Abstract
With a rapidly aging society it becomes increasingly important to counter normal age-related decline in cognitive functioning. Growing evidence suggests that cognitive training programs may have the potential to counteract this decline. On the basis of a growing body of research that shows that meditation has positive effects on cognition in younger and middle-aged adults, meditation may be able to offset normal age-related cognitive decline or even enhance cognitive function in older adults. In this paper, we review studies investigating the effects of meditation on age-related cognitive decline. We searched the Web of Science (1900 to present), PsycINFO (1597 to present), MEDLINE (1950 to present), and CABI (1910 to present) to identify original studies investigating the effects of meditation on cognition and cognitive decline in the context of aging. Twelve studies were included in the review, six of which were randomized controlled trials. Studies involved a wide variety of meditation techniques and reported preliminary positive effects on attention, memory, executive function, processing speed, and general cognition. However, most studies had a high risk of bias and small sample sizes. Reported dropout rates were low and compliance rates high. We conclude that meditation interventions for older adults are feasible, and preliminary evidence suggests that meditation can offset age-related cognitive decline.
Keywords: aging, cognitive decline, meditation, mindfulness
Introduction
The world population is rapidly aging, in part due to increases in life expectancy and the baby boom generation getting older.1,2 In the United States, the population of individuals aged 65 years and older has grown by 18% in the last decade and is expected to almost double to 79 million in 2040, making up 20% of the U.S. population.2
Although aging is associated with increased social satisfaction and well-being (for reviews, see Refs. 3–5), it is also well established that normal healthy aging is accompanied by a decline in cognitive function6–8 and related declines in neural structure and activity.9–11 The decline in cognition is rather specific. While crystallized mental capabilities such as vocabulary increase steadily until midlife, remain relatively stable throughout midlife, and then only slowly decline at an older age, fluid capabilities, speed, and memory decrease sooner and faster with aging.12,13 The decline can progress to mild cognitive impairment (MCI) and dementia, which are characterized by limitations in daily functioning and typically result in a reduced quality of life. Therefore, cognitive decline is an often feared aspect of aging and is not only a burden for the affected individual but also for relatives and society.14,15 Some preliminary literature has suggested that a number of lifestyle factors can affect the rate of normal decline (for reviews, see Refs. 15–17). A variety of cognitive training programs18–20 and aerobic exercise21,22 have also recently been suggested to slow cognitive decline. However, on the basis of the limited body of research trials, no clear recommendations can be established so far, and further rigorous research is needed to more clearly address this question.23
A growing body of research suggests that meditation can enhance various cognitive functions, including attention, memory, and executive function,24–28 and that it positively affects brain function and structure relevant to cognition.29–35 Despite this evidence, the effects of meditation on cognition and cognitive decline in aging have not been extensively studied. In this paper, we will review what is currently known about this young field at the intersection of gerontology and contemplative sciences. While we focus here on the effects of meditation on cognitive decline, a review of the effects of meditation on brain function and structure in aging has been done by Luders et al.36
Methods
This systematic review followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.37
Eligibility criteria
As the field of contemplative gerontology is still in its infancy and part of our aim was to provide a broad overview of the attempts to investigate the effect of meditation on age-related cognitive decline rather than drawing firm conclusions about this relationship, eligibility criteria were kept rather broad. Original studies of all designs investigating the effects of meditation on cognition and cognitive decline in the context of aging were eligible. The focus was on formal meditation or interventions describing formal meditation as part of their curriculum, but without substantial exercise components to avoid exercise as a confound.21 Therefore, studies involving mind–body interventions, such as yoga and tai chi, were not considered eligible, even though yoga, for example, clearly involves meditation.38 Reports were required to be written in English and published in peer-reviewed journals.
Study search and selection
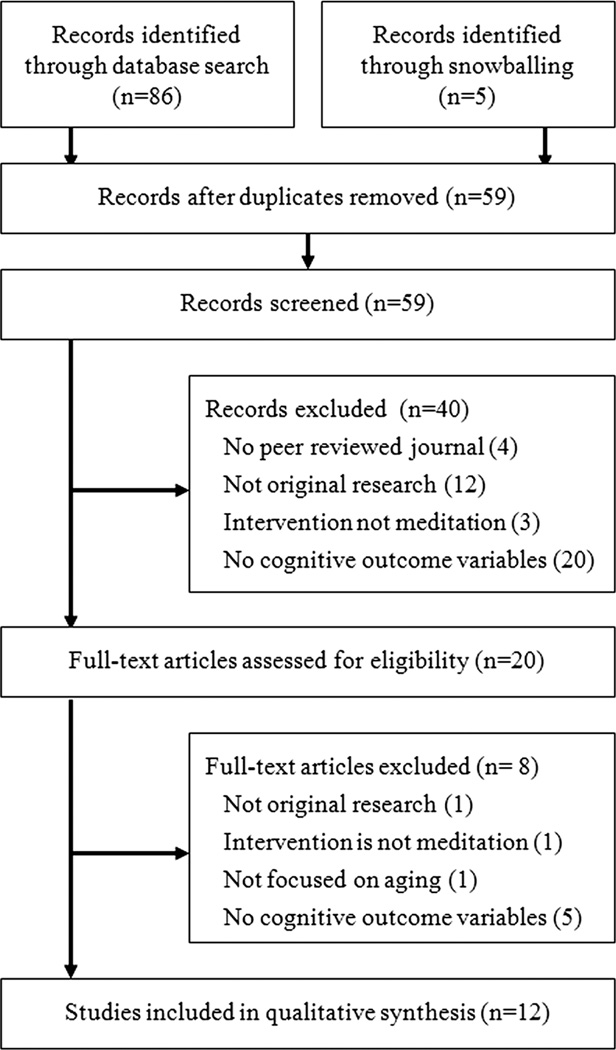
Studies were identified by searching the databases Web of Science (1900 to present), CABI (1910 to present), PsycINFO (1597 to present), and MEDLINE (1950 to present), without restrictions. To avoid excluding potentially relevant articles at the search stage, a relatively broad search strategy was used. The query “(Meditation OR Mindfulness) AND Aging AND Decline” was used in the topic field of Web of Scinece, CABI, and MEDLINE, and in all fields in PsycINFO. The search was not restricted to also include “cognition” or related search terms, as we also wanted to identify studies that did not primarily focus on cognition but still included some measures of it. Searches were first conducted on May 29, 2013 and last updated on November 14, 2013. In addition to database searches, additional relevant studies were identified from the reference lists of examined articles. Eligibility of articles was assessed on the basis of eligibility criteria—first at the level of the title, abstract, and source, and then the full article (Fig. 1). Search and study selection were conducted by T.G.
Figure 1.
Study search and selection.
Data extraction and risk of bias assessment
A data extraction form was developed in which data on study design, population and intervention characteristics, and study results are summarized (Table 1). The risk of bias was assessed with the Cochrane Risk of Bias Tool39 (Table 2). Data extraction was conducted by T.G. and the risk-of-bias evaluation by T.G. and B.K.H.
Table 1.
Characteristics and results of reviewed studies
| Publication | Design | N (% male) | Population | Mean age (SD) | Interventions/ meditation type |
Main cognitive findings + relevant other findings |
|---|---|---|---|---|---|---|
| Alexander et al.40 | RCT Four arms, two time points: pre- and postintervention |
73 (18) TM: 20 MFL: 21 MR: 21 NT: 11 |
Normal to poor cognitive functioning | 80.7 (n.r.) | TM MFL MR All 12 weeks of 40-min daily home practice, 30 min/week instruction NT |
The postintervention contrast 3TM+1MFL-2MR-2NT was sig. for DST ALS, DST WFS, OVT, but not for DST total and Stroop TM scored sig. better on OVT than MFL, MR, or NT postintervention |
| Lavretsky et al.47 | RCT Four arms, two time points: pre- and postintervention |
39 (5) EXP: 23 CTL: 16 |
Family dementia caregivers Mild to moderate depression Normal cognitive functioning |
60.3 (10.2) | EXP: KKYM CTL: Relaxation (listening to instrumental music) Both cond.: 8 weeks, 12-min daily home practice with audio recordings |
Improved overall (MANCOVA) cognitive functioning in EXP relative to CTL. Individually, improvements on Trails B, and MMSE, but not on Trails A and CVLT in EXP relative to CTL Improved mental health (SF-36, Ham-D24) in EXP relative to CTL |
| McHugh et al.52 | RCT Two arms, one time point |
24 (50) EXP: 12 CTL: 12 |
Normal cognitive functioning | 78.58 (n.r.) | EXP: MF instruction CTL: mind wandering instruction Both conditions: one time 15 min |
Sig. lower stimulus overselectivity in EXP than in CTL |
| Moynihan et al.54 | RCT Two arms, four time points: pre-, post-, at 3 and 24 weeks after the intervention |
201 (38) EXP: 101 (38) CTL: 100 (38) |
Elderly without cognitive impairment, uncorrected sensory impairments, psychosis, and stable if on medication | EXP: 73.3 (6.7) CTL: 73.6 (6.7) |
EXP: MBSR: 8 weeks, weekly 120-min sessions, retreat day, 40-min daily home practice CTL: wait list |
Sig. better executive function (Trails B/A) in EXP versus CTL at post but not FU1 and FU2. No sig. differences in Trails A or B at any time Sig. greater leftward frontal alpha asymmetry in EXP versus CTL No sig. difference between groups in depression (CES-D) and perceived stress (PSS) at any time point |
| Oken et al.58 | RCT Three arms, two time points: pre- and postintervention |
31 (20) EXP: 10 Act. CTL: 11 Pass. CTL: 10 |
Family dementia caregivers Normal cognitive functioning Stressed |
64.55 (9.41) | EXP: MF based on MBCT Act. CTL: powerful tools for caregivers education program Both interventions: 7 weeks, weekly 90-min sessions Pass. CTL: no intervention but same amount of respite care as in interventions |
Sig. group differences in postintervention Stroop and ANT alerting, driven by higher ANT scores in EXP and act. CTL than in pass. CTL. No sig. group differences in word learning or ANT executive functioning or when correcting for multiple comparisons Sig. lower perceived stress in EXP and act. CTL than in pass. CTL, no difference between EXP and act. CTL |
| Sun et al.66 | RCT Two arms, four time points: pre-, at 3, 6, 12 months of training |
80 (25) EXP: 40 CTL: 40 |
Chinese Reduced sleep quality |
69.69 (7.89) | EXP: Self-relaxation: PMR + MED (guided visual imagery), 12 months; four initial group sessions and daily home practice with audiotapes CTL: sleep hygiene education brochure |
Improved cognitive functioning (MMSE, WMS) in EXP, worsening in CTL Increased sleep quality in EXP, worsening in CTL |
| Ernst et al.70 | LGT NR Two groups, two time points: pre- and postintervention |
22 (27) EXP: 15 (20) CTL: 7 (43) |
German nursing home residents | Median age 83.5 EXP: 80 CLT: 89 |
EXP: modified MBSR: 8 weeks, weekly 90-min sessions, no retreat day, reduced home practice CTL: no intervention |
No sig. difference in MMSE change between groups Sig. greater improvement in physical health (SF-12), depression (GDS-12R), and severity of major complaints |
| Newberg et al.75 | LGT NR Two groups, two time points: pre- and postintervention |
EXP: 15 (33) CTL: 5 (0) |
Memory problems: MMI (7 EXP, 3 CTL) MCI (5 EXP, 2 CTL) AD (3 EXP, 0 CLT) |
EXP: 64 (8) CTL: 65 (10) |
EXP: KKYM CTL: listening to classical music Both conditions: 8 weeks, 12-min daily home practice with audio recordings |
Sig. increases on Trails B, WAIS Symbol Substitution Test, and Logical Memory Delayed but not on MMSE, Category Fluency, and Trails A in EXP when not corrected for multiple comparisons. No sig. increases after correction and in CTL |
| Pagnoni et al.77 | CROSSC Two groups | 26 MED: 13 (77) CTL: 13 (77) |
Experienced MED CTL matched for age, sex, education |
MED: 37.2 (6.9) CTL: 35.5 (5.7) |
Zen >3 years’ experience |
Sustained attention sig. decreases with age in CTL but not in MED Gray matter volume sig. decreases in left putamen in CTL but increases in MED |
| Prakash et al.87 | CROSSC Two groups | 40 MED: 20 (100) CTL: 20 (100) |
Mentally healthy Normal cognitive functioning Experienced MED CTL mateched for age, sex, education, SES |
MED: 59.45(3.86) CTL: 60.36(4.51) |
Vihangam yoga meditation, >10 years’ experience (mean 15.90, SD 6.81) | MED performed sig. better than CTL on Digit Span forward, DSST correct, Stroop, Trails A, B, and B-A, LCT total and omission error, RSCT 2, but not on Digit span backward, DSST errors, LCT commission error, and RSCT test time |
| Van Leeuwen et al.81 | CROSSC Three groups | 51 MED: 17 (59) CTL old: 17 (59) CTL young: 17 (41) |
Experienced MED old CTL matched for age, sex, education | MED: 49.8 (5) CTL old: 50 (5.4) CTL young: 24.3 (2.3) |
14 Shamatha Vipashayana (FA, OM) 3 Zen 1–29 years’ experience |
Overall sig. smaller attentional blink in MED than in old CTL and no difference between MED and young CTL Sig. smaller blink in MED than even young CTL at 200 ms lag |
| Jedrczak et al.83 | CROSSC One group | Some tests 150 (57), some 87 (56) | Experienced TM-Sidhi MED | 38.74 (n.r.) | TM (mean 108 months) and TM Sidhi (mean 44 months) | Amount TM Sidhi practice predicted verbal intelligence (WAIS: DSST, PAT) and psychomotor speed (LC and RT but not PMS) |
AD, Alzheimer’s disease; ALS, Associate Learning Scale; CES-D, Center for Epidemiologic Studies Depression Scale; Cond., condition; CROSSC, cross-sectional; CTL, control; CVLT, California Verbal Learning Test; DSST, Digit Symbol Substitution Test; DST, Dementia Screening Test; EXP, experimental; FA, focused attention; FU, follow-up; GDS-12R, Geriatric Depression Scale Residential; HRSD-24, Hamilton Rating Scale for Depression (24 items); LGT, longitudinal; KKYM, Kirtan Kriya yogic meditation (finger tapping, chanting, and brief breathing relaxation along with visualization of light); LC, Line Crossing; LCT, Letter Cancellation Task; MBCT, mindfulness-based cognitive therapy; MBSR, mindfulness-based stress reduction; MCI, mild cognitive impairment; MED, meditation; MF, mindfulness; MFL, mindfulness as defined by Langer;41,42 MMI, mild age-related memory impairment; MMSE, Mini-Mental State Examination; MR, mental relaxation; NR, not randomized; n.r., not reported; NT, no treatment; OM, open monitoring; OVT, Overlearned Verbal Task; PAT, Picture Arrangement Test; PMR, progressive muscle relaxation; PMS, psychomotor speed; PSS, Perceived Stress Scale; RCT, randomized controlled trial; RSCT, Rule Shift Card Test; RT, reaction time; SF-12, Short-Form General Health Survey; TM, transcendental meditation; WAIS, Wechsler Adult Intelligence Scale; WFS, Word Fluency Scale.
Table 2.
Risk of bias of reviewed studies
| Selection bias | Detection bias | Attrition bias |
Reporting bias |
Performance bias | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication | Random sequence generation |
Allocation concealment |
Other: baseline/ group differences |
Blinding of outcome assessment |
Incomplete outcome data |
Selective reporting |
Blinding of participants and personnel |
Other: control condition/ group |
Other: treatment fidelity |
Other: compliance |
Other: credibility expectancy |
| Alexander et al.40 | ? | ? | + | + | ? | ? | ? | + | ? | + | ? |
| Lavretsky et al.47 | + | ? | + | ? | ? | ? | ? | + | ? | ? | ? |
| McHugh et al.52 | ? | ? | + | ? | + | ? | ? | + | + | + | ? |
| Moynihan et al.54 | + | ? | + | ? | ? | − | ? | − | + | + | ? |
| Oken et al.58 | + | + | + | + | + | ? | ? | + | ? | + | + |
| Sun et al.66 | + | ? | + | ? | + | ? | ? | − | ? | ? | ? |
| Ernst et al.70 | N/A | N/A | − | ? | − | ? | ? | − | ? | + | ? |
| Newberg et al.75 | N/A | N/A | N/A | ? | ? | ? | ? | N/A | ? | + | N/A |
| Pagnoni et al.77 | N/A | N/A | ? | ? | N/A | ? | N/A | N/A | N/A | N/A | N/A |
| Prakash et al.87 | N/A | N/A | ? | + | N/A | ? | N/A | N/A | N/A | N/A | N/A |
| Van Leeuwen et al.81 | N/A | N/A | ? | ? | N/A | ? | N/A | N/A | N/A | N/A | N/A |
| Jedrczak et al.83 | N/A | N/A | N/A | ? | N/A | ? | N/A | N/A | N/A | N/A | N/A |
Note: +, low risk; −, high risk; ?, unknown risk.
Results
The literature search resulted in a total of 91 records, 86 through the databases (Web of Science 30, CABI 2, MEDLINE 37, PsycINFO 17), and five through snowballing examined articles. After screening and full-text assessment, 12 articles were included in the qualitative synthesis (Fig. 1). Characteristics and results of individual studies are summarized below and in Table 1. Results of the risk-of-bias assessment are presented in Table 2. For all ratings, a consensus was reached between the two evaluators. Each individual study will first be summarized briefly before characteristics and findings will be discussed in an integrative way.
Alexander et al.40 investigated the effects of transcendental meditation (TM), a program developed by Ellen Langer that used techniques termed mindfulness (MFL)41,42 (although it should be noted that this form of mindfulness does not use meditation and is different than the mindfulness popularized by Jon Kabat-Zinn; Ref. 43), and the effects of a mental relaxation (MR) condition on a number of health-related and cognitive variables. Cognitive function was assessed with the Dementia Screening Test (DST),44 the Stroop Color and Word test,45 and the Overlearned Verbal Task (OVT; see Ref. 40), which is intended to measure cognitive flexibility. It should be mentioned here that the OVT was developed for the study, but no validation data were reported. The DST total score and the Associative Learning and Word Fluency subtest scores were used as outcome measures.
Seventy-three older participants (mean age = 80.7 years) with normal to poor cognitive functioning were randomized to the four conditions such that the TM, MFL, and MR groups had 20, 21, and 21 participants, respectively, and the no-intervention (NT) control group had 11 participants. All three interventions lasted for 12 weeks and consisted of 30 min of individual teaching per week and 2 × 20 min of home practice per day. The TM practice involves focusing on a mantra and “a specific procedure for using it mentally without effort,”40 to turn “inwards towards subtler levels of a thought until the mind transcends the subtlest state of thought and arrives at the source of thought,”46 which is experienced as content-free pure consciousness.40 The mindfulness intervention was meant to increase mindfulness as defined by Langer.41,42 According to this definition mindfulness is described as “a general style or mode of functioning through which the individual actively engages in reconstructing the environment through creating new categories or distinctions, thus directing attention to new contextual cues that may be consciously controlled or manipulated as appropriate.”40
To increase mindfulness, participants were trained in two tasks, a word production and creative mental activity task. The word production task involved thinking of a word and finding a new word that begins with the last letter of the previous word. The level of difficulty was increased throughout the intervention by restricting words to specific categories such as animals or foods. For the creative mental activity task, participants were asked to think in new ways, for example, of new uses of an object or to argue against one’s usual opinions on certain topics. In the MR condition, participants were asked to sit with eyes closed, as in the other two conditions, and to silently repeat a self-chosen mental stimulus. Each week a new stimulus could be chosen. To test the main hypothesis that TM would have the strongest effect on outcome variables, followed by MFL as compared to MR and NT, a contrast with contrast weights 3, 1, −2, and −2 for the respective conditions was tested. This contrast was significant for the following outcome variables: mental health, systolic blood pressure, and ratings of behavioral flexibility, perceived control, perceived age, and treatment efficacy. Most impressively, groups also significantly differed in survival rate at a 36-month follow-up. This was driven by a greater survival rate in TM than in MR and NT and greater survival of MFL than MR. For the cognitive variables, the main contrast was not significant for DST total and there was only a trend toward significance (P < 0.10) for the Stroop Test. However, the contrast was significant for the Associate Learning Scale, OVT, and Word Fluency, indicating that TM and MFL combined resulted in increased performance on these tests compared to MR and NT combined. Follow-up tests revealed that the TM group had significantly greater improvement than the NT group on the Associate Learning Scale and significantly greater improvements than MFL, MR, and NT on the OVT.
A strength of the study is that interventions were matched for form (sitting eyes closed) and time spent. Furthermore, expectancy of the effectiveness of the intervention, instructor likeability, and competence were assessed and did not differ among groups, and all teachers believed that their intervention was the most effective one. A weakness of the study is that the sample size was rather small for a four-arm study. The authors did not report whether and how they corrected for multiple comparisons. This study also included participants with compromised cognitive functions; however, it is not reported how many participants were impaired, how severe the impairment was, and whether results were driven by the impaired participants.
Lavretsky et al.47 investigated the effects of Kirtan Kriya, a form of yogic meditation from the Kundalini yoga tradition, on mental health, cognition, and telomerase activity in older family dementia caregivers with mild depression (mean age = 60.3, SD 10.2 years). Participants were randomized to either Kirtan Kriya or a relaxation condition. Both interventions lasted for 8 weeks and took the form of 12 min of daily home practice with audio recordings. Kirtan Kriya consisted of mudras (finger gestures), chanting of a mantra, and a short breathing relaxation accompanied by the visualization of light. The relaxation condition consisted of listening to instrumental music. Mental health, cognition, and telomerase activity were assessed before and after the interventions. Mental health was assessed with the mental health scale of the SF-3648 and the Hamilton Rating Scale for Depression;49 cognitive functioning with the Mini-Mental State Examination (MMSE)50 and the Trail-Making Test A (attention, processing speed) and B (executive function).51 The researchers reported that the number of responders (improvement of ≥ 50%) on both mental health variables was greater in the experimental condition than in the control condition. Furthermore, when all the tests were combined (multivariate analysis of covariance, MANCOVA), there were significant group differences in changes in cognitive function. When analyzed individually (analysis of covariance, ANCOVA) there was a significantly greater improvement on the MMSE and Trail-Making Test B in the Kirtan Kriya condition than in the relaxation condition. The same was true for telomerase activity. These findings suggest that Kirtan Kriya is more effective than relaxation alone in improving mental health and cognition in older dementia caregivers. Again, in this study it is not clear if the effects on cognition were directly dependent on the meditation or the result of reductions in depression and improvements in mental health in general.
McHugh et al.52 studied the effects of a 15-min mindfulness intervention on stimulus overselectivity in mentally healthy older adults. Stimulus overselectivity refers to hyperattentiveness to limited stimuli in the environment while ignoring others. This attentional abnormality is common in Autism Spectrum Disorders and has recently been shown to increase with age.53 Twenty-four older participants (mean age = 78.58 years) first completed a battery of psychometric tests, including the MMSE,50 Trail-Making Test A and B,51 and others, to ensure normal cognitive function and to evaluate group differences on these baseline measures. Participants were then randomized to either a 15-min mindfulness or 15-min mind wandering intervention. Participants in the mindfulness intervention were instructed to “focus on the actual sensations of breath entering and leaving the body. There is no need to think about the breath—just experience the sensations of it. When you notice that your awareness is no longer on the breath, gently bring your awareness back to the sensations of breathing,” and participants in the mind wandering condition were instructed to “simply think about whatever comes to mind. Let your mind wander freely without trying to focus on anything in particular.”52 Participants of the meditation intervention had significantly lower stimulus overselectivity than those of the mind wandering condition, indicating that a short mindfulness intervention can decrease this age-related attention bias. These findings seem impressive, as the intervention was very brief. However, as the authors acknowledge, testing was done immediately after the intervention/induction, and it would be interesting and relevant for future studies to investigate longer term effects of mindfulness interventions on stimulus overselectivity.
Moynihan et al.54 investigated the effects of mindfulness-based stress reduction (MBSR) on executive function, frontal alpha asymmetry, and immune function in a community sample of relatively healthy older adults (mean age = 73.4, SD 6.7 years). One hundred and eight participants were randomized to MBSR and 112 to a wait list control (WLC) group. Of those, 201 participants completed measurements before the intervention, right after the intervention, and 3 and 24 weeks after completion of the course. MBSR participants had a significantly lower Trails B/A ratio51 than WLCs immediately after the intervention, indicating improved executive function. This effect was not maintained at the follow-up time points and there were no significant group differences on Trails A and B at any time point. MBSR participants also had greater left lateralized frontal alpha asymmetry than WLCs right after the intervention. Furthermore, the MBSR group had significant increases in mindfulness as assessed with the Mindful Attention Awareness Scale (MAAS)55 after the intervention and after 24 weeks of follow-up. Depression as measured with the Center for Epidemiologic Studies Depression Scale Revised (CESD-R)56 and perceived stress as assessed with the Perceived Stress Scale (PSS)57 were not affected by the intervention. Against expectations, immune response at the 24-week follow-up was lower in the MBSR group than in the WLC group. Despite the fact that this study is the largest randomized controlled trial (RCT) that we have reviewed here, it also has several limitations. The control group did not receive any intervention, some subjects were randomized pair-wise, and baseline differences were present in perceived stress, depression, and mindfulness.
Oken et al.58 investigated the effects of a mindfulness meditation intervention on older family dementia caregivers. Thirty-one participants with normal cognitive functioning (mean age = 64.55, SD = 9.41) and some stress were included in the study. Participants were randomized to either a mindfulness training, the Powerful Tools for Caregivers (PTC) education program,59 or did not participate in any program but received the same amount of respite care as participants of the interventions. Both programs lasted for 7 weeks and consisted of weekly 90-min sessions and daily homework. The mindfulness intervention was developed according to the mindfulness-based cognitive therapy (MBCT) protocol60 and consisted of psycho-education, mindfulness practice, and group discussions. The PTC is based on the Chronic Disease Self-Management Program61 and consists of weekly lectures and the development of self-care tools by the participants. Before and after the intervention, a large number of measurements were taken, including the primary outcome measure, caregiver perceived stress as evaluated with the reaction rating of the Revised Memory and Behavior Problems Checklist (RMBPC).62 Other measures included mood, fatigue, self-efficacy, mindfulness, cortisol, cytokines, and of most interest for this review, cognitive function. Cognitive function was assessed with a 10-word list-learning task,63 Stroop Test,64 and a shortened Attention Network Test (ANT)65 that included only cued/noncued and congruent/incongruent conditions to calculate an alerting and executive control score. In addition, participants rated expectancy and intervention credibility.
The researchers reported significantly lower caregiver perceived stress in the mindfulness and education group after the intervention than in the respite-only group, while there were no differences between the mindfulness and education group. When correcting for multiple comparisons, there were no group differences on any of the secondary outcome measures at the post time point. However, when not correcting for multiple comparisons, group differences were significant on the Stroop Test and on the ANT alerting score (reaction time noncued–cued). The group difference in the ANT alerting score was driven by higher scores in the meditation and education groups than in the respite-only group. The comparison between the meditation and the education groups was not reported, nor was any post hoc test for the Stroop Test. Although this pilot study had a rather small sample size, it had a solid design with an active comparison condition. A further strength of the study is the measurement of credibility and expectancy. This study suggests that mindfulness is superior to respite care in reducing caregiver perceived stress. However, the absence of a difference in perceived stress between the mindfulness and the active control condition suggests that stress reduction was not specific to the mindfulness intervention in this study. The fact that the effects of mindfulness and education on executive function and alerting attention did not survive when correcting for multiple comparisons might be due to the small sample size. Furthermore, it is not clear whether the effects were directly or indirectly mediated by stress.
Sun et al.66 studied the effects of self-relaxation on quality of sleep and cognitive functions in older people with reduced quality of sleep (Pittsburg Sleep Quality Index, PSQI > 5). Eighty Chinese community-dwelling older adults (mean age = 69.69, SD 7.89 years) without mental illness that could potentially result in cognitive impairment were randomized to either a self-relaxation intervention or a sleep hygiene education condition. The sleep hygiene education condition included only a brochure with descriptions/explanations of healthy and unhealthy sleep habits and the instruction to maintain healthy habits and avoid unhealthy ones. The self-relaxation condition on the other hand consisted of a 12-month program of progressive muscle relaxation and guided imagery meditation. The 12-month program consisted of four group sessions of 90 min during the first month and 3 × 30 min of daily home practice with audio tapes and weekly phone interviews for the remaining 11 months. Before the intervention, and at months 3, 6, and 12, sleep quality and cognitive functions were assessed. Sleep quality was assessed with the PSQI67 and the Epworth Sleepiness Scale,68 and cognitive function was assessed with the MMSE50 and the number memory, picture memory, associative memory, and understanding memory subtests of the Chinese adaptation of the Wechsler Memory Scale Revised.69 The authors reported significant group-by-time interactions that were driven by decreased quality of sleep and cognitive function over time in the control group but increases in the experimental group. These results suggest that the self-relaxation program can not only offset the decline in sleep quality and cognition (that occurred in the control group during the year) but also can even enhance sleep quality and cognition. Unfortunately, it is not clear whether the positive effect on cognitive function was direct or indirect, or mediated by improvements in sleep quality. Furthermore, it is not clear how much of the effectiveness of the self-relaxation intervention can be attributed to its meditation component.
Ernst et al.70 conducted a feasibility study to investigate the effects of an MBSR43 program on the quality of life of German nursing home residents. Fifteen of the 22 participants (median age = 83.5) completed assessments before and after an 8-week modified MBSR program while seven controls did not participate in any intervention but completed assessments with an interval of 8 weeks. The modification of the original MBSR program included shortening of the weekly sessions to 90 min, simplification of physical practices, reduction of homework, and removal of the retreat day. Per-protocol analysis (only including the nine completers and seven controls) revealed significantly greater improvement in the experimental group than in the control group in physical health as measured with the Short-Form General Health Survey (SF-12),71 depression as measured with the Geriatric Depression Scale Residential (GDS-12R),72 and in severity of major complaints. There were no differences between the groups in changes in mental health as assessed with the SF-12,71 intensity of pain, satisfaction with life, activities of daily living as measured with the Barthel Index,73,74 or cognition as measured with the MMSE.50 Although the difference in MMSE change was not significant, there seemed to be no change (median pre-29, post-29) in the experimental group but a decline (median pre-27, post-24) in the control group. The study has several limitations, including a small sample size, no randomization, no active control intervention, a relatively high dropout rate of 40%, and baseline differences between the groups in age and nursing status.
Newberg et al.75 conducted a study to investigate the effects of Kirtan Kriya on cerebral blood flow and cognition in older participants (mean age = 64, SD 8 years) with memory problems ranging from normal age-related memory impairment (n = 7) to MCI (n = 5) to Alzheimer’s disease (AD, n = 3). Participants underwent single-photon emission computed tomography (SPECT) scans while listening to neutral information (baseline) or while doing a guided meditation before and after an 8-week meditation intervention. Before and after the intervention, participants’ cognitive performance was assessed with the MMSE,50 Category Fluency Test (see Ref. 75), Trail-Making Test A and B,51 Digit Symbol Substitution Test (DSST) of the Wechsler Adult Intelligence Scale (WAIS),76 and a logical memory task (see Ref. 75). The meditation intervention consisted of daily practice of a 12-min guided Kirtan Kriya meditation for 8 weeks. Five additional participants were recruited to participate in a music listening control intervention, but this group was not used for statistical comparisons with the Kirtan Kriya group and is not discussed further. After the intervention, participants had increased blood flow in frontal and parietal brain regions, specifically in the right inferior, bilateral superior frontal, right superior parietal, right sensorimotor, and right dorsolateral prefrontal cortex (DLPFC) in a baseline scan. During the meditation scan, there was decreased blood flow in the right DLPFC. In cognitive performance, participants showed significant improvements on Trails B,51 WAIS Symbol Substitution Test,76 and Logical Memory Delayed (see Ref. 75) when not correcting results for multiple comparisons. With correction for multiple comparisons, there was only a significant improvement on the Category Fluency Test. These findings suggest that an 8-week Kirtan Kriya intervention might improve cognitive function and increase cerebral blood flow in brain regions associated with cognitive functions in older adults with memory impairment. Unfortunately, the study is lacking statistical comparison with a control group and it is not clear whether the improvements are driven by participants with normal age-related memory problems or by those with MCI or AD.
Pagnoni et al.77 investigated the effects of age on gray matter volume and sustained attention in 13 experienced Zen meditators and 13 controls that were matched for age, gender, and education. Participants were middle aged (meditators’ average age = 37.2, SD 6.9 years; controls’ average age = 35.5, SD 5.7 years). To determine gray matter volume, participants underwent structural magnetic resonance imaging (MRI) scanning. Furthermore, they completed the Rapid Visual Information Processing (RVIP) task from the Cambridge Neuropsychological Test Automated Battery78 (CANTAB) to assess sustained attention capacity. This task involved rapid presentation of numbers on a screen and responding to specific target sequences. Performance was evaluated with respect to reaction time and “A,” a sensitivity index that indicates the ability to detect targets correctly while taking errors into account. The researchers reported a significant group × age interaction for reaction time, which was driven by a significant decrease in performance (i.e., increase in reaction time) with age in the controls but not in the meditators. While the same pattern was found for A, the group × age interaction only showed a trend toward significance here. Similarly, a significant group × age interaction was reported for gray matter volume in the left putamen, which also was driven by decreases in controls and a borderline significant increase in meditators. For total gray matter volume group × age interaction, follow-up tests followed the same pattern but only showed a trend toward significance. This study suggests an offset in the decline in sustained attention and gray matter volume. However, as the authors acknowledge, the cross-sectional design and the small sample size are clear limitations.
Prakash et al.79 investigated cognitive performance in 20 older Vihangam yoga meditators (mean age = 59.45, SD 3.86) as compared to 20 age- (mean age = 60.36, SD 4.51), gender-, and education-matched controls. Vihangam yoga meditation involves focusing attention on an external visual object. Meditators had an average meditation practice of 15.40 years (SD 1.18). They performed significantly better than controls on the Digit Span Forward Test, which is a measure of working memory;76 the Trail-Making Test A, B, and B-A;51 Stroop Test color reading and color word interference;45 Digit Symbol number correct, a measure of information processing speed;76 Letter Cancellation total and omission errors, measures of sustained attention;80 and errors on the Rule Shift test, another measure of executive function. Groups did not differ on Digit Span backward, Stroop word reading, Digit Symbol errors, Letter Cancellation Commission errors, and time on the Rule Shift test. While a strength of the study is the well-matched sample, even including socioeconomic status (SES), the cross-sectional design and relatively small sample size are weaknesses. It is also not reported if and how correction for multiple comparisons was done, which is of particular importance with the large number of statistical tests performed here.
Van Leeuwen et al.81 investigated attentional blink in experienced meditators and nonmeditators in a cross-sectional study. For this study, 17 middle-aged meditators (mean age = 49.8, SD 5.0 years) with 1–29 years of meditation experience, were compared to 17 age-, sex-, and education-matched adults (mean age = 50, SD 5.4 years) without meditation experience and 17 young adults (mean age = 24.3, SD 2.3) without meditation experience. Thirteen of the meditators were trained in Shamatha and Vipassana, also referred to as focused attention and open monitoring,82 and three were trained in Zazen. In the attentional blink task, letters are rapidly presented on a screen and in this stream of letters, two numbers were presented with a lag of 100–700 ms in increments of 100 (lag 1–7) between the numbers. The task was to correctly identify the first and the second number. The main outcome measure of this study was the proportion of correctly identified second numbers. When considering all seven lags, meditators outperformed age-matched controls while there was no difference between meditators and younger controls. On lag 2, meditators even outperformed younger controls. The authors conclude that, “the practice of meditation aids in overcoming age-related attentional deficits in the temporal domain.”81 While these results are impressive, an obvious limitation of the study that the authors also acknowledge is the cross-sectional design, which does not allow for causal inferences.
Jedrczak et al.83 studied nonverbal intelligence and perceptual motor speed in a group of TM practitioners who also practiced the TM Sidhi program, which is described as an extension of the TM program. This preliminary cross-sectional study did not include a control group, but was simply correlational. The DSST and Picture Arrangement Test of the WAIS subscales76 were employed to test nonverbal intelligence. Perceptual motor speed was tested with the line crossing task,84 a reaction task to a visual stimulus, and the psychomotor speed variable of the Test of Behavioral Rigidity.85 The sample consisted of 87 participants who completed all five tests and 150 participants who completed only the three group-administered tests (DSST, line crossing, and psychomotor speed). Participants were in the age range of 20–79 years (mean age = 38.74 years) and had practiced TM Sidhi for a mean of 44.07 months and TM for 108.39 months (no standard deviations provided). Multiple regression analyses controlling for age, sex, education, motivation, and TM practice time revealed that TM Sidhi practice time significantly predicted nonverbal intelligence (both WAIS tests) as well as two of the tests for perceptual motor speed, the line crossing, and simple reaction time tests. Psychomotor speed was not predicted by TM Sidhi meditation practice. Owing to its strong methodological limitations, including the absence of a control group, the study has very limited relevance.
Discussion
Here we reviewed 12 studies investigating the effects of meditation on age-related cognitive decline and cognition in middle-aged and older adults.
Different types of meditation
The studies reviewed here employed a wide range of meditation techniques, ranging from mantra-based meditations such as TM,40 Kirtan Kriya yogic meditation (KKYM),47,75 and meditations that involve focusing on a visual object,79 to Buddhist-based mindfulness practices, including Shamata-Vipassana,81 Zen,77 and nonsecularized forms, including MBCT,58 MBSR,54,70 and others.52 Two studies did not clearly describe what the meditation techniques they used involved.66,83 Researchers who investigate different meditation traditions have not referenced each others’ work much in the past. Relating findings from different techniques to one another seems important though to better understand the exact mechanisms underlying their effects.
Summary of evidence
With this common ground of focused attention, it makes sense that there is overlap in outcome measures and results between the different studies. The authors used a variety of neuropsychological tests to investigate the effect of meditation on a range of cognitive domains, including attention, memory, executive function, and processing speed. The most commonly used tests were the MMSE,47,66,70,75 the Trail-Making Task,47,54,75,79 the Stroop Test,58,79,86 and the DSST.74,82,85 Although three of the reviewed studies also reported meditation-related changes in brain structure77 and function54,75 that are consistent with the changes in cognition, we have restricted this review to cognitive findings. Eileen Luders has previously reviewed the effects of meditation on the aging brain.36 In the future, it will be important to increasingly bring these areas together and to establish what neural changes underlie the effects of meditation on cognitive function. While a few studies have begun to investigate this relationship,54,75,77 it is still too early to reach clear conclusions.
While the studies reviewed in this paper form the beginning of an emerging field at the intersection of meditation research and gerontology and provide a first orientation in this field, the results so far must be evaluated with caution. Only six of the 12 reviewed studies were RCTs, and of these six studies only one study had more “low” risk of bias ratings than “unclear” or “high” risk58 (Table 2). However, this study that was best evaluated with respect to risk of bias suffered from low precision owing to the small sample size. The categorization of the results into general cognition, attention, executive function, memory, and processing speed in the following sections is not rigid, as not all tests fit clearly or exclusively into one category.
General cognition
Two studies, one examining Kirtan Kriya yoga meditation and another investigating self-relaxation, including guided visual imagery–based meditation, revealed significant improvements in overall cognitive function.46,65 However, three other studies, examining TM, KKYM, and MBSR, did not find significant effects of meditation on overall cognitive function.40,70,75 Both Newberg et al.75 and Lavretsky et al.47 studied the same intervention protocol for KKYM and used the same instrument (MMSE) to measure overall cognitive function. However, Lavretsky et al.47 investigated individuals with mild depression but normal cognitive function, whereas Newberg et al.75 studied older adults with memory problems. These data may suggest that KKYM may be more useful for preserving normal functioning than for countering declines related to dementia.
Attention
The clearest significant finding in a more specific cognitive domain was improved attention related to mindfulness meditation.52,77,81 This conclusion is based on two cross-sectional studies77,81 and one study with a brief mindfulness induction.52 A longitudinal study with a 7-week mindfulness intervention also found improvements in attention when compared to a passive control group, but not after correcting for multiple comparisons, or when compared to an active intervention.58 That older Vipassana meditators outperformed younger nonmeditators on attention suggests that mindfulness meditation not only increases cognitive reserve or resilience but also perhaps even enhances attentional capabilities.81 Significant improvements in attention were also reported for Vihangam yoga meditation.87
Executive function
Executive function was significantly improved as the result of KKYM,47 Vihangam yoga meditation,79 and MBSR.54 Other studies also reported positive effects of KKYM,75 MBCT,58 and TM86 on executive functions. However, when correcting for multiple comparisons, these findings were no longer significant, or the correction was not reported.
Memory
One RCT suggested memory improvement as a result of a self-relaxation meditation program.66 A cross-sectional study showed that Vihangam yoga meditation practitioners performed better on Digit Span Forward, but not Backward, than did matched controls.79 This study also revealed unclear group differences on the DSST, which is thought to involve memory. Jedrczak et al.83 found a correlaton between TM Sidhi practice and DSST performance, which may be related to the memory component of the test. KKYM75 also had positive effects on memory but not significantly after correcting for multiple comparisons. Alexander et al.40 reported significant effects of TM on memory and verbal fluency but did not report multiple comparison correction.
Processing speed
The DSST that involves memory also involves processing speed. Hence, the study of Prakash et al.79 involving Vihangam yoga meditation practitioners is also inconclusive regarding processing speed. The effects of KKYM75 on this task also might be related to processing speed but did not hold after correcting for multiple comparisons. Besides a positive relationship between TM Sidhi practice and DSST performance, Jedrczak et al.83 also reported a positive relationship between practice and two of three measures of psychomotor speed.
Limitations
As mentioned earlier, the field of contemplative gerontology is in its infancy, clearly reflected by the limitations of the reviewed studies. Only half of the sudies were RCTs and even these studies had high risk of bias (Table 2). Most studies also had small sample sizes, resulting in imprecision. Furthermore, the large number of outcome variables and multiple control groups are problematic. These factors in combination with the small sample sizes resulted in underpowered studies that often did not reveal significant findings after correcting for multiple comparisons. Interestingly, the studies investigating mindfulness were mainly cross-sectional with only one or two outcome measures per study.
While the reviewed studies have investigated specific cognitive domains, the question remains how changes in these domains influence the real life of the elderly. Therefore, it might be of value to also have direct measures of quality of life and real-life functioning or to measure the higher order cognitive variable of intelligence, which has real-life relevance.88,89 As it has been suggested that meditation might not only increase quality of life by reducing cognitive decline but also by allowing one to learn how to successfully cope with life,90 future studies might also want to explore that possibility.
Future studies will also need to elucidate potential mediators and moderators of improved cognition following meditation (e.g., reductions in stress or depressive and anxiety symptoms). Depression is very common in old age,91 and depressive symptoms have been linked to impaired cognitive performance.92 Through an increase in glucocorticoid levels, chronic stress and depression can significantly affect cognition by altering prefrontal, amygdaloid, and hippocampal functioning.93,94 Meditation reliably reduces stress95 and improves depressive and anxiety symptoms.96 We have found in adults that improvements in stress reduction and anxiety are associated with alterations of the structure and function of frontolimbic regions.97,98 Future research should, therefore, investigate whether a reduction in stress and improvements in mental health serve as mediating variables for the effects of meditation on cognition in older adults, determine their relative effects as compared to direct training of attentional performance, and investigate neural correlates (e.g., stress hormone levels, brain volume and functioning). While some of the studies that were reviewed here have investigated changes in depression, stress, and other mental health variables in the elderly,47,54,58,70 it has not been established how such changes relate to cognitive improvements. It will also be important to more thoroughly differentiate between different types of meditation and their relative effects on these mediating and outcome variables.
Furthermore, to really allow causal inference, future studies should employ longitudinal designs. Particularly RCTs with long-term follow-up measures are required to investigate if improvements in cognitive function are lasting and how much they depend on continuous meditation practice.
An important conclusion that can be drawn from the published studies is that meditation interventions for the elderly are feasible. First of all, dropout rates were mostly low. Five of the seven reviewed intervention studies reported dropout rates, which were 5%,54 9%,47 20%,58 33.5%,40 and 40%.70 Second, compliance rates were exceptionally high. The four studies that reported them found intervention compliance rates of 75%,75 88%,58 88%,70 and 98% (attended more than five of eight classes).54 However, in one study it was noted that an AD patient with an MMSE of 16 was not able to properly perform the meditation, indicating that a certain level of cognitive function is required to practice meditation, at least KKYM.75 This is not surprising as meditation relies on cognitive functions, and this limitation should be taken into consideration when designing future meditation-based interventions for the elderly.
Conclusions
This review of the literature on the effects of meditation on age-related cognitive decline revealed that although this field is still young and small, the effects of a wide range of practices have been investigated. While most studies were small underpowered pilot studies, they provide preliminary evidence that a variety of meditation techniques may be able to offset age-related cognitive decline and perhaps even increase cognitive capabilities in older adults. Furthermore, these studies show that it is feasible to effectively teach these techniques to the elderly. Future studies should continue to use rigorous RCT designs with active control groups, but with sufficiently large samples and long-term follow-up measures. While the neuropsychological measures used so far should be good predictors of quality of life and real-life functioning, more direct measures of these variables might help to investigate the true relevance of these interventions for the elderly. As a next step, investigating the (neural) mechanisms of preservation and enhancement of cognition in aging through meditation is of great interest.
Acknowledgments
There was no dedicated funding for this review. While working on the review, S.W.L. was supported by Grants R01AT006344 and R01AT006464; B.K.H. by the Kusala Foundation, Germany; and T.G. by the Kripalu Center for Yoga and Health, and the Brach Family Charitable Foundation.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.United Nations. New York: United Nations; 2002. World Population Ageing, 1950–2050. [Google Scholar]
- 2.Administration on Aging. Washington DC: Department of Health and Human Services Retrieved; 2012. A Profile of Older Americans Vol. June 18. [Google Scholar]
- 3.Charles ST, Carstensen LL. Social and emotional aging. Annu. Rev. Psychol. 2010;61:383–409. doi: 10.1146/annurev.psych.093008.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn. Sci. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Scheibe S, Carstensen LL. Emotional aging: recent findings and future trends. J. Gerontol. B Psychol. Sci. Soc. Sci. 2010;65:135–144. doi: 10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker-Drob EM. Global and domain-specific changes in cognition throughout adulthood. Dev. Psychol. 2011;47:331. doi: 10.1037/a0021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salthouse TA. When does age-related cognitive decline begin? Neurobiol. Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson RS, et al. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Arch. Neurol. 1999;56:1274. doi: 10.1001/archneur.56.10.1274. [DOI] [PubMed] [Google Scholar]
- 9.Persson J, et al. Structure-function correlates of cognitive decline in aging. Cereb. Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- 10.Morrison J, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 11.Salat DH, et al. Thinning of the cerebral cortex in aging. Cereb. Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 12.Salthouse TA. Decomposing age correlations on neuropsychological and cognitive variables. J. Int. Neuropsychol. Soc. 2009;15:650. doi: 10.1017/S1355617709990385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salthouse TA. Localizing age-related individual differences in a hierarchical structure. Intelligence. 2004;32:541–561. doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deary IJ, et al. Age-associated cognitive decline. Br. Med. Bull. 2009;92:135–152. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- 15.Plassman BL, et al. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann. Intern. Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 16.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat. Rev. Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 17.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn. Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Willis SL, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. J. Am. Med. Assoc. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belleville S, et al. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain. 2011;134:1623–1634. doi: 10.1093/brain/awr037. [DOI] [PubMed] [Google Scholar]
- 20.Nyberg L, et al. Neural correlates of training-related memory improvement in adulthood and aging. Proc. Nat. Acad. Sci. U. S. A. 2003;100:13728. doi: 10.1073/pnas.1735487100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry. 2013;18:864–874. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- 22.Colcombe SJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc. Nat. Acad. Sci. U. S. A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daviglus ML, et al. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer disease and cognitive decline. Ann. Intern. Med. 2010;153:176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 24.Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn. Affect. Behav. Neurosci. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 25.Tang YY, et al. Short-term meditation training improves attention and self-regulation. Proc. Nat. Acad. Sci. U. S. A. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeidan F, et al. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious. Cogn. 2010;19:597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Cranson RW, Orme-Johnson DW, Gackenbach J, et al. Transcendental meditation and improved performance on intelligence-related measures: a longitudinal study. Pers. Individ. Dif. 1991;12:1105–1116. [Google Scholar]
- 28.Jha AP, et al. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10:54–64. doi: 10.1037/a0018438. [DOI] [PubMed] [Google Scholar]
- 29.Brefczynski-Lewis JA, et al. Neural correlates of attentional expertise in long-term meditation practitioners. Proc. Nat. Acad. Sci. U. S. A. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hölzel BK, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hölzel BK, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci. Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 32.Lazar SW, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gard T, et al. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb. Cortex. 2012;22:2692–2702. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagnoni G. Dynamical properties of BOLD activity from the ventral posteromedial cortex associated with meditation and attentional skills. J. Neurosci. 2012;32:5242–5249. doi: 10.1523/JNEUROSCI.4135-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen M, et al. Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J. Neurosci. 2012;32:15601–15610. doi: 10.1523/JNEUROSCI.2957-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luders E. Exploring age-related brain degeneration in meditation practitioners. Ann. N. Y. Acad. Sci. 2014;1307:82–88. doi: 10.1111/nyas.12217. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gard T, et al. Effects of a yoga-based intervention for young adults on quality of life and perceived stress: the potential mediating roles of mindfulness and self-compassion. J. Posit. Psychol. 2012;7:165–175. [Google Scholar]
- 39.Higgins JP, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br.Med. J. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander CN, et al. Transcendental meditation, mindfulness, and longevity: an experimental study with the elderly. J. Pers. Soc. Psychol. 1989;57:950–964. doi: 10.1037//0022-3514.57.6.950. [DOI] [PubMed] [Google Scholar]
- 41.Langer EJ. Matters of mind: mindfulness/mindlessness in perspective. Conscious. Cogn. 1992;1:289–305. [Google Scholar]
- 42.Langer EJ, Moldoveanu M. The construct of mindfulness. J. Soc. Issues. 2000;56:1–9. [Google Scholar]
- 43.Kabat-Zinn J. Full Catastrophe Living. New York: Delta Publishing; 1990. [Google Scholar]
- 44.Farmer ME, et al. Neuropsychological test performance in Framingham: a descriptive study. Psychol. Rep. 1987;60:1023–1040. doi: 10.1177/0033294187060003-201.1. [DOI] [PubMed] [Google Scholar]
- 45.Stroop JR. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- 46.Yogi MM. On the Bhagavad-Gita: A new translation and commentary. Baltimore: Penguin Books; 1969. [Google Scholar]
- 47.Lavretsky H, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int. J. Geriatr. Psychiatry. 2013;28:57–65. doi: 10.1002/gps.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 49.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folstein MF, Folstein SE, McHugh PR. Minimental state: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 51.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8:271–276. [Google Scholar]
- 52.McHugh L, Simpson A, Reed P. Mindfulness as a potential intervention for stimulus over-selectivity in older adults. Res. Dev. Disabil. 2010;31:178–184. doi: 10.1016/j.ridd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 53.McHugh L, Reed P. Age trends in stimulus overselectivity. J. Exp. Anal. Behav. 2007;88:369–380. doi: 10.1901/jeab.2007.88-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moynihan JA, et al. Mindfulness-based stress reduction for older adults: effects on executive function, frontal alpha asymmetry and immune function. Neuropsychobiology. 2013;68:34–43. doi: 10.1159/000350949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J. Pers. Soc. Psychol. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 56.Lewinsohn PM, et al. Center for epidemiologic studies depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol. Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 57.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 58.Oken BS, et al. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. The Journal of Alternative and Complementary Medicine. 2010;16:1031–1038. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Services L. The Caregiver Helpbook: Powerful Tools for Caregivers. Portland, OR: Legacy Health Systems; 2006. [Google Scholar]
- 60.Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: The Guilford Press; 2002. [Google Scholar]
- 61.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Ann. Behav. Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 62.Teri L, et al. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol. Aging. 1992;7:622–622. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 63.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am. J. Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 64.Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 65.Fan J, et al. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 66.Sun J, et al. Self-relaxation training can improve sleep quality and cognitive functions in the older: a one-year randomised controlled trial. J. Clin. Nur. 2013;22:1270–1280. doi: 10.1111/jocn.12096. [DOI] [PubMed] [Google Scholar]
- 67.Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 68.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep. 1994;17:703–710. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 69.Gong Y. Handbook of Wechsler Memory Scale-Revised. Changsha: Hunan Medical College; 1989. [Google Scholar]
- 70.Ernst S, et al. Effects of mindfulness-based stress reduction on quality of life in nursing home residents: a feasibility study. Forschende Komplementarmedizin (2006) 2008;15:74–81. doi: 10.1159/000121479. [DOI] [PubMed] [Google Scholar]
- 71.Gandek B, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA project. J. Clin. Epidemiol. 1998;51:1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 72.Sutcliffe C, et al. A new version of the geriatric depression scale for nursing and residential home populations: the geriatric depression scale (residential) (GDS-12R) Int. Psychogeriatr. 2000;12:173–181. doi: 10.1017/s104161020000630x. [DOI] [PubMed] [Google Scholar]
- 73.Mahoney FI. Functional evaluation: the Barthel index. Maryland State Med. J. 1965;14:61–65. [PubMed] [Google Scholar]
- 74.Heuschmann P, et al. Untersuchung der Reliabilität der deutschen Version des Barthel-Index sowie Entwicklung einer postalischen und telefonischen Fassung für den Einsatz bei Schlaganfall-Patienten. Fortschr. Neurol. Psychiatr. 2005;73:74–82. doi: 10.1055/s-2004-830172. [DOI] [PubMed] [Google Scholar]
- 75.Newberg AB, et al. Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: a preliminary study. J. Alzheimers Dis. 2010;20:517–526. doi: 10.3233/JAD-2010-1391. [DOI] [PubMed] [Google Scholar]
- 76.Wechsler D. Manual for the Wechsler Adult Intelligence Scale. Oxford, England: The Psychological Corporation; 1955. [Google Scholar]
- 77.Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol. Aging. 2007;28:1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Sahakian B, Owen A. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J. R. Soc. Med. 1992;85:399. [PMC free article] [PubMed] [Google Scholar]
- 79.Prakash R, et al. Long-term concentrative meditation and cognitive performance among older adults. Neuropsychol. Dev. Cogn. B. Aging Neuropsychol. Cogn. 2012;19:479–494. doi: 10.1080/13825585.2011.630932. [DOI] [PubMed] [Google Scholar]
- 80.Dawson DR, TannerCohen C. Visual scanning patterns in an adult Chinese population: preliminary normative data. Occup. Ther. J. Res. 1997;17:264–279. [Google Scholar]
- 81.van Leeuwen S, Müller NG, Melloni L. Age effects on attentional blink performance in meditation. Conscious. Cogn. 2009;18:593–599. doi: 10.1016/j.concog.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Lutz A, et al. Cognitive-emotional interactions—attention regulation and monitoring in meditation. Trends Cogn. Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jedrczak A, Toomey M, Clements G. The TM-Sidhi Program, age, and brief tests of perceptual-motor speed and nonverbal intelligence. J. Clin. Psychol. 1986;42:161–164. doi: 10.1002/1097-4679(198601)42:1<161::aid-jclp2270420127>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 84.Botwinick J, Storandt M. Speed functions, vocabulary ability, and age. Percept. Mot. Skills. 1973;36:1123–1128. doi: 10.2466/pms.1973.36.3c.1123. [DOI] [PubMed] [Google Scholar]
- 85.Schaie KW, Parham I. Manual for the Test of Behavioral Rigidity. 2nd revised ed. Palo Alto, CA: Consulting Psychologists Press; 1975. [Google Scholar]
- 86.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 87.Prakash RS, et al. Cardiorespiratory fitness and attentional control in the aging brain. Front. Hum. Neurosci. 2011;4:229. doi: 10.3389/fnhum.2010.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deary IJ. Intelligence. Annu. Rev. Psychol. 2012;63:453–482. doi: 10.1146/annurev-psych-120710-100353. [DOI] [PubMed] [Google Scholar]
- 89.Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nat. Rev. Neurosci. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- 90.Petkus AJ, Wetherell JL. Acceptance and commitment therapy with older adults: rationale and considerations. Cogn. Behav. Pract. 2013;20:47–56. doi: 10.1016/j.cbpra.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luppa M, et al. Age- and gender-specific prevalence of depression in latest-life—systematic review and meta-analysis. J. Affect. Disord. 2012;136:212–221. doi: 10.1016/j.jad.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 92.Reppermund S, et al. The relationship of current depressive symptoms and past depression with cognitive impairment and instrumental activities of daily living in an elderly population: the Sydney Memory and Ageing Study. J. Psychiatr. Res. 2011;45:1600–1607. doi: 10.1016/j.jpsychires.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 93.Lupien SJ, et al. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Lupien SJ, et al. Increased cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev. Neurosci. 1999;10:117–139. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- 95.Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J. Altern. Complement Med. 2009;15:593–600. doi: 10.1089/acm.2008.0495. [DOI] [PubMed] [Google Scholar]
- 96.Hofmann SG, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J. Consult. Clin. Psychol. 2010;78:169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hölzel BK, et al. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect. Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hölzel BK, et al. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clin. 2013;2:448–458. doi: 10.1016/j.nicl.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]