Abstract
Cocaine abuse is associated with a high prevalence of nicotine dependence. In animals, nicotinic antagonists have been reported to block the development of cocaine behavioral sensitization and to attenuate cocaine place preference or self-administration. In the present study, we have determined: (1) changes in the locomotor responses to nicotine challenge during the first week of withdrawal from daily cocaine pretreatment; and (2) effects of the non-selective nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine given during the first 5 days of cocaine withdrawal on maintenance of cocaine behavioral sensitization. Male Sprague-Dawley rats were pretreated with daily saline (SI) or cocaine (CI) injections for 14 days. In Experiment 1, separate animals in the SI and CI groups received a single nicotine challenge on day 1, 3, or 7 of withdrawal from their respective pretreatments. The CI group displayed enhanced locomotor responses to nicotine as compared to SI controls on days 3 and 7 of withdrawal, but not day 1. In Experiment 2, SI and CI animals were treated once a day with either saline or mecamylamine during the first 5 days of withdrawal, and were subsequently challenged with single cocaine injections on both withdrawal days 7 and 14. Mecamylamine treatment significantly attenuated expression of cocaine behavioral sensitization on both withdrawal days 7 and 14. Time-dependent changes in nicotinic responses occur during the first week of cocaine withdrawal, and intact nAChR neurotransmission during this period may be necessary for maintenance of cocaine behavioral sensitization.
Keywords: cocaine abuse, cocaine sensitization, cocaine withdrawal period, mecamylamine, nicotinic antagonist
Introduction
Epidemiological studies have consistently reported high rates of comorbidity between cocaine abuse and nicotine dependence [1, 2, 3]. Cocaine abusers smoke tobacco more often than non-abusers [4, 5] and the magnitude of nicotine dependence predicts time to cocaine relapse among abstinent addicts who smoke [6]. It has been also hypothesized that nicotine replacement therapy in cocaine abusers, who are trying to quit smoking, may be detrimental to the achievement of cocaine abstinence [2]. Given these considerations, a greater understanding of the role of nicotinic acetylcholinergic receptors (nAChRs) in cocaine abuse is warranted.
Mecamylamine (Inversine®) is the first orally available antihypertensive agent and a nAChR antagonist, which has been shown to clinically reduce smoking [7], subjective effects of cocaine and nicotine [8, 9], and cue-induced cocaine craving in humans [10]. In animals, mecamylamine can decrease cocaine place preference and disrupt development of cocaine sensitization when given with daily cocaine injections; in contrast, responses to acute cocaine administration in naive animals are not altered [11]. In addition to cocaine place preference and behavioral sensitization, mecamylamine treatment has been shown to attenuate cocaine self-administration in rats [12].
The above considerations indicate that nAChR-dependent processes may partly underlie development and maintenance of cocaine sensitization in animals and cocaine abuse in humans. In the present study we sought to assess: (1) changes in behavioral sensitivity to nicotine during the first week of withdrawal from chronic intermittent cocaine pretreatment; and (2) effect of mecamylamine on the maintenance of cocaine behavioral sensitization when administered once a day between cocaine withdrawal days (WD) 1 and 5.
Methods and Materials
Animals
Male Sprague Dawley rats, initially weighing 175–200 g (Charles River Laboratories, Raleigh, NC), were acclimated to the vivarium on a 12 h light/dark cycle (light 7 AM-7 PM) for 1 week prior to the experiments. Rats were housed in pairs in plastic cages and cared for in accordance with NIH and the Duke IACUC guidelines. Food and water were available ad libitum. This study was approved by Duke University Institutional Animal Care and Use Committee.
Drug Treatments and Behavioral Measurements
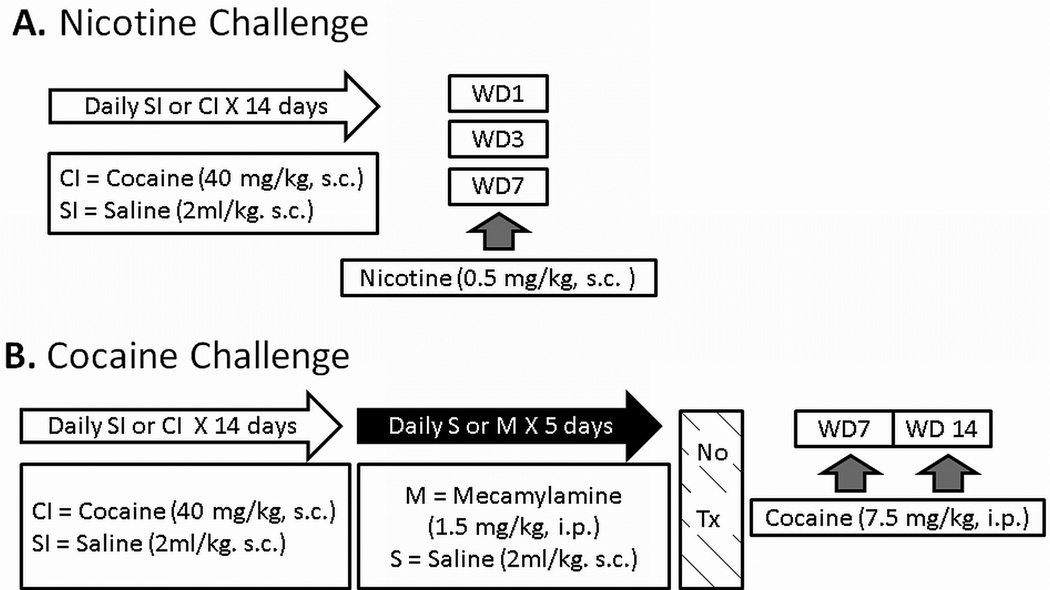
All animals were injected once a day for 14 days with either 40 mg/kg, s.c. cocaine (CI) or equivalent volume (2 ml/kg) of saline (SI). In Experiment 1, separate groups of rats were challenged with nicotine (0.5 mg/kg, s.c.) on day 1, 3 or 7 of saline or cocaine withdrawal (WD1, WD3 or WD7) to determine time-dependent alterations in nicotine-induced stimulation of locomotor activity (see Fig. 1 for illustration of procedural time lines and experimental group designations). In Experiment 2, both the SI and CI groups were treated with daily injections of saline or mecamylamine (1.5 mg/kg, i.p.) during the first 5 days of withdrawal. On WD7, all animals were challenged with cocaine (7.5 mg/kg, i.p.), and locomotor activity and behavioral rating scores were determined (Fig. 1). These rats were again challenged with cocaine on WD14 to determine whether or not the earlier mecamylamine treatment had sustained effects on cocaine sensitization.
Fig. 1.
Schematic representation of experimental designs for Experiment 1 (A) and Experiment 2 (B). For both experiments, animals were injected once a day with cocaine (CI) or saline (SI) for 14 days (open arrow). A: On day 1 (WD1), day 3 (WD3), and day 7 (WD7) of withdrawal from SI or CI treatment, separate groups of rats were injected with nicotine (grey arrows), and locomotor activity was recorded for 60 min. B: Animals were treated with daily mecamylamine (M) or saline injection (S) during the first 5 days of cocaine withdrawal (black arrow). These rats were challenged with cocaine (grey arrow) on WD7 and again on WD14. Locomotor activity was counted using an automated monitoring system and behavioral rating scores were simultaneously recorded using the Ellinwood/Balster scale [13]. See Davidson et al. [25] for additional descriptions of cocaine sensitization regimen and behavioral assessment.
Locomotor responses to either nicotine or cocaine challenge were measured using an automated activity monitor. Activity counting was accomplished in individual cages (28 × 18 × 12 cm) placed inside Opto-Varimex photobeam monitors (8 × 8 beams, Columbus Instruments, OH, U.S.A.). Since nicotine-induced locomotor stimulation depends on the animals being well acclimatized, rats were transferred to the testing facility 18 h prior to testing and were left undisturbed until nicotine challenge assessment in their home cages.
Following a 15 min stable baseline activity measurement, animals were injected with nicotine (0.5 mg/kg, s.c.; Experiment 1) or cocaine (7.5 mg/kg, i.p.; Experiment 2) and activity over 60 min were measured. Each animal was challenged with nicotine only once on WD1, WD3 or WD7 (i.e., between-subject design), while animals in Experiment 2 underwent testing on both WD7 and WD14 (i.e., within-subject design). In addition to locomotor responses, behavioral responses to cocaine (Experiment 2) were simultaneously rated by a blind observer using the modified Ellinwood and Balster behavioral rating scale [13] (see table 1). Exposure to high doses of psychostimulant can lead to restriction of the behavioral repertoire of animals in the open field, such that locomotor activity is replaced by in-place stereotypy [13]. Therefore, the rating scale was used to determine whether a decrease in locomotion counts following a cocaine challenge was not due to emergence of stereotypy (indicating increased cocaine sensitization).
Table 1.
Modified Ellinwood and Balster (1974) rating scale. Each rat is examined for 20 s at 5 min intervals and its behavior noted.
| score | classification | definition |
|---|---|---|
| 1 | asleep | lying down, eyes closed |
| 2 | almost asleep | lying down, eyes partially shut |
| 3 | dystonia | lying down, abnormal posture, tense muscles |
| 4 | inactive | lying down, eyes open, infrequent sniffing |
| 5 | in-place oral behavior | lying down, oral movements e.g. yawning |
| 6 | grooming | grooming of face, body or groin |
| 7 | normal active movement | investigation or sniffing of cage, rearing |
| 8 | hyperactive | running characterized by rapid jerky positional changes |
| 9 | slow patterned movement | repetitive exploration of the cage at normal activity level |
| 10 | fast patterned movement | intense, rapid repetitive exploration of cage |
| 11 | stereotypy | the types of stereotypy are noted |
Data Analysis
All experimental data were normally distributed. Data from Experiment 1 were analyzed using between-subject, one-way analysis of variance (ANOVA). Since the three SI groups (SI-WD1, SI-WD3 and SI-WD7) were not statistically different from one another, they were combined into a single SI control group for ANOVA and determination of individual group differences (Dunnett’s test with SI as the control). In Experiment 2, where animals were challenged with cocaine on both days 7 and 14, locomotor and behavioral rating scores were separately analyzed using mixed, two-way ANOVA (SI-S, SI-M, CI-S and CI-M groups × cocaine challenge days) followed by Newman-Keul’s test for individual group comparisons. Statistical results of p ≤ 0.05 were considered significant.
Results
In both Experiment 1 and 2, all animals exhibited minimal baseline locomotor activities during the 15-min baseline measurement period (<25 beam interruptions) prior to drug challenges, indicating that they were well-acclimatized. In Experiment 2, baseline behavioral rating scores ranged between 1 (asleep) and 4 (inactive).
Changes in locomotor responsivity to nicotine challenge during the first week of cocaine withdrawal
A one-way ANOVA revealed significant overall treatment effects (F(3, 54)=3.78, p < 0.016; Fig. 2). Compared to the combined SI group, animals pretreated with cocaine exhibited greater locomotor counts following nicotine challenge on days 3 and 7 (CI-WD3 and CI-WD7), but not on day 1 (CI-WD1; Dunnett’s test). These results suggest that a time-dependent increase in nicotinic responsivity occurs during the first 3 days of cocaine withdrawal and that this increase remains stable for at least 7 days following cocaine discontinuation.
Fig. 2.
Animals were injected once a day with cocaine (CI) or saline (SI) for 14 days, and were challenged with nicotine (0.5 mg/kg, s.c.) on cocaine withdrawal day 1 (WD1), day 3 (WD3), or day 7 (WD7), and the locomotor activity in the 60 min period following the challenge was recorded. The three SI groups were not statistically different from one another and were combined into a single SI control group (SI #). n = 36, 14, 9 and 10 for SI #, CI-WD1, CI-WD3 and CI-WD7 groups, respectively. *, p < 0.05 compared to SI # group, Dunnett’s test.
Blockade of cocaine behavioral sensitization expression on WD7 and WD14 by daily mecamylamine treatment between WD1 and WD5
In Experiment 2, we determined whether the nicotinic antagonist mecamylamine (1.5 mg/kg, i.p.) given once a day during the first 5 days of cocaine withdrawal can alter behavioral responsivity to subsequent cocaine challenges on WD7 and WD14. Overall, a mixed two-way ANOVA (SI-S, SI-M, CI-S and CI-M treatment groups × challenge days) revealed that earlier mecamylamine treatment had significant effects on locomotor counts and behavioral rating scores (F(3,20) = 12.47, p < 0.001 and F(3,20) = 11.92, p < 0.001 respectively; Fig. 3). No significant effects for cocaine challenge days (F(1,20) = 0.89, p > 0.35 and F(1,20) = 0.78, p > 0.38 respectively for locomotor and behavioral rating scores) or treatment × challenge day interaction (F(3,20) = 0.40, p > 0.75 and F(3,20) = 1.40, p > 0.27, respectively) was observed. The CI-S group exhibited significantly greater locomotor responses to cocaine than the SI-S, SI-M or CI-M groups on both WD7 (Fig. 3A) and WD14 (Fig. 3B), while the CI-M group was statistically indistinguishable from the SI-S and SI-M groups on both days (Newman Keul’s test). These results indicate that treatment with mecamylamine during the first 5 days of cocaine withdrawal blocks maintenance of behavioral sensitization.
Fig. 3.
Following a 14-day saline (SI) or cocaine (CI) pretreatment, animals were treated with daily mecamylamine (M) or saline injection (S) during the first 5 days of cocaine withdrawal. These animals were subsequently challenged with cocaine (7.5 mg/kg, i.p.) on withdrawal days 7 and 14, and the locomotor activity and behavioral rating scores in the 60 min period following the challenge were simultaneously recorded. A: Cumulative locomotor counts. B: Cumulative behavioral rating scores. n = 6 in each experimental group. *, p < 0.05, Newman-Keul’s test.
Discussion
One of the main findings in the present study is that nicotine-induced stimulation of locomotor activity increases in a time-dependent manner during the first week of withdrawal from intermittent cocaine injections (Fig 2). Earlier studies have demonstrated that the first week of withdrawal from chronic cocaine administration is characterized by alterations in regulation of mesoaccumbens DA neurotransmission by various receptor systems [14, 15, 16]. The present results are consistent with the hypothesis that changes in nAChR-dependent neurotransmission, as indicated by increased locomotor stimulation by nicotine on WD3 and WD7, but not on WD1, may also contribute to neuroadaptation underlying cocaine behavioral sensitization. A recent study by Levine et al. [2] reported that animals pretreated with chronic cocaine injections did not exhibit enhanced responsivity to nicotine administered in drinking water on day 1 of cocaine withdrawal. The present study is consistent with their results and extends this finding by demonstrating that nicotinic responses are enhanced with longer cocaine withdrawal durations (i.e., the enhancement is time-dependent).
Altered modulation of mesoaccumbens DA neurotransmission by nAChR-dependent mechanisms may involve direct effects on terminal DA release in the nucleus accumbens through somatodendritic and terminal nAChR on DA neurons [17, 18, 19]. Indirect effects via nicotinic heteroreceptors on excitatory amino acid (EAA) and GABA terminals in the ventral tegmental area (VTA) may also play a role. For example, enhancement of nAChR stimulation of glutamate release during early cocaine withdrawal is expected to contribute to establishment of neuroadaptation underlying cocaine sensitization maintenance (see [20] for review of the glutamate role in behavioral sensitization). In addition, alterations in somatodendritic nAChR on DA neurons (see above) may induce neuronal membrane depolarization and further potentiate the impact of increased glutamate release in the VTA by relieving DA neurons of voltage-dependent NMDA receptor blockade. The resulting enhancement of NMDA-dependent Ca2+ currents, in turn, may lead to intracellular changes necessary for early neuroadaptation of the underlying neuronal plasticity [e.g., 21]. Additional studies on alterations in nAChR function during the early cocaine withdrawal period may add further mechanistic insight to these findings.
The present study demonstrates that a short-term treatment with the nAChR antagonist mecamylamine during the first 5 days of cocaine withdrawal can prevent subsequent expression of cocaine behavioral sensitization up to WD14 (Fig 3). These findings provide additional support for an important role of nAChR-dependent neuroadaptive changes during early cocaine withdrawal in the maintenance of cocaine behavioral sensitization. Furthermore, they complement previous studies that have demonstrated blockade of cocaine behavioral sensitization and self-administration using concurrent administration of a nAChR antagonist [12]. To the extent behavioral sensitization is generally considered as a model of neuroadaptation associated with dependence on psychostimulants and other drugs of abuse [22, 23, 24], treatment with mecamylamine or other nicotinic antagonists during early abstinence periods might confer a protective effect in cocaine abuse.
A limitation of the present study is that changes in nicotine responsivity and blockade of behavioral effects of chronic cocaine administration by mecamylamine were shown using only a behavioral sensitization model of cocaine abuse. Additional studies utilizing a self-administration model and inclusion of other time points (e.g., > 14 days of withdrawal) are expected to provide further insights into the potential role of nAChR-dependent mechanisms in cocaine abuse. Testing additional mecamylamine doses, utilization of more selective nAChR antagonists, and detailed investigations of nAChR modulation of DA/glutamate interactions using behavioral and other investigational tools may also contribute to elucidating nAChR-dependent processes following chronic cocaine administration. In summary, we have demonstrated time-dependent increases in locomotor responsivity to nicotine during early cocaine withdrawal, and a blockade of cocaine sensitization maintenance by mecamylamine treatment during this period. Our results suggest that mecamylamine and other nAChR antagonists may be useful pharmacological agents in treatment of cocaine abuse.
Highlights.
Changes in nicotinic neurotransmission may play a role in cocaine behavioral sensitization.
Locomotor stimulation by nicotine increases during early cocaine withdrawal in a time-dependent manner.
Daily mecamylamine during this period prevents subsequent maintenance of cocaine sensitization.
Nicotine receptors may be an effective target for treatment of chronic cocaine abuse.
Acknowledgement
Research supported by National Institute on Drug Abuse (DA-06519 and DA-10327). We acknowledge Dr. Qiang Chen and Mr. Daniel Marks for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the American College of Neuropsychopharmacology (ACNP) 2012, Waikoloa, HI.
Conflict of Interest
Authors report no conflict of interests.
References
- 1.Gorelick DA, Simmons MS, Carriero N, Tashkin DP. Characteristics of smoked drug use among cocaine smokers. Am J Addict. 1997;6:237–245. [PubMed] [Google Scholar]
- 2.Levine A, Huang Y, Drisaldi B, Griffin EA, Jr, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3003062. 107ra109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schorling JB, Gutgesell M, Klas P, Smith D, Keller A. Tobacco, alcohol and other drug use among college students. J Subst Abuse. 1994;6:105–115. doi: 10.1016/s0899-3289(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 4.Patkar AA, Sterling RC, Leone FT, Lundy A, Weinstein SP. Relationship between tobacco smoking and medical symptoms among cocaine-, alcohol-, and opiate-dependent patients. Am J Addict. 2002;11:209–218. doi: 10.1080/10550490290087974. [DOI] [PubMed] [Google Scholar]
- 5.Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend. 1996;40:195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- 6.Patkar AA, Vergare MJ, Thornton CC, Weinstein SP, Murray HW, Leone FT. Nicotine dependence and treatment outcome among African American cocaine-dependent patients. Nicotine Tob Res. 2003;5:411–418. doi: 10.1080/1462220031000094178. [DOI] [PubMed] [Google Scholar]
- 7.Rose JE. Nicotine addiction and treatment. Annu Rev Med. 1996;47:493–507. doi: 10.1146/annurev.med.47.1.493. [DOI] [PubMed] [Google Scholar]
- 8.Wiseman EJ, McMillan DE. Combined use of cocaine with alcohol or cigarettes. Am J Drug Alcohol Abuse. 1996;22:577–587. doi: 10.3109/00952999609001682. [DOI] [PubMed] [Google Scholar]
- 9.Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- 10.Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacol. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- 11.Zachariou V, Thome J, Parikh K, Picciotto MR. Upregulation of galanin binding sites and GalR1 mRNA levels in the mouse locus coeruleus following chronic morphine treatments and precipitated morphine withdrawal. Neuropsychopharmacol. 2000;23:127–137. doi: 10.1016/S0893-133X(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 12.Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71:565–570. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- 13.Ellinwood EH, Jr, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- 14.Gao WY, Lee TH, King GR, Ellinwood EH. Alterations in Baseline Activity and Quinpirole Sensitivity in Putative Dopamine Neurons in the Substantia Nigra and Ventral Tegmental Area After Withdrawal From Cocaine Pretreatment. Neuropsychopharmacol. 1998;18:222–232. doi: 10.1016/S0893-133X(97)00132-2. [DOI] [PubMed] [Google Scholar]
- 15.King GR, Xiong Z, Ellinwood EH. Blockade of the Expression of Sensitization and Tolerance By Ondansetron, a 5-HT3 Receptor Antagonist, Administered During Withdrawal Front Intermittent and Continuous Cocaine. Psychopharmacology (Berl) 1998;135:263–269. doi: 10.1007/s002130050508. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. JPET. 1997;281:699–706. [PubMed] [Google Scholar]
- 17.el-Bizri H, Clarke PB. Blockade of nicotinic receptor-mediated release of dopamine from striatal synaptosomes by chlorisondamine administered in vivo. Br J Pharmacol. 1994;111:414–418. doi: 10.1111/j.1476-5381.1994.tb14750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994;75:348–352. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 19.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 21.Ferrario CR, Goussakov I, Stutzmann GE, Wolf ME. Withdrawal from cocaine selfadministration alters NMDA receptor-mediated Ca2+ entry in nucleus accumbens dendritic spines. PLoS One. 2012;7:e40898. doi: 10.1371/journal.pone.0040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TH, Szabo ST, Fowler JC, Mannelli P, Mangum OB, Beyer WF, Patkar A, Wetsel WC. Pharmacologically-mediated reactivation and reconsolidation blockade of the psychostimulantabuse circuit: a novel treatment strategy. Drug Alcohol Depend. 2012;124:11–18. doi: 10.1016/j.drugalcdep.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia KS, Szabo ST, Fowler JC, Wetsel WC, Lee TH. Reversal of long-term methamphetamine sensitization by combination of pergolide with ondansetron or ketanserin, but not mirtazapine. Behav Brain Res. 2011;223:227–232. doi: 10.1016/j.bbr.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson C, Lee TH, Xiong Z, Ellinwood EH. Ondansetron given either in the acute or chronic withdrawal from repeated cocaine sensitization dosing regimens reverses the expression of sensitization and inhibits self-administration. Neuropsychopharmacol. 2002;27:542–553. doi: 10.1016/S0893-133X(02)00336-6. [DOI] [PubMed] [Google Scholar]