cells (right). B. CCL-4 (left) and CCL-8 (right) from cultured supernatant of each population at 24 h. Data are means ± SEM (n = 4-6/group) combined from 2 independent experiments. C. CCL-4 (left) and CCL-8 (right) from cultured supernatant of BMDCs with and without Df stimulation for 12 h. Data are means ± SEM (n = 4) combined from 2 independent experiments. D-F. WT or Clec4n−/− mice were sensitized with WT BMDCs and challenged with Df with or without addition of 5 μg CCL8 30 min prior to each Df challenge. D. Experimental diagram. E. Cytospins from BAL fluid were stained and counted. Data are means ± SEM (n = 7-9 mice/group) combined from 2 independent experiments. F. Pulmonary mononuclear cells were isolated, stimulated, fixed, stained for CD3 and intracellular cytokines, and analyzed by flow cytometry, as in Fig. 1 C&D. Data are means ± SEM (n = 4-7 mice/group) combined from 2 independent experiments.
CCL8 is a ligand for CCR8 found on Th2 cells and plays a critical role in promoting allergic inflammation in the skin (31). To determine whether restoring CCL8 in the airway of deficient Clec4n−/− recipients would be sufficient to reconstitute allergic airway inflammation, we sensitized WT and Clec4n−/− mice by adoptive transfer of WT Df-pulsed BMDCs, challenged them with Df, and provided 5 ng CCL8 by intranasal injection at the time of each Df challenge (Fig. 7D). WT recipients had recruitment of inflammatory cells to the BAL fluid including lymphocytes, eosinophils, and neutrophils, while these were nearly absent in Clec4n−/− mice (Fig. 7E). However, addition of CCL8 to Df airway challenge restored BAL fluid inflammation with recruitment of macrophages, lymphocytes, eosinophils, and neutrophils, as compared to Clec4n−/− mice challenged with Df alone.
Pulmonary mononuclear cells from each treatment group were isolated, stimulated with PMA and ionomycin, and assessed for the numbers of CD3+ cells that produced IL-5, IL-17A, and IFN-γ (Fig. 7F). WT mice sensitized with Df-pulsed BMDCs and challenged with Df recruited more CD3+IL-5+, CD3+IL-17A+, and CD3+IFN-γ+ subpopulations, as compared to Clec4n−/− recipients. Addition of CCL8 to the Df airway challenge of Clec4n−/− mice significantly increased the CD3+IL-5+ cell population to 3 fold that of Clec4n−/− mice injected with Df alone. There was a trend to increasing the CD3+IL-17A+ population that was not statistically significant, and no increase in CD3+IFN-γ+ cells. These results demonstrate that restoration of CCL8 alone is sufficient to reconstitute BAL fluid inflammation and Th2 cell recruitment to the lung in deficient Clec4n−/− mice.
Discussion
Despite recent attention, the inflammatory pathways activated by native allergens are still incompletely understood and the magnitude of the contribution of innate signaling to allergic pulmonary inflammation remains unclear. We previously identified a role for the Dectin-2/cys-LT pathway in DC priming for Th2 immunity to the house dust mite Df (6). As Dectin-2 can initiate inflammatory signaling via both the cys-LT pathway and the CARD9/cytokine pathway, we considered that Dectin-2 may have a role not only in canonical Th2 sensitization, but also in the inflammatory events in the effector phase of Df-elicited pulmonary inflammation. By using Dectin-2 blocking antibody during Df challenges of sensitized WT mice, and by using the adoptive transfer of WT Df-pulsed BMDCs to sensitize Clec4n−/− mice, we established a role for Dectin-2 in the effector phase of Df-elicited pulmonary inflammation. Furthermore, in each model, deficiency of Dectin-2 only in the effector phase caused striking reductions in pulmonary Th1, Th2, and Th17 cells, but little or no reduction in systemic immunity, as characterized by serum levels of IgE and by the number of lung-draining lymph node cells and their cytokine generation. These findings indicate that Dectin-2 regulation of allergen-induced effector phase responses is specific to the pulmonary microenvironment.
In the lung of naïve mice, we found Dectin-2 expression on multiple CD11c+ cell populations. Dectin-2 was expressed on non-autofluorescent CD11c+CD11b− and CD11c+CD11b+ DCs and on autofluorescent CD11c+CD11b− macrophages, but not on CD11c+CD103+ DCs. A prior study demonstrated Dectin-2 uniquely expressed on tissue macrophages in naive lung using immunohistochemistry with the same Ab on fixed tissue (32). Our detection of additional Dectin-2-expressing cell populations is likely due to the increased sensitivity of flow cytometry on unfixed cells and the availability of Clec4n−/− mice as negative controls. The absence of Dectin-2 on CD11c+CD103+ DCs in the lung is consistent with the distinct developmental lineage of this uniquely Batf1-dependent DC subset (33). The CD11c+CD11b− lung macrophage population is reported to control the generation of antigen-induced T regulatory cells and this function is abrogated by dust mite activation via the MyD88/Trif pathway (34). Although we found that this population expresses high levels of Dectin-2, the role of Dectin-2 signaling in macrophage-mediated tolerance is unknown.
In sensitized and challenged mice, we found Dectin-2 on CD11c+CD11b−, CD11c+CD11b+, and on CD11c+CD11b+Ly6C+ ‘monocyte-derived DCs’, but not on the CD11c−CD115+Ly6C+ monocytes from which they derive (30, 35, 36), suggesting that Dectin-2 is upregulated during the maturation of these monocytes in the inflammatory setting of the lung. This is consistent with a study of Dectin-2 expression in a zymosan peritonitis model, where Dectin-2 was noted to be absent on newly recruited inflammatory monocytes, upregulated on maturing inflammatory monocytes, and later downregulated on the monocyte-derived macrophage population (32). This tight regulation was reproduced in the peritoneum by injection of thioglycollate and of BIOgel, and in the spleen by i.v. injections of LPS and pam3Cys. In murine BMDCs, we have found that Dectin-2 cell surface expression is upregulated by IL-33 in a dose-dependent and ST2-dependent fashion (data not shown), suggesting again that Dectin-2 is coordinately expressed during the maturation of inflammatory DCs (37, 38). Further studies on IL-33 regulation of Dectin-2 function will be important, as IL-33 is recently recognized as a critical regulator of DC function in allergic inflammation induced by HDM (39, 40).
In cultured DCs and macrophages, Dectin-2 signaling triggers the generation of IL-6, IL-23, and TNF-alpha via a Syk/CARD9-dependent pathway (13, 17, 18) and cys-LTs via a Syk-dependent/CARD9-independent pathway (16) (and unpublished). Nonetheless, we found that Clec4n−/− mice sensitized with WT Df-pulsed BMDCs and challenged with Df, had levels of lung transcript for TNF-alpha, IL-6, and IL-23 that were comparable to that of WT recipients, despite their impaired inflammatory response. While TNF-alpha and IL-6 in Clec4n−/− mice may be generated by activation of the TLR4 pathway in DCs (41) by HDM, normal Df-induced IL-23 levels in Clec4n−/− mice indicates signaling from an additional receptor system, which may be Dectin-1 or the C3a receptor (C3aR) (42, 43). WT and Clec4n−/− recipients sensitized with Df-pulsed BMDCs had similar levels of cys-LTs in the BAL 24 h after the last Df challenge, although the levels detected were not greatly elevated above PBS control, and likely reflect the rapid metabolism of cys-LTs in tissue (44) or their inactivation by the respiratory burst of myeloid cells (45).
We found impairments in pulmonary chemokine production in the lung of Clec4n−/− mice after Df-pulsed BMDC sensitization and Df challenge, including CCL2, CCL4, and CCL8, ligands for monocytes, CCR5-expressing Th1 cells, and CCR8-expressing Th2 cells, respectively (46). Furthermore, deficient CCL4 and CCL8 chemokine production in the lung of Clec4n−/− mice was mapped to pulmonary CD11c+CD11b+ Ly6C+ and CD11c+CD11b+ Ly6C−CD64+ MDDCs. This confirms and extends a prior study demonstrating chemokine generation in the lung of HDM challenged mice is specific to recruited CD11c+CD11b+Ly6C+ MDDCs (30). We found Df-induced CCL4 production from BMDCs was also dependent on Dectin-2, demonstrating that Dectin-2 can directly mediate chemokine production. In the lung of Clec4n−/− mice, we did not find significant differences in the generation of CCL17 or CCL22, generated from CD103+ DCs (30), nor did we see a difference in CXCL1 or CXCL2, chemokines generated from another FcRγ chain-associated CLR, Mincle (47). These results suggest that one way in which Dectin-2 promotes inflammation is via the specific activation of CC chemokines.
Prior studies on the role of CCL8 in allergic pulmonary inflammation have come to discrepant conclusions. The receptor for CCL8, CCR8, is expressed on both murine and human Th2 cells (48, 49) and participates in Th2 cell recruitment to CCL8-expressing skin in a model of atopic dermatitis (31). CCL8 is not expressed in naive lung (31). Moreover, pulmonary models of allergic inflammation elicited with ovalbumin, which failed to demonstrate a role for CCR8, did not establish induction of CCL8 in the tissue (50, 51). In our studies using house dust mite, we observed robust generation of CCL8 in both whole lung and isolated MDDCs. Moreover, pharmacologic addition of CCL8 to the airways of Clec4n−/− mice increased both eosinophilic pulmonary inflammation and Th2 cell recruitment. This is consistent with models of allergic pulmonary inflammation elicited by cockroach or Aspergillus where a role for CCR8 was shown (52, 53) along with the induction of the ligand, CCL8 (52). While our results do establish a role for Dectin-2 in regulating allergic pulmonary inflammation at the effector stage and in regulating chemokine production from MDDCs, we cannot exclude the possibility that Dectin-2 mediates additional functions of CD11c+ populations in the effector phase.
Although our studies showed a dominant effect of Dectin-2 in specifically regulating lung inflammation, we did note some reduction in lymph node cell numbers and their generation of IL-5, IL-13, and IL-17A with Dectin-2 mAb during the challenge phase. This presumptively reflects an additional influence of Dectin-2 on memory Th2 and Th17 responses to Df challenge, in accordance with our earlier work on Dectin-2, in which antibody blockade during both sensitization and challenge dramatically reduced lymph node cell expansion and cytokine production elicited by Df (6).
In sum, our findings suggest that Dectin-2 on CD11c+ cells regulates the effector phase of allergic pulmonary inflammation by promoting polarized T cell recruitment and CC chemokine generation in the lung. Furthermore, these findings indicate that native allergens such as HDM may induce significant pulmonary inflammation by activating innate signaling pathways independently from their role in sensitization, and that therapies directed to these innate activities may be a novel approach for allergic asthma.
Acknowledgments
This work is supported by National Institutes of Health Grants U19AI095219, R21 AI107425 (to Y.K.), and K08AI080948 and the Joycelyn C. Austen Fund for the Career Development of Women Physician Scientists (to N.A.B.).
The abbreviations used in this paper
- BAL
bronchoalveolar lavage
- BMDC
bone marrow-derived dendritic cell
- CLR
C-type lectin receptor
- cys-LT
cysteinyl leukotriene
- DC
dendritic cell
- DC-SIGN
Dendritic Cell-specific Intercellular Adhesion Molecule-3-grabbing Non-integrin
- Df
extracts from Dermatophagoides farinae
- Dp
extracts from Dermatophagoides pteronyssinus
- HDM
house dust mite
- hMDDC
human monocyte-derived dendritic cell
- LT
leukotriene
- MDDC
monocyte-derived dendritic cell
- WT
wild-type
References
- 1.Arbes SJ, Jr, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2007;120:1139–1145. doi: 10.1016/j.jaci.2007.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 3.Celedon JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TAE, Gold DR. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007;120:144–149. doi: 10.1016/j.jaci.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, Kanaoka Y. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208:593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RH, Maruoka S, Whitehead GS, Foley JF, Flake GP, Sever ML, Zeldin DC, Kraft M, Garantziotis S, Nakano H, Cook DN. The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens. Nat Med. 2012;18:1705–1710. doi: 10.1038/nm.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phipps S, Lam CE, Kaiko GE, Foo SY, Collison A, Mattes J, Barry J, Davidson S, Oreo K, Smith L, Mansell A, Matthaei KI, Foster PS. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 responses. Am J Respir Crit Care Med. 2009;179:883–893. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 9.Royer PJ, Emara M, Yang C, Al-Ghouleh A, Tighe P, Jones N, Sewell HF, Shakib F, Martinez-Pomares L, Ghaemmaghami AM. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol. 2010;185:1522–1531. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- 10.Deslee G, Charbonnier AS, Hammad H, Angyalosi G, Tillie-Leblond I, Mantovani A, Tonnel AB, Pestel J. Involvement of the mannose receptor in the uptake of Der p 1, a major mite allergen, by human dendritic cells. J Allergy Clin Immunol. 2002;110:763–770. doi: 10.1067/mai.2002.129121. [DOI] [PubMed] [Google Scholar]
- 11.Emara M, Royer PJ, Mahdavi J, Shakib F, Ghaemmaghami AM. Retagging identifies dendritic cell-specific intercellular adhesion molecule-3 (ICAM3)-grabbing non-integrin (DC-SIGN) protein as a novel receptor for a major allergen from house dust mite. J Biol Chem. 2012;287:5756–5763. doi: 10.1074/jbc.M111.312520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang HJ, Lin YL, Liu CF, Kao HF, Wang JY. Mite allergen decreases DC-SIGN expression and modulates human dendritic cell differentiation and function in allergic asthma. Mucosal Immunol. 2011;4:519–527. doi: 10.1038/mi.2011.17. [DOI] [PubMed] [Google Scholar]
- 13.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T, Yamasaki S. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe. 2013;13:477–488. doi: 10.1016/j.chom.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, Tschopp J, Layland LE, Prazeres da Costa C. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A. 2010;107:20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Reis e Sousa C. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, Iwakura Y, Ohno N, Koseki H, Yoshida H, Penninger JM, Saito T. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol. 2007;8:619–629. doi: 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- 19.LeibundGut-Landmann S, Grosz O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 20.Gringhuis SI, Wevers BA, Kaptein TM, van Capel TMM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TBH. Selective C-Rel Activation via Malt1 Controls Anti-Fungal Th17 Immunity by Dectin-1 and Dectin-2. PLoS Pathog. 2011;7:e1001259. doi: 10.1371/journal.ppat.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke DL, Davis NH, Campion CL, Foster ML, Heasman SC, Lewis AR, Anderson IK, Corkill DJ, Sleeman MA, May RD, Robinson MJ. Dectin-2 sensing of house dust mite is critical for the initiation of airway inflammation. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 23.Barrett NA, Fernandez JM, Maekawa A, Xing W, Li L, Parsons MW, Austen KF, Kanaoka Y. Cysteinyl Leukotriene 2 Receptor on Dendritic Cells Negatively Regulates Ligand-Dependent Allergic Pulmonary Inflammation. J Immunol. 2012;189:4556–4565. doi: 10.4049/jimmunol.1201865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannattasio G, Ohta S, Boyce JR, Xing W, Balestrieri B, Boyce JA. The purinergic G protein-coupled receptor 6 inhibits effector T cell activation in allergic pulmonary inflammation. J Immunol. 2011;187:1486–1495. doi: 10.4049/jimmunol.1003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohta S, Imamura M, Xing W, Boyce JA, Balestrieri B. Group V secretory phospholipase A2 is involved in macrophage activation and is sufficient for macrophage effector functions in allergic pulmonary inflammation. J Immunol. 2013;190:5927–5938. doi: 10.4049/jimmunol.1203202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 27.Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh FH, Lam BK, Penrose JF, Austen KF, Boyce JA. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C4 synthase expression by interleukin 4. J Exp Med. 2001;193:123–133. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaty SR, Rose CE, Sung SSJ. Diverse and Potent Chemokine Production by Lung CD11bhigh Dendritic Cells in Homeostasis and in Allergic Lung Inflammation. J Immunol. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- 30.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, Lira SA, Charo IF, Luster AD. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat Immunol. 2011;12:167–177. doi: 10.1038/ni.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor, Philip R, Reid Delyth M, Heinsbroek SEM, Brown Gordon D, Gordon S, Wong Simon YC. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur J Immunol. 2005;35:2163–2174. doi: 10.1002/eji.200425785. [DOI] [PubMed] [Google Scholar]
- 33.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8 alpha conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103- pulmonary dendritic cell populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 37.Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- 38.Su Z, Lin J, Lu F, Zhang X, Zhang L, Gandhi NB, de Paiva CS, Pflugfelder SC, Li DQ. Potential autocrine regulation of interleukin-33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol. 2013;6:921–930. doi: 10.1038/mi.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013;131:187–200. e181–188. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan AM, Chen HC, Pochard P, Eisenbarth SC, Herrick CA, Bottomly HK. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J Immunol. 2010;184:3535–3544. doi: 10.4049/jimmunol.0900340. [DOI] [PubMed] [Google Scholar]
- 42.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, Adema GJ, Kullberg BJ, Schweighoffer E, Tybulewicz V, Mora-Montes HM, Gow NA, Williams DL, Netea MG, Brown GD. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krilis S, Lewis RA, Corey EJ, Austen KF. Bioconversion of C-6 sulfidopeptide leukotrienes by the responding guinea pig ileum determines the time course of its contraction. J Clin Invest. 1983;71:909–915. doi: 10.1172/JCI110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CW, Lewis RA, Tauber AI, Mehrotra M, Corey EJ, Austen KF. The myeloperoxidase-dependent metabolism of leukotrienes C4, D4, and E4 to 6-trans-leukotriene B4 diastereoisomers and the subclass-specific S-diastereoisomeric sulfoxides. J Biol Chem. 1983;258:15004–15010. [PubMed] [Google Scholar]
- 46.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18:705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 48.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D'Ambrosio D, O'Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 49.D'Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 50.Chung CD, Kuo F, Kumer J, Motani AS, Lawrence CE, Henderson WR, Jr, Venkataraman C. CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J Immunol. 2003;170:581–587. doi: 10.4049/jimmunol.170.1.581. [DOI] [PubMed] [Google Scholar]
- 51.Mikhak Z, Fukui M, Farsidjani A, Medoff BD, Tager AM, Luster AD. Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. J Allergy Clin Immunol. 2009;123:67–73. e63. doi: 10.1016/j.jaci.2008.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckland KF, O'Connor E C, Coleman EM, Lira SA, Lukacs NW, Hogaboam CM. Remission of chronic fungal asthma in the absence of CCR8. J Allergy Clin Immunol. 2007;119:997–1004. doi: 10.1016/j.jaci.2006.12.660. [DOI] [PubMed] [Google Scholar]
- 53.Chensue SW, Lukacs NW, Yang TY, Shang X, Frait KA, Kunkel SL, Kung T, Wiekowski MT, Hedrick JA, Cook DN, Zingoni A, Narula SK, Zlotnik A, Barrat FJ, O'Garra A, Napolitano M, Lira SA. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J Exp Med. 2001;193:573–584. doi: 10.1084/jem.193.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]