Abstract
Though the cortical contributions to age-related declines in motor and cognitive performance are well-known, the potential contributions of the cerebellum are less clear. The diverse functions of the cerebellum make it an important structure to investigate in aging. Here, we review the extant literature on this topic. To date, there is evidence to indicate that there are morphological age differences in the cerebellum that are linked to motor and cognitive behavior. Cerebellar morphology is often as good as -- or even better -- at predicting performance than the prefrontal cortex. We also touch on the few studies using functional neuroimaging and connectivity analyses that further implicate the cerebellum in age-related performance declines. Importantly, we provide a conceptual framework for the cerebellum influencing age differences in performance, centered on the notion of degraded internal models. The evidence indicating that cerebellar age differences associate with performance highlights the need for additional work in this domain to further elucidate the role of the cerebellum in age differences in movement control and cognitive function.
Keywords: cerebellum, aging, neuroimaging, cognition, motor performance
1.1 Introduction
Daily life requires a wide variety of motor and cognitive behaviors. Take for example a trip to the grocery store to purchase ingredients for dinner. One needs to navigate the store, execute a series of reach and grasp movements to place ingredients into a grocery cart, and rely upon memory to ensure that all of the necessary ingredients have been purchased. With healthy aging, there are performance declines in both the motor and cognitive domains. As the population rapidly ages, understanding the factors that contribute to these age-related performance declines is critical for the development of appropriate targeted interventions and preventative measures. While much progress has been made to date with respect to our understanding of these performance declines, the majority of this work has focused on the cerebral cortex. However, accumulating evidence supports a role for the cerebellum in both motor and cognitive behaviors (eg. Leiner et al. 1986, 1993; Stoodley and Schmahmann 2009; Stoodley et al. 2012), and study of this structure may also provide important insights into aging. Here, we provide an overview of the cognitive and motor deficits in aging with respect to cortical structure and function. Importantly, we also provide a review of the extant literature on the role of the cerebellum in these functional age differences. While much of this work has focused on cross-sectional morphological comparisons, we will also briefly touch upon task-based functional magnetic resonance imaging (fMRI) studies and work using resting state functional connectivity MRI (fcMRI). In concert, this literature supports an important role for the cerebellum in the cognitive and motor performance declines associated with aging, perhaps due to degraded internal models of motor and cognitive behaviors. Lastly, we will discuss potential targets for both future basic science research and applied interventions focusing on the cerebellum in order to mitigate these declines.
1.1.2 Cognitive and Motor Function and Aging
With advanced age, individuals begin to exhibit performance declines in a variety of domains, including working memory, processing speed, spatial processing, and long-term memory, though notably crystallized knowledge, such as vocabulary, remains intact (Park et al., 2001). Neural functional changes have been associated with performance declines (cf. Reuter-Lorenz and Park 2010; Seidler et al. 2010). It has been suggested that the brain relies upon neural scaffolding to try to maintain performance (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2010). That is, additional prefrontal cortical resources are recruited to compensate for functional declines, and though this activation may not be as neurally efficient as that seen in young adults, the additional brain activation may allow older adults to maintain higher levels of performance (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2010). Indeed, over-activation in healthy aging is thought to perhaps be compensatory in nature, given that in prefrontally-mediated cognitive tasks, activation of additional brain regions is often associated with better task performance for older adults (Reuter-Lorenz and Cappell, 2008).
Bilateral prefrontal cortical recruitment during cognitive task performance in older adults was first demonstrated by Reuter-Lorenz and colleagues (2000) during the performance of verbal and spatial working memory tasks. Subsequent to this finding, such activation patterns were conceptualized within the hemispheric asymmetry reduction in older adults (HAROLD) framework (Cabeza, 2002). Since these initial findings, this pattern has been repeatedly demonstrated numerous times, indicating that such bilateral patterns of activation are a robust difference between young and older adults during cognitive task performance (for reviews see Reuter-Lorenz and Cappell 2008; Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2010).
In addition to the known cognitive performance declines associated with advanced age, deficits are also seen in the motor domain (reviewed in Seidler et al. 2010). There are gait and balance declines associated with advanced age (Bohannon et al., 1984; Holviala et al., 2012; Laughton et al., 2003; Maki et al., 1990; Prince et al., 1997), and older adults show less learning on sensorimotor adaptation (Anguera et al., 2011) and motor sequence learning tasks (Bo et al., 2011a, 2009). Perhaps not surprisingly, much like during cognitive task performance, there are also differences in neural recruitment during the performance of motor tasks.
Using a simple button-pressing task, Mattay and colleagues demonstrated that there is greater activation in the motor cortex as well as in the cerebellum in older adults (Mattay et al., 2002). Similarly, during a finger-to-thumb opposition task Naccarato and colleagues (2006) also showed decreased laterality in motor cortical activation with advanced age. The extent of this bilateral activation was positively correlated with age (Naccarato et al., 2006). More recently, multi-voxel pattern analysis was employed to analyze a tapping task in young and older adults, with the results showing decreased distinctiveness in motor representations in the motor cortex and cerebellum in older adults (Carp et al., 2011). More bilateral motor cortical representations in older adults has also been demonstrated at rest in the absence of task performance using transcranial magnetic stimulation (Bernard and Seidler, 2012).
Along with more bilateral activation in motor cortical regions during motor task performance, older adults also recruit additional prefrontal cortical regions (Heuninckx et al., 2008, 2005). Indeed, motor performance (alternating hand and foot movements) was positively correlated with the degree of activation in the prefrontal cortex in older adults (Heuninckx et al., 2008). Finally, dual-task motor and cognitive paradigms have shown greater motor performance declines in older adults (Huxhold et al., 2006; Li et al., 2001; Lindenberger et al., 2000; Lövdén et al., 2008) indicating an increased reliance on cognitive resources during motor performance.
Taken together, the literature to date provides a great deal of evidence for age-related declines in cognitive and motor function. Notably however, the work investigating the neural underpinnings of these declines has focused largely on the cerebral cortex. A complete overview of this literature is beyond the scope of the current review; the reader is referred to other articles for additional information (Park and Reuter-Lorenz, 2009; Reuter-Lorenz and Cappell, 2008; Seidler et al., 2010). While previous work focusing on age differences in the cerebral cortex has greatly advanced our knowledge and understanding of the aging mind and brain, an emerging literature points to the cerebellum as an important area of interest in aging. This is particularly pertinent given work implicating the cerebellum in both cognitive and motor tasks (eg. Leiner et al. 1986, 1989, 1991, 1993; Chen and Desmond 2005a, 2005b; Stoodley and Schmahmann 2009). The majority of this work to date has focused on cerebellar morphology, but evidence from both fMRI and resting state fcMRI also point to a potential role for the cerebellum in age related cognitive and motor performance declines.
2.1 Cerebellar Morphology in Aging
As briefly noted above, the majority of the literature investigating the cerebellum in advanced age is focused on cerebellar structure. Within this literature, several methodological approaches have been used. These include stereological studies done on the post-mortem brain, neuroimaging methods quantifying the volume of hand-traced regions of interest, voxel-based morphometry (VBM), and diffusion tensor imaging (DTI) which focuses on white matter structural integrity. Across these different methodologies, the overall finding however remains the same – the volume of the cerebellum is smaller and white matter integrity is reduced in older adults. The specificities of these findings and work across these methods will be described in turn below.
2.1.1 Stereology
Stereological studies require the precise counting of cell types typically done in the post-mortem brain, though there are also methods based on in vivo neuroimaging. This line of work has been very informative for increasing our understanding of age differences in the cerebellum at the cellular level. For example, Andersen and colleagues (Andersen et al., 2003), found that with advanced age, there were significantly fewer Purkinje and granule cells in the anterior cerebellum. There was also a significant age-related association in the volume of the anterior cerebellum, and overall lower volume of cerebellar white matter (Andersen et al., 2003). More recently, similar methods have revealed differences in cerebellar volume in the brains of Alzheimer's Disease patients versus healthy age-matched controls, though there were no differences in the number of Purkinje nor granule cells in the two groups (Andersen et al., 2012). This latter finding highlights the potential impact of age-related disease on the cerebellum, and the need for further study in this domain.
Recent stereological work in mice has also provided interesting data with respect to the aging cerebellum. Though the hippocampus is often implicated in aging, the impact of age on both the hippocampus and cerebellum was recently compared in a mouse model from 4 to 24 months of age (Woodruff-Pak et al., 2010). There was a significant age-related loss of Purkinje cells in the cerebellum, though pyramidal cell numbers in the hippocampus were stable. In addition, both cerebellar-mediated (eye-blink conditioning) and hippocampal-mediated (contextual fear conditioning) tasks were tested in these mice. At 24 months, the mice showed worse performance on the cerebellar eyeblink conditioning task, but there was no age effect for the hippocampal fear conditioning task (Woodruff-Pak et al., 2010). Taken together, the authors suggest that the cerebellum shows earlier senescence than the hippocampus, and this might have crucial implications for aging (Woodruff-Pak et al., 2010). Overall, across stereological studies in both humans and animal models, there is evidence to indicate age-related decreases in the numbers of cerebellar cells, and as such, a decrease in cerebellar volume.
2.1.2 Cerebellar Volume
Evidence of age differences in cerebellar volume assessed using MRI came starting in the early 1990s (Shah et al., 1991), and since that time a large body of literature has supported the idea that cerebellar volume is smaller in older individuals. First, we review work looking at the cerebellum as a whole, investigations using large divisions of the cerebellum (the entire cerebellar vermis, or cerebellar hemispheres), and those exploring gray matter globally using VBM. We will then focus on work, including our own, that has taken a finer grained approach to subdivide the cerebellum to investigate regional volumes with respect to aging.
Stereological techniques, similar to those previously described in animal models, have also been used in humans with neuroimaging approaches. Tang and colleagues (2001) conducted a longitudinal study of brain volume, and investigated the cerebral cortex, ventricles, and the cerebellum. Scans were completed about 4 years apart, on average. For each participant, scans were carefully investigated using point counting to determine the brain area, and subsequently the volume. Significant decreases in cerebellar volume were found, on the order of 1.2% per year with increased age (Tang et al., 2001).
Total cerebellar volume has been investigated primarily using automated techniques, with several studies reporting smaller cerebellar volume with advanced age (Hoogendam et al., 2012; Jernigan et al., 2001; Lee et al., 2009; Walhovd et al., 2011, 2005). Interestingly, cerebellar volume does not seem to associate linearly with age. In increasingly older individuals, cerebellar volume got smaller at a faster rate and was better described by a quadratic function (Hoogendam et al., 2012; Walhovd et al., 2011, 2005). Compared to other brain regions, the cerebellum showed significant negative correlations with advanced age, similar in magnitude to the prefrontal cortex and second only to correlations between age and the hippocampus (Jernigan et al., 2001). However, in both the cerebrum and the cerebellum in this sample white matter declines were greater in magnitude than gray matter declines (Jernigan et al., 2001), and more recent work has also indicated that white matter drives age differences in cerebellar volume (Hoogendam et al., 2012). However, they also noted that the underlying physiological factors that influence brain aging may differ across regions (Hoogendam et al., 2012).
Using only a mid-sagittal MRI in a cross-sectional study of adults between 26 and 79 years old, Shah and colleagues (Shah et al., 1991) revealed a negative correlation between age and volume of the cerebellar vermis. Additional studies have since confirmed this finding of smaller cerebellar vermis volume in older adults (Raz et al., 2001, 1998; Rhyu et al., 1999; Sullivan et al., 2000) and have indicated that the vermis lobules VI and VII as well as VIII-X may be particularly vulnerable to the effects of age (Raz et al., 1998). However, in a follow-up study by Raz and colleagues (2001) this finding was not replicated. Sex differences in cerebellar vermis volume have also been reported in advanced age (Rhyu et al., 1999).
In the cerebellar hemispheres age differences in volume have also been observed. In two studies with large cross-sectional samples from young adulthood through old age Raz and colleagues (Raz et al., 2001, 1998) found a reduction in volume of the cerebellar hemispheres with advanced age. However, the volume of the pons remained stable, perhaps indicating that the cerebellar cortex is selectively impacted by aging (Raz et al., 2001, 1998).
In addition to the studies that used either automatic segmentation methods (eg. Walhovd et al. 2005, 2011) or hand tracing (eg. Raz et al. 1998, 2001), whole brain methods of measuring gray matter (and white matter) density and methods that investigate regional covariance in volume have also been employed. VBM is a relatively automatic method of investigating gray matter that provides a comparison of the density of gray matter in a particular region (at the voxel-level) across subjects or groups (Ashburner and Friston, 2000). Typically completed as part of whole-brain analyses, VBM has indicated that gray matter in the cerebellum is impacted by aging. However, across multiple studies, this cerebellar impact is in addition to widespread effects in the prefrontal cortex and motor cortical areas (Abe et al., 2008; Giorgio et al., 2010; Kalpouzos et al., 2009). Within the cerebellum, the posterior lobe, specifically lobules Crus I and Crus II seem to be especially impacted by aging (Abe et al., 2008).
VBM data have also provided insight into the cerebellum in middle age as well. While our primary focus is on the cerebellum in advanced age, understanding the cerebellum over the course of the lifespan, particularly during middle age when many changes may begin to occur is important. Using a sample ranging from young adulthood to middle age, Terribilli and colleagues (Terribilli et al., 2011) found that regions of the prefrontal cortex as well as the cerebellum were negatively correlated with age; older individuals had less gray matter density.
Using VBM, covariance in gray matter between brain regions has also been investigated in aging (Alexander et al., 2006). The network of gray matter covariance related to age included bilateral frontal, temporal, and thalamic gray matter along with gray matter in the right cerebellum located in the regions of Crus I and II (Alexander et al., 2006). Interestingly these regions are similar to those associated with the resting state networks of Crus I and II (Bernard et al., 2012) and are consistent with targets of white matter from these lobules mapped using DTI (Salmi et al., 2010). Together, this may speak to deficits in cerebello-cortical networks that are associated with cognitive function, which may be causally associated with age-related cognitive declines.
Su and colleagues (2012) used multi-voxel pattern analysis (MVPA) to differentiate gray matter covariance patterns between young and older adults. MVPA is a method that allows researchers to compare the similarity of neural representations between groups and under different conditions, quantifying the distinctiveness of the patterns of brain activity or structure (Haxby, 2012; Haxby et al., 2001). Using this method, two patterns of covariance were able to classify individuals by age. The first group of regions was made up of the caudate and the pre- and post-central gyri, while the second group included the cerebellum, thalamus and inferior frontal gyrus (Su et al., 2012). Again, it is interesting that a pattern of brain covariance that is able to differentiate young from older adults includes cerebellar and prefrontal regions, which are together involved in cognitive function via cerebellar-thalamo-cortical loops.
Finally, though much of the work to date has been cross-sectional allowing for little inference about the changes in volume of the cerebellum in older adults over time, there are now several published longitudinal studies also supporting cerebellar volumetric declines with age (Raz et al., 2013, 2005; Smith et al., 2007). Within the five-year period, the volume of the cerebellum showed decreases from time point one to time point two, supporting that age per se rather than cohort effects underlie age differences in cerebellar volume (Raz et al., 2005). Additionally, it was noted that the rate of cerebellar shrinkage decreased from young to middle adulthood but then increased again with advanced age, and that the volumetric decreases in both the cerebellum and the hippocampus accelerated with age (Raz et al., 2005). Changes in cerebellar volume with advanced age have recently been shown in a time period as short as 6 months, though cognitive training may help mitigate these changes (Raz et al., 2013). Furthermore, longitudinal VBM investigations of the aging brain have also provided evidence for decreased cerebellar gray matter, in addition to decreases in total brain gray matter, prefrontal, parietal, and temporal cortical gray matter (Smith et al., 2007).
2.1.3 Regional Cerebellar Volume
It is clear that with age there are decreases in cerebellar volume in advanced age. However, it is notable that the majority of the work in this domain has focused on the volume of the entire cerebellum, or large subdivisions (vermis and hemispheres), despite the distinct lobular organization and known diverse functions of the structure. A role for the cerebellum in both cognitive and motor behavior has been postulated for quite some time (Leiner et al., 1993, 1989, 1986; Schmahmann and Sherman, 1998); moreover, recent work in non-human primates has demonstrated segregated cognitive and motor cerebello-cortical closed-loop circuits (Kelly and Strick 2003; Dum and Strick 2009; for a review see Strick et al. 2009). Similar distinct circuits have also been demonstrated in the human brain using fcMRI (Bernard et al., 2012; Bernard et al., 2013; Habas et al., 2009; Krienen and Buckner, 2009; O'Reilly et al., 2010), and crucially, a functional topography has been demonstrated in the cerebellum with fMRI and meta-analysis (Stoodley and Schmahmann, 2010, 2009; Stoodley et al., 2012). Together, this work underscores the importance of investigations of cerebellar sub-regions, as aging may differentially impact regions of the cerebellum associated with cognitive and motor function, and indeed some work has begun to address this question.
As noted above, using stereological methods, regional effects of aging were found in the anterior lobe of the cerebellum (Andersen et al., 2003). Using neuroimaging, Luft and colleagues (1999) investigated a group of individuals ranging in age from 19 to 73 years old. The authors subdivided the cerebellum into the medial and lateral hemispheres, and the former was further divided into three sub-regions. Additionally, they also subdivided the vermis into three sub-regions. Total cerebellar volume remains relatively stable until age 50, after which volumes are negatively correlated with age, but regionally, the vermis was markedly impacted by age, while there were minimal trend-level changes in the medial hemisphere, and the lateral hemisphere was not impacted by age at all (Luft et al., 1999). Luft and colleagues therefore suggested a medio-lateral gradient of age decreases in cerebellar volume. More recently, age differences in volume of the cerebellar vermis and the whole cerebellum were demonstrated by Paul and colleagues (2009). Though they subdivided the vermis and found that the posterior superior region (lobules VI, Crus I, Crus II, and VIIb) was particularly impacted by age, they did not compare the vermis to other more lateral regions of the cerebellum.
Most recently, we took a lobular approach to investigating age differences in cerebellar volume (Bernard and Seidler, 2013). Using cerebellar lobules as defined by the SUIT atlas (Diedrichsen, 2006; Diedrichsen et al., 2009), we found overall differences in cerebellar volume such that volume was significantly smaller in older adults. Interestingly however, we also found a significant age by lobule interaction, with a general pattern indicating that the anterior cerebellum and Crus I were most strongly impacted by aging (Bernard and Seidler, 2013). Importantly, this further underscores the necessity of work investigating lobular regions of the cerebellum in aging. Though we did not find evidence to support a medio-lateral gradient of age differences in cerebellar volume as in Luft et al. (1999), it is important to consider that we used different methodologies for subdividing the cerebellum and investigating volume. Taken together, both studies indicate that regional cerebellar morphology may be differentially impacted by aging. Table 1 provides a summary of the studies investigating cerebellar morphological differences in young and older adults, and highlights important patterns across these studies.
Table 1.
Summary of studies investigating morphological differences in the cerebellum between young and older adults. Both gray and white matter findings are included. DTI: diffusion tensor imaging; MVPA: multi-voxel pattern analysis; VBM: voxel based morphometry; Anterior cerebellum: lobules I-V; Posterior cerebellum: lobules VI-X.
| Study | Method | Volumetric Findings |
|---|---|---|
| Gray Matter | ||
| Andersen et al. 2003 | Post-mortem Stereology | -Significant age-associated loss of Purkinje and granule cells in anterior cerebellum -Age-related decreases in volume of anterior cerebellum, and overall decrease in cerebellar white matter volume |
| Andersen et al. 2012 | Post-mortem Stereology | - Differences in cerebellar volume in Alzheimer's patients compared to age-matched controls |
| Tang et al. 2001 | In vivo Stereology (Neuroimaging) | - Longitudinal findings indicating a 1.2% decrease in cerebellar volume with increasing age |
| Hoogendam et al. 2012 | Automatic Labeling | -Smaller cerebellar gray and white matter with increased age -Age differences in cerebellar volume largely driven by white matter |
| Jernigan et al. 2001 | Hand Tracing | - Significant negative correlations between total cerebellar volume and age, similar in magnitude to those in pre-frontal cortex, and second only to those in the hippocampus |
| Lee et al. 2009 | Automatic Labeling | - Total cerebellar volume significantly smaller in older adults |
| Walhovd et al. 2005 | Automatic Labeling | - Declines in gray and white matter volume with age that were best fit using a curvilinear function indicating greater declines with advanced age |
| Walhovd et al. 2011 | Automatic Labeling | - Cerebellar gray matter negatively associated with age, and further evidence for accelerated declines in cerebellar white matter based on a curvilinear function |
| Shah et al. 1991 | Hand Tracing | - Significant negative correlation between age and volume of anterior cerebellar vermis |
| Raz et al. 1998 | Hand Tracing | -Significant negative correlations with age in the cerebellar vermis and hemispheres -Vermis lobules VI, VII, and VIII-X may be especially vulnerable to age effects |
| Raz et al. 2001 | Hand Tracing | -Significant negative correlations with age in the cerebellar vermis and hemispheres. -Estimate that volume declines in the cerebellum occur at a rate of about 2% per decade - Does not replicate regional findings of Raz et al. 1998 |
| Rhyu et al. 1999 | Hand Tracing | - Smaller cerebellar vermis volume only in women over 50, and no differences in total cerebellar volume |
| Sullivan et al. 2000 | Hand Tracing combined with Automatic Segmentation | -Lower gray matter volume of the cerebellar vermis and hemispheres in advanced age -Also the case for lobules I-VIII |
| Abe et al. 2008 | VBM | - Negative age-related associations with gray matter density of Crus I and Crus II as well as in the prefrontal cortex |
| Kalpouzos et al. 2009 | VBM | - Lower gray matter density in widespread brain regions including the cerebellum |
| Giorgio et al. 2010 | VBM | - General widespread reduction in gray matter, including in the cerebellum, along with prefrontal and motor cortical regions |
| Teribilli et al. 2011 | VBM | - In middle age there are also negative relationships between age and gray matter and the cerebellum and prefrontal cortex (subjects 18-50 years old) |
| Alexander et al. 2006 | VBM and Gray Matter Covariance | - Network of gray matter covariance associated with advanced age that included Crus I and II in the cerebellum, along with bilateral frontal, temporal, and thalamic gray matter |
| Su et al. 2012 | MVPA | -Two patterns of volumetric covariance differentiate age groups -One of the two patterns includes gray matter of the cerebellum, thalamus, and inferior frontal gyrus |
| Raz et al. 2005 | Hand Tracing | - 5 year longitudinal study demonstrating volumetric loss over time in the cerebellar hemispheres |
| Smith et al. 2007 | VBM | - Bi-annual longitudinal scanning indicated a significant decline in overall gray matter volume with changes in the cerebellum, along with the frontal, parietal and temporal cortices and basal ganglia |
| Raz et al. 2013 | Hand Tracing | -6-month longitudinal study indicating declines in cerebellar volume, but they are attenuated with cognitive training -Also showed volumetric shrinkage in the lateral prefrontal cortex, hippocampus, and caudate |
| Luft et al. 1999 | Hand Tracing & Automatic Labeling | -Stable total cerebellar volume until about age 50, after which volume is smaller -Regional differences such that the vermis was greatly influenced by age, trend level decreases in the medial hemispheres, and no age differences in the lateral hemispheres |
| Paul et al. 2009 | Automatic Labeling | - Volumes of vermis lobules VI, Crus I, Crus II, and VIIb were especially impacted by advanced age |
| Bernard & Seidler 2013 | Automated Lobular Volume Measurement | - Older adults showed smaller total cerebellar volume, but an age by lobule interaction further indicated that the anterior cerebellum and Crus I were especially impacted by age |
| White Matter | ||
| Giorgio et al. 2010 | DTI | - Linear negative association between cerebellar white matter integrity and age |
| Fjell et al. 2013 | Automatic Segmentation & Labeling | - Non-linear decrease in cerebellar white matter volume with advanced age that was also confirmed with a longitudinal sample |
| Bennett et al. 2010 | DTI | -Lower cerebellar white matter integrity, as well as in other cortical brain regions in older adults -Impact on cerebellar white matter indicates that anterior-posterior gradient of decline is not fully followed |
| Abe et al. 2008 | DTI | - Lower FA with age in right cerebellum, and overall increase in diffusivity in all cerebellar white matter, indicative of age-related loss of integrity |
| Pagani et al. 2008 | DTI-based Volume Calculations | - Negative correlations between age and white matter volume in the left superior cerebellar peduncle |
| Cavallari et al. 2013 | DTI | -White matter integrity of cerebellar peduncles were negatively correlated with age -Mobility measures were also associated with white matter in the cerebellar peduncles |
| Kafri et al. 2013 | DTI | - White matter integrity of the superior cerebellar peduncles was lower in older adults with gait disorder |
2.1.4 Diffusion Tensor Imaging of White Matter
Diffusion tensor imaging (DTI) assesses the structural integrity of white matter in the brain. DTI measures the diffusion of water along the axons in the brain, and is quantified as fractional anisotropy (FA), radial diffusivity (RD) -- which is interpreted as a measure of myelination -- and axial diffusivity which indexes axonal integrity. DTI in older adults has provided a wealth of information about the aging brain, and in general, white matter integrity is decreased with advanced age (for a review see Sullivan and Pfefferbaum 2006). In reviewing the literature on white matter structural integrity in aging, Sullivan and Pfefferbaum noted that white matter differences with age follow an anterior to posterior gradient, such that prefrontal white matter is typically most impacted by age, along with the corpus callosum. However, they also noted that the cerebellum, possibly due to its interactions with the prefrontal cortex and its role in cognitive function, may also contribute significantly to age-related performance deficits (Sullivan and Pfefferbaum, 2006). The consistent findings of decreased white matter integrity in the prefrontal cortex and corpus callosum have been replicated repeatedly, and are beyond the scope of this review (e.g. Fling et al., 2012, 2011; Head et al., 2004; O'Sullivan et al., 2001; Ota et al., 2006). Importantly, however, though cerebello-cortical networks and brainstem white matter have been delineated using DTI in young adults (Habas and Cabanis, 2007; Salmi et al., 2010), new work has only just begun to investigate cerebellar white matter volume and structural integrity in aging.
A whole brain analysis of gray and white matter volume as well as white matter structural integrity revealed linear relationships between white matter volume and age in the cerebellum such that white matter volume was smaller in older adults (Giorgio et al., 2010). However, a large cross-sectional study demonstrated that cerebellar white matter actually follows non-linear patterns of decline with advanced age and this pattern was confirmed in a longitudinal follow-up sample (Fjell et al., 2013). Using DTI, Bennett and colleagues (2010) also demonstrated age-related decreases in cerebellar white matter integrity (both FA and RD) along with decreases in other brain regions. However, they argue that the degree to which the cerebellum was impacted by aging indicates that patterns of age-related white matter integrity decreases are likely more complex than just following the hypothesized anterior-posterior gradient (Bennett et al., 2010). Finally, decreased FA in the right cerebellum and an overall increase in diffusivity in cerebellar white matter were detected by Abe and colleagues (2008).
Using a voxel based technique to investigate changes in white matter fiber bundles with age, Pagani and colleagues (2008) found correlations between white matter volume (calculated based on DTI measures of FA) and age in the left superior cerebellar peduncle. Older adults had smaller volume in the left cerebellar peduncle. Age differences were also found in the corona radiata, the cingulum, and in the fornix (Pagani et al., 2008).
Two recent studies focusing on mobility and gait in aging have also implicated the white matter integrity of the cerebellar peduncles (Cavallari et al., 2013; Kafri et al., 2013). The cerebellar peduncles are white matter tracts of the cerebellum. The superior cerebellar peduncle is the primary source of output for the cerebellum to the midbrain, while the middle and inferior cerebellar peduncles connect the cerebellum with the cortex and spinal and vestibular regions, respectively. In a large sample of older adults between the ages of 75 and 90, with no other known signs of cerebellar dysfunction, both mobility (self-paced maximum walking speed, typical walking velocity, standing balance) and white matter structural integrity of the cerebellar peduncles (superior, inferior, middle) were investigated (Cavallari et al., 2013). Participants were grouped based on their mobility (high versus low mobility scores), and there were significant differences in white matter integrity of the cerebellar peduncles between the high and low mobility groups. Furthermore, within this sample of older adults, multiple measures of white matter integrity (including FA and RD, along with both axial and mean diffusivity) in the cerebellar peduncles were correlated with age and mobility measures (Cavallari et al., 2013). This work highlights the importance of cerebellar white matter for mobility in older adults, and provides some evidence that age does negatively impact cerebellar white matter structural integrity.
Kafri and colleagues (2013) acquired DTI scans on a sample of older adults with high-level gait disorder along with older and middle aged controls. Regions of interest in the superior cerebellar peduncles, internal capsule (white matter tract in the brain), and cerebral peduncles (white matter in the midbrain) were compared across the groups. The older adults with high-level gait disorder had significantly lower FA in all of the investigated regions except for the left internal capsule. Thus, age-related declines in balance and gait may be linked to cerebellar white matter structural integrity, though further work is needed to investigate this directly, and in comparison to young adult control groups. Furthermore, work investigating cognitive behaviors with respect to these white matter tracts would be particularly informative and provide support for the framework presented by Sullivan and Pfefferbaum (2006) suggesting that the cerebellum may be important for age-related declines in cognitive performance due to the rich connections between the cerebellum and prefrontal cortex.
3.1 Cerebellar Morphology and Performance
The large body of research demonstrating age differences in cerebellar morphology is illuminating and points to the cerebellum as a potentially important area of study in aging. Further support for the role of the cerebellum in aging comes from work linking morphological differences to behavioral performance. While the cerebellum is not causally implicated in age-related deficits, these relationships provide insight into the potential contributions of the cerebellum to age-related motor and cognitive declines.
Several studies have linked the volume of the cerebellar hemispheres to motor function in older adults. In one instance, adults between 22 to 80 years old completed a procedural learning task (pursuit rotor) and two working memory tasks (verbal and non-verbal). Volumes of the cerebellar hemispheres, striatum, prefrontal cortex and the hippocampus were all investigated as well (Raz et al., 2000). Not surprisingly, the young adults performed the pursuit rotor task better than the older adults. Smaller volumes in the cerebellar hemispheres and putamen were associated with worse performance (less time on target), and the authors speculate that shrinkage of the cerebellum and putamen mediate the impact of age on procedural learning (Raz et al., 2000). During the later stages of learning, the putamen was no longer related to performance, though the cerebellum and non-verbal working memory were both important factors (Raz et al., 2000). It is also of note that the volume of the cerebellar hemispheres was correlated with performance on both the verbal and non-verbal working memory tasks (Raz et al., 2000). Together this work indicates that reduced cerebellar volume may contribute to procedural learning deficits in older adults, and that it also plays a role in working memory performance.
Eye-blink conditioning is also an implicit form of learning. In this paradigm, a puff of air is administered to the eye resulting in a blink. Prior to the air-puff a tone is played. After learning the association between the tone and air-puff individuals make anticipatory eye-blinks (cf. Woodruff-Pak et al. 2000). The degree to which young adults learn this task, quantified by anticipatory eye-blinks, is correlated with total cerebellar volume (Woodruff-Pak et al. 2000). Older adults also show an association between total cerebellar volume and eye blink conditioning, but have smaller cerebellar volumes than young adults and do not form as strong of an association between the tone and air puff resulting in fewer anticipatory eye-blinks (Woodruff-pak et al., 2001). Again, this implicates the cerebellum in deficits of implicit learning in older adults.
Walking and balance in older adults have also been investigated in relation to the cerebellum. Rosano and colleagues (2007) measured walking speed and standing balance (the amount of time spent standing with the feet in tandem) along with regional gray matter volume determined using automatic labeling methods. Slower walking speed was associated with smaller volume in both the cerebellum and the prefrontal cortex whereas poorer balance was associated with volume of the cerebellum, putamen, and the posterior parietal lobe (Rosano et al., 2007). Importantly this work also included numerous covariates to take into account physical health and cognitive abilities. Though for both balance and walking speed multiple brain regions are implicated, these findings further implicate the cerebellum in motor declines in older adults and also support the idea that cerebellar-prefrontal circuits may be especially important for motor and cognitive performance in older age.
Cognitive function has also been investigated, particularly with respect to the volume of the cerebellar vermis (MacLullich et al., 2004; Miller et al., 2013; Paul et al., 2009). Both MacLullich and colleagues and Miller and colleagues investigated the volume of four subregions of the cerebellar vermis with respect to a large battery of cognitive tests (MacLullich et al., 2004; Miller et al., 2013). In the earlier study the authors found that the vermis region associated with lobule VI, Crus I and Crus II was correlated with processing speed, memory, and visual reproduction, while a more anterior region of the vermis (lobules IV and V) was correlated with visual reproduction. In all instances, larger volume was associated with better performance (MacLullich et al., 2004). The results from Miller and colleagues (2013) are consistent with the earlier study. However, they also found relationships between vermis lobules VIII-X and general cognitive function. There were several additional relationships with vermis lobules IV and V including reading ability, speed of processing and executive function (Miller et al., 2013). Generally, preserved cerebellar volume was indicative of better cognitive functioning in older adults.
Paul and colleagues also investigated cognitive function and the cerebellar vermis along with the prefrontal cortex in a large adult sample (Paul et al., 2009). In the older adults, both the prefrontal cortex and the cerebellar vermis were smaller than in young adults. Furthermore, volume of both the prefrontal cortex and vermis were associated with cognitive performance (Paul et al., 2009). However, when the authors controlled for the prefrontal cortex in their analysis of the vermis, the relationships were no longer significant (Paul et al., 2009). Thus, while the cerebellum likely plays an important role in cognitive performance in older adults, it is also important to consider additional brain regions, as well as the potential interactions between the cerebellum and cortical regions.
Cerebellar and cortical gray matter as measured using VBM have also been investigated with respect to cognition in older adults. Lee and colleagues looked at gray matter with respect to performance on the Weschler adult intelligence scale (WAIS), a measure of general intelligence, in older adults (Lee et al., 2005). Though they initially expected the relationships between intelligence and gray matter to be in the prefrontal cortex, the significant associations were actually found in the posterior cerebellum, roughly in the region of Crus I (Lee et al., 2005). The authors also noted that age was negatively correlated with gray matter density in this area of the cerebellum (and in the prefrontal cortex), when controlling for education levels.
Consistent with these findings, cerebellar gray matter indexed with VBM has also been linked to general cognitive ability as quantified with non-verbal reasoning, speed of processing, and both short and long term memory (Hogan et al., 2011). Regions in the cerebellum as well as the cortex were positively correlated with general intelligence. Interestingly, within the cerebellum alone, when prefrontal cortex gray and white matter were controlled for, this relationship remained significant, further implicating the cerebellum in general cognitive function in older adults (Hogan et al., 2011). Taken together both Hogan and colleagues (2011) and Lee and colleagues (2005) have provided converging evidence indicating the potential importance of the cerebellum in general cognitive function in advanced age.
In a unique approach looking at structural covariance between brain regions, gray and white matter variation in both the cerebellum and prefrontal cortex were associated with processing speed declines in older adults (Eckert et al., 2010). This approach allowed the authors to look at relationships between brain regions and their links with behavior. The relationships between the prefrontal cortex and cerebellum with respect to performance not only highlight the importance of both of these regions for processing speed in older adults, but also underscore the utility of looking at brain regions together and as part of networks that influence behavior.
Finally, we have recently investigated regional cerebellar volume with respect to both motor and cognitive performance in young and older adults (Bernard and Seidler, 2013), given the functional topography of the human cerebellum (Stoodley and Schmahmann, 2009; Stoodley et al., 2012). As described above, we looked at individual lobular volumes in both the cerebellar hemispheres and the vermis. In addition, young and older adult participants completed a battery of motor and cognitive assessments including working memory, executive function measures, balance, motor learning (visuomotor adaptation), and timing. When pooling across both age groups, we found that working memory performance was positively associated with volume in the posterior cerebellum (Crus II through lobule X), whereas motor performance (timing, balance, choice reaction time) was often negatively associated with volumes in the posterior cerebellum and Crus I (Bernard and Seidler, 2013). While this was somewhat surprising at first, we postulate that this is due to interference from cognitive processing given that the cerebellar regions in question are often associated with cognitive function and networks involving the prefrontal cortices (Bernard and Seidler, 2013). Additionally, we looked at differential relationships between regional volume and behavior in young and older adults. We found evidence of differential relationship across several tasks, indicating that the cerebellum may be differentially engaged in these tasks in older adults, perhaps due to volumetric differences in these regions (Bernard and Seidler, 2013).
Across this emerging literature investigating cerebellar morphology with respect to behavior in older adults, several clear patterns emerge. Table 2 provides a summary of these findings. First and most obviously is that the cerebellum is related to performance across a variety of motor and cognitive task domains in older adults, indicating that volumetric differences in older adults may be a key factor contributing to the age-related declines seen in both the motor and cognitive task domains. Second, sub-regions of the cerebellum seem to be distinctly related to performance in older adults. Though there is relatively little work to date investigating cerebellar sub-regions and behavior, this is an important area of future inquiry, and thus far seems to be an important approach to take when investigating the cerebellum with respect to behavior. Third, the notion of covariance between the cerebellum and additional cortical regions, particularly the prefrontal cortex is an important one. Considering networks with respect to behavior is important for our understanding of the aging mind and brain, and doing so by investigating covariance between brain volumes provides important insights into this important scientific question. Finally, across studies investigating the cerebellum and the prefrontal cortex, in several cases, the cerebellum is as good a predictor of behavioral performance declines in older adults as the prefrontal cortex, if not better (Eckert et al., 2010; Hogan et al., 2011; Lee et al., 2005). While it is unclear whether or not the stronger associations with the cerebellum and behavioral performance are due to ease of measurement in the cerebellum relative to the prefrontal cortex, or because the cerebellum is indeed the better predictor of performance declines remains unknown. However, the fact that the cerebellum closely tracks the patterns and relationships seen in the prefrontal cortex is important, and makes future investigation of this structure with respect to aging especially informative.
Table 2.
A summary of relationships between cerebellar morphology and motor and cognitive performance in older adults. Notably, in several instances, the magnitude of these relationships were comparable to, or larger than those between behavior and prefrontal cortex morphology. WAIS: Weschler Adult Intelligence Scale; STM: short-term memory; LTM: long-term memory; VBM: voxel-based morphometry; Anterior cerebellum: lobules I-V; Posterior cerebellum: lobules VI-X.
| Study | Method | Task(s) | Relationships with Cerebellum |
|---|---|---|---|
| Raz et al. 2000 | Hand Tracing | -Procedural Learning -Verbal and Non-Verbal Working Memory |
-Cerebellar hemisphere and putamen volume positively associated with early learning across sample from ages 22-80 -Cerebellar hemisphere volume remained correlated during late learning and was also associated with working memory measures |
| Woodruff-Pak et al. 2000 and 2001 | Hand Tracing | - Eye-blink conditioning | - Positive correlation between total cerebellar volume and eye-blink conditioning in young adults alone, and when collapsing across young and older adults |
| Rosano et al. 2007 | Automatic Labeling | -Walking speed -Standing balance |
-Slower walking speed negatively correlated with cerebellum and PFC volumes -Standing balance negatively correlated with cerebellum, putamen, and posterior parietal lobe volume |
| MacLullich et al. 2004 | Hand Tracing | -Raven's Matrices -Paragraph recall -Memory -Visuospatial memory -Verbal fluency -Processing speed -WAIS |
-Vermis (lobules VI, Crus I and Crus II) volume associated with processing speed, memory, and visuospatial memory -Anterior vermis volume also associated with visuospatial memory |
| Miller et al. 2013 | Hand Tracing | - Battery comparabley to MacLullich et al. 2004 | - Results generally consistent with MacLullich et al. 2004 -In addition, relationships between vermis lobules VIII-X and general cognitive function along with vermis lobules IV and V with reading ability, speed of processing, and executive function -Across both studies, preserved volume associated with better performance |
| Paul et al., 2009 | Automatic Labeling | -Motor tapping -Reverse digit span -Attention switching -Verbal interference -Spatial processing (maze task) -Verbal Fluency -Timing |
-Prefrontal cortical and cerebellar vermis volumes associated with cognitive performance (attention & spatial processing) -When controlling for prefrontal cortical volume, relationships with cerebellar vermis were no longer significant |
| Lee et al. 2005 | VBM | - WAIS | -Gray matter density in Crus I was correlated with general intelligence assessed by the WAIS -No relationships with PFC, which was counter to the authors’ hypotheses |
| Hogan et al. 2011 | VBM | -Speed of Processing -Non-Verbal Reasoning -STM -LTM |
-Positive correlations with cerebellum and cortex gray matter (general intelligence) -When controlling for PFC cerebellum alone remained as a strong predictor of general intelligence |
| Eckert et al. 2010 | Structural Covariance (source based morphometry; SBM) | - Speed of Processing | - Structural covariance between gray and white mater of the PFC and cerebellum associated with declines in processing speed |
| Bernard and Seidler 2013 | Automated Lobular Volume Measurement | -Verbal Working Memory -Executive Function -Balance -Choice RT -Motor Adaptation -Timing |
- Across age groups working memory performance positively associated with posterior cerebellum -Timing, balance, & choice RT were negatively associated with posterior cerebellum volume across both groups -Differential engagement of cerebellum across age groups for some tasks |
4.1 Functional Investigations of the Aging Cerebellum
4.1.1 fMRI Investigations
During the performance of both motor and cognitive tasks, older adults show different patterns of brain activation (Cabeza, 2002; Mattay et al., 2002; Naccarato et al., 2006; Reuter-Lorenz et al., 1999). These differences are manifest as increased bilateral activation in the motor and prefrontal cortices during motor and cognitive tasks, respectively (Mattay et al., 2002; Naccarato et al., 2006; Reuter-Lorenz et al., 1999). This pattern of more bilateral activation seems to extend to the cerebellum as well. In their finger tapping study in young and older adults, Mattay and colleagues (2002) also found bilateral activation in the cerebellum in older adults relative to young adults, indicating that this bilateral activation pattern extends to the cerebellum.
Similarly, when older adults were asked to perform memorized sequences of finger movements, though older individuals were able to match the performance of young individuals, they did so at a slower pace (Wu and Hallett, 2005). Functional activation during the performance of these sequences of movements was also greater in older adults. There was more bilateral activation in motor cortical areas, as well as in the anterior cerebellum (lobules I-V; Wu and Hallett, 2005), consistent with the findings of Mattay and colleagues (2002). Interestingly however, the authors also found areas of greater deactivation in the young adults, which correspond largely to the default mode network (Wu and Hallett, 2005). Thus, not only do older adults recruit additional cortical motor and cerebellar brain resources during motor task performance, they also fail to deactivate the default mode network to the same extent as young adults, which may be detrimental to performance.
More recently, new imaging analysis methods have been used to investigate these patterns of bilateral activation during motor tasks in the cortex and subcortical regions. Using multi-voxel pattern analysis to compare the neural representations associated with unimanual finger tapping in young and older adults, a decrease in the distinctiveness of finger representations was revealed in older adults compared to young adult controls (Carp et al., 2011). Importantly, the decrease in distinctiveness was seen in both cortical motor regions as well as in the cerebellum.
Finally, evidence for increased bilateral motor activation in the cerebellum is also seen when participants complete grip force tasks (Ward and Frackowiak, 2003). Here, young and older adult participants completed an isometric force task with both the dominant and the non-dominant hand. Consistent with finger tapping and sequencing paradigms, the older adults again showed a more bilateral pattern of activation in the cerebral cortex and the cerebellum (Ward and Frackowiak, 2003).
In addition to the finding of increased bilateral cerebellar activation during motor tasks in older adults, there is also evidence for decreased activation in the cerebellum. In an investigation of internally and externally generated hand movements, though there was greater activity in the primary motor cortex of older adults, Taniwaki and colleagues also found decreased activity in the cerebellum in old relative to younger adults (Taniwaki et al., 2007). Additionally, because they were interested in the interactions between cerebellar and basal ganglia loops, the authors used structural equation modeling to look at these networks. They found significant age differences in the cerebellar and basal ganglia networks such that connectivity decreases in the cerebellar network were found during the externally triggered task whereas basal ganglia decreases were associated with the internally generated task (Taniwaki et al., 2007). Together, this work further demonstrates that the cerebellum is functionally impacted with advanced age.
Relatedly, we (Bo et al., 2011a) recently demonstrated that older adults show less activation relative to young adults in lobule VI of the cerebellum during symbolic motor learning. In young adults this region was preferentially activated under conditions in which movements were symbolically cued, and activation was correlated with the degree of learning over the course of the test session (Bo et al., 2011b). A similar correlation between lobule VI activation magnitude and learning was seen in older adults, but along with their overall decreased activation, they also showed less learning when compared to young adults (Bo et al., 2011a).
Work on sensory perception has also demonstrated changes in cerebellar functional activation with advanced age. Using an odor discrimination task and a test of olfactory threshold, Ferdon and Murphy ( 2003) investigated olfaction in older adults with a specific focus on Crus I and II as well as lobule VI. The older adults showed poorer performance on the odor identification test, and also had decreased functional activity in Crus I and II when compared to the young adults (Ferdon and Murphy, 2003). This work implicates the cerebellum in sensory function in older adults. Furthermore, though this was not a cognitive task, age differences were seen in areas of the cerebellum (Crus I and II) often associated with cognitive functions and networks with the prefrontal cortex (Bernard et al., 2012; Chen and Desmond, 2005b; Stoodley and Schmahmann, 2009; Stoodley et al., 2012) and this may indicate that there would be decreased activation in similar areas in older adults during cognitive task performance. Crucially, it is notable that past work may have found such differences in the cerebellum during cognitive performance, but they were not the main interest of the investigation. As a result, cerebellar findings are perhaps lost in tables reporting foci of activation. Both meta-analysis of the extant literature as well as direct investigations of the cerebellum during the performance of non-motor tasks in older adults are needed in order to more fully understand the role of the cerebellum in age-related cognitive performance declines.
4.1.2 Resting state functional connectivity
Resting state functional connectivity MRI (fcMRI) measures correlations in the blood oxygen level dependent (BOLD) signal between different brain regions at rest (Biswal et al., 1995, 2010). fcMRI is particularly useful for studying aging as the results are not confounded by task demands which may differ between young and older adults. Furthermore, the scan is relatively short which is beneficial for older individuals who may be uncomfortable in the scanner environment. To date, particular attention has been paid to the default mode network and motor networks in aging populations (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Langan et al., 2010; Wu et al., 2007). Across these studies, a general decrease in resting state connectivity was seen with advanced age. Furthermore, these changes in default mode network connectivity are also associated with behavioral performance declines in older adults (Andrews-Hanna et al., 2007). A complete overview of the findings of studies investigating connectivity in aging is beyond the scope of this review, and it has recently been reviewed elsewhere (Ferreira and Busatto, 2013). However, the utility of fcMRI to better understand the aging brain is clear, though there has been relatively little work to date investigating cerebello-cortical networks, with the exception of one study (Bernard et al., 2013). With that said, some of the work investigating resting state networks in the aging brain has implicated the cerebellum.
In addition to the aforementioned investigation of the motor network by Wu and colleagues which demonstrated a decrease in resting state connectivity with age (Wu et al., 2007), this group also investigated regional homogeneity (ReHo) in young and older adults (Wu et al., 2007). ReHo measures the similarity of the time series of a voxel in the resting state with respect to its neighbors. When comparing ReHo within regions of the motor network in young and older adults, they found decreased ReHo in motor cortical regions, the thalamus, and both the left and right cerebellum (Wu et al., 2007). The authors suggest that perhaps this is due in part to histological changes with age. This work further indicates changes in motor networks at rest, and also implicates the cerebellum to some degree.
Recently, we directly investigated cerebello-cortical resting state networks in young and older adults (Bernard et al., 2013). Using masks of each lobule of the right hemisphere and vermis, we were able to compare resting state networks in older adults to those that we had previously defined in young adults (Bernard et al., 2012), while controlling for lobular volume. In general, our findings were consistent with the overall pattern of decreased functional connectivity in older adults (Andrews-Hanna et al. 2007; Wu, Zang, Wang, Long, Hallett, et al. 2007; Damoiseaux et al. 2008; Langan et al. 2010; reviewed in Ferreira and Busatto 2013), as this was the case across all of the networks we investigated (Bernard et al., 2013). Interestingly however, connectivity between the cerebellum and both the basal ganglia and medial temporal lobe were particularly impacted by advanced age. Importantly, decreased connectivity in the cerebello-cortical networks was also behaviorally relevant. That is, connectivity strength was correlated with both motor (manual dexterity, timing, balance confidence) and cognitive (working memory) performance in older adults (Bernard et al., 2013), with greater connectivity strength associated with better performance. While more work investigating cerebello-cortical networks in older adults is needed, these findings further highlight the role of the cerebellum in aging. The decreased connectivity of functional networks in older adults and their implications for behavior indicate the cerebellum may play a role in many of the performance declines seen with advanced age.
5.1 A Framework for the Cerebellum in Aging
It is clear that the cerebellum in older adults differs from that of young adults not only in morphology, but also with respect to resting state networks and functional activation during task performance. Furthermore, these differences seem to contribute, at least in part, to many of the age related motor and cognitive performance declines. However, though the cerebellum is different in older adults and these differences also contribute to behavioral outcomes, the question of how the cerebellum impacts behavior in older adults remains open. Certainly smaller volume may directly impact the performance of a variety of tasks, but the cerebellum must not be considered as an independently acting structure, and its networks with cortical and other subcortical regions such as the basal ganglia are perhaps the more important feature when conceptualizing the role of the cerebellum in motor and cognitive aging.
We know now that the cerebellum has distinct networks with the cerebral cortex, forming closed-loop circuits with motor and cognitive cortical regions (Dum and Strick, 2009; Kelly and Strick, 2003; Strick et al., 2009) and also with the basal ganglia (Bostan and Strick, 2010; Hoshi et al., 2005). Altered interactions between the cerebellum and the cortex or basal ganglia due in part to the process of normal aging have the potential to result in a variety of behavioral disruptions. In particular, the disrupted cortico-cerebellar networks (Bernard et al., 2013), along with both the morphological and functional differences between the cerebella of young and older adults (eg. Bernard and Seidler, 2013; Bo et al., 2011b; Carp et al., 2011; Eckert et al., 2010; Raz et al., 2000) may affect the formation of new internal models, and/or result in the degradation of existing internal models of both motor and cognitive behaviors.
Because of the role of the cerebellum in such diverse behaviors in both the motor and cognitive domain, in conjunction with the distinct closed-loop cerebello-cortical circuits, it has been proposed that the cerebellum is important for the formation of internal models of behavior (Ito, 2008; Ramnani, 2006). In the motor domain, it is thought that the cerebellum helps control movements through inverse and forward models that allow for one to predict the trajectory of a movement and then engage in rapid online corrections (Ito, 2008). Indeed, over the course of learning a new motor task, there are changes in the engagement of the cerebellum (Imamizu et al., 2000) and in the excitability of the cerebellum (Jayaram et al., 2011) both of which may be indicative of the formation of new internal models of actions. A similar framework with respect to internal models for cognitive behaviors has also been proposed. However in this case, the cerebellum forms internal models of the mental processes that are associated with said tasks (Ito, 2008) and these models allow for fluid cognitive performance. Though the study of the cerebellum in advanced age is still an emerging field, we speculate that the age-related differences in morphology, functional activation, and networks of the cerebellum may result in the degradation of capacity to predict behavioral outcomes (cf. Miall et al., 1993), and impaired formation of new internal models resulting in a wide array of performance declines. Specifically, we propose that in advanced age, forward models are especially impacted, though inverse models may also be impacted.
As discussed in detail above, there are morphological differences in the cerebellum between young and older adults, and we and others have linked cerebellar morphology to behavioral performance in older adults (Bernard and Seidler, 2013; Paul et al., 2009; Raz et al., 2000). To a certain degree it is not surprising that cerebellar morphological differences with age are associated with behavioral performance deficits. However, why these volumetric differences result in behavioral deficits is still unexplored, and the most promising suggestion is due to difficulty in forming or accessing internal models for a given task. Thus, in advanced age, the inability to appropriately monitor motor and cognitive behavior through the cerebellum (using forward models) may result in performance declines. Indeed Ito makes a similar argument with respect to pathology (Ito, 2008). While healthy aging is certainly not a pathological state, similar processes may be occurring on a smaller scale in the aging brain resulting in degraded internal models of behavior. We suggest that the forward models that allow for the monitoring of behavior through a corollary discharge that allows for the comparison of ongoing behavior with the predicted behavior (Ito, 2008) are degraded. Further supporting this notion is the evidence pointing to decreased functional connectivity between the cerebellum and cerebral cortex with advanced age (Bernard et al., 2013) along with data demonstrating decreased structural integrity of cerebellar white matter (Bennett et al., 2010; Fjell et al., 2013; Giorgio et al., 2010). If in advanced age, the cerebellum is further unable to communicate with the cerebral cortex due to degraded connections, important updates about performance due to monitoring of the actual behavior (either a movement or an unseen mental process) cannot be transmitted as effectively resulting in behavioral deficits. In a similar vein, this would also impede the formation of precise internal models of behavior negatively impacting behavioral performance in both the motor and cognitive domains during the learning of new tasks (Figure 1). In fact, recent behavioral evidence in the domain of proprioception supports the notion that internal models are different in older adults when compared to young adults (Boisgontier and Nougier, 2013).
Figure 1.
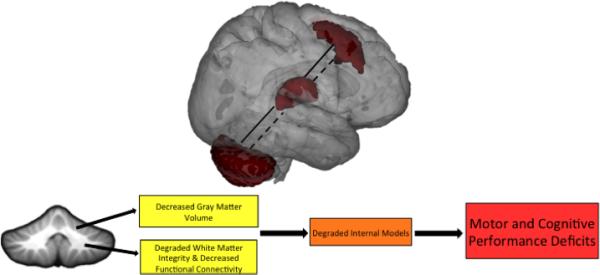
Cerebellar-prefrontal interactions (via the thalamus) are illustrated as an example circuit that is disrupted in advanced age. In young adults (solid lines), we see strong functional connectivity between these regions. In older adults (dashed lines), connectivity is decreased. We suggest that age effects on these cerebellar-prefrontal interactions may play a critical role in motor and cognitive performance declines in advanced age. Disruption of additional cerebello-cortical circuits, including those with the motor cortex and basal ganglia, contribute to the wide variety of motor and cognitive performance declines seen in age. The bottom schematic illustrates the hypothesized framework linking the cerebellum to age-related declines in motor and cognitive performance. With advanced age, there is a loss of cerebellar volume, and there are also degraded connections, both structural and functional, between the cerebellum and cerebral cortex. Together, we suggest that this results in degraded internal models that have a negative impact across task domains, given the closed-loop circuitry of the cerebellum. Ultimately, this contributes to the motor and cognitive performance declines seen in older adulthood. Non-invasive brain stimulation and behavioral interventions targeting the cerebellum may help mitigate some of these declines.
Indeed, such a framework might explain, at least in part, the associations between the cerebellum and behavioral performance in older adults (Bernard et al., 2013; Bernard and Seidler, 2013; MacLullich et al., 2004; Miller et al., 2013; Raz et al., 2000; Rosano et al., 2007), particularly given that as discussed above, these relationships are often comparable to or larger than behavioral associations with the prefrontal cortex. Degraded internal models would have a broad impact on behavior generally, and would also do so across task domains. Such a broad impact on behavior may explain at least in part why the cerebellum is so strongly associated with performance decrements in older adults, even above and beyond the prefrontal cortex. However, further work is needed to test this notion directly, and investigations of the interactions between the cerebellum and prefrontal cortex are also warranted.
In addition to evidence indicating that the cerebellum is important for the formation of internal models of behavior, others have argued that the cerebellum is important for the timing of behaviors (Keele and Ivry, 1990; Spencer and Ivry, 2013), or that the main function of the cerebellum is in sequence processing (Molinari and Leggio, 2013; Molinari and Petrosini, 1997). Neither of these hypotheses are mutually exclusive of internal models, and indeed they may be indicative of the computations made by the cerebellum as part of these internal models. Both timing and sequencing deficits of the cerebellum may also explain the contributions of the cerebellum to age-related performance declines; several studies have reported age deficits in timing (Baudouin et al., 2006; Block et al., 1998; Craik and Hay, 1999; McCormack et al., 2002). There is also substantial evidence to indicate that older adults have deficits in sequence learning (Bo and Seidler, 2010; Bo et al., 2011a, 2009; Curran, 1997), and as we noted above, in our recent work we found decreased activation in the cerebellum in old relative to younger adults, that was correlated with learning (Bo et al., 2011a). These sequencing deficits in advanced age may be indicative of a broader cerebellar deficit in sequencing, which may extend to the non-motor domain, contributing to the declines in motor and cognitive processing seen in advanced age. Thus the aging of the cerebellum may produce timing and/or sequencing deficits, which in turn result in declines in both motor and cognitive task performance. Future work directly comparing these possible accounts is needed to better understand the role of the cerebellum in advanced age.
Finally, altered cerebellar-basal ganglia interactions may be an important contributor to performance declines in older adults. Work in animal models has demonstrated anatomical connections between the cerebellum and the basal ganglia (Bostan and Strick, 2010; Hoshi et al., 2005), and we have found similar connections in the resting state (Bernard et al., 2012, 2013; Kwak et al., 2010). However, in older adults, there are decreases in the connectivity between these regions in the resting state, and connectivity strength is correlated with behavioral performance on both motor and cognitive tasks (Bernard et al., 2013). In addition to the proposed deficits with respect to internal models in older adults, the age differences in cerebellar-basal ganglia interactions may be particularly important for behavior as well. The specific information shared between these two structures is not clear, but given that the cerebellum monitors behaviors and detects errors within the internal model framework (Ito, 2008) and that the basal ganglia play a role in goal driven behavior, it may be the case that the cerebellum is sending error information to the basal ganglia. In turn, this may result in the updating of goals. If this system of communication is interrupted or degraded due to age-related changes in connectivity, then behavior could in turn be affected across multiple domains. Admittedly however, all of these hypotheses at this point remain speculative. Future work is clearly needed to better understand and elucidate the role of the cerebellum in aging, along with the specific mechanisms resulting in age-related performance declines.
6.1 Future Directions
6.1.2. Cerebellar Interventions in Aging
Though it is obvious that there is still a great deal of work to be done investigating the functional brain changes in the cerebellum with advanced age, investigations into cerebellar targets for interventions are also needed. Given that the evidence to date from the volumetric literature points to a role for the cerebellum in behavioral declines associated with aging (e.g. Bernard & Seidler, 2013; Hogan et al., 2011; Raz, Williamson, Gunning-Dixon, Head, & Acker, 2000; Woodruff-pak et al., 2001), interventions targeting the cerebellum may help mitigate some of the motor and cognitive deficits in older adults.
Broadly speaking, such interventions may benefit from taking into account the scaffolding theory of aging and cognition (STAC) (Park and Reuter-Lorenz, 2009; Reuter-lorenz and Park, 2010). According to this theory, physical changes in the brain due to age result in altered brain function (with respect to both resting state networks and functional activation). But, to compensate for this the brain creates new circuits that may not be as efficient as those in young adults (such as the increased bilateral activation seen in older adults), allowing for some maintenance of cognitive function in late life (Park and Reuter-Lorenz, 2009; Reuter-lorenz and Park, 2010). The increased bilateral activation in the cerebellum in advanced age that was recently reported (Carp et al., 2011) may perhaps contribute to this scaffolding. However, targeting the cerebellum is also important for improving this scaffolding. As we have discussed, there are structural changes in the cerebellum with advance age, and by targeting the structure and its networks, we may be able to improve these scaffolds in older adults, allowing for continued maintenance of performance in older adults. To date, there is work to indicate that targeting the cerebellum may be effective.
Recently, Raz and colleagues (2013) demonstrated that older adults who completed 100 days of cognitive training over 6 months showed smaller declines in cerebellar volume compared to controls. However, the mechanisms underlying this finding are unclear as there were no correlations between cerebellar volume and cognitive performance or change. Somewhat relatedly, providing explicit cues about the environment and context can improve performance and prediction (Imamizu et al., 2007). Using a similar approach in older adults, and harnessing this notion for training may also help ameliorate age related performance declines. Further work in this domain is needed, though Raz and colleagues (2013) have highlighted the potential for behavioral interventions. In addition to targeting the cerebellum and cortico-cerebellar circuits through behavioral paradigms, non-invasive brain stimulation is a particularly promising method (Reis et al., 2008). Transcranial direct current stimulation (tDCS) is an especially interesting technique given its ease of use and the fact that it is well-tolerated by participants.
tDCS is similar to transcranial magnetic stimulation (TMS) in that an electrical current is applied to the scalp and induces changes in brain function. However, unlike TMS which can cause discomfort when stimulating over the cerebellum due to the neck musculature, tDCS is much more comfortable and generally well-tolerated by participants due to the low amplitude of stimulation. The ease of use and comfort make it a reasonable method to consider in future interventions, and indeed the potential benefits of tDCS in rehabilitation have recently been discussed (Block and Celnik, 2012). Most importantly however, is the recent work using tDCS over the cerebellum demonstrating a variety of behavioral benefits in both the motor and cognitive domains (for a review see Ferrucci and Priori 2013).
Within the motor domain, across several studies the benefits of cerebellar tDCS on adaptation learning have been demonstrated. Using a visuomotor adaptation paradigm with reaching movements combined with a 30 degree visual transformation, Galea and colleagues (2011) demonstrated that individuals that received cerebellar tDCS adapted to the perturbation more quickly than with primary motor cortex stimulation. However, stimulation to the primary motor cortex did help with the retention of the learning (Galea et al., 2011). This finding has been recently replicated by Block and Celnik (2013), though they also demonstrated that while tDCS speeds the adaptation process, it does not aid in intermanual transfer of the learning. Together however, this work highlights the potential of tDCS to improve motor learning.
Relatedly, adaptation has also been looked at with respect to gait. Jayaram and colleagues (2012) used cerebellar tDCS during a gait adaptation task on a split-belt treadmill. Like the upper limb adaptation work, cerebellar tDCS improved locomotor adaptation. Finally, visuomotor tracking and adaptation of the ankle was investigated with cerebellar tDCS (Shah et al., 2013). Consistent with both the upper limb and locomotor findings, individuals were able to better track a sine wave with flexion of their non-dominant ankle when they received tDCS over the cerebellum.
While tDCS investigations in the motor domain have consistently demonstrated the benefits of cerebellar tDCS to learning, work on cognitive function has been somewhat mixed, though notably there has also been much less work done in this domain. tDCS applied to the cerebellum has been shown to be detrimental to working memory (Boehringer et al., 2012; Ferrucci et al., 2008). However, Pope and Miall (2012) have shown benefits of tDCS on cognitive performance. In their work, participants performed the paced auditory serial addition task, the paced auditory serial subtraction task, and a verb generation task. In the more complex subtraction task, performance after receiving tDCS was improved, as was performance on the verb generation task. However, the easier addition task was not improved with tDCS (Pope and Miall, 2012). This work indicates that there may be potential cognitive benefits to be gleaned from cerebellar tDCS, but they are dependent upon task difficulty (Pope and Miall, 2012). Clearly, further work is needed to investigate the cognitive benefits of cerebellar tDCS given the mixed results to date, but this may be potentially beneficial to older adults.
With that in mind, cerebellar tDCS may be particularly helpful for older individuals who have demonstrated performance declines due to the natural changes that occur with advanced age. Investigating the possible motor and cognitive benefits of cerebellar stimulation is an interesting new research direction that has the potential to open up new treatment and intervention options for older adults in order to improve quality of life and day-to-day function. Future work investigating cerebellar stimulation will not only improve our understanding of cerebellar contributions to age-related motor and cognitive declines, but it also has the potential to lead to new treatment interventions for aging individuals.
6.1.2. Cerebellar Contributions to Age-Related Pathology
In addition to pursuing cerebellar targets for interventions to improve behavioral performance in older adults, more in-depth investigations of the cerebellum with respect to age-related pathologies are warranted. In Parkinson's disease, studying the cerebellum has been an emerging area of great interest (cf. Wu and Hallett, 2013). Ballanger and colleagues (2008) found that during a motor task where Parkinson's patients made button presses, there was increased neural activity in the cerebellum. They speculate that this additional cerebellar activation may be an attempt to compensate for the basal ganglia deficits so that patients could move more quickly (Ballanger et al., 2008). Similarly, hyperactivation in the cerebellum of Parkinson's patients when performing a motor task is negatively correlated with activation in the putamen (Yu et al., 2007). That is, individuals who had less activation in the putamen also had more activation in the cerebellum, providing further support for the notion that the cerebellum compensates for basal ganglia dysfunction in Parkinson's disease. However, the exact role of the cerebellum in Parkinson's disease has yet to be delineated, and a pathological role of the cerebellum has also been proposed in addition to the evidence for compensation (Wu and Hallett, 2013).
Also of note is work pointing to a potential role for the cerebellum in Alzheimer's disease (AD). A post-mortem stereological study comparing women with AD and age-matched controls found that though there were no differences in the number of Purkinje and granule cells in the cerebellum, women with AD had smaller cerebellar volume (Andersen et al., 2012). Furthermore, additional post-mortem work demonstrated that out of 100 cases of AD, 47 individuals had plaques in the cerebellum, while none of the control individuals had such plaques (Joachim et al., 1989). Together, this work indicates that the cerebellum is perhaps impacted by AD, and this may contribute to the deficits seen in these patients.
Recent neuroimaging research also highlights the cerebellum in AD. A group of older individuals were classified based on the severity of their dementia, and volumes of the cerebellar hemispheres, vermis and posterior lobe were investigated along with the volume of the temporal lobe (Baldaçara et al., 2010). Smaller volumes in these regions were seen in patients with the most severe deficits, and interestingly, volumes of both the temporal lobe and cerebellum were positively associated with dementia rating scales and self-reported activity performance. That is, larger volumes indicated better function. Finally, it was also noted that smaller volume in the temporal lobe, posterior cerebellum, and cerebellar vermis were predictive of dementia in a subset of individuals that were healthy at the initial point of study (Baldaçara et al., 2010). While the temporal lobe is also implicated along with the cerebellum, this work further underscores the potential role of the cerebellum in the pathophysiology of AD. Finally, fcMRI investigations in patients with amnestic mild cognitive impairment have demonstrated that altered connectivity between the hippocampus and a variety of brain regions including the cerebellum is associated with episodic memory retrieval (Bai et al., 2009). Thus it appears that cerebellar-hippocampal interactions may at least to some degree underlie memory deficits associated with AD. Across this work, evidence is increasingly pointing to a potential role for the cerebellum in AD, and at the very least, a better understanding of the cerebellum in the aging brain may provide important insights into age-related pathology making this a fruitful area of future study.
7.1 Limitations
While it is clear that the cerebellum is decreased in volume with age, that there are functional network differences in the cerebellum between young and older adults, and that these volumetric and functional network differences are associated with both motor and cognitive function, it is important to note that considering individual structures, or subregions of a structure (in this case, individual cerebellar lobules) may not provide us with the most accurate picture of brain aging. Regional analyses of lobular cerebellar volume have certainly provided us with greater insight into cerebellar function, and its contributions to aging, but investigating interactions between multiple regions is also of great importance. The cerebellum has anatomical and functional connections with both prefrontal and motor cortical areas (Kelly and Strick 2003; Dum and Strick 2009; Habas et al. 2009; Krienen and Buckner 2009; Strick et al. 2009; Bernard et al. 2012, 2013, In Press). It is therefore important to consider the interactions between these regions in aging populations, and to determine which brain region (or combination of brain regions) best explains the performance declines seen in healthy aging. While some studies have begun to do this (Eckert et al., 2010), a multifactorial approach will be an important future step in our investigations of the neural underpinnings of cognitive and motor aging.
8.1 Conclusion
Age differences in overall cerebellar volume, as well as in regional volumes, are the most consistent and well-documented phenomena related to cerebellar aging. In addition, age differences in cerebellar functional activation as well in resting state functional connectivity have also been reported. Taken together it is clear that the cerebellum is impacted by aging, and given its role in a wide variety of task domains, it is an important area of research for understanding the neural underpinnings of age-related motor and cognitive performance declines. Indeed, both cerebellar morphology and resting state network connectivity have been linked to behavioral performance in older adults. We propose that the functional and morphological age differences in the cerebellum result in degraded internal models, and impede the formation of new internal models contributing, at least in part, to the wide variety of motor and cognitive deficits seen in older adults. Future work investigating the cerebellum, as well as interactions between the cerebellum and the cerebral cortex in older adults is warranted. Such work will provide further new insights into the aging mind and brain, by taking into account the contributions of the cerebellum to a wide variety of behaviors.
Highlights.
- Significant age differences in total and regional cerebellar gray matter volume
- Evidence also indicates decreased white matter structural integrity and functional connectivity
- Cerebellar volume is strongly correlated with behavior, often more so than prefrontal cortex volume
- We propose that cerebellar structural changes with advanced age result in degraded internal models
- Degraded internal models may underlie age-related motor and cognitive performance declines
Acknowledgements
J.A.B was previously supported by T32AG000279 (R. Schwartz, PI) and is currently supported by F32MH102989-01. J.A.B. is an alumna and R.D.S is a faculty member of the International Max Planck Research School on the Life Course (LIFE; www.imprs-life.mpg.de; including: MPI for Human Development, Humboldt-Universität zu Berlin, Freie Universität Berlin, University of Michigan, University of Virginia, University of Zurich).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29:102–16. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Merkley TL, Reiman EM, Caselli RJ, Aschenbrenner M, Santerre-Lemmon L, Lewis DJ, Pietrini P, Teipel SJ, Hampel H, Rapoport SI, Moeller JR. Regional network of magnetic resonance imaging gray matter volume in healthy aging. Neuroreport. 2006;17:951–6. doi: 10.1097/01.wnr.0000220135.16844.b6. [DOI] [PubMed] [Google Scholar]
- Andersen BB, Gundersen HJG, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466:356–65. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- Andersen K, Andersen BB, Pakkenberg B. Stereological quantification of the cerebellum in patients with Alzheimer's disease. Neurobiol Aging. 2012;33:197.e11–20. doi: 10.1016/j.neurobiolaging.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J Cogn Neurosci. 2011;23:11–25. doi: 10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry -- The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Yuan Y, Zang Y, Zhu C, Qian Y. Abnormal functional connectivity of hippocampus during episodic memory retrieval processing network in amnestic mild cognitive impairment. Biol Psychiatry. 2009;65:951–8. doi: 10.1016/j.biopsych.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Baldaçara L, Guilherme J, Borgio F, André W, Luiz A, Lacerda T, Beatriz M, Macedo M, Tufik S, Bressan RA, Ramos LR, Jackowski AP. Cerebellar volume in patients with dementia Volume cerebelar em pacientes com demência. Rev Bras Psiquiatr. 2010;33:122–129. doi: 10.1590/s1516-44462011000200006. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Baraduc P, Broussolle E, Le Bars D, Desmurget M, Thobois S. Motor urgency is mediated by the contralateral cerebellum in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2008;79:1110–6. doi: 10.1136/jnnp.2007.141689. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Pouthas V, Isingrini M. Age-related changes in duration reproduction: involvement of working memory processes. Brain Cogn. 2006;62:17–23. doi: 10.1016/j.bandc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–90. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Peltier SJ, Wiggins JL, Jaeggi SM, Buschkuehl M, Fling BW, Kwak Y, Jonides J, Monk CS, Seidler RD. Disrupted cortico-cerebellar connectivity in older adults. Neuroimage. 2013b;83:103–119. doi: 10.1016/j.neuroimage.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. Evidence for motor cortex dedifferentiation in older adults. Neurobiol Aging. 2012;33:1890–9. doi: 10.1016/j.neurobiolaging.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Jaeggi SM, Buschkuehl M, Monk CS, Jonides J, Peltier SJ. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6:31. doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Peltier SJ, Benson BL, Wiggins JL, Jaeggi SM, Buschkuehl M, Jonides J, Monk CS, Seidler RD. Dissociable Functional Networks of the Human Dentate Nucleus. Cereb Cortex. 2013 doi: 10.1093/cercor/bht065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. Relationships Between Regional Cerebellar Volume and Sensorimotor and Cognitive Function in Young and Older Adults. Cerebellum. 2013;12:721–737. doi: 10.1007/s12311-013-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski A-M, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li S-J, Lin C-P, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts S. a R.B., Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng G-J, Veijola J, Villringer A, Walter M, Wang L, Weng X-C, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang Y-F, Zhang H-Y, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block H, Celnik P. Stimulating the Cerebellum Affects Visuomotor Adaptation but not Intermanual Transfer of Learning. Cerebellum. 2013 doi: 10.1007/s12311-013-0486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block HJ, Celnik P. Can cerebellar transcranial direct current stimulation become a valuable neurorehabilitation intervention? Invited Editorial. Expert Rev Neurother. 2012;12:1275–1277. doi: 10.1586/ern.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block R. a, Zakay D, Hancock P. a. Human aging and duration judgments: a meta-analytic review. Psychol Aging. 1998;13:584–96. doi: 10.1037//0882-7974.13.4.584. [DOI] [PubMed] [Google Scholar]
- Bo J, Borza V, Seidler RD. Age-related declines in visuospatial working memory correlate with deficits in explicit motor sequence learning. J Neurophysiol. 2009;102:2744–54. doi: 10.1152/jn.00393.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo J, Peltier SJ, Noll DC, Seidler RD. Age differences in symbolic representations of motor sequence learning. Neurosci Lett. 2011a;504:68–72. doi: 10.1016/j.neulet.2011.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo J, Peltier SJ, Noll DC, Seidler RD. Symbolic representations in motor sequence learning. Neuroimage. 2011b;54:417–26. doi: 10.1016/j.neuroimage.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo J, Seidler RD. Spatial and symbolic implicit sequence learning in young and older adults. Exp Brain Res. 2010;201:837–51. doi: 10.1007/s00221-009-2098-5. [DOI] [PubMed] [Google Scholar]
- Boehringer A, Macher K, Dukart J, Villringer A, Pleger B. Cerebellar transcranial direct current stimulation modulates verbal working memory. Brain Stimul. 2012:1–5. doi: 10.1016/j.brs.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Larkin P. a, Cook a C., Gear J, Singer J. Decrease in timed balance test scores with aging. Phys Ther. 1984;64:1067–70. doi: 10.1093/ptj/64.7.1067. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP, Nougier V. Ageing of internal models: From a continuous to an intermittent proprioceptive control of movement. Age (Omaha) 2013;35:1339–1355. doi: 10.1007/s11357-012-9436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–70. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Carp J, Park J, Hebrank A, Park DC, Polk T. a. Age-related neural dedifferentiation in the motor system. PLoS One. 2011;6:e29411. doi: 10.1371/journal.pone.0029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari M, Moscufo N, Skudlarski P, Meier D, Panzer VP, Pearlson GD, White WB, Wolfson L, Guttmann CRG. Mobility impairment is associated with reduced microstructural integrity of the inferior and superior cerebellar peduncles in elderly with no clinical signs of cerebellar dysfunction. NeuroImage Clin. 2013;2:332–340. doi: 10.1016/j.nicl.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005a;43:1227–37. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005b;24:332–8. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Craik FI, Hay JF. Aging and judgments of duration: effects of task complexity and method of estimation. Percept Psychophys. 1999;61:549–60. doi: 10.3758/bf03211972. [DOI] [PubMed] [Google Scholar]
- Curran T. Effects of aging on implicit sequence learning: Accounting for sequence structure and explicit knowledge. Psychol Res. 1997;60:24–41. doi: 10.1007/BF00419678. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts S. a R.B. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An Unfolded Map of the Cerebellar Dentate Nucleus and its Projections to the Cerebral Cortex. J Neurophysiol. 2009;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- Eckert M. a, Keren NI, Roberts DR, Calhoun VD, Harris KC. Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci. 2010;4:10. doi: 10.3389/neuro.09.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdon S, Murphy C. The cerebellum and olfaction in the aging brain: a functional magnetic resonance imaging study. Neuroimage. 2003;20:12–21. doi: 10.1016/s1053-8119(03)00276-3. [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF. Resting-state functional connectivity in normal brain aging. Neurosci Biobehav Rev. 2013;37:384–400. doi: 10.1016/j.neubiorev.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Zago S, Barbieri S, Priori a. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci. 2008;20:1687–97. doi: 10.1162/jocn.2008.20112. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Priori A. Transcranial cerebellar direct current stimulation (tcDCS): Motor control, cognition, learning and emotions. Neuroimage. 2013:1–6. doi: 10.1016/j.neuroimage.2013.04.122. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol Aging. 2013;34:2239–47. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Kwak Y, Peltier SJ, Seidler RD. Differential relationships between transcallosal structural and functional connectivity in young and older adults. Neurobiol Aging. 2012;33:2521–2526. doi: 10.1016/j.neurobiolaging.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Walsh CM, Bangert AS, Reuter-Lorenz P. a, Welsh RC, Seidler RD. Differential callosal contributions to bimanual control in young and older adults. J Cogn Neurosci. 2011;23:2171–85. doi: 10.1162/jocn.2010.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry J-JO, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 2011;21:1761–70. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–51. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Cabanis EA. Anatomical parcellation of the brainstem and cerebellar white matter: a preliminary probabilistic tractography study at 3 T. Neuroradiology. 2007;49:849–63. doi: 10.1007/s00234-007-0267-4. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–94. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J., V Multivariate pattern analysis of fMRI: the early beginnings. Neuroimage. 2012;62:852–5. doi: 10.1016/j.neuroimage.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J, V, Gobbini MI, Furey ML, Ishai a, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–30. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential Vulnerability of Anterior White Matter in Nondemented Aging with Minimal Acceleration in Dementia of the Alzheimer Type: Evidence from Diffusion Tensor Imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25:6787–96. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–9. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Murray AD, Ahearn TS, Deary IJ, Whalley LJ. Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex. 2011;47:441–50. doi: 10.1016/j.cortex.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Holviala J, Kraemer WJ, Sillanpää E, Karppinen H, Avela J, Kauhanen a, Häkkinen a, Häkkinen K. Effects of strength, endurance and combined training on muscle strength, walking speed and dynamic balance in aging men. Eur J Appl Physiol. 2012;112:1335–47. doi: 10.1007/s00421-011-2089-7. [DOI] [PubMed] [Google Scholar]
- Hoogendam YY, van der Geest JN, van der Lijn F, van der Lugt A, Niessen WJ, Krestin GP, Hofman A, Vernooij MW, Breteler MMB, Ikram MA. Determinants of cerebellar and cerebral volume in the general elderly population. Neurobiol Aging. 2012;33:2774–81. doi: 10.1016/j.neurobiolaging.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Féger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–3. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Huxhold O, Li S-C, Schmiedek F, Lindenberger U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res Bull. 2006;69:294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Pütz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–5. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Sugimoto N, Osu R, Tsutsui K, Sugiyama K, Wada Y, Kawato M. Explicit contextual information selectively contributes to predictive switching of internal models. Exp Brain Res. 2007;181:395–408. doi: 10.1007/s00221-007-0940-1. [DOI] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–13. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex. 2011;21:1901–9. doi: 10.1093/cercor/bhq263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G, Tang B, Pallegadda R, Vasudevan EVL, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012;107:2950–7. doi: 10.1152/jn.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Joachim CL, Morris JH, Selkoe DJ. Diffuse Senile Plaques Occur Commonly in the Cerebellum in Alzheimer ' s Disease. Am J Pathol. 1989;135:309–319. [PMC free article] [PubMed] [Google Scholar]
- Kafri M, Sasson E, Assaf Y, Balash Y, Aiznstein O, Hausdorff JM, Giladi N. High-level gait disorder: associations with specific white matter changes observed on advanced diffusion imaging. J Neuroimaging. 2013;23:39–46. doi: 10.1111/j.1552-6569.2012.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron J-C, Landeau B, Mevel K, Godeau C, Barré L, Constans J-M, Viader F, Eustache F, Desgranges B. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30:112–24. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Keele SW, Ivry RB. Does the cerebellum provide a common computation for diverse tasks? A timing hypothesis. Ann N Y Acad Sci. 1990;6-8:179–211. doi: 10.1111/j.1749-6632.1990.tb48897.x. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–97. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Müller MLTM, Dayalu P, Seidler RD. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front Syst Neurosci. 2010;4:143. doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J, Peltier SJ, Bo J, Fling BW, Welsh RC, Seidler RD. Functional implications of age differences in motor system connectivity. Front Syst Neurosci. 2010;4:17. doi: 10.3389/fnsys.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz L. a., Collins JJ. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture. 2003;18:101–108. doi: 10.1016/s0966-6362(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Lee J, Lyoo INK, Kim S. Intellect declines in healthy elderly subjects and cerebellum. Psychiatry Clin Neurosci. 2005;59:45–51. doi: 10.1111/j.1440-1819.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- Lee NJ, Park IS, Koh I, Jung TW, Rhyu IJ. No volume difference of medulla oblongata between young and old Korean people. Brain Res. 2009;1276:77–82. doi: 10.1016/j.brainres.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner a L., Dow RS. Reappraising the cerebellum: what does the hindbrain contribute to the forebrain? Behav Neurosci. 1989;103:998–1008. doi: 10.1037//0735-7044.103.5.998. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner a L., Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16:444–7. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100:443–54. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44:113–128. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- Li KZH, Lindenberger U, Freund a. M., Baltes PB. Walking While Memorizing: Age-Related Differences in Compensatory Behavior. Psychol Sci. 2001;12:230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–36. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Schaefer S, Pohlmeyer AE, Lindenberger U. Walking variability and working-memory load in aging: a dual-process account relating cognitive control to motor control performance. J Gerontol B Psychol Sci Soc Sci. 2008;63:P121–8. doi: 10.1093/geronb/63.3.p121. [DOI] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Schulz JB, Kolb R, Bürk K, Klockgether T. Patterns of Age-related Shrinkage in Cerebellum and Brainstem Observed In Vivo Using Three-dimensional MRI Volumetry. Cereb Cortex. 1999;9:712–721. doi: 10.1093/cercor/9.7.712. [DOI] [PubMed] [Google Scholar]
- MacLullich AMJ, Edmond CL, Ferguson KJ, Wardlaw JM, Starr JM, Seckl JR, Deary IJ. Size of the neocerebellar vermis is associated with cognition in healthy elderly men. Brain Cogn. 2004;56:344–8. doi: 10.1016/j.bandc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Fernie GR. Aging and postural control. A comparison of spontaneous and induced-sway balance tests. J Am Geriatr Soc. 1990;38:1–9. doi: 10.1111/j.1532-5415.1990.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore a., Hariri a. R., Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- McCormack T, Brown GDA, Maylor EA, Richardson LB, Darby RJ. Effects of aging on absolute identification of duration. Psychol Aging. 2002;17:363. [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a smith predictor? J Mot Behav. 1993;25:203–16. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- Miller TD, Ferguson KJ, Reid LM, Wardlaw JM, Starr JM, Seckl JR, Deary IJ, Maclullich AMJ. Cerebellar vermis size and cognitive ability in community-dwelling elderly men. Cerebellum. 2013;12:68–73. doi: 10.1007/s12311-012-0397-z. [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG. Cerebellar sequencing for cognitive processing. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N, editors. Handbook of the Cerebellum and Cerebellar Disorders. Springer Netherlands; Dordrecht: 2013. pp. 1701–1716. [Google Scholar]
- Molinari M, Petrosini L. Is sequence-in/sequence-out a cerebellar mode of operation in cognition too? Behav Brain Sci. 1997;20:259–260. [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter T. a, Baron J-C. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage. 2006;32:1250–6. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–65. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–52. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Pagani E, Agosta F, Rocca M. a, Caputo D, Filippi M. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage. 2008;41:657–67. doi: 10.1016/j.neuroimage.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels JA, Taylor SF, Marshuetz C. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues Clin Neurosci. 2001;3:151–165. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz PA. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, Clark CR, Kukla M, Mulligan R, Gordon E. Relative contributions of the cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiol Aging. 2009;30:457–65. doi: 10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Pope P. a, Miall RC. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul. 2012;5:84–94. doi: 10.1016/j.brs.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince F, Hkbert R, Winter A. Gait in the elderly. Gait Posture. 1997;5:128–135. [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–22. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD. Differential effects of age and sex on the cerebellar hemispheres and the vermis: a prospective MR study. AJNR Am J Neuroradiol. 1998;19:65–71. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson a, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol. 2001;22:1161–7. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Schmiedek F, Rodrigue KM, Kennedy KM, Lindenberger U, Lövdén M. Differential brain shrinkage over 6months shows limited association with cognitive practice. Brain Cogn. 2013;82:171–80. doi: 10.1016/j.bandc.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Williamson a, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, Wassermann E, Pascual-Leone A, Hummel F, Celnik P. a, Classen J, Floel A, Ziemann U, Paulus W, Siebner HR, Born J, Cohen LG. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?”. Brain Stimul. 2008;1:363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell K. a. Neurocognitive Aging and the Compensation Hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller a. C. Neural Recruitment and Cognitive Aging: Two Hemispheres Are Better Than One, Especially as You Age. Psychol Sci. 1999;10:494–500. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-lorenz PA, Park DC. Human Neuroscience and the Aging Mind: A New Look at Old Problems. J Gerontol Psychol Sci. 2010;65B:405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu IJ, Cho TH, Lee NJ, Uhm CS, Kim H, Suh YS. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci Lett. 1999;270:149–52. doi: 10.1016/s0304-3940(99)00487-5. [DOI] [PubMed] [Google Scholar]
- Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–55. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22:2663–76. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121Pt 4:561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–33. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B, Nguyen TT, Madhavan S. Polarity Independent Effects of Cerebellar tDCS on Short Term Ankle Visuomotor Learning. Brain Stimul. 2013:1–3. doi: 10.1016/j.brs.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Shah S. a, Doraiswamy PM, Husain MM, Figiel GS, Boyko OB, McDonald WM, Ellinwood EH, Krishnan KR. Assessment of posterior fossa structures with midsagittal MRI: the effects of age. Neurobiol Aging. 1991;12:371–4. doi: 10.1016/0197-4580(91)90025-f. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt F. a, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–87. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Ivry RB. Cerebellum and timing. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N, editors. Handbook of the Cerebellum and Cerebellar Disorders. Springer; New York: 2013. pp. 1201–1219. [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–44. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–70. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and Nonmotor Function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Su L, Wang L, Chen F, Shen H, Li B, Hu D. Sparse representation of brain aging: extracting covariance patterns from structural MRI. PLoS One. 2012;7:e36147. doi: 10.1371/journal.pone.0036147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E, V, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar Volume Decline in Normal Aging , Alcoholism , and Korsakoff s Syndrome: Relation to Ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Sullivan E, V, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–61. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Tang Y, Whitman GT, Lopez I, Baloh RW. Brain Volume Changes on Longitudinal Magnetic Resonance. J Neuroimaging. 2001;11:393–400. doi: 10.1111/j.1552-6569.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Taniwaki T, Okayama A, Yoshiura T, Togao O, Nakamura Y, Yamasaki T, Ogata K, Shigeto H, Ohyagi Y, Kira J-I, Tobimatsu S. Age-related alterations of the functional interactions within the basal ganglia and cerebellar motor loops in vivo. Neuroimage. 2007;36:1263–76. doi: 10.1016/j.neuroimage.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Terribilli D, Schaufelberger MS, Duran FLS, Zanetti M, V, Curiati PK, Menezes PR, Scazufca M, Amaro E, Leite CC, Busatto GF. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol Aging. 2011;32:354–368. doi: 10.1016/j.neurobiolaging.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–70. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–8. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011;32:916–32. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KPT, Comalli DM, Kennard J. a, Agelan A, Thompson RF. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci U S A. 2010;107:1624–9. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-pak DS, Goldenberg CAG, Downey-lamb MM, Boyko OB, Lemieux SK. Cerebellar volume in humans related to magnitude of classical conditioning. Cogn Neurosci Neuropsychol. 2000;11:609–615. doi: 10.1097/00001756-200002280-00035. [DOI] [PubMed] [Google Scholar]
- Woodruff-pak DSRWV, Iii, Ewers M, Coffey J, Boyko OB, Lemieux SK. MRI-Assessed Volume of Cerebellum Correlates with Associative Learning. Neurobiol Learn Mem. 2001;357:342–357. doi: 10.1006/nlme.2001.4026. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. The influence of normal human ageing on automatic movements. J Physiol. 2005;562:605–15. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain. 2013 doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Hallett M, Chen Y, Li K, Chan P. Aging influence on functional connectivity of the motor network in the resting state. Neurosci Lett. 2007a;422:164–8. doi: 10.1016/j.neulet.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Wu T, Zang Y, Wang L, Long X, Li K, Chan P. Normal aging decreases regional homogeneity of the motor areas in the resting state. Neurosci Lett. 2007b;423:189–93. doi: 10.1016/j.neulet.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. 2007;35:222–33. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]


