Abstract
Background
The therapeutic role of L-carnitine (LC) on the anemia of chronic hemodialized patients is still controversial. In order to clarify the long-term effects of LC administration on renal anemia, an open, observational 12-month study was performed.
Methods
Twenty stable outpatients undergoing hemodialysis were administered LC 900 mg p.o. daily for 12 months. The recombinant human erythropoietin (rHuEPO) dose was adjusted monthly when necessary to maintain the target hemoglobin (Hb) levels.
Results
The free LC level increased, while the acyl/free LC ratio decreased significantly 3 months after administration and was then maintained until the end of the study. There was no difference in Hb levels and the erythropoietin resistance index (ERI) during the study period. However, it was observed that ERI decreased significantly in 7 out of 18 patients (responders) 5 months after LC administration and was maintained thereafter (almost 40% reduction of the rHuEPO dose). The acyl/free carnitine ratio at baseline was the most contributing factor distinguishing responders from nonresponders.
Conclusion
Although the beneficial effect of LC supplementation on renal anemia was not observed in all patients, at least 40% of the patients (responders) showed a significant improvement in ERI after long-term LC administration.
Key Words : L-Carnitine, Anemia, Hemodialysis, Erythropoietin
Introduction
Anemia is a common complication of chronic hemodialized patients, and the efficacy of recombinant human erythropoietin (rHuEPO) in the treatment of renal anemia has been well established [1]. Although the relative deficiency of the intrinsic erythropoietin production is a major factor of renal anemia, other mechanisms such as iron deficiency, secondary hyperparathyroidism, inflammation, malignancy, aluminum intoxication and hemolysis have also been reported [2].
L-Carnitine (LC) is an essential factor for the membrane transport of acyl-CoA compounds, especially for the intramitochondrial transport of long-chain fatty acids. Despite the absence of mitochondria in mammalian red blood cells (RBC), evidence for a role of LC in RBC metabolism is suggested by the presence of LC in RBC.
The concentration of LC of chronic hemodialyzed patients in plasma and tissues decreased because of the impaired synthesis in kidney and liver and the great loss across the dialysis membrane during hemodialysis [3,4,5].
Although the possibility of the improvement of renal anemia has been emphasized by means of LC supplementation, the results of clinical trials were conflicting [4,6,7,8,9,10]. This is an open observational study to evaluate the long-term effects of LC on renal anemia.
Materials and Methods
Study Patients
Twenty stable and ambulatory hemodialysis patients were studied. The inclusion criteria were as follows: chronic hemodialysis for more than 2 years, dialysis frequency or duration remained unchanged for the previous 6 months, stable hemoglobin (Hb) levels between 9 and 12 g/dl with a stable rHuEPO dose and a normal iron status (according to serum Fe, ferritin and transferrin saturation levels). The exclusion criteria were the following: recent LC treatment, transfusion within the last 6 months, severe hyperparathyroidism, signs of aluminum intoxication, severe liver disease or causes of anemia other than uremia. Uremia was well controlled by dialysis prescription, as demonstrated by Kt/V (1.54 ± 0.29), duration of each dialysis session (4 h three times weekly), high-performance membrane dialyzer (1.73 ± 0.22 m2) and blood flow (203 ± 24 ml/min). All patients were administered LC (900 mg LC p.o. daily) for 12 months because of the evaluation of long-term effects of LC on renal anemia. The study protocol was approved by the local ethics committee. Written informed consent was obtained from each patient.
Laboratory Investigations
In all patients, the RBC count was performed monthly, and the rHuEPO dose was adjusted monthly according to the RBC results. The rHuEPO dose adjustments were determined by the target Hb level (10-11 g/dl), the patient's Hb level, the observed rate of increase or decrease in the Hb level and clinical circumstances. The rHuEPO resistance index (erythropoietin resistance index (ERI): weekly rHuEPO dose per gram of Hb adjusted by dry weight) was calculated to provide a more individualized measure of the rHuEPO requirement. Plasma levels of total and free carnitine were determined at baseline, 3 months and 12 months after the administration, according to the enzyme cycling method. Other hematological parameters were measured every month during the study period.
Statistics
Descriptive results are shown as the mean value ± standard deviation. Data were statistically analyzed by Student's t test for paired or unpaired samples, one-way ANOVA and discriminant analysis. The optimal discriminant formula was obtained when Ru was maximized: Ru = 1 – (1 – R2) × (n + k + 1)/(n – k – 1), where R is the multiple regression coefficient, n the observation number and k the degree of freedom. p < 0.05 was regarded as significant.
Results
Patient Characteristics and Laboratory Data
After study entry, one patient suffered from gastric cancer and another one experienced bone fracture accidentally. Eighteen patients were enrolled in this study.
Free and acyl carnitine and the acyl/free carnitine ratio were 22.0 ± 7.2 μmol/l, 16.5 ± 4.9 μmol/l, 0.78 ± 0.2% at baseline, respectively, 140.3 ± 57.5 μmol/l, 94.8 ± 47.8 μmol/l, 0.67 ± 0.13% after 3 months and 139.3 ± 43 μmol/l, 91.6 ± 47.3 μmol/l, 0.64 ± 0.18% after 12 months, respectively. Differences between free carnitine and acyl carnitine levels at baseline versus those after 3 and 12 months were highly significant (p < 0.001). The acyl/free carnitine ratio decreased significantly after 3 and 12 months of treatment (p < 0.05).
Hb and ERI did not change significantly during the study period, and there was no difference in other parameters either (table 1).
Table 1.
Patient characteristics
| Baseline | 3 months | 12 months | p value | |
|---|---|---|---|---|
| Duration of dialysis, months | 135.9 ± 101.3 | |||
| Age, years | 66.7 ± 8.2 | |||
| Male/female | 8/10 | |||
| Free carnitine, μmol/l | 22.0 ± 7.2 | 140.3 ± 57.5 | 139.3 ± 43 | <0.001 |
| Acyl carnitine, μmol/l | 16.5 ± 4.9 | 94.8 ± 47.8 | 91.6 ± 47.3 | <0.001 |
| Acyl/free carnitine ratio | 0.78 ± 0.2 | 0.67 ± 0.13 | 0.64 ± 0.18 | <0.05 |
| Hematocrit, % | 34.4 ± 2.9 | 34.9 ± 2.9 | 33.8 ± 2.9 | NS |
| Hb, g/dl | 10.9 ± 1.1 | 10.8 ± 0.95 | 10.5 ± 0.85 | NS |
| Serum iron, μg/dl | 77.2 ± 26.8 | 75.9 ± 29 | 70.4 ± 28.4 | NS |
| Ferritin, ng/ml | 203.3 ± 196.6 | 160.3 ± 202.2 | 179.7 ± 189.7 | NS |
| P, mg/dl | 6.0 ± 1.2 | 5.3 ± 1.0 | 5.5 ± 0.85 | NS |
| Al, μg/l | 10.8 ± 3.5 | 11.1 ± 3.7 | 11 ± 4 | NS |
| i-PTH, pg/ml | 150.6 ± 94.7 | 202.6 ± 138.6 | 236 ± 186 | NS |
| CRP, mg/dl | 0.32 ± 0.63 | 0.27 ± 0.41 | 0.38 ± 0.66 | NS |
| Albumin, g/dl | 3.64 ± 0.2 | 3.69 ± 0.22 | 3.60 ± 0.19 | NS |
| Epo dosage, IU/week | 3,194 ± 3,293 | 3,486 ± 3,243 | 3,013 ± 2,951 | NS |
| ERI, IU/week/g Hb/kg BW | 6.1 ± 6.0 | 6.7 ± 6.5 | 6.6 ± 5.1 | NS |
Values are mean ± standard deviation. P = Phosphate; Al = aluminum; i-PTH = intact parathormone; CRP = C-reactive protein; BW = body weight; NS = not significant.
Significant p values: baseline vs. 3 months and 12 months.
Responders and Nonresponders
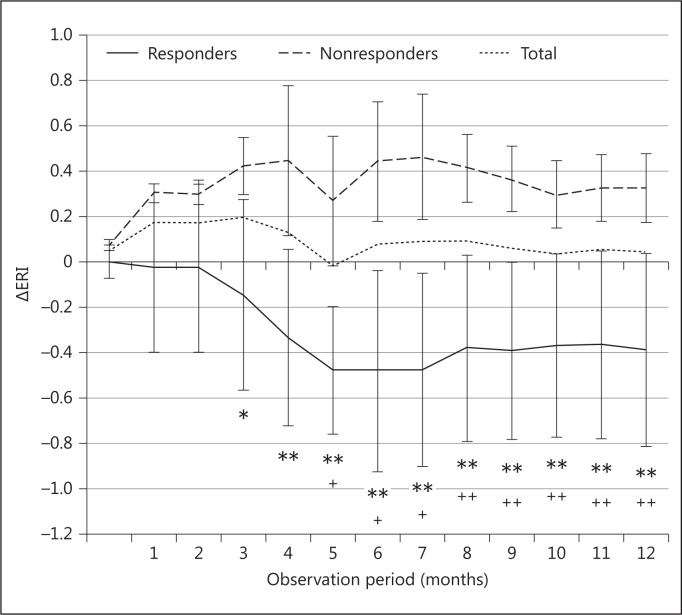
After LC treatment, 7 patients from the treated group showed a marked decrease in their rHuEPO requirement (responders), while another 11 patients showed no change in their rHuEPO requirement (nonresponders). ERI decreased significantly in responders 5 months after LC administration and was maintained thereafter (-39.2 ± 15.3% at 12 months, p < 0.005), which meant almost 40% reduction of the rHuEPO dose (fig. 1). Using statistical analysis, the acyl/free carnitine ratio and plasma calcium concentrations were significantly different between responders and nonresponders. However, no differences were found in other parameters (table 2).
Fig. 1.
Changes of ERI in responders and nonresponders. ERI (weekly rHuEPO dose per gram of Hb adjusted by dry weight). ** p < 0.01, * p < 0.05 responders vs. nonresponders. ++ p < 0.01, + p < 0.05 baseline vs. each period.
Table 2.
Comparison of each parameter between responders and nonresponders
| Nonresponders (n = 11) | Responders (n = 7) | p value | |
|---|---|---|---|
| Duration of hemodialysis, months | 144.9 ± 75.9 | 121.7 ± 124.7 | NS |
| Age, years | 68.9 ± 8.8 | 63.3 ± 4.7 | NS |
| Free carnitine (baseline), μmol/l | 20.6 ± 7.1 | 23.5 ± 6.4 | NS |
| Acyl carnitine (baseline), μmol/l | 16.7 ± 4.8 | 15.9 ± 2.4 | NS |
| Acyl/free carnitine ratio (baseline) | 0.83 ± 0.12 | 0.7.± 0.09 | <0.05 |
| Total cholesterol, mg/dl | 168.7 ± 41.3 | 167.9 ± 42.7 | NS |
| Triglyceride, mg/dl | 93.6 ± 59.3 | 83.6 ± 50.3 | NS |
| HDL cholesterol, mg/dl | 57.5 ± 12.6 | 63.6 ± 20.5 | NS |
| LDL cholesterol, mg/dl | 82.8 ± 39.9 | 80.0 ± 27.8 | NS |
| Hematocrit, % | 33.4 ± 4.0 | 34.1 ± 2.2 | NS |
| Hb, g/dl | 10.4 ± 1.2 | 10.8 ± 1.0 | NS |
| Serum iron, % | 68.3 ± 16.1 | 62.3 ± 7.9 | NS |
| Ferritin, ng/ml | 211.4 ± 230.3 | 229.3 ± 194.2 | NS |
| Ca, mg/dl | 8.6 ± 0.6 | 9.4 ± 0.7 | <0.05 |
| P, μg/l | 6.2 ± 1.6 | 5.4 ± 0.3 | NS |
| PTH, pg/ml | 158.4 ± 106.9 | 163.7 ± 91.2 | NS |
| CRP, mg/dl | 0.31 ± 0.41 | 0.46 ± 0.94 | NS |
| Albumin, g/dl | 3.6 ± 0.2 | 3.7 ± 0.2 | NS |
| Ch-e, U/l | 242.8 ± 50.8 | 254.7 ± 67.4 | NS |
| ERI (baseline), IU/week/g Hb/kg BW | 8.29 ± 6.81 | 5.73 ± 3.42 | NS |
Values are mean ± standard deviation. HDL = High-density lipoprotein; LDL = low-density lipoprotein; Ca = calcium; P = phosphate; PTH = parathormone; CRP = C-reactive protein; Ch-e = choline esterase; BW = body weight; NS = not significant.
Significant p values: responders vs. nonresponders.
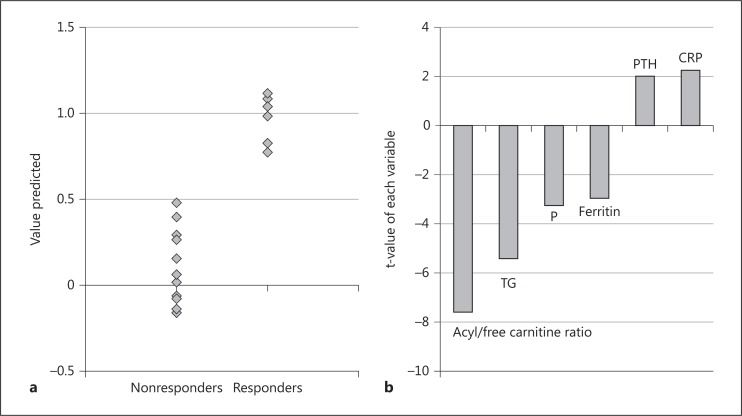
In order to clarify the factors influencing the responsiveness of LC on renal anemia, stepwise linear discriminant analysis was applied in this study. The separation of responders from nonresponders was well achieved by means of the optimal discriminant analysis (fig. 2a). Six independent factors were obtained as explainable variables. It is suggested that the acyl/free carnitine ratio was the most contributing factor in distinguishing responders from nonesponders (fig. 2b).
Fig. 2.
Stepwise linear discriminant analysis. a The separation of responders from nonresponders was well accomplished without overlaps. b The acyl/free carnitine ratio was the most contributing factor distinguishing responders from nonresponders. TG = Triglyceride; P = phosphate; PTH = parathormone; CRP = C-reactive protein.
Discussion
LC has been shown to increase hematocrit in hemodialysis patients without rHuEPO therapy and to reduce rHuEPO requirements in hemodialysis patients undergoing rHuEPO therapy [4,6]. However, several studies could not demonstrate the beneficial effect of LC on renal anemia [11,12,13]. Thus, the therapeutic role of LC in the anemia of chronic hemodialysis patients is still debated [4,6,7,8,9,10].
The variability of observation periods might be one of the reasons to explain the heterogeneity of numerous studies. In order to evaluate not only the short-term but also the long-term effect of LC, we assessed the effect of LC on anemia for 12 months.
Bellinghieri et al. [14] reported that the hematocrit values rose at the end of a 2-month treatment with LC (2 g/day orally, p < 0.02). Labonia [15] reported that LC treatment promoted a 38.1% reduction in the rHuEPO requirement of the active group (1 g intravenously after every dialysis session for 6 months, p < 0.02). This active group (responders) was composed of 7 out of 13 patients. Kletzmayr et al. [16] revealed that after 4 months of co-administration of intravenous iron and LC, the rHuEPO requirement decreased in 8 out of 19 evaluable hemodialysis patients. In these responders, the weekly rHuEPO dose was decreased significantly by 36.9 ± 23.3% (p < 0.001). However, the rHuEPO requirement was unchanged when all carnitine-treated patients were compared between study entry and after 4 months of treatment. This result was consistent with that found by Labonia [15] and the present study. Vaux et al. [13] reported that LC had no statistically significant effect on the hematologic parameters measured in the treated patients (20 mg/kg intravenously three times weekly after hemodialysis for 16 weeks). On the other hand, Emami Naini et al. [17] demonstrated that the increase in Hb concentrations was not significant, but the erythropoietin dose was significantly decreased in the carnitine group (1 g/day orally for 16 weeks, p = 0.001). Therefore, LC supplementation might have beneficial effects in certain patients (responders) in terms of the improvement of renal anemia.
The NKF-K/DOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure [18] reveal that the most promising of the proposed applications for LC in dialysis patients is the treatment of rHuEPO-resistant anemia. A 4-month trial of LC (20 mg/kg intravenously administered after dialysis) is suggested. In our study, an improvement of ERI was observed within 5 months after carnitine administration in responders. The 4-month trial of LC supplementation might make sense with regard to the detection of responders.
There are some studies that found a significant negative correlation of plasma total carnitine levels and rHuEPO requirement in hemodialyzed patients [19,20]. In our study, there was no significant difference in the plasma carnitine levels before or after treatment between responders and nonresponders. There was no correlation between plasma carnitine concentrations and reduction of ERI. Therefore, it would be suspected that the level of carnitine was not an important factor to determine responsiveness to carnitine in our study.
Reuter et al. [20] suggested that the apparent dichotomy of patients between responders and nonresponders might depend on the proportion of nonacetyl acylcarnitines within the total carnitine pool. This might be coincident with our study, because the acyl/free carnitine ratio is a strong predicting factor distinguishing responders from nonresponders in this work. It might be speculated that a low acyl-free carnitine ratio indicates a better tendency to erythropoietin responsiveness.
The several actions of LC on circulating RBC were reviewed by Bonomini et al. [4]. It is suggested that LC and carnitine palmitoyltransferase in RBC play a role in terms of membrane phospholipid fatty acid turnover. LC may improve the viscoelastic properties of RBC by intervening on both the outer and the inner side of the erythrocyte membrane [4]. The anti-inflammatory activity of LC is also suggested to play a role in the prevention of apoptosis [21]. A marked reduction in RBC survival in patients undergoing intermittent hemodialysis compared with healthy controls (RBC half-life is 14.5 days in patient vs. 23.5 days in controls) is reported [22].
In addition, 51Cr labeling procedure, a gold standard methodology in RBC survival studies, showed a strong trend towards an improved RBC survival in the LC-treated group [23]. The erythrocyte life span is well known to be almost 120 days in normal subjects. Because the reductions of ERI in responders were observed to be gradually larger until 5 months in our study, it is strongly speculated that the treatment might expand the erythrocyte life span.
The KDIGO guideline suggests not using adjuvants to an erythrocyte sedimentation rate treatment that includes vitamin C, vitamin D, vitamin E, folic acid, LC and pentoxifylline because the evidence level is not sufficient [24]. Although the beneficial effect of LC supplementation on renal anemia was not observed in all patients, at least 40% of them (responders) showed a significant improvement of ERI (almost 40% reduction of the rHuEPO dose) in our study.
Conclusions
Long-term LC supplementation might make sense in certain patients (responders) with regard to the EPO dose-sparing effect. The acyl/free carnitine ratio at baseline was the most contributing factor in distinguishing responders from nonresponders.
References
- 1.Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2:1175–1178. doi: 10.1016/s0140-6736(86)92192-6. [DOI] [PubMed] [Google Scholar]
- 2.Drueke TB. R-HuEPO hyporesponsiveness – who and why? Nephrol Dial Trasplant. 1995;10(suppl 2):S62–S68. doi: 10.1093/ndt/10.supp2.69. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber B. Levocarnitine and dialysis: review. Nutr Clin Pract. 2005;20:218–243. doi: 10.1177/0115426505020002218. [DOI] [PubMed] [Google Scholar]
- 4.Bonomini M, Zammit V, Pusey CD, Vecchi AD, Arduini A. Pharmacological use of L-carnitine in uremic anemia: has its full potential been exploited? Pharmacol Res. 2011;63:157–164. doi: 10.1016/j.phrs.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012;51:553–572. doi: 10.1007/BF03261931. [DOI] [PubMed] [Google Scholar]
- 6.Golper TA, Goral S, Becker BN, Langman CB. L-Carnitine treatment of anemia. Am J Kidney Dis. 2003;41(suppl 4):S27–S34. doi: 10.1016/s0272-6386(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 7.Steinman TI. L-Carnitine supplementation in dialysis patients: does the evidence justify its use? Semin Dial. 2005;18:1–2. doi: 10.1111/j.1525-139X.2005.18104.x. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber B. Common misconceptions about levocarnitine and dialysis. Semin Dial. 2005;18:349–354. doi: 10.1111/j.1525-139X.2005.18412.x. [DOI] [PubMed] [Google Scholar]
- 9.Handelman GJ. Debate forum: carnitine supplements have not been demonstrated as effective in patients on long-term dialysis therapy. Blood Purif. 2006;24:140–142. doi: 10.1159/000089450. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber BD. Debate forum: levocarnitine therapy is rational and justified in selected dialysis patients. Blood Purif. 2006;24:128–139. doi: 10.1159/000089449. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson-Ehle P, Cederblad G, Fagher B, Monti M, Thysell H. Plasma lipoproteins, liver function and glucose metabolism in hemodialysis patients: lack of effect of L-carnitine supplementation. Scand J Clin Lab Invest. 1985;45:179–184. doi: 10.3109/00365518509160992. [DOI] [PubMed] [Google Scholar]
- 12.Lilien MR, Duran M, Quak JME, Frankhuisen JJ, Schroider CH. Oral L-carnitine does not decrease erythropoietin requirement in pediatric dialysis. Pediatr Nephrol. 2000;15:17–20. doi: 10.1007/s004670000423. [DOI] [PubMed] [Google Scholar]
- 13.Vaux E, Taylor DJ, Altman P, Rajagopalan B, Graham K, Cooper R, Bonomo Y, Styles P. Effect of carnitine supplementation on muscle metabolism by the use of magnetic resonance spectroscopy and near-infrared spectroscopy in end-stage renal disease. Nephron Clin Pract. 2004;97:c41–c48. doi: 10.1159/000078399. [DOI] [PubMed] [Google Scholar]
- 14.Bellinghieri G, Savica V, Mallamace A, Stefano CD, Console F, Spagnoli LG, Villaschi S, Palmieri G, Corsi M, Maccari F. Correlation between increased serum and tissue L-carnitine levels and improved muscle symptoms in hemodialyzed patients. Am J Clin Nutr. 1983;38:523–531. doi: 10.1093/ajcn/38.4.523. [DOI] [PubMed] [Google Scholar]
- 15.Labonia WD. L-Carnitine effects on anemia in hemodialyzed patients treated with erythropoietin. Am J Kidney Dis. 1995;26:757–764. doi: 10.1016/0272-6386(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 16.Kletzmayr J, Mayer G, Legenstein E, Heinz-Peer G, Leitha T, Hori WH, Kovarik J. Anemia and carnitine supplementation in hemodialyzed patients. Kidney Int Suppl. 1999;69:S93–S106. [PubMed] [Google Scholar]
- 17.Emami Naini A, Moradi M, Mortazavi M, Amini Harandi A, Hadizadeh M, Shirani F, Basir Ghafoori H, Emami Naini P. Effects of oral L-carnitine supplementation on lipid profiles, anemia, and quality of life in chronic renal disease patients under hemodialysis: randamized, double-blinded, placebo-controlled trial. J Nutr Metab. 2012;2012:510483. doi: 10.1155/2012/510483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopple JD. National Kidney Foundation K/DOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. Am J Kidney Dis. 2001;37(suppl 2):S66–S70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 19.Kooistra MP, Struyvenberg A, Es AV. The response to recombinant human erythropoietin in patients with the anemia of end-stage renal disease is correlated with serum carnitine levels. Nephron. 1991;57:127–128. doi: 10.1159/000186237. [DOI] [PubMed] [Google Scholar]
- 20.Reuter SE, Faull RJ, Ranieri E, Evans AM. Endogenous plasma carnitine pool composition and response to erythropoietin treatment in chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24:990–996. doi: 10.1093/ndt/gfn588. [DOI] [PubMed] [Google Scholar]
- 21.Fortin G, Yurchenko K, Collette C, Rubio M, Villani AC, Bitton A, Sarfati M, Franchimont D. L-Carnitine, a diet component and organic cation transporter OCTN ligand, displays immune suppressive properties and abrogates intestinal inflammation. Clin Exp Immunol. 2009;156:161–171. doi: 10.1111/j.1365-2249.2009.03879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ly J, Maticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis. 2004;44:715–719. [PubMed] [Google Scholar]
- 23.Arduini A, Bonomini M, Clutterbuck EJ, Laffan MA, Pusey CD. Effects of L-carnitine administration on erythrocyte survival in haemodialysis patients. Nephrol Dial Transplant. 2006;21:2671–2672. doi: 10.1093/ndt/gfl155. [DOI] [PubMed] [Google Scholar]
- 24.Drueke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s) Kidney Int. 2012;82:952–960. doi: 10.1038/ki.2012.270. [DOI] [PubMed] [Google Scholar]