Abstract
Background
Cardiovascular calcification represents a marker of cardiovascular risk in chronic dialysis patients. In the general population, aortic arch calcification (AAC) can predict cardiovascular mortality. We conducted a prospective study to investigate factors associated with AAC in hemodialysis patients and examined its prognostic value in long-term outcome.
Methods
A total of 712 hemodialysis patients were enrolled. AAC was identified on postero-anterior chest X-ray films and classified as grade (Gr.) 0, 1, 2 or 3. Demographic data including age, gender, dialysis vintage, co-morbidity and biochemical data were reviewed and recorded. The patients were followed for 10 years.
Results
AAC was present in 164 patients (23%) as Gr. 1, in 116 patients (16.3%) as Gr. 2 and in 126 patients (17.7%) as Gr. 3. An increase in the severity of calcification was associated with older patients who had lower albumin, higher calcium and glucose levels. During the follow-up period of 10 years, we found that the grade of AAC was directly related to cardiovascular mortality (Gr. 0: 5.3%; Gr. 1: 12.7%; Gr. 2: 18.9%, and Gr. 3: 24.4%; p < 0.05) and all-cause mortality (Gr. 0: 19.9%; Gr. 1: 31.1%; Gr. 2: 44.8%, and Gr. 3: 53.2%; p < 0.001). Multivariate Cox proportional hazards analysis revealed that high-grade calcification was associated with cardiovascular and all-cause mortality. Patients with AAC were associated with a worse outcome in survival analysis. The severity of AAC also influenced their survival.
Conclusion
Calcification of the aortic arch detected in plain chest radiography was an important determinant of cardiovascular as well as all-cause mortality in chronic hemodialysis patients. The presence and severity of AAC predicted long-term survival.
Key Words : Vascular calcification, Hemodialysis, Survival
Introduction
Cardiovascular disease is the leading cause of death in chronic dialysis patients. For adults on dialysis therapy, the cardiovascular mortality rate is 20-fold higher than that in the general population [1]. One of the major factors contributing to the markedly increased cardiovascular mortality and morbidity is generalized vasculopathy [2]. In dialysis patients, vascular calcification in the aorta and coronary arteries has been identified as an important risk factor of cardiovascular disease [3]. Although vascular calcification is considered an aging process in nature, the presence of calcification in any arterial wall is associated with a three-to-four-fold higher risk for cardiovascular events and mortality [4]. Notably, in young dialysis patients, coronary artery calcification is very common and can progress rapidly [5].
Previous studies have demonstrated the association between the presence and extent of vascular or valvular calcification and outcome in the dialysis population [6,7]. Plain radiography is a convenient and inexpensive tool for the identification of vascular calcification. In the general population, aortic arch calcification (AAC) identified in chest radiography has been shown to correlate with cardiovascular mortality in long follow-up periods [8,9].
Plain chest X-ray films are frequently arranged for chronic dialysis patients in different clinical settings. A calcium deposit over the aortic arch is a visible abnormality on a plain radiographic film that is rarely ignored. Nevertheless, the clinical implication of calcification of the aortic arch has not been studied thoroughly in chronic hemodialysis patients [10]. In the present study, we aimed to investigate the prevalence and extent of AAC in hemodialysis patients. We also analyzed the association between AAC and outcome in a longitudinal follow-up study.
Methods
Patient Selection
Seven hundred and twelve adult uremic patients undergoing maintenance hemodialysis for at least 6 months were enrolled. Patients were eligible if they were free of any active infection, malignancy or acute illness. The underlying causes of renal failure were chronic glomerulonephritis (n = 234), diabetes (n = 242), hypertension (n = 114), polycystic kidney (n = 49), interstitial nephritis (n = 37) and unknown etiology (n = 36). Hemodialysis was performed in three 4-hour sessions per week. Demographic data including age, gender and duration on hemodialysis therapy as well as co-morbidity such as diabetes and hypertension were reviewed and recorded. A chest X-ray film was obtained for each participant during the period from September to October 2001. The patients were then followed prospectively for 10 years. Biochemical data such as monthly albumin, nonfasting glucose, blood urea nitrogen, creatinine, calcium, phosphorous, uric acid, alkaline phosphates, total cholesterol, triglyceride and intact parathyroid hormone were collected for 6 months prior to study enrollment and then averaged. The adequacy of dialysis was assessed by Kt/Vurea using the urea kinetic model of Gotch [11]. Causes of death either due to cardiovascular or noncardiovascular events were also reviewed from the medical records. This study was approved by the Institutional Review Board of Chang-Gung Memorial Hospital (97-0598B).
Evaluation of AAC in Chest Radiography
A simple classification reported by Symeonidis et al. [12] was introduced to evaluate AAC in the present study. Two independent nephrologists interpreted the X-ray films. The severity of calcification was classified as grade (Gr.) 0 (no calcification visible), Gr. 1 (single thin or small spots of calcification), Gr. 2 (one or more areas of thick calcification, but ≤50% of the circular area of the aortic knob) and Gr. 3 (circular calcification with >50% of circular area of the aortic knob). The cardiothoracic ratio was calculated as the ratio of the maximal transverse diameter of the cardiac silhouette (heart diameter) to the distance between the internal margins of the ribs at the level of the right hemidiaphragm (transverse thoracic diameter).
Statistical Analysis
All data were analyzed for normality of distribution by the Kolmogorov-Smirnov test. The results are expressed as mean ± SD for normally distributed data, unless otherwise specified. A comparison between the groups was performed using the ANOVA method. Categorical data were compared between the groups by the χ2 test. Variables relevant to survival were identified by the univariate Cox proportional hazards method. Significant variables were then selected for further analysis using multivariate Cox proportional hazard models. The Kaplan-Meier method was used to estimate survival probabilities using the log-rank test. All analyses were performed using SPSS for Windows (version 17). A p value <0.05 was considered statistically significant.
Results
Calcification of the Aortic Arch at Baseline
The mean age of the study subjects was 55.6 ± 14.3 years. The average duration of hemodialysis therapy was 4.3 ± 3.7 years. Male patients accounted for 43% of all enrolled patients. About one third of the patients had diabetes and hypertension. Interpretations of the chest radiography found that 306 patients were categorized as Gr. 0 (43.0%), 164 as Gr. 1 (23.0%), 116 as Gr. 2 (16.3%) and 126 as Gr. 3 (17.7%). Table 1 displays the baseline demographic characteristics of all patients according to the severity of AAC. There was no difference in gender distribution and duration of hemodialysis across all groups. The patients with calcification were older than those without calcification (p < 0.001), and a proportional increase in diabetes and hypertension with an increasing severity of calcification was noted. The cardiac-thoracic ratio also increased gradually with progression of calcification (p < 0.001). We did not find significant differences in dialysis adequacy as assessed by Kt/V among all groups.
Table 1.
Baseline demographic and biochemical data of the hemodialysis patients
| Gr. 0 | Gr. 1 | Gr. 2 | Gr. 3 | All | p value | |
|---|---|---|---|---|---|---|
| Patient, n (%) | 306 (43.0) | 164 (23.0) | 116 (16.3) | 126 (17.7) | 712 (100) | |
| Duration, years | 4.2 ± 3.7 | 4.6 ± 3.6 | 4.0 ± 3.5 | 4.5 ± 3.9 | 4.3 ± 3.7 | 0.547 |
| Age, years | 47.5 ± 13.1 | 57.5 ± 12.0 | 64.4 ± 10.9 | 64.8 ± 11.2 | 55.6 ± 14.3 | <0.001 |
| Gender, M/F | 134/172 | 75/89 | 49/67 | 48/78 | 306/406 | 0.606 |
| Diabetes, n (%) | 89 (29.1) | 49 (29.9) | 56 (48.3) | 52 (41.3) | 246 (34.6) | <0.001 |
| Hypertension, n (%) | 89 (29.1) | 51 (31.1) | 46 (39.7) | 53 (42.1) | 239 (33.6) | 0.027 |
| KT/V | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.7 ± 0.3 | 0.643 |
| CTR | 0.49 ± 0.07 | 0.52 ± 0.07 | 0.54 ± 0.08 | 0.55 ± 0.08 | 0.52 ± 0.08 | <0.001 |
| Hemoglobin, g/dl | 9.2 ± 1.2 | 9.1 ± 1.4 | 9.1 ± 1.2 | 9.5 ± 1.6 | 9.2 ± 1.3 | 0.90 |
| Creatinine, mg/dl | 11.8 ± 2.4 | 11.3 ± 2.5 | 10.6 ± 2.5 | 10.7 ± 2.7 | 11.3 ± 2.5 | <0.001 |
| Glucose, mg/dl | 139.8 ± 65.4 | 152.2 ± 78.2 | 161.8 ± 80.3 | 156.1 ± 65.9 | 149.2 ± 71.5 | 0.019 |
| Albumin, g/dl | 3.9 ± 0.4 | 3.8 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.3 | 3.8 ± 0.4 | <0.001 |
| Calcium, mg/dl | 9.4 ± 0.7 | 9.5 ± 0.7 | 9.5 ± 0.7 | 9.7 ± 0.7 | 9.5 ± 0.7 | 0.001 |
| Phosphorus, mg/dl | 5.4 ± 1.4 | 5.2 ± 1.2 | 5.0 ± 1.2 | 5.2 ± 1.2 | 5.2 ± 1.3 | 0.150 |
| Ca × P | 50.4 ± 13.7 | 49.1 ± 11.9 | 48.2 ± 12.5 | 50.9 ± 12.5 | 49.9 ± 12.9 | 0.298 |
| Alk-p, U/l | 85.9 ± 75.4 | 84.5 ± 53.5 | 90.7 ± 64.7 | 82.3 ± 43.6 | 85.7 ± 64.1 | 0.786 |
| IPTH, pg/ml | 328.7 ± 357.9 | 255.6 ± 266.8 | 271.5 ± 327.4 | 307.1 ± 395.6 | 298.7 ± 342.1 | 0.124 |
| Total cholesterol, mg/dl | 181.9 ± 36.6 | 189.5 ± 39.1 | 187.7 ± 40.1 | 180.5 ± 39.5 | 185.4 ± 38.3 | 0.181 |
| Triglyceride, mg/dl | 164.6 ± 97.7 | 187.5 ± 128.9 | 174.4 ± 104.8 | 173.6 ± 105.3 | 173.1 ± 108.2 | 0.188 |
| Uric acid, mg/dl | 8.0 ± 1.4 | 7.9 ± 1.2 | 7.9 ± 1.4 | 7.9 ± 1.4 | 7.9 ± 1.3 | 0.569 |
Values are expressed as mean ± SD, unless otherwise specified. CTR = Cardiothoracic ratio; Ca × P = Calcium and phosphorus product; Alk-p = alkaline phosphatase; IPTH = intact parathyroid hormone.
Patients with AAC had lower creatinine and albumin levels. ACC was also associated with higher nonfasting glucose and calcium levels. With the increase in the severity of AAC, there was a parallel change in creatinine, albumin, nonfasting glucose and calcium levels. No significant differences in phosphorus, calcium phosphorus product and intact parathyroid hormone was noted in all groups. The lipid profile did not differ across all groups.
Factors Associated with Outcomes
During a follow-up period of 10 years, there were 231(32.4%) deaths in this study cohort. Cardiovascular death was noted in 87 deaths (12.2%), which accounted for 37.7% of all deaths, followed by infectious disease (34.6%) and malignancy (20.7%). Cardiovascular mortality increased as the severity of calcification increased (Gr. 0: 5.3%; Gr. 1: 12.7%; Gr. 2: 18.9%, and Gr. 3: 24.4%; p < 0.05). All-cause mortality was closely related to the severity of AAC (Gr. 0: 19.9%; Gr. 1: 31.1%; Gr. 2: 44.8%, and Gr. 3: 53.2%; p < 0.001). As shown in table 2, univariate analysis disclosed that age, male gender, diabetes, cardio-thoracic ratio, nonfasting glucose, albumin, creatinine, phosphorus, calcium phosphorus product, intact parathyroid hormone and AAC (Gr. 2 and 3) were significant associates of cardiovascular mortality. In multivariate analysis, age, male gender, nonfasting glucose and Gr. 3 AAC independently correlated with cardiovascular mortality. Table 3 displays factors associated with all-cause mortality. Age, diabetes, cardiothoracic ratio, albumin, creatinine, nonfasting glucose, phosphorus, calcium phosphorus product, total cholesterol, intact parathyroid hormone, alkaline phosphatase and AAC (Gr. 2 and 3) all were significant factors related to all-cause mortality. Multivariate analysis revealed that age, albumin, nonfasting glucose, alkaline phosphatase and Gr. 3 AAC were independent factors associated with overall survival.
Table 2.
Univariate and multivariate Cox proportional hazards analysis for cardiovascular mortality
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| hazard ratio | 95% confidence interval | p | hazard ratio | 95% confidence interval | p | |
| Age | 1.080 | 1.062 – 1.099 | <0.001 | 1.057 | 1.032 – 1.082 | <0.001 |
| Male gender | 1.617 | 1.061 – 2.465 | 0.025 | 2.354 | 1.371 – 4.042 | 0.002 |
| Diabetes | 1.993 | 1.309 – 3.307 | 0.001 | 0.927 | 0.549 – 1.568 | 0.779 |
| Hypertension | 0.993 | 0.633 – 1.557 | 0.976 | |||
| Kt/V | 0.494 | 0.233 – 1.048 | 0.066 | |||
| CTR | 2.546 | 1.276 – 5.079 | 0.008 | 5.851 | 0.280 – 122.323 | 0.255 |
| Hemoglobin | 0.962 | 0.813 – 1.138 | 0.652 | |||
| Albumin | 0.248 | 0.156 – 0.394 | <0.001 | 0.652 | 0.323 – 1.310 | 0.230 |
| Glucose | 1.006 | 1.003 – 1.008 | <0.001 | 1.005 | 1.002 – 1.007 | 0.002 |
| Creatinine | 0.081 | 0.733 – 0.874 | <0.001 | 0.986 | 0.848 – 1.106 | 0.634 |
| Calcium | 0.838 | 0.629 – 1.114 | 0.224 | |||
| Phosphorus | 0.664 | 0.561 – 0.786 | <0.001 | 0.520 | 0.244 – 1.106 | 0.089 |
| Ca × P | 0.959 | 0.943 – 0.976 | <0.001 | 1.046 | 0.969 – 1.129 | 0.247 |
| Alk-p | 1.002 | 0.999 – 1.005 | 0.193 | |||
| IPTH | 0.998 | 0.998 – 0.999 | 0.002 | 0.999 | 0.998 – 1.000 | 0.246 |
| Cholesterol | 0.995 | 0.989 – 1.000 | 0.067 | |||
| Triglyceride | 1.000 | 0.998 – 1.002 | 0.790 | |||
| Uric acid | 0.890 | 0.753 – 1.051 | 0.169 | |||
| AAC | ||||||
| Gr. 1 | 2.586 | 1.340 – 4.991 | 0.005 | 1.749 | 0.876 – 3.493 | 0.113 |
| Gr. 2 | 4.288 | 2.236 – 8.223 | <0.001 | 1.435 | 0.680 – 3.026 | 0.343 |
| Gr. 3 | 5.856 | 3.190 – 10.753 | <0.001 | 2.497 | 1.237 – 5.043 | 0.011 |
CTR = Cardiothoracic ratio; Ca × P = calcium and phosphorus product; Alk-p = alkaline phosphatase; IPTH = intact parathyroid hormone.
Table 3.
Univariate and multivariate Cox proportional hazards analysis for all-cause mortality
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| hazard ratio | 95% confidence interval | p | hazard ratio | 95% confidence interval | p | |
| Age | 1.067 | 1.056 – 1.078 | <0.001 | 1.047 | 1.031 – 1.062 | <0.001 |
| Male gender | 1.158 | 0.894 – 1.501 | 0.266 | |||
| Diabetes | 1.956 | 1.511 – 2.533 | <0.001 | 1.084 | 0.788 – 1.491 | 0.622 |
| Hypertension | 1.155 | 0.883 – 1.511 | 0.294 | |||
| Kt/V | 0.785 | 0.487 – 1.182 | 0.222 | |||
| CTR | 2.327 | 1.535 – 3.528 | <0.001 | 1.695 | 0.258 – 11.146 | 0.583 |
| Hemoglobin | 0.948 | 0.853 – 1.054 | 0.323 | |||
| Albumin | 0.221 | 0.167 – 0.293 | <0.001 | 0.523 | 0.342 – 0.828 | 0.005 |
| Glucose | 1.005 | 1.004 – 1.006 | <0.001 | 1.004 | 1.002 – 1.005 | <0.001 |
| Creatinine | 0.786 | 0.745 – 0.830 | <0.001 | 0.943 | 0.924 – 1.007 | 0.997 |
| Calcium | 0.873 | 0.731 – 0.041 | 0.873 | |||
| Phosphorus | 0.697 | 0.627 – 1.773 | <0.001 | 0.839 | 0.545 – 1.292 | 0.425 |
| Ca × P | 0.964 | 0.954 – 0.974 | <0.001 | 1.004 | 0.961 – 1.048 | 0.863 |
| Alk-p | 1.003 | 1.002 – 1.004 | <0.001 | 1.003 | 1.002 – 1.005 | <0.001 |
| IPTH | 0.999 | 0.999 – 1.000 | 0.003 | 1.000 | 0.999 – 1.000 | 0.588 |
| Cholesterol | 0.995 | 0.991 – 0.999 | 0.006 | 0.997 | 0.994 – 1.001 | 0.158 |
| Triglyceride | 0.999 | 0.998 – 1.001 | 0.234 | |||
| Uric acid | 0.907 | 0.820 – 1.004 | 0.059 | |||
| AAC | ||||||
| Gr. 1 | 1.713 | 1.181 – 2.485 | 0.005 | 1.165 | 0.781 – 1.783 | 0.453 |
| Gr. 2 | 2.733 | 1.887 – 3.959 | <0.001 | 0.939 | 0.600 – 1.456 | 0.778 |
| Gr. 3 | 3.419 | 2.415 – 4.839 | <0.001 | 1.604 | 1.058 – 2.431 | 0.026 |
CTR = Cardiothoracic ratio; Ca × P = calcium and phosphorous product; Alk-p = alkaline phosphatase; IPTH = intact parathyroid hormone.
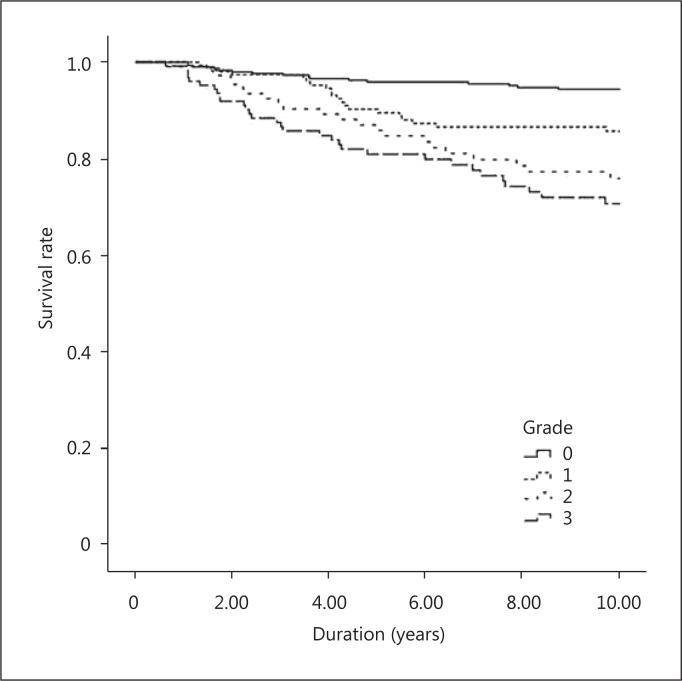
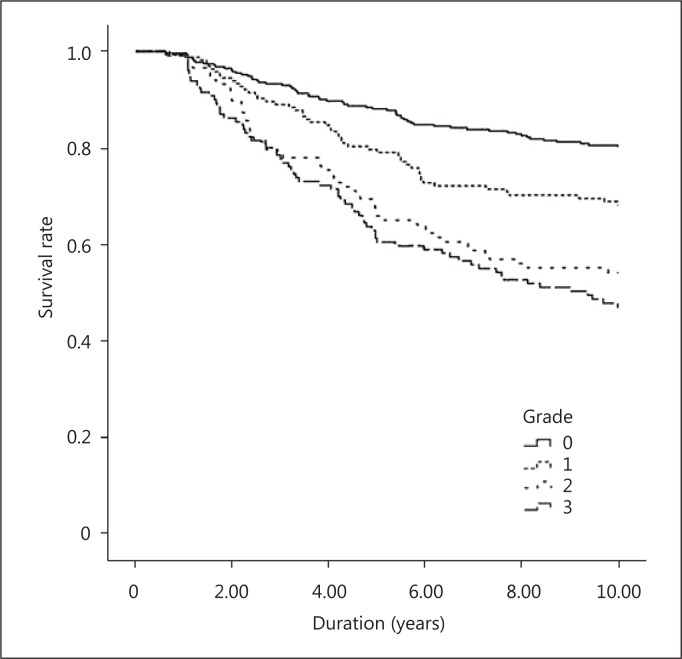
Survival Analysis
Kaplan-Meier survival analysis of cardiovascular mortality is presented in figure 1. Patients with AAC had less cardiovascular survival than those without AAC. A higher mortality rate was observed in patients with high-grade ACC (Gr. 3). Figure 2 shows the survival curve of different groups according to the severity of AAC. Significant differences were noted between patients with and without AAC. Furthermore, patients with marked calcification (Gr. 2 and 3) had an increased mortality compared to those with mild calcification (Gr. 1). There was no significant difference between Gr. 2 and Gr. 3.
Fig. 1.
Kaplan-Meier analysis for cardiovascular mortality of 712 hemodialysis patients. log-rank p of Gr. 0 and 1: 0.004; Gr. 0 and 2: <0.001; Gr. 0 and 3: <0.001; Gr. 1 and 2: 0.096; Gr. 1 and 3: 0.004, and Gr. 2 and 3: 0.276.
Fig. 2.
Kaplan-Meier analysis for all-cause mortality of 712 hemodialysis patients. log-rank p of Gr. 0 and 1: 0.004; Gr. 0 and 2: <0.001; Gr. 0 and 3: <0.001; Gr. 1 and 2: 0.018; Gr. 1 and 3: <0.001, and Gr. 2 and 3: 0.229.
Discussion
Our study demonstrated a high prevalence of vascular calcification in chronic hemodialysis: more than 50% of the patients presented with AAC and about one third of them had high-grade calcification. AAC was associated with old age, diabetes and hypertension. Serum levels of creatinine, nonfasting glucose, albumin and calcium were significant associates of AAC. Furthermore, we found that AAC was a significant predictor of cardiovascular and all-cause mortality. Patients with a more severe calcification had a worse outcome.
Cardiovascular calcification has emerged as one of the risk factors of cardiovascular mortality in patients with chronic kidney disease. Calcification can increase stiffness and reduce elasticity of large arteries such as the aorta, which may result in substantial mortality and morbidity by impairing cardiovascular hemodynamics and vascular compliance [7]. A variety of methods is available to detect cardiovascular calcification in dialysis patients. These methods differ in the risk, sensitivity, availability and cost [13,14]. The current guidelines recommend echocardiography and plain X-ray films of the lumbar spine for the detection and assessment of cardiovascular calcification in chronic kidney disease [15]. In the general population, AAC identified in plain chest X-ray is associated with an increased risk of coronary artery disease and is linked to cardiovascular risk factors such as age, hypertension, dyslipidemia and diabetes mellitus [9,12,16]. Moreover, compared with traditional risk factors, AAC is an independent determinant of cardiovascular outcome [17]. Our study supports the additional value of plain chest films in hemodialysis patients to predict their long-term outcome.
The pathogenesis of cardiovascular calcification in dialysis patients is multiple and complex. In addition to dysregulation of calcium and phosphate, hyperparathyroidism, underling disease, dialysis vintage and age are all relevant factors [6,18,19]. In the present study, AAC correlated with age, diabetes and hypertension. Interestingly, we found that the cardio-thoracic ratio was directly related to the severity of aortic calcification. Calculation of the cardio-thoracic ratio is often used to detect cardiomegaly and evaluate fluid status in dialysis patients. A higher ratio was associated with poor outcome [20]. The association between the cardio-thoracic ratio and AAC suggests more advanced cardiovascular disease in these patients. Both serum creatinine and albumin levels were inversely related to the severity of AAC in our patients. It has been reported that lower serum creatinine levels were associated with poor outcomes in chronic dialysis patients [21,22]. Poor nutrition and chronic inflammation contribute to lower levels of albumin and creatinine, and thus lead to increased mortality. Our results suggest a close relationship between inflammation and vascular calcification in dialysis patients [23].
One of the important findings in our study is the association between AAC and long-term outcome. Our results indicate the importance to screen for cardiovascular calcification in hemodialysis patients. In multivariate analysis, patients with high-grade AAC had a 2.49-fold risk for cardiovascular death and a 1.6-fold risk for all-cause mortality. Among factors relevant to survival, nonfasting sugar was independently associated with both cardiovascular and all-cause mortality. It has been reported that pre-dialysis fasting glucose levels correlate with short-term outcome in hemodialysis patients [24]. Although the blood samples we collected in the present study were nonfasting and random, this association may represent the abnormal glucose metabolism in dialysis patients. The so-called malnutrition, inflammation and atherosclerosis syndrome represents an overwhelming inflammatory status with increased oxidative stress and high cardiovascular risk in dialysis patients [25]. In our study, albumin was significantly associated with all-cause death, indicating the critical role of the nutrition-inflammation axis in dialysis patients. We found that the serum level of alkaline phosphatase predicted survival. In a large cohort of hemodialysis patients, Kalantar-Zadeh et al. [26] also reported an incremental association with mortality. Both liver disease and renal osteodystrophy may contribute to this relationship.
Survival analysis further revealed that the presence of AAC predicts that the worse outcome and greater severity of AAC was associated with mortality. Only one small-scale study demonstrated the association between AAC and survival in dialysis patients [10]. Whether the severity of AAC can further aggravate outcome is not clear. One recent study showed that the presence and progression of AAC was related to both cardiovascular and all-cause mortality in peritoneal dialysis patients [27]. However, the follow-up period was rather short.
There are several limitations of the present study. First, information on calcium administration and usage of vitamin D was not available; second, pharmacological agents such as lipid-lowering drugs and calcimimetics with the potential to modify vascular calcification were not included for analysis, and third, longitudinal change of AAC is lacking. Moreover, the factors relevant to calcification progression are not clear.
In conclusion, AAC detected in plain chest X-ray films was significantly associated with cardiovascular and all-cause mortality. The severity of AAC was an important determinant of survival in chronic hemodialysis patients.
Acknowledgements
This work was supported by a grant from the National Science Council, Republic of China (NSC101-2314-B-182A-053-MY3).
References
- 1.Collins AJ, Li S, Ma JZ, Herzog C. Cardiovascular disease in end-stage renal disease patients. Am J Kidney Dis. 2001;38:S26–S29. doi: 10.1053/ajkd.2001.27392. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre CW. The functional cardiovascular consequences of vascular calcification. Semin Dial. 2007;20:122–128. doi: 10.1111/j.1525-139X.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 3.Cannata-Andia JB, Rodriguez-Garcia M, Carrillo-Lopez N, Naves-Diaz M, Diaz-Lopez B. Vascular calcifications: pathogenesis, management, and impact on clinical outcomes. J Am Soc Nephrol. 2006;17:S267–S273. doi: 10.1681/ASN.2006080925. [DOI] [PubMed] [Google Scholar]
- 4.Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5:185–197. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 6.Wang AY, Wang M, Woo J, Lam CW, Li PK, Lui SF, Sanderson JE. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: a prospective study. J Am Soc Nephrol. 2003;14:159–168. doi: 10.1097/01.asn.0000038685.95946.83. [DOI] [PubMed] [Google Scholar]
- 7.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens. 2005;14:525–531. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]
- 8.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 9.Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–2815. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa T, Ishida H, Akamatsu M, Matsuda N, Fujiu A, Ito K, Ando Y, Nitta K. Progression of aortic arch calcification and all-cause and cardiovascular mortality in chronic hemodialysis patients. Int Urol Nephrol. 2010;42:187–194. doi: 10.1007/s11255-009-9574-5. [DOI] [PubMed] [Google Scholar]
- 11.Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS) Kidney Int. 1985;28:526–534. doi: 10.1038/ki.1985.160. [DOI] [PubMed] [Google Scholar]
- 12.Symeonidis G, Papanas N, Giannakis I, Mavridis G, Lakasas G, Kyriakidis G, Artopoulos I. Gravity of aortic arch calcification as evaluated in adult Greek patients. Int Angiol. 2002;21:233–236. [PubMed] [Google Scholar]
- 13.Karohl C, Raggi P. Cardiovascular imaging in patients with chronic kidney disease. Blood Purif. 2011;31:130–137. doi: 10.1159/000321839. [DOI] [PubMed] [Google Scholar]
- 14.Bellasi A, Ferramosca E, Muntner P, Ratti C, Wildman RP, Block GA, Raggi P. Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int. 2006;70:1623–1628. doi: 10.1038/sj.ki.5001820. [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009:S1-S130. [DOI] [PubMed]
- 16.Li J, Galvin HK, Johnson SC, Langston CS, Sclamberg J, Preston CA. Aortic calcification on plain chest radiography increases risk for coronary artery disease. Chest. 2002;121:1468–1471. doi: 10.1378/chest.121.5.1468. [DOI] [PubMed] [Google Scholar]
- 17.Iijima K, Hashimoto H, Hashimoto M, Son BK, Ota H, Ogawa S, Eto M, Akishita M, Ouchi Y. Aortic arch calcification detectable on chest X-ray is a strong independent predictor of cardiovascular events beyond traditional risk factors. Atherosclerosis. 2010;210:137–144. doi: 10.1016/j.atherosclerosis.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CT, Tsai YC, Su CY, Ng HY, Hsu CY, Ko SF, Chen TC, Kuo CC, Yang CC, Chiou TT, Lee WC, Yang YK, Lam KK. Interleukin 10 and residual kidney function are associated with risk of vascular calcification in patients undergoing peritoneal dialysis. Clin Nephrol. 2011;75:397–402. doi: 10.5414/cnp75397. [DOI] [PubMed] [Google Scholar]
- 20.Ozkahya M, Ok E, Toz H, Asci G, Duman S, Basci A, Kose T, Dorhout Mees EJ. Long-term survival rates in haemodialysis patients treated with strict volume control. Nephrol Dial Transplant. 2006;21:3506–3513. doi: 10.1093/ndt/gfl487. [DOI] [PubMed] [Google Scholar]
- 21.Pifer TB, McCullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, Young EW. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–2245. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38:1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 23.Tsirpanlis G. Is inflammation the link between atherosclerosis and vascular calcification in chronic kidney disease? Blood Purif. 2007;25:179–182. doi: 10.1159/000099011. [DOI] [PubMed] [Google Scholar]
- 24.Lin-Tan DT, Lin JL, Wang LH, Wang LM, Huang LM, Liu L, Huang JY, Huang YL. Fasting glucose levels in predicting 1-year all-cause mortality in patients who do not have diabetes and are on maintenance hemodialysis. J Am Soc Nephrol. 2007;18:2385–2391. doi: 10.1681/ASN.2006121409. [DOI] [PubMed] [Google Scholar]
- 25.Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome – the heart of the matter. Nephrol Dial Transplant. 2002;17(suppl 11):28–31. doi: 10.1093/ndt/17.suppl_11.28. [DOI] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ, Shin DH, Kim SJ, Oh HJ, Yoo DE, Ko KI, Koo HM, Kim CH, Doh FM, Park JT, Han SH, Yoo TH, Choi KH, Kang SW. Progression of aortic arch calcification over 1 year is an independent predictor of mortality in incident peritoneal dialysis patients. PLoS One. 2012;7:e48793. doi: 10.1371/journal.pone.0048793. [DOI] [PMC free article] [PubMed] [Google Scholar]