Abstract
Hypoxic ischemic encephalopathy (HIE) in neonates is a leading cause of neurological impairment. Significant progress has been achieved investigating the pathologic contributions of excitotoxicity, oxidative stress, and neuroinflammation to cerebral injury in HIE. Less extensively investigated has been the contribution of vascular dysfunction, and whether modulation of cerebral perfusion may improve HIE outcome. Here, we investigated the function of the prostaglandin E2 (PGE2) EP4 receptor, a vasoactive Gαs-protein coupled receptor (GPCR), in rodent models of neonatal HIE. The function of PGE2 signaling through the EP4 receptor was investigated using pharmacologic and conditional knockout genetic strategies in vivo in rodent models of HIE. Pharmacologic activation of the EP4 receptor with a selective agonist was significantly cerebroprotective both acutely and after 7 days. Measurement of cerebral perfusion during and after hypoxia-ischemia demonstrated that EP4 receptor activation improved cerebral perfusion in both the contralateral and ipsilateral hypoxic-ischemic hemispheres. To test whether vascular EP4 signaling exerted a critical function in HIE injury, cell specific conditional knockout mouse pups were generated in which endothelial EP4 receptor was selectively deleted postnatally. VE-Cadherin Cre-ERT2;EP4lox/lox pups demonstrated significant increases in cerebral injury as compared to VE-Cadherin CreERT2;EP4+/+ control littermates, indicating that endothelial EP4 signaling is protective in HIE. Our findings identify vascular PGE2 signaling through its EP4 receptor as protective in HIE. Given the pharmacologic accessibility of endothelial EP4 GPCRs, these data support further investigation into novel approaches to target cerebral perfusion in neonatal HIE.
Keywords: PGE2 EP4 receptor, hypoxic ischemic encephalopathy, endothelium, cerebral protection, conditional knockout
INTRODUCTION
Hypoxic-Ischemic Encephalopathy (HIE) develops as a result of systemic asphyxia and occurs in 0.2–0.4% of full-term and 60% of premature live births. 25% of neonates who survive HIE develop permanent neurological deficits, including mental retardation, cerebral palsy, and/or epilepsy. Events precipitating HIE can occur at any point during gestation or parturition and include maternal infection, hypertension, cardiopulmonary anomalies, or complications of the birth process. In HIE, diminished placental or pulmonary gas exchange leads to reduced blood pressure, reduced oxygen and glucose delivery to the brain, and consequent cerebral hypoxia and infarction (Vannucci and Hagberg, 2004). At present, the only therapeutic intervention for perinatal HIE is hypothermia, which improves survival and reduces neurological sequelae in a proportion of neonates when administered within 6 hours of birth (Johnston et al., 2011; Pietrini et al., 2012). Additional refinement of treatment, potentially with the addition of adjunctive therapies, may be important to further improve functional recovery after HIE.
Major pathophysiologic processes that contribute to permanent brain injury from HIE include vascular insufficiency, excitotoxicity and oxidative stress, and secondary inflammation (Baburamani et al., 2012; Gonzalez and Ferriero, 2009; Johnston et al., 2000; McLean and Ferriero, 2004; Vexler and Yenari, 2009). Of these pathologic processes, significant progress has been achieved in understanding mechanisms of excitotoxicity and oxidative stress, where energy failure induced by hypoxia in neonatal brain results in excessive release and decreased reuptake of the glutamate by glial cells. NMDA receptor overactivation leads to unregulated Ca2+ entry into neurons, activation of nitric oxide synthase, mitochondrial dysfunction and production of reactive oxygen species, and ultimately cell death. Recent studies have also demonstrated the importance of secondary neuroinflammatory processes triggered by cerebral injury in the neonate, and its role in exacerbation of cerebral injury. Less extensively studied has been the regulation of cerebrovascular flow in neonatal HIE, and the identification of signaling pathways that could modulate this process and optimize cerebral perfusion.
The prostaglandin PGE2 is a potent lipid messenger that is derived from membrane stores of arachidonic acid by the sequential action of the cyclooxygenase and PGE2 synthase enzymes. PGE2 regulates vascular tone through activation of its four G-protein coupled receptors, EP1-EP4 (reviewed in (Swan and Breyer, 2011)). Studies in ex vivo preparations and in vivo have shown that the Gαs-coupled EP2 and EP4 receptors are vasodilatory, whereas the Gαq-coupled EP1 and Gαi-coupled EP3 receptor promote vasoconstriction. In a murine model of adult transient focal cerebral ischemia, the PGE2 EP4 receptor is significantly cerebroprotective via dual neuronal and endothelial mechanisms of action (Liang, 2011). Moreover, in models of innate immune neuroinflammation, EP4 signaling exerts potent anti-inflammatory effects (Shi et al., 2010), suggesting that in HIE, where there is prominent vascular, neuronal, and inflammatory dysfunction, EP4 signaling may be beneficial. Herein, we examine the function of the PGE2 EP4 receptor in perinatal brain hypoxia-ischemia using a combination of pharmacological and genetic strategies.
MATERIALS AND METHODS
Animals and surgical procedures
This study was conducted in accordance with the National Institutes of Health guidelines for the use of experimental animals and the Institutional Animal Care and Use Committee at Stanford University. The rodent model of neonatal rat HIE was carried out according to the protocol established by Rice et. al. (Rice et al., 1981; Taniguchi and Andreasson, 2008). Briefly, 7 day old Sprague-Dawley male and female rat pups (Charles River, Wilmington, MA) were anesthetized with 2–4% isoflurane (Aerrane, Baxter, Deerfield, IL) and the left carotid artery was surgically ligated. Following ligation, pups were randomized and returned to their dam for 1–2 h, and placed in a hypoxic chamber (8% oxygen-92% nitrogen) for 100 min. Initial studies confirmed maintenance and stability of rectal temperature before and after hypoxia, and rectal temperatures were monitored before, during, and after hypoxia in the LDF cohorts. The temperature inside the hypoxia chamber was maintained at 37°C. Pups were returned to their dam. Sham treated animals were generated by ligating the carotid artery but not subjecting them to hypoxia. The neonatal mouse HIE model was carried out as previously described (Taniguchi et al., 2007), except that mice underwent HI at postnatal day 9, and were subjected to hypoxia for 30 min. C57BL/6 EP4lox/lox mice (Schneider et al., 2004) were kindly provided by Drs. Richard and Matthew Breyer (Vanderbilt University School of Medicine, Nashville, TN). C57BL/6 VE-CadherinCreERT2 mice (abbreviated VE-CadCreERT2) were kindly provided by Dr. Luisa Iruela Arispe (UCLA, Los Angeles, CA) (Monvoisin et al., 2006). EP4lox/lox mice and VE-CadCreERT2 mice were intercrossed to yield VE-CadCreERT2; EP4+/+ and VE-CadCreERT2;EP4lox/lox conditional knockout mice. Tamoxifen (Sigma, St Louis, MO) was administered to all VE-CadCreERT2 genotypes to induce translocation of Cre and excision of floxed EP4 sequences (Monvoisin et al., 2006). Filtered tamoxifen stock solution at 10 mg/ml was prepared in sunflower seed oil (10:1 oil:ethanol) and tamoxifen was injected subcutaneously from postnatal day 3 to postnatal day 5 at a dose of 33μg/kg/mouse pup. Mice then underwent the HIE protocol by an experimenter who was blinded to genotype.
Reagents and materials
The EP4 receptor specific agonist AE1-329 (Maruyama et al., 2001) [16-(3-methoxymethyl) phenyl-omega-tetranor-3,7-dithia prostaglandin E1] was a generous gift from Ono Pharmaceuticals Co. (Osaka, Japan), and was solubilized in ethanol and kept in 10 mM stock solution at −80°C. The selectivity of AE1-329 (Ki =0.4 nM) for the EP4 receptor has been previously established (Maruyama et al., 2001; Suzawa et al., 2000).
Morphometric analysis of the infarct area
For rat HIE experiments, 24h after HI, pups were lethally anesthetized and brain tissue was harvested for infarct quantification using 1% 2,3,5-triphenyl-tetrazolium chloride staining (TTC) (Taniguchi and Andreasson, 2008). TTC stained slices were measured by two investigators who were blinded to treatment group. The percent infarct area per slice and percent volume of infarct representing edema and infarct were calculated for TTC stained sections. For mouse HIE and 7-day rat HIE experiments, brains were processed for Cresyl violet staining and morphometry. Under deep isoflurane anesthesia, pups were perfused with phosphate-buffered saline (PBS pH7.4), followed by 4% paraformaldehyde/PBS pH 7.4. Brains were postfixed overnight, and coronal brain sections were processed into paraffin blocks. Brain sections were examined at bregma +2.0, +1.0, 0. −1.0, −2.0, −3.0 and −4.0 mm according to the Mouse Brain Atlas (Paxinos and Franklin, 2001). For cresyl violet stained paraffin sections, which include the 7 day post HIE cohorts and VE-Cadherin Cre-ERT2:EP4lox/lox and control cohorts, we used the indirect method (Lin et al., 1993) that allows quantification of infarcted area and not edema. Quantification of infarct volume was carried out by an examiner blinded to genotype.
Measurement of cerebral blood flow (CBF)
Laser Speckle Imaging (Briers, 2001) was used to obtain two-dimensional spatio-temporal resolution of changes in CBF (Dunn et al., 2001) in both hemispheres following initiation of HI (LS; FLPI system, Moor Instruments, Wilmington, DE). Laser Doppler flowmetry (LDF; Perimed model 5000, Stockholm, Sweden) was used in subsequent experiments to determine regional CBF changes in both hemispheres in response to vehicle or EP4 agonist. LDF analysis was used in subsequent studies to examine effects of EP4 receptor agonist because mortality was high in EP4 agonist treated cohorts in the LS compared to the LDF groups (35–40% for LS and ~10% for LDF); it is possible that there is a negative interaction between the anesthesia and AE1-329. Isofluorane anesthesia was maintained at 1.0% for the LS study and 0.5% for the LDF study and body temperature was maintained at 37°C with a heating pad. For the LS study, repeated images of the region of interest (ROI; delineated by the coronal, saggital, and lambdoid sutures) were taken every 10 seconds beginning 5 min before initiation of hypoxia to 60 min after termination of hypoxia. Cerebral perfusion was recorded using arbitrary Perfusion Units (PUs); PUs were averaged for each minute and normalized to the average PU value 3 minutes before onset of hypoxia. For LDF studies, CBF, flow velocity, and flow volume were measured by a laser probe with a polyacrylamide sheath and an inner diameter of 0.8 mm that was attached with dental cement to the intact skull 3 mm posterior and 3 mm lateral to the bregma. After confirming a stable flow wave of the ipsilateral hemisphere for 15 min, pups were subjected to hypoxia; CBF was continuously monitored until 15–20 min after the start of reoxygenation. Data points were collected every 0.1 s (Perisoft software, Perimed) and were averaged every 1 min.
Quantification of acute changes in cerebral blood volume
To determine cerebral volume at risk, cohorts were injected with Evans Blue dye (Renic et al., 2009). Evans blue (EB) was diluted in PBS to a final concentration of 2% (Sigma, St. Louis, MO) and was injected subcutaneously (200 mg/kg) 5 min before the start of hypoxia or 5 min after the end of hypoxia +/− AE1-329 (50μg/kg) or vehicle. Mice were euthanized and brains were harvested, weighed, and stored at −80°C until the time of analysis. Brain hemispheres were incubated in 1 ml of formamide at 60°C for 48h and the EB concentration was measured by spectrophotometer as μg per gram tissue protein (Spectra Max M5, Molecular Devices, Sunnyvale, CA).
Immunocytochemistry
Postnatal day 7 mouse C57B6 pups were deeply anesthetized with isoflurane and transcardially perfused with normal saline followed by 4% PFA and overnight fixation in PFA at 4°C. Brains were processed for paraffin sections (5 μm thickness). Following antigen retrieval (in citrate pH 6.0, boiled in a microwave for 20 min) and permeabilization with 0.3% triton together with blocking with 10% normal goat serum for 1h, immunostaining for EP4 was carried out in wild type C56BL/6 mouse pups using anti-EP4 polyclonal antibodies (1/500; Cayman Chemical, Ann Arbor, MN) overnight at 4°C followed by secondary antibody (biotinylated anti rabbit IgG, VECTOR, Burlingame, CA) and detection reagents (DAB: 3′,3′-diaminobenzidine tetrahydrochloride, Polysciences Inc, Warrington, PA). Images were acquired with a Leica DM5000B microscope and a Leica DC500 camera and digitized using software PictureFrame 2.3 (Optronics, Goleta, CA).
Statistical Analysis
Statistical analysis was performed by Student’s t-test or analysis of variance (ANOVA), followed by Tukey post hoc analysis. All data are reported as mean±standard error of the mean (SEM). p values <0.05 were considered significant.
RESULTS
EP4 receptor activation is cerebroprotective in HIE
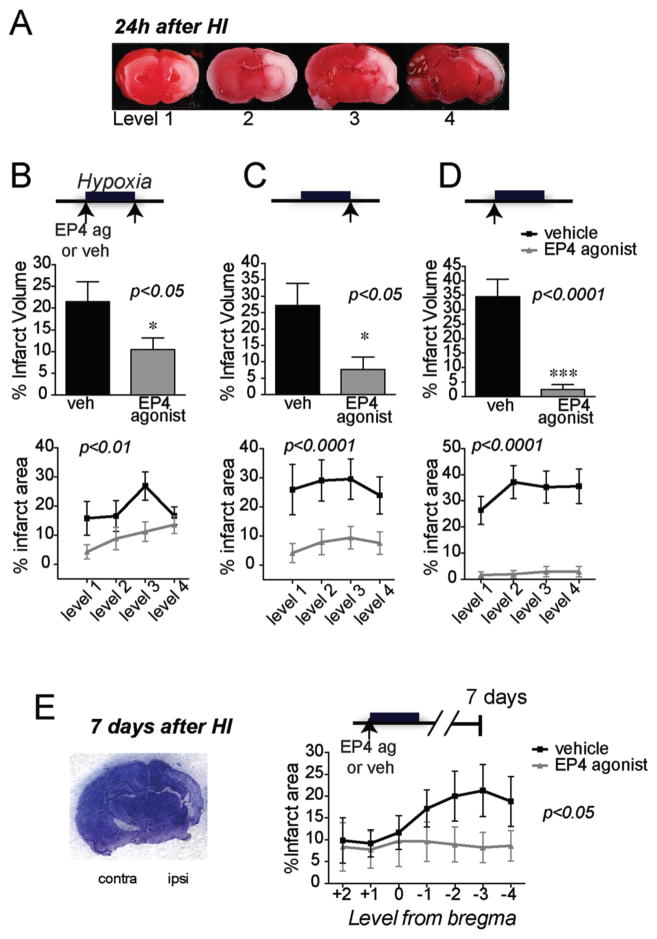
The rodent postnatal HIE model is a widely used model that recapitulates cerebral hypoxia-ischemia occurring in the perinatal period in newborns. Histological and immunocytochemical characterization studies have demonstrated that the 7 day old rodent has the analogous brain maturity to model a third trimester human fetus (Clancy et al., 2001). To determine whether EP4 receptor activation may be cerebroprotective in HI injury, we examined the effect of the selective EP4 agonist AE1-329 on infarct volume in 7 day old Sprague Dawley rat pups 24 h after HI; AE1-329 (50 μg/kg sub-cutaneously) was given either 5 min before or 5 min after 100 min of hypoxia (Figure 1). EP4 receptor activation before and after hypoxia reduced hemispheric cerebral injury by 51.11% (p<0.05, n=12–14 per group; Figure 1B), and reduced infarct area across the four levels examined (effect of treatment [F(1,93)=9.99], p=0.002). A second experiment tested a single dose immediately after hypoxia that rescued infarct volume by 72.02% (p=0.017, n=11–12 per group; Figure 1C), and reduced infarct area across levels (effect of treatment [F(1,84)=24.25] p<0.0001). Finally, a single dose of EP4 agonist administered right before onset of hypoxia very significantly rescued volume of cerebral injury (n=20–21 per group; p<0.001; Figure 1D) and significantly reduced infarct area across levels (effect of treatment [F (1, 156) = 92.45], p<0.0001). The difference between single and double administrations of EP4 agonist is interesting, and the lower efficacy of administration of EP4 agonist before and after HI may be due in part to toxic effects of repeated administration. A beneficial long-term effect of EP4 receptor activation was further confirmed in postnatal rat pups subjected to HI that survived 7 days to postnatal day 14 (Figure 1E). Cresyl violet histochemistry was used to quantify percent infarct area from +2 to −4 from the bregma, and demonstrated a rescue of brain tissue with EP4 agonist (effect of treatment [F (1, 133) = 6.473], p=0.021). Thus, selective activation of EP4 signaling was cerebroprotective, both acutely at 24h and after 7 days.
Figure 1. Administration of the selective EP4 agonist AE1-329 reduces cerebral injury at 24h in a model of HIE.
Administration of EP4 agonist AE1-329 (50 μg/kg subcutaneously) significantly reduced cerebral injury as measured by TTC staining when given at different time points before and/or after hypoxia. (A) Representative coronal TTC stained brain sections illustrating ischemic tissue (in white) over four levels of 3 mm in width; in this model, cerebral cortex, striatum, and hippocampus are significantly affected. (B) Administration of the EP4 agonist AE1-329 before and after 100 min hypoxia reduced percent hemispheric infarct volume (n=12–14 per group; *p<0.05, upper panel) and percent area across all four levels (p<0.01, bottom panel). (C) Administration of EP4 agonist after hypoxia similarly elicited significant protection (n=11–12; *p<0.05 for percent hemispheric infarct volume and p<0.001 for percent infarcted area across levels). (D) A single dose of AE1-329 right before hypoxia significantly rescued infarct injury (n=20–21 per group; ***p<0.001 for percent hemispheric volume, and p<0.0001 for percent area across levels). (E) A representative coronal section of post-natal day 14 rat brain stained with cresyl violet is shown 7 days after hypoxia-ischemia. Administration of EP4 agonist before onset of hypoxia at postnatal day 7 resulted in significant cerebroprotection 7 days later at postnatal day 14 (n=9–12 per group; p=0.012).
EP4 signaling increases cerebral blood volume during and after hypoxia-ischemia (HI)
An important physiologic response to hypoxia in neonatal HI is a decrease in cardiac output and blood pressure, a process that leads to brain hypoperfusion. In rodent HIE, the predominant cerebral injury occurs in the sensorimotor cerebral cortex, striatum, and hippocampus. In this model, before hypoxia, reduced cerebral blood flow (CBF) in the hemisphere ipsilateral to the ligated carotid artery may be partially compensated by collateral blood flow from the circle of Willis; however with addition of hypoxia, the CBF in the ipsilateral hemisphere decreases further, resulting in cerebral injury, but is restored over time with reoxygenation (Grow and Barks, 2002). EP4 receptor signaling can mediate vasodilatory effects of PGE2 in vascular beds (Audoly et al., 1999; Liang, 2011; Zhang et al., 2000). The significantly protective effect of EP4 receptor activation when given right before initiation of hypoxia (Figure 1C), a period characterized by maximal reduction in cardiac output, suggested that cerebral perfusion to the ischemic ipsilateral hemisphere might be increased, resulting in diminished short- and long-term cerebral injury.
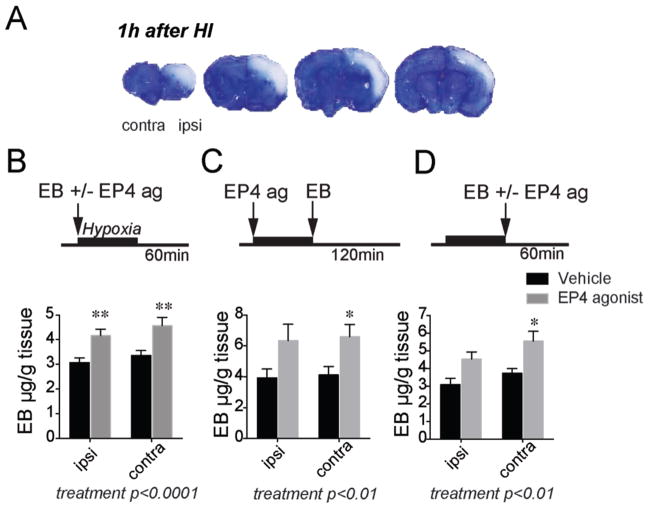
We therefore tested whether EP4 agonist increased cerebral perfusion in postnatal rat pups initially by measuring retention of Evans Blue (EB) in brain acutely 1–2 h after hypoxia (Renic et al., 2009). At early stages after cerebral hypoxia-ischemia, before there is significant blood-brain barrier breakdown, levels of retained EB dye reflect the amount of cerebral perfusion. EB retention can be measured using image analysis quantification which provides a sensitive assessment of hypoperfused regions, as shown in Figure 2A. Absolute levels of EB retention can also be measured in whole hemispheric lysates (Yepes et al., 2003); in this case, the EB concentration will reflect EB retention in hypo-, normo-, and hyper-perfused regions of forebrain. Because we found a higher variability in EB quantification with the image analysis method following HIE, we used the spectrophotometric method to assess absolute EB concentrations in ipsilateral and contralateral hemispheric lysates.
Figure 2. Effects of EP4 receptor activation on Evans Blue (EB) levels in brain.
(A) Post natal rat pups were administered EB before 100 min hypoxia, and brains were harvested at the end of hypoxia; representative sections of brain show lack of EB signal in hypoxic-ischemic areas. EB is excluded from non-perfused regions in the cerebral cortex acutely after hypoxia. (B) EB concentration (μg/g protein) was increased in both ischemic ipsilateral hemisphere and non-ischemic contralateral hemisphere with EP4 agonist treatment; here AE1-329 and EB were administered before hypoxia and brain tissue was harvested 60 min after 100 min HI (n=9 per group; 2-way ANOVA for effect of treatment ****p<0.0001, Sidak’s multiple comparisons test comparing mean of vehicle and EP4 agonist was **p=0.0087 for ipsilateral hemisphere and **p=0.0036 for contralateral hemisphere. (C) EP4 agonist or vehicle were administered before 100 minutes of hypoxia, EB was given immediately after hypoxia, and brains were analyzed 2h later (n=5–7 per group; effect of treatment **p<0.01, Sidak’s multiple comparisons test comparing mean of vehicle and EP4 agonist was p=0.057 for ipsilateral hemisphere and *p=0.05 for contralateral hemisphere). (D) EP4 agonist or vehicle were co-administered with EB immediately after 100 min hypoxia, and brains were analyzed 60 min later (n=6–7 per group; effect of treatment [F (1, 21) = 13.87, **p<0.01], Sidak’s multiple comparisons test comparing mean of vehicle and EP4 agonist was p=0.051 for ipsilateral hemisphere and *p=0.0185 for contralateral hemisphere).
Three separate experiments tested whether EP4 receptor activation resulted in altered EB retention. In the first experiment, administration of EP4 agonist before hypoxia resulted in a significant increase in EB retention both in ipsilateral ischemic and contralateral hemispheres when EB concentrations were measured 60 min after hypoxia (effect of treatment [F (1, 32) = 20.97], p<0.0001; post-hoc p<0.01 for differences in ipsilateral and contralateral hemispheres; Figure 2B). This effect was partially retained when brain was analyzed later at 120 min after hypoxia (effect of treatment [F (1, 20) = 11.40], **p<0.01, post-hoc p=0.05 for contralateral hemisphere; Figure 2C) and when EP4 agonist was administered immediately after hypoxia (effect of treatment [F (1, 21) = 13.87], **p<0.01, post-hoc p<0.05 for contralateral hemisphere; Figure 2D). Taken together, levels of EB were retained to a greater extent with EP4 agonist administration, suggesting an increase in cerebral perfusion with EP4 receptor activation. Interestingly, both the ipsilateral as well as contralateral hemispheres responded in this manner to EP4 agonist, suggesting a potential role of contralateral blood flow in the cerebroprotection seen with EP4 receptor activation.
EP4 signaling alters cerebral perfusion in HIE
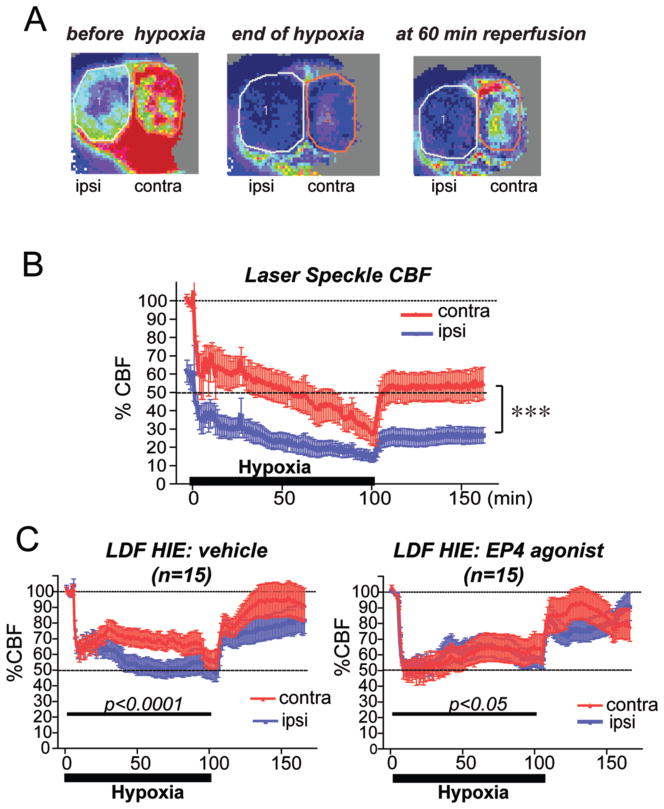
Accordingly, we investigated the spatio-temporal dynamics of cerebral perfusion following hypoxia in both contralateral and ipsilateral hemispheres using laser Speckle flow measurements (Figure 3A and B). Before onset of hypoxia, flow was lower on the ipsilateral side (left-most panel, Figure 3A) because of the ligated left carotid artery. With hypoxia, flow was reduced further in the ipsilateral hemisphere, but also substantially in the contralateral hemisphere, reflecting decreased cardiac output (middle panel, Figure 3A). At 60 minutes after reoxygenation, the ipsilateral hemisphere remained in a low-flow state, although the contralateral hemisphere had begun to recover (right panel, Figure 3A). Pixels were quantified for each hemisphere to generate a temporal course of %CBF (Figure 3B) that demonstrated a marked decrease in CBF in the ipsilateral hemiphere of about 60% before hypoxia. With hypoxia, both ipsilateral and contralateral hemispheres demonstrated a marked reduction in CBF that recovered incompletely after the start of reoxygenation. Thus the LS technique enabled the visualization and direct comparison of ipsilateral and contralateral hemispheric blood flow and demonstrated that there is a marked decrease in perfusion not only in the hypoxic-ischemic ipsilateral hemisphere, but the contralateral hemisphere as well in this HIE model.
Figure 3. Contralateral and ipsilateral cerebral blood flow is changed in HI and in response to EP4 agonist.
(A) Representative Laser Speckle (LS) images of both ipsilateral (ipsi) and contralateral (contra) hemispheres before hypoxia, at the end of 100 min hypoxia, and at 60 min of reoxygenation are shown, where red indicates normal flow, and blue denotes low flow states. Cerebral perfusion is decreased at baseline in the ipsilateral hemisphere as compared to the contralateral side from ligation of the carotid artery on the left side. Blood flow is decreased in the contralateral (contra) as well as ipsilateral (ipsi) hemispheres at the end of hypoxia. Following reoxygenation for 60 minutes, CBF recovers mostly in the contralateral hemisphere, but not in the ipsilateral hemisphere. The total number of pixels in the circled areas (1: ipsilateral; 2: contralateral) was used for quantification in panel (B). Here, temporal changes of % CBF demonstrate a decrease of both contralateral and ipsilateral perfusion during hypoxia that recovers with reoxygenation (n=4 per group; 2-way ANOVA shows effect of ipsilateral vs contralateral hemispheres as well as effect of time, ****p<0.0001 for both). (C) The EP4 agonist AE1-329 (50 μg/kg) was administered immediately prior to hypoxia, and laser Doppler flow was quantified in ipsilateral and contralateral hemispheres during 100 min of hypoxia and for 70 min after end of hypoxia (level of 2 mm caudal and 2 mm lateral from the bregma). Vehicle treatment showed a significant difference in perfusion during the hypoxic period whereas EP4 agonist treatment reduces this difference (n=15 per group; differences between hemispheres for vehicle treatment, 2-way ANOVA [F(1,3146)=186.6, ****p<0.0001 and for EP4 agonist treatment[F(1,3402)=4.572; *p=0.032].
To quantify effects of EP4 receptor activation on cerebral perfusion, we turned to Laser Doppler fluometry (LDF); this method was adopted in lieu of LS as it was better tolerated with lower mortality (see Methods). Vehicle or EP4 agonist was administered immediately before hypoxia (as in Figure 1D) and relative cerebral perfusion was measured simultaneously using two probes over motor cortex of ipsilateral and contralateral hemispheres. For LDF studies, CBF was measured for 5 min before hypoxia for each hemisphere, averaged, and set at 100%, with subsequent measurements normalized to this value. Because separate normalizations are used for ipsilateral and contralateral hemispheres, comparison of ipsilateral to contralateral hemispheric CBF is indirect, unlike the LS method, where they can be compared at the same time. In vehicle treated rat pups, LDF again demonstrated a significant decrease of cerebral blood perfusion with initiation of 100 min of hypoxia by about 40% for each hemisphere, which decreased during the course of hypoxia to ~50% on the ipsilateral side (Figure 3B). There was a decrease in CBF in both hemispheres relative to starting CBF of ~40% with initiation of hypoxia; this is similar to the drop seen in LS in contralateral hemisphere (100% to 60%) and ipsilateral hemisphere (60% to 35%), similar to previous studies (Vannucci et al., 1988) which showed an overall ~60% decrease in CBF ipsilateral to the occluded carotid artery. During hypoxia, there was a significant difference in CBF between ipsilateral and contralateral hemispheres (Figure 3C; p<0.0001), consistent with significantly decreased ipsilateral vs contralateral CBF in vehicle control groups. However with EP4 agonist administration, the difference between hemispheric perfusion was reduced (p<0.05), suggesting a redistribution of CBF from contralateral to ipsilateral hemispheres. EP4 agonist treatment altered perfusion such that flow was more synchronized between ipsilateral and contralateral hemispheres.
Conditional deletion of EP4 receptor in endothelial cells worsens outcome in mouse HIE
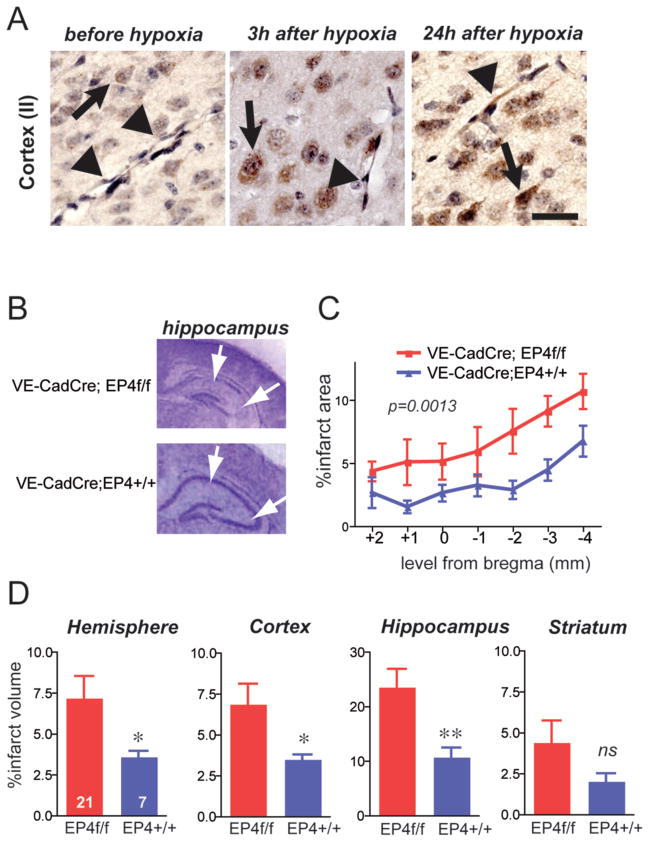
The EB and LDF findings suggested that the site of cerebroprotective action of EP4 signaling in HIE may be at the level of the cerebral vasculature. Immunostaining of postnatal mouse cerebral cortex revealed that EP4 is expressed in neurons as well as endothelial cells at low levels basally, but increases in both cell types 24 h after hypoxia (Figure 4A).
Figure 4. Conditional deletion of endothelial EP4 receptor increases cerebral injury 24h after hypoxia-ischemia (HI).
(A) Immunostaining of EP4 receptor before HI, 3h after HI, and 24h after HI. EP4 receptor is expressed in neurons (arrows) at low levels basally, with induction after HI; EP4 receptor is also weakly expressed basally in endothelium, and is induced by 24h after HI (arrowheads). Scale bar=20μm. (B) Representative sections of cresyl violet stained sections of VE-CadCreERT2;EP4+/+ and VE-CadCreERT2;EP4lox/lox post-natal HI tissue demonstrating infarct injury at 24h with loss of hippocampal pyramidal CA1 and CA3 regions (white arrows). (C) Endothelial deletion of EP4 receptor increased percent infarct area across 7 sections from +2 to −4 of the bregma (n=7–21 per group; 2-way ANOVA, effect of genotype [F1,182)=10.74], **p=0.0013). (D) Percent infarct volume was calculated for individual structures, and is significantly increased 24 h after HI in VE Cad Cre ER;EP4lox/lox mouse pups compared to control VE Cad Cre ER; EP4+/+ pups (*p<0.05; **p<0.01).
To test whether endothelial EP4 signaling contributes to acute outcome of HIE, we examined the role of endothelial EP4 signaling using the Cre-loxP system to selectively delete the EP4 receptor only in endothelial cells. To avoid developmental effects of EP4 deletion (Dumont et al., 1999), we generated endothelial EP4 receptor conditional knockout (cKO) pups using the tamoxifen-inducible endothelial VE-CadCreERT2 line (Monvoisin et al., 2006). Previous studies have demonstrated that tamoxifen triggers excision of “floxed” sequences in cerebral vasculature (Liang, 2011; Monvoisin et al., 2006). Specifically, VE-Cadherin Cre-ERT2 activity in ROSAR26R reporter mice efficiently excised floxed sequences at embryonic and postnatal developmental stages (Monvoisin et al., 2006). During embryonic development, tamoxifen injections at E10.5, E12.5, and E14.5 resulted in 95% excision by E18.5; postnatal injection of tamoxifen for 3 days resulted in 65–89% recombination 7 days after injection. We accordingly adopted a similar tamoxifen strategy and carried out 3 days of tamoxifen administration from P3 to P5 and performed HIE at P9 for 30 minutes; pups were examined 24 h after hypoxia using cresyl violet quantification of infarct injury (Figure 4B). Selective deletion of endothelial EP4 receptor significantly worsened cerebral injury in cerebral cortex, hippocampus, and hemisphere, confirming an endogenous cerebroprotective function for endothelial EP4 signaling in postnatal brain (Figure 4C and D). Taken together, these studies indicate a critical role for endothelial EP4 signaling in early postnatal HI cerebral injury.
DISCUSSION
The rodent HIE model has been very informative for understanding mechanisms of brain injury from perinatal hypoxia (Vannucci and Vannucci, 2005). The ability to reproducibly carry out HIE in rodents has allowed for pharmacologic and genetic approaches to identify potential mechanisms of cerebroprotection. In this study, pharmacologic activation of the PGE2 EP4 receptor elicited short and long-term cerebroprotection, and was associated with an increase acutely in cerebral perfusion of both ipsilateral and contralateral hemispheres, as measured by retention of Evans Blue dye. LDF measurements during and after hypoxia-ischemia demonstrated that EP4 receptor activation altered perfusion dynamics in contralateral and ischemic ipsilateral hemispheres suggestive of a redistribution of blood flow towards the hypoxic-ischemic hemisphere. Validation for a role for vascular EP4 signaling was obtained using a conditional knockout approach, wherein VE-CadCreERT2;EP4lox/lox mice demonstrated a significant worsening in cerebral injury compared to control VE-CadCreERT2;EP4+/+ mice. Taken together, these data indicate an important role for vascular EP4 receptor signaling in acute and long-term outcomes after neonatal hypoxia-ischemia.
The responses of the immature cerebrovasculature to hypoxia are not well understood, however are likely to play a critical role in HIE outcome, as levels of cerebral perfusion correlate inversely with the extent of later cerebral injury in rodent models (Ohshima et al., 2012). In models of systemic vascular function, PGE2 can enhance blood perfusion via EP4 or EP2 receptor signaling (Audoly et al., 1999; Kennedy et al., 1999; Tilley et al., 1999; Zhang et al., 2000); both EP2 and EP4 are positively coupled to cAMP production, as opposed to the EP1 and EP3 receptors, which are coupled to Gq and Gi, with activation resulting in increased intracellular Ca2+ levels and decreased cAMP production, respectively. In rodent models, the EP1 receptor in particular has been shown to play a role in cerebral perfusion (Saleem et al., 2007) as well as systemic hypertension (Guan et al., 2007). Activation of the EP4 receptor, on the other hand, elicits vascular relaxation that is dependent on endothelium-derived nitric oxide production by eNOS. Generation of NO by eNOS is protective in settings of hypoxia and ischemia in part through induction of cGMP-dependent smooth muscle relaxation (Dalkara et al., 1994; Huang et al., 1996; Morikawa et al., 1994; Zhang et al., 1994). Indeed, studies in brain microvessels demonstrate increases in eNOS protein and phosphorylation of Ser1177 eNOS in response to pharmacologic EP4 receptor stimulation that are abolished with genetic deletion of EP4 (Liang, 2011). Thus, vasoactive signaling pathways to improve perfusion may represent a promising and novel strategy for therapeutic intervention in HIE. Our findings support the fact that vascular EP4 signaling improves cerebral perfusion, as measured by Evans Blue concentrations, elicits a redistribution of cerebral perfusion by LDF, and reduces cerebral injury. Our data do not indicate whether EP4 signaling elicits vasodilation or vasoconstriction, only that cerebral perfusion is improved. Moreover, in spite of the significant cardiac depression that occurs in HIE pups, EP4 signaling improved brain perfusion and outcome.
Regarding measurements of CBF, there was a decrease in CBF after carotid ligation in the ipsilateral hemisphere by LS, as well as a decrease in contralateral CBF by LDF. Previous studies using radio-isotopic methods to measure CBF have not shown decreases in ipsilateral CBF following carotid artery ligation or decreases in contralateral CBF (Vannucci et al., 1988), which we find by LS and LDF, respectively. We suggest that differences in our findings have to do with the techniques used to measure CBF. The radio-isotopic iodo-antipyrene (IAP) signal reflects the flow of a diffusible indicator in plasma, and is measured over coronal sections of the brain. LDF and LS studies measure the velocity of red blood cell flow over the surface of the cerebral cortex. Recent studies using LS and LDF (Ohshima et al., 2012; Wainwright et al., 2007) show decreases in ipsilateral CBF with carotid artery ligation as well as decreases in contralateral CBF, in line with our data.
A consideration that was not specifically tested in this study is the possibility that EP4 receptor activation may improve cardiac function during HI, and thus improve cerebral perfusion. A role for EP4 receptor activation in cerebral endothelium has been identified in an adult model of transient focal ischemia, wherein systemic administration of an EP4 agonist resulted in increased levels of eNOS and phospho-eNOS in brain microvessels isolated from treated mice (Liang, 2011). Moreover, in addition to its effects on cerebral perfusion, the EP4 receptor exerts beneficial effects that were not specifically tested in this study, notably potent neuroprotective and anti-inflammatory effects. EP4 signaling can rescue neurons in vivo in settings of excitotoxicity and ischemia via cAMP-PKA dependent mechanisms (Liang, 2011). In models of innate immune inflammation, EP4 receptor signaling is potently anti-inflammatory through mechanisms that reduce NF-kappaB activation and nuclear translocation and subsequent upregulation of inflammatory genes (Minami et al., 2008; Shi et al., 2010; Takayama et al., 2006). Thus, in addition to vascular effects of EP4 signaling, where cerebroprotection may be occurring via enhanced arteriolar and/or capillary perfusion, EP4 receptor activation in the setting of HIE may also be beneficial and reduce excitotoxicity and neuroinflammatory responses, two prominent pathologic processes that contribute to neonatal brain injury.
In conclusion, this study demonstrates that PGE2 EP4 receptor activation enhances cerebral perfusion acutely after hypoxia-ischemia and results in significant protection both acutely and long-term after hypoxia-ischemia. Although neuroprotective and anti-inflammatory effects of EP4 signaling may be occurring in parallel, our findings indicate a prominent and beneficial effect of EP4 signaling on cerebral perfusion during hypoxia-ischemia, suggesting that targeting perfusion via endothelial EP4 signaling may be beneficial in neonatal HIE. These findings also reinforce the clinical relevance of prostaglandin receptor signal transduction in models of HIE (Taniguchi et al., 2011; Taniguchi et al., 2007).
Acknowledgments
This work was supported by the Tashi and John Morgridge Endowed Postdoctoral Fellowship Foundation (HT), the American Heart Association (HT and KA), March of Dimes (KA), NIH R01NS045727 (KA), Weston Havens Foundation (KA), International Max Planck Research School (CA), and the German Academic Foundation (CA). The authors thank C. Maier, J. Myer, P. Narasimhan, P. H. Chan, M. Daadi, A. Arac, and G. Steinberg for their generous assistance and use of equipment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audoly LP, Tilley SL, Goulet J, Key M, Nguyen M, Stock JL, McNeish JD, Koller BH, Coffman TM. Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol. 1999;277:H924–930. doi: 10.1152/ajpheart.1999.277.3.H924. [DOI] [PubMed] [Google Scholar]
- Baburamani AA, Ek CJ, Walker DW, Castillo-Melendez M. Vulnerability of the developing brain to hypoxic-ischemic damage: contribution of the cerebral vasculature to injury and repair? Frontiers in physiology. 2012;3:424. doi: 10.3389/fphys.2012.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiological measurement. 2001;22:R35–66. doi: 10.1088/0967-3334/22/4/201. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Morikawa E, Panahian N, Moskowitz MA. Blood flow-dependent functional recovery in a rat model of focal cerebral ischemia. Am J Physiol. 1994;267:H678–683. doi: 10.1152/ajpheart.1994.267.2.H678. [DOI] [PubMed] [Google Scholar]
- Dumont I, Hou X, Hardy P, Peri KG, Beauchamp M, Najarian T, Molotchnikoff S, Varma DR, Chemtob S. Developmental regulation of endothelial nitric oxide synthase in cerebral vessels of newborn pig by prostaglandin E(2) J Pharmacol Exp Ther. 1999;291:627–633. [PubMed] [Google Scholar]
- Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Gonzalez FF, Ferriero DM. Neuroprotection in the newborn infant. Clinics in perinatology. 2009;36:859–880. vii. doi: 10.1016/j.clp.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grow J, Barks JD. Pathogenesis of hypoxic-ischemic cerebral injury in the term infant: current concepts. Clinics in perinatology. 2002;29:585–602. v. doi: 10.1016/s0095-5108(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, Wei M, Fan X, Carmosino M, Hao C, Imig JD, Breyer RM, Breyer MD. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10:372–382. doi: 10.1016/S1474-4422(11)70016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Trescher WH, Ishida A, Nakajima W. Novel treatments after experimental brain injury. Semin Neonatol. 2000;5:75–86. doi: 10.1053/siny.1999.0116. [DOI] [PubMed] [Google Scholar]
- Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- Liang X, Lin L, Wang Q, Woodling NS, Anacker C, Pan T, Merchant M, Andreasson K. Neuronal and vascular protection by the prostaglandin E2 EP4 receptor in a mouse model of cerebral ischemia. J Clin Invest. 2011;121:4362–4371. doi: 10.1172/JCI46279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Asada M, Shiraishi T, Ishida A, Egashira H, Yoshida H, Ohuchida S, Nakai H, Kondo K, Toda M. Design and synthesis of a highly selective EP4-receptor agonist. Part 1: 3,7-dithiaPG derivatives with high selectivity. Bioorg Med Chem Lett. 2001;11:2029–2031. doi: 10.1016/s0960-894x(01)00364-x. [DOI] [PubMed] [Google Scholar]
- McLean C, Ferriero D. Mechanisms of hypoxic-ischemic injury in the term infant. Seminars in perinatology. 2004;28:425–432. doi: 10.1053/j.semperi.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Minami M, Shimizu K, Okamoto Y, Folco E, Ilasaca ML, Feinberg MW, Aikawa M, Libby P. Prostaglandin E receptor type 4-associated protein interacts directly with NF-kappaB1 and attenuates macrophage activation. J Biol Chem. 2008;283:9692–9703. doi: 10.1074/jbc.M709663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. VE-cadherin-CreERT2 transgenic mouse: a model for inducible recombination in the endothelium. Dev Dyn. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- Morikawa E, Moskowitz MA, Huang Z, Yoshida T, Irikura K, Dalkara T. L-arginine infusion promotes nitric oxide-dependent vasodilation, increases regional cerebral blood flow, and reduces infarction volume in the rat. Stroke. 1994;25:429–435. doi: 10.1161/01.str.25.2.429. [DOI] [PubMed] [Google Scholar]
- Ohshima M, Tsuji M, Taguchi A, Kasahara Y, Ikeda T. Cerebral blood flow during reperfusion predicts later brain damage in a mouse and a rat model of neonatal hypoxic-ischemic encephalopathy. Exp Neurol. 2012;233:481–489. doi: 10.1016/j.expneurol.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Pietrini D, Piastra M, Luca E, Mancino A, Conti G, Cavaliere F, De Luca D. Neuroprotection and hypothermia in infants and children. Curr Drug Targets. 2012;13:925–935. doi: 10.2174/138945012800675641. [DOI] [PubMed] [Google Scholar]
- Renic M, Klaus JA, Omura T, Kawashima N, Onishi M, Miyata N, Koehler RC, Harder DR, Roman RJ. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2009;29:629–639. doi: 10.1038/jcbfm.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Saleem S, Li RC, Wei G, Dore S. Effects of EP1 receptor on cerebral blood flow in the middle cerebral artery occlusion model of stroke in mice. J Neurosci Res. 2007;85:2433–2440. doi: 10.1002/jnr.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Guan Y, Zhang Y, Magnuson MA, Pettepher C, Loftin CD, Langenbach R, Breyer RM, Breyer MD. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis. 2004;40:7–14. doi: 10.1002/gene.20048. [DOI] [PubMed] [Google Scholar]
- Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184:7207–7218. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- Swan CE, Breyer RM. Prostaglandin E2 modulation of blood pressure homeostasis: studies in rodent models. Prostaglandins Other Lipid Mediat. 2011;96:10–13. doi: 10.1016/j.prostaglandins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Sukhova GK, Chin MT, Libby P. A novel prostaglandin E receptor 4-associated protein participates in antiinflammatory signaling. Circ Res. 2006;98:499–504. doi: 10.1161/01.RES.0000204451.88147.96. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Anacker C, Suarez-Mier GB, Wang Q, Andreasson K. Function of prostaglandin E2 EP receptors in the acute outcome of rodent hypoxic ischemic encephalopathy. Neurosci Lett. 2011;504:185–190. doi: 10.1016/j.neulet.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Andreasson K. The hypoxic-ischemic encephalopathy model of perinatal ischemia. J Vis Exp. 2008 doi: 10.3791/955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Mohri I, Okabe-Arahori H, Aritake K, Wada K, Kanekiyo T, Narumiya S, Nakayama M, Ozono K, Urade Y, Taniike M. Prostaglandin D2 protects neonatal mouse brain from hypoxic ischemic injury. J Neurosci. 2007;27:4303–4312. doi: 10.1523/JNEUROSCI.0321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, Coffman TM, Koller BH. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC, Lyons DT, Vasta F. Regional cerebral blood flow during hypoxia-ischemia in immature rats. Stroke. 1988;19:245–250. doi: 10.1161/01.str.19.2.245. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Developmental neuroscience. 2005;27:81–86. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Developmental neuroscience. 2009;31:378–393. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright MS, Grundhoefer D, Sharma S, Black SM. A nitric oxide donor reduces brain injury and enhances recovery of cerebral blood flow after hypoxia-ischemia in the newborn rat. Neurosci Lett. 2007;415:124–129. doi: 10.1016/j.neulet.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, White JG, Iadecola C. Nitric oxide donors increase blood flow and reduce brain damage in focal ischemia: evidence that nitric oxide is beneficial in the early stages of cerebral ischemia. J Cereb Blood Flow Metab. 1994;14:217–226. doi: 10.1038/jcbfm.1994.28. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guan Y, Schneider A, Brandon S, Breyer RM, Breyer MD. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension. 2000;35:1129–1134. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]