Abstract
Cardiovascular disease (CVD), including heart disease and stroke, is the leading cause of death in the USA, regardless of self-determined race/ethnicity, and largely driven by cardiometabolic risk (CMR) and cardiorenal metabolic syndrome (CRS). The primary drivers of increased CMR include obesity, hypertension, insulin resistance, hyperglycemia, dyslipidemia, chronic kidney disease as well as associated adverse behaviors of physical inactivity, smoking, and unhealthy eating habits. Given the importance of CRS for public health, multiple stakeholders, including the National Minority Quality Forum (the Forum), the American Association of Clinical Endocrinologists (AACE), the American College of Cardiology (ACC), and the Association of Black Cardiologists (ABC), have developed this review to inform clinicians and other health professionals of the unique aspects of CMR in racial/ethnic minorities and of potential means to improve CMR factor control, to reduce CRS and CVD in diverse populations, and to provide more effective, coordinated care. This paper highlights CRS and CMR as sources of significant morbidity and mortality (particularly in racial/ethnic minorities), associated health-care costs, and an evolving index tool for cardiometabolic disease to determine geographical and environmental factors. Finally, this work provides a few examples of interventions potentially successful at reducing disparities in cardiometabolic health.
Key Words : Cardiorenal metabolic syndrome, Cardiometabolic risk, Metabolic syndrome, Racial/ethnic minorities, Disparities
Cardiometabolic Risk and Health Care Burden
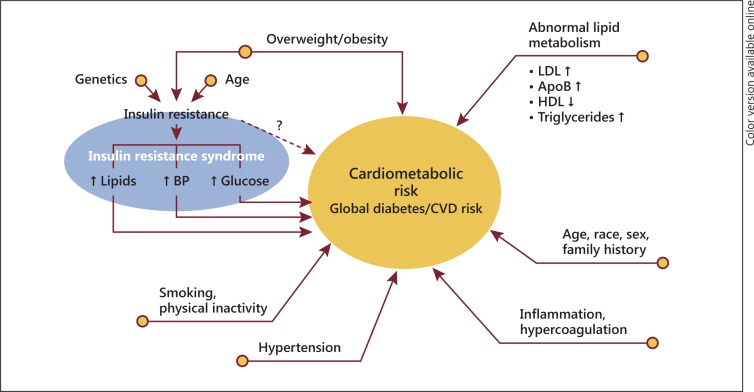
Cardiovascular disease (CVD), including heart disease and stroke, is the leading cause of death in the United States, regardless of self-determined race/ethnicity [1]. Furthermore, multiple risk factors significantly contribute to CVD morbidity and mortality, including the constellation of risk factors termed cardiometabolic risk (CMR) [2] (fig. 1). CMR refers to interrelated traditional risk factors for metabolic syndrome (MetS) such as dyslipidemia, hypertension, obesity, insulin resistance, and hyperglycemia as well as behavioral factors such as physical inactivity, smoking, and unhealthy eating patterns [2]. Family history, age, sex, and race/ethnicity also contribute to CMR. More recently, the concept of CMR has been expanded to the cardiorenal metabolic syndrome (CRS) also including microalbuminuria and reduced renal function [3]. This improved category is especially relevant to understanding and approaching the disparities in chronic kidney disease and end-stage renal disease in US racial/ethnic minorities.
Fig. 1.
Multiple risk factors significantly contribute to CVD morbidity and mortality, including the constellation of risk factors termed CMR. LDL = Low-density lipoprotein; HDL = high-density lipoprotein; ApoB = apolipoprotein B; ↑ = elevated; ↓ = reduced. Reproduced with permission from the ACC [2].
Importantly, obesity has served as the primary driver of the dramatic rise in CRS and CMR during the past 20 years. More than one third of the adults (35.7%) and approximately 17% (12.5 million) of the youth are obese [4,5], and diabetes mellitus (DM) tripled in adults between 1980 and 2010, from 5.5 million to 20.7 million [6]. These trends, and enlarging numbers of individuals with CMR, represent a significant health-care burden. Furthermore, while significant improvements in coronary heart disease (CHD) and CVD risk continued from 1999 to 2010, especially for Whites, the mean predicted risk for CHD and CVD increased among African Americans. The lack of significant improvements in mean blood pressure (BP) and total cholesterol coupled with strong increases in prevalent DM among African Americans largely account for the observed racial differences [7].
By 2012, US health care spending was estimated at USD 2.8 trillion or about 18% of the total goods and services spending [8]. Direct obesity costs are astounding – approximately USD 147 billion in 2008 [9] – and the estimated total economic cost of diagnosed DM in 2012 was USD 245 billion, a 41% increase from the previous estimate of USD 174 billion [10]. Overall, the total cardiovascular costs in the year 2010 were approximately USD 444 billion [1], largely attributable to CMR treatment.
Although US life expectancy and health care improved dramatically over the last century, these benefits have not occurred equitably, largely due to disparities in CRS and CVD mortality. Although having become smaller since 2003, the black-white life expectancy gap remains large, reflecting social inequities and adverse life experiences [11]. Eliminating racial/ethnic disparities would prevent approximately 1 million hospitalizations and save USD 6.7 billion yearly. Preventable hospitalizations for Blacks for all conditions occur at twice the rate of Whites, with approximately 430,000 yearly excess hospitalizations that waste USD 3.4 billion. For Hispanics, approximately 110,000 excess hospitalizations yearly waste USD 900 million [12].
Defining the Need for Identification of Racial/Ethnic Disparities in Cardiometabolic Health
The National Health and Nutrition Examination Survey (NHANES) age-adjusted MetS prevalence is 35.1% in men and 32.6% in women [1]. However, MetS prevalence among racial/ethnic groups varies, and the burden of CVD, chronic kidney disease, and DM disproportionately affects African Americans, Hispanics, American Indians/Alaskan Natives, and perhaps other minorities such as Americans of Asian Indian (South Asian), East Asian and Middle Eastern descent [13,14,15]. Therefore, especially in minorities, the need to identify CRS and associated CV morbidity, mortality, and all-cause mortality remains of critical importance.
Unique Racial/Ethnic Aspects of CMR and Associated CVD
National disease data are limited in describing the unique and diverse tapestry of contemporary America. Critically missing is a national, coordinated surveillance system to integrate current and emerging data, including understudied minorities [16]. Since the early 1960s, NHANES has provided the most robust data to assess the health and nutritional status of adults and children [17]. However, in order to produce reliable statistics, NHANES oversamples African Americans and Hispanics (mainly Mexican Americans), thereby including limited data from other growing subpopulations.
Nevertheless, MetS varies among subpopulations [15,18]. Among men, 37% of the non-Hispanic Whites meet the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III MetS criteria, compared to 33% of the Mexican Americans and 25% of the non-Hispanic Blacks [19,20]. For Mexican American women, the prevalence of MetS is 40.6%, compared to 38.8% in non-Hispanic black and 31.5% in non-Hispanic white women.
Additionally, the national prevalence of type 2 DM in adults was 7.1% in non-Hispanic Whites, 8.4% in Asian Americans, 11.8% in Hispanics, and 12.6% in non-Hispanic Blacks between 2007 and 2009. Among Hispanics, the rates were 7.6% for Cubans and Central and South Americans, 13.3% for Mexican Americans, and 13.8% for Puerto Ricans [21]. Despite higher hemoglobin A1c levels in Blacks than in Whites at any given glycemic level, retinopathy prevalence in Blacks increases at a lower hemoglobin A1c level [22]. The unexpectedly lower MetS rates in Blacks, especially in men, are a conundrum and contradict their higher DM, hypertension, obesity (in women), CHD and stroke mortality, and premature CVD rates. Possible explanations for the lower MetS rates in Blacks include lower triglycerides and higher high-density lipoprotein C levels [23]. This MetS paradox in Blacks, despite high CVD morbidity and mortality, is one of the primary reasons for the expanded terms CMR and CRS (table 1) [3,24].
Table 1.
CRS, CMR and CVD: the African American paradox
| Higher CVD mortality rates, contributing significantly to reduced longevity |
| Higher proportion of black women and men (45 – 74 years) have premature CHD |
| Higher overweight/obesity rates (women) |
| Sedentary lifestyle |
| Relatively less MetS (especially men) |
| Earlier onset, more severe, and poorly controlled hypertension |
| Less prevalent hypertriglyceridemia, low high-density lipoprotein C levels, or both |
| Higher type 2 DM rates and associated complications |
| High chronic kidney disease/end-stage renal disease prevalence |
On the other hand, studies have documented that Hispanics in the USA may experience an aggregate CVD risk and a mortality rate similar to or lower than that experienced by their white counterparts. This epidemiological phenomenon of better than expected outcomes in CVD mortality among Hispanics, despite lower levels of socioeconomic metrics such as education and income and often higher levels of overall risk factors, has been coined the Hispanic paradox. For example, Mexican Americans demonstrate lower hypertension rates as compared to non-Hispanic Whites and Blacks, despite highly prevalent DM and obesity as well as less awareness, access to treatment, and adequate BP control [25]. Therefore, there is an inherent inaccuracy in determining CVD risk across racial/ethnic populations by using the conventional ATP III MetS definition, with somewhat arbitrary cutoff points, which may be different for diverse population subgroups.
Recent findings from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) indicate that previous Hispanic CVD prevalence data, primarily from Mexican Americans, may underestimate the CVD risk burden and heterogeneity among US Hispanics/Latinos [25]. For instance, foreign-born Hispanics with lower levels of acculturation may demonstrate less CVD despite a lower income and education [26]. In the HCHS/SOL, adverse CVD risk factors were more prevalent in participants of Puerto Rican descent, in subjects with a lower socioeconomic status, and in those reporting greater acculturation. Gender-matched hypertension prevalence was highest among Dominican men and Puerto Rican women; obesity was highest among Puerto Rican men and women. The prevalence of CMR factors in both sexes was highest among Puerto Ricans and lowest among South Americans. Overall, CVD was more prevalent in second- or third-generation immigrants who prefer English as their primary language and in those with longer lengths of residency in the USA [25]. These findings highlight the importance of considering the heterogeneity of CVD risk among Hispanic populations by country of origin and other metrics of acculturation.
Significant heterogeneity in Asian subpopulations warrants group disaggregation, particularly for studying CMR factors and associated CVD. Data from 94,423 Asians (Asian Indians, Chinese, Filipinos, Japanese, Korean, and Vietnamese) show considerable variation in CVD prevalence; CVD is highest among Asian Indians and Filipinos [27] and lowest for Chinese participants. Despite a lower BMI and a different definition of overweight/obesity than that conventionally used in Whites, Asian Americans may have higher MetS rates [28]. US Filipinos, the largest Southeast Asian and second largest Asian American subpopulation after Chinese Americans, have a higher prevalence of type 2 DM than Caucasians, and Filipino American women have high rates of central obesity [29].
Asian Indians, including younger individuals, have a high CHD mortality [30] and may have less favorable lipid, glucose, insulin, and C-reactive protein profiles as compared to Europeans despite having the same BMI, which is primarily due to greater visceral adiposity [31]. A cross-sectional study of 150 Asian Indian Americans (aged 45-79 years) demonstrated that 37% had prediabetes and 29% had DM. Interestingly, prediabetes and DM have been associated with stronger traditional Indian cultural beliefs – in part due to traditional diets rich in carbohydrates [32].
There may be a higher prevalence of DM in Middle Eastern or Arab Americans as compared to European Americans. DM affects about 18% of the Arab Americans aged 20-75 years in southeastern Michigan, with about half of them being unaware of their disease and not undergoing treatment [33]. Furthermore, obesity prevalence is 34% (with 26% MetS prevalence), and low high-density lipoprotein C is the most common criterion [34].
Classification of Race/Ethnicity and CMR: Limitations in Utility and Pitfalls
Race, a crude proxy for any disease, is a social construct and not a scientific category. Ethnicity, federally defined as the binary ‘Hispanic/Latino’ or ‘not Hispanic/Latino’, is also inexact. Race/ethnicity categories, including multiracial identity, were proposed to assess various health-related data, including surveillance and research. However, race categories are arbitrary, as White can reflect origins in Europe, the Middle East, or North Africa, and Asian can reflect origins ranging from India to Japan.
Moreover, there are greater genetic differences within than between certain racial/ethnic groups and genetic variations within all populations [35,36,37]. Hence, CRS and CMR with associated obesity are due to a complex interplay of behavioral, genetic, environmental, and social factors. Racial/ethnic differences in disease prevalence and responses to medical products may be only partially attributable to intrinsic factors (e.g., genetics, metabolism, and elimination) and include extrinsic factors (e.g., diet, environmental exposure, and sociocultural issues) or their interactions. Therefore, social determinants of health may be particularly important contributors to CRS and CMR disparities [38]. For example, inadequate community level, healthy food sources, or safe environments for physical activity may contribute to disproportionate obesity levels among minorities.
The Framingham Risk Score is the most widely employed tool to estimate CVD risk, incorporating age, sex, total cholesterol, high-density lipoprotein cholesterol, BP, and cigarette smoking [39,40]. Overall, the Framingham Risk Score calculator can determine a patient's 10-year absolute risk of CHD but may have some limitations and perhaps underestimate the risk in diverse populations [41,42,43]. The strength of associations between individual CVD risk factors differs significantly by race/ethnicity, with a greater risk for CVD mortality at younger ages in some minority individuals, suggesting that CVD risk prediction models should be validated in increasingly diverse, contemporary cohorts [43].
Reducing Health Disparities in Cardiometabolic Health
While a comprehensive review of interventions to address and overcome CMR disparities is beyond this review's scope, several potentially beneficial approaches are highlighted herein.
The National Minority Quality Forum's (the Forum) Disease-Based Index and Cardiometabolic Index
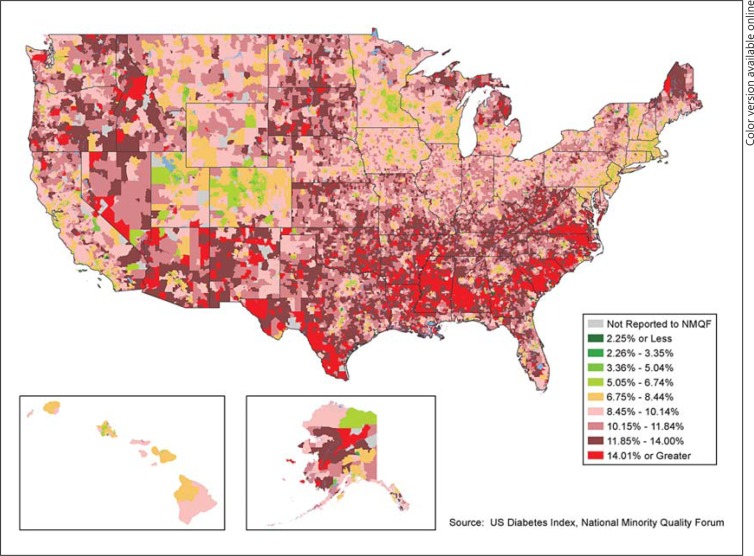
The National Health Index, built by the Forum, is a comprehensive database of patient records allowing users to map chronic diseases by prevalence, cost, outcomes, comorbidities, socioeconomic status, prescription drug use, etc. for any zip code, state, and congressional and state legislative districts. This enables users to identify unmet needs, including undiagnosed and uncontrolled populations, and to forecast trends. The recent 2013 index [44] provides a repository of zip code level data to compare, contrast, and define multiple variables related to CRS, CMR, MetS, and associated diseases by race/ethnicity, age, and gender (fig. 2). Nevertheless, the specificity of translating geocoding to the individual level remains a concern potentially limiting the widespread use of geocoding.
Fig. 2.
The figure indicates geographic variation in the overall prevalence of diabetes (BRFSS, 2011). Reproduced with permission from the Forum; National Health Index [44].
The Need for Interdisciplinary Approaches
This review is an interdisciplinary collaboration to improve CMR factor control in diverse and high-risk populations and provide more effective, coordinated care for established cardiometabolic disease [45]. The multiple associations and organizations herein seek to identify communities in which acute events from CRS and CMR factors as well as disease are higher than national average. Moreover, they aim at providing clinical profiles of typical cardiometabolic patients with acute events in high-risk communities to contrast them with patients in low-risk communities. This review and subsequent collaborations will ensure recommendations to prevent progression by raising public and provider awareness, developing research opportunities, supporting national or local quality improvement, investigating and advancing health policy as well as addressing and reducing barriers confronting minorities and at-risk patients. The overall goal is to reduce acute events (inpatient admissions, emergency department visits, and deaths) associated with CRS and CVD, particularly among minority and at-risk populations.
Advances in Patient-Centered Care: Self-Management of Cardiometabolic Health
To address patient communication, education, and engagement, the ACC initiated the CardioSmart website [46] in March 2008, which provides comprehensive and authoritative information as well as interactive management and compliance tools for heart disease patients and their families, including racial/ethnic minorities and focusing on patient-centered care. For example, the web-based Diabetes Education Center contains culturally and linguistically appropriate modules, including novel tools such as text messaging for medication and dietary adherence reminders.
Another innovative program is e-HealthyStrides© [47], managed by the Morehouse School of Medicine and designed to improve DM self-management via an integrated system that empowers and engages patients, their social network, health coach, and care team. After 12 weeks of intervention, 260 African Americans had a significantly improved BP, physical activity, and overall blood glucose level. Similarly, the Latino Diabetes Initiative at Joslin Diabetes Center, affiliated with Harvard Medical School, comprehensively includes patient care and education, research, outreach, and professional education to tackle the enormous challenges of DM and CVD [48].
Importantly, the use of electronic medical records can be leveraged to reduce disparities [49]. By recording race/ethnicity and language preference, health systems can better track efforts at reducing disparities and monitor preventive service utilization data to identify gaps for minority patients and develop targeted interventions. Increasingly, other programs and/or tools are being developed to address CRS and CMR in racial/ethnic populations at local, regional, and national levels.
Therapeutic Lifestyle Modifications
Importantly, obesity is a primary driver of the CRS and CMR epidemic. Therefore, comprehensive, therapeutic lifestyle modification through physical activity and weight loss promotion is the cornerstone of any prevention program aimed at reducing CRS and CMR. Nationally, physical activity remains suboptimal, with approximately 60% of all adults not engaging in the physical activity recommended and 25% not engaging in physical activity at all. Fourteen percent of the youth report no recent physical activity [50]. Given the rising rates of pediatric obesity and CMR, future studies should focus on the youth from diverse racial/ethnic and socioeconomic backgrounds. The diverse Diabetes Prevention Program, comprising 3,234 adults with impaired glucose tolerance, demonstrated that intensive lifestyle modification reduced progression to type 2 DM by 58%, with a positive impact on multiple CV risk factors (p < 0.001) [51].
Comprehensive CMR and Diabetes Management: Unmet Clinical Needs
All CRS patients should receive a comprehensive treatment program tailored to their unique medical history, behaviors and risk factors, ethno-cultural background, and environment. For subjects with prediabetes, including MetS, type 2 DM can be prevented or delayed by lifestyle modification. Regular glycemic status monitoring is recommended: at least one annual fasting plasma glucose and/or an oral glucose tolerance test [52], with extensive dietary counseling and increased physical activity. Minority populations, often with multiple risk factors, need more widespread screening and primary prevention (table 2). Healthy lifestyle habits should start as early as possible in life, involving the whole family [53].
Table 2.
Prediabetes risk factors indicating screening [42]
| Family history of DM |
| CVD |
| Overweight or obesity |
| Sedentary lifestyle |
| Non-white ancestry |
| Prior impaired glucose tolerance, impaired fasting glucose, and/or MetS |
| Hypertension |
| Increased levels of triglycerides, low high-density lipoprotein C, or both |
| Gestational DM history |
| Delivery of babies weighing >4 kg (9 lb) |
| Polycystic ovary syndrome |
| Antipsychotic therapy (schizophrenia and/or severe bipolar disease) |
Reproduced with permission from the American Association of Clinical Endocrinologists [52].
Multiple federal agencies and a broad range of private-sector partners support the ‘Million Hearts’ (MH) initiative to prevent heart attacks and strokes and reduce disparities by implementing proven, effective, and inexpensive interventions [54]. The National Forum for Heart Disease and Stroke Prevention, a coalition of over 65 organizations, spearheads multisector action to support the MH initiative and build the collective voice for CVD prevention [55].
The Affordable Care Act (ACA) may increase the access to insurance and utilization of healthy lifestyles by creating new incentives, enlarging existing employer wellness programs, supporting fitness center memberships, and rewarding health education or health risk assessment. As many as 129 million (approx. 50%) non-elderly Americans have some preexisting health condition, including DM or CVD, affecting their insurability, renewal, or treatment [56].
Conclusions
Recent dramatic improvements in life expectancy and health care have not occurred equitably, and disparities in quality care, morbidity, and mortality between Whites and certain racial/ethnic minorities, including the white-black mortality gap, persist. Furthermore, CRS and CMR account for substantial CVD and health care costs; starting in early childhood, gene-environment interactions disparately influence obesity and associated CMR. The costs of CVD and CRS disparities are tremendous and must be addressed through culturally appropriate lifestyle modification, therapeutic programs, and public health initiatives. This review promotes an interdisciplinary collaboration to improve CRS and CMR factor identification and control in diverse populations as well as strategies to provide effective, coordinated care.
Disclosure Statement
K.C.F. is a consultant for Roche, Merck, Forest, Takeda, and AstraZeneca, and received research grants from Novartis and Daiichi Sankyo. A.E.C. is a consultant for Merck, Roche, Sanofi, Bristol-Myers Squibb/AstraZeneca, and Eli Lilly & Co. G.A.P. has a research relationship with Boehringer Ingelheim and is President of the National Minority Quality Forum. J.M.F. is a consultant for Aegerion, Amarin, Bristol-Myers Squibb, Abbott Laboratories, Gilead, Pfizer, and Merck, and has a relationship with the ACC. P.E.P. receives a salary from the National Center for Research Resources and has received research funding from the National Institute on Aging and the National Heart Lung and Blood Institute. E.O.O. is a consultant for Abbott Laboratories, Abor Pharmaceuticals, Merck and Co., the Advisory Board of Novartis, and the Advisory Board of Sanofi. In addition, she is a trustee for the Global Healthcare Alliance, has received research funding from the NIH, and is a principal investigator of the WARFARIN Study, funded by Iverson Genetic Diagnostics. All other authors report that they have no relevant relationships to disclose.
Acknowledgements
E.O.O. and P.E.P. are supported in part by the National Institute on Minority Health and Health Disparities (NIMHD, grant No. 8U54MD007588 and 8R25MD007589-10). Moreover, they are also supported in part by the National Center for Advancing Translational Sciences (NCATS, grant No. UL1TR000454). Both NIMHD and NCATS are components of the National Institutes of Health (NIH). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of NIMHD, NCATS, or NIH.
Boehringer Ingelheim provided funding to the ACC for the CardioMetabolic Health Alliance.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with CMR: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. Jama. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and extreme obesity among adults in the United States, trends 1960-1962 through 2009-2010. NCHS Health E-Stat 2012. http://www.cdc.gov/nchs/data/hestat/obesity_adult_09_10/obesity_adult_09_10.htm (accessed March 4, 2013).
- 6.Centers for Disease Control (CDC) and Prevention National diabetes surveillance system. 2012. http://www.cdc.gov/diabetes/statistics (accessed March 4, 2013).
- 7.Ford ES. Trends in predicted 10-year risk of coronary heart disease and cardiovascular disease among US adults from 1999 to 2010. J Am Coll Cardiol. 2013;61:2249–2252. doi: 10.1016/j.jacc.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emanuel E, Tanden N, Altman S, et al. A systemic approach to containing health care spending. N Engl J Med. 2012;367:949–954. doi: 10.1056/NEJMsb1205901. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper S, Rushani D, Kaufman JS. Trends in the black-white life expectancy gap, 2003-2008. Jama. 2012;307:2257–2259. doi: 10.1001/jama.2012.5059. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, Office of Minority Health and Health Equity. Minority health. 2012. http://www.cdc.gov/minorityhealth/ (accessed March 4, 2013).
- 13.Boden-Albala B, Sacco RL, Lee HS, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke. 2008;39:30–35. doi: 10.1161/STROKEAHA.107.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 16.Committee on a National Surveillance System for Cardiovascular and Select Chronic Diseases . A Nationwide Framework for Surveillance of Cardiovascular and Chronic Lung Diseases. Washington: National Academies Press; 2011. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). National health and nutrition examination survey data. 2012. http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l11_b_doc.pdf (accessed January 3, 2013).
- 18.Meigs JB, Wilson PW, Nathan DM, D'Agostino RB, Sr, Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52:2160–2167. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 19.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009;5:1–7. [PubMed] [Google Scholar]
- 20.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Jama. 2002;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) National diabetes fact sheet. 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf (accessed January 3, 2013).
- 22.Tsugawa Y, Mukamal KJ, Davis RB, Taylor WC, Wee CC. Should the hemoglobin A(1c) diagnostic cutoff differ between blacks and whites? A cross-sectional study. Ann Intern Med. 2012;157:153–159. doi: 10.7326/0003-4819-157-3-201208070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SX, Carnethon M, Szklo M, Bertoni A. Racial/ethnic differences in the association of triglycerides with other metabolic syndrome components: the Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord. 2011;9:35–40. doi: 10.1089/met.2010.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61:943–947. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daviglus ML, Talavera GA, Aviles-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. Jama. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimbro RT, Bzostek S, Goldman N, Rodriguez G. Race, ethnicity, and the education gradient in health. Health Aff (Millwood) 2008;27:361–372. doi: 10.1377/hlthaff.27.2.361. [DOI] [PubMed] [Google Scholar]
- 27.Holland AT, Wong EC, Lauderdale DS, Palaniappan LP. Spectrum of cardiovascular diseases in Asian-American racial/ethnic subgroups. Ann Epidemiol. 2011;21:608–614. doi: 10.1016/j.annepidem.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palaniappan LP, Wong EC, Shin JJ, Fortmann SP, Lauderdale DS. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond) 2011;35:393–400. doi: 10.1038/ijo.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sales CS, Lee RY, Agadzi AK, Hee MR, Singh K, Lin SC. Prevalence of diabetes mellitus and diabetic retinopathy in Filipino vs. Caucasian Americans: a retrospective cross-sectional epidemiologic study of two convenience samples. Ethn Dis. 2012;22:459–465. [PubMed] [Google Scholar]
- 30.Palaniappan LP, Araneta MR, Assimes TL, et al. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122:1242–1252. doi: 10.1161/CIR.0b013e3181f22af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lear SA, Chockalingam A, Kohli S, Richardson CG, Humphries KH. Elevation in cardiovascular disease risk in South Asians is mediated by differences in visceral adipose tissue. Obesity (Silver Spring) 2012;20:1293–1300. doi: 10.1038/oby.2011.395. [DOI] [PubMed] [Google Scholar]
- 32.Kanaya AM, Wassel CL, Mathur D, et al. Prevalence and correlates of diabetes in South Asian Indians in the United States: findings from the Metabolic Syndrome and Atherosclerosis in South Asians Living in America Study and the Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord. 2010;8:157–164. doi: 10.1089/met.2009.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin D and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33:1373–1375. doi: 10.2337/dc09-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaber LA. Diabetes and the metabolic syndrome in Arab Americans: a community-based sample. Ethn Dis. 2005;15:S1-26-8. [PubMed] [Google Scholar]
- 35.Kanakamedala P, Haga SB. Characterization of clinical study populations by race and ethnicity in biomedical literature. Ethn Dis. 2012;22:96–101. [PMC free article] [PubMed] [Google Scholar]
- 36.Caballero AE. Diabetes in minority populations; in Joslin's Diabetes Mellitus. ed 14. New York: Lippincott Williams & Wilkins; 2008. pp. 505–524. [Google Scholar]
- 37.Armstrong K. Genomics and health care disparities: the role of statistical discrimination. Jama. 2012;308:1979–1980. doi: 10.1001/2012.jama.10820. [DOI] [PubMed] [Google Scholar]
- 38.Cheng JK. Confronting the social determinants of health – obesity, neglect, and inequity. N Engl J Med. 2012;367:1976–1977. doi: 10.1056/NEJMp1209420. [DOI] [PubMed] [Google Scholar]
- 39.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 40.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 41.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. Jama. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 42.Hurley LP, Dickinson LM, Estacio RO, Steiner JF, Havranek EP. Prediction of cardiovascular death in racial/ethnic minorities using Framingham risk factors. Circ Cardiovasc Qual Outcomes. 2010;3:181–187. doi: 10.1161/CIRCOUTCOMES.108.831073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger JS, Jordan CO, Lloyd-Jones D, Blumenthal RS. Screening for cardiovascular risk in asymptomatic patients. J Am Coll Cardiol. 2010;55:1169–1177. doi: 10.1016/j.jacc.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 44.Health in America Series. ed 1. National Minority Quality Forum Amenia: Grey House Publishing, Inc.; 2013. Diabetes in America – a geographic and demographic analysis of an epidemic by state and congressional districts. [Google Scholar]
- 45.Ferdinand KC.CardioMetabolic health alliance. http://www.cardiometabolica.org/2013
- 46.American College of Cardiology CardioSmart. http://www.cardiosmart.org/2008 (accessed on March 4, 2013).
- 47.Pemu PE, Quarshie AQ, Josiah-Willock R, Ojutalayo FO, Alema-Mensah E, Ofili EO. Socio-demographic psychosocial and clinical characteristics of participants in e-HealthyStrides©: an interactive e-health program to improve diabetes self-management skills. J Health Care Poor Underserved. 2011;22:146–164. doi: 10.1353/hpu.2011.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caballero AE.Diabetes initiative for Latinos, 2012. http://www.joslin.org/latino (accessed June 16, 2013).
- 49.von Sternberg T, Averbeck B, McClure N. Collection of data on race and ethnic group by physician practices. N Engl J Med. 2010;363:96. doi: 10.1056/NEJMc1004036. author reply 97. [DOI] [PubMed] [Google Scholar]
- 50.U.S. Department of Health and Human Services Physical activity and health: a report of the surgeon general executive summary. Atlanta, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc gov/nccdphp/sgr/summary.htm (accessed June 16, 2013).
- 51.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(suppl 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 53.Cortes DE, Millan-Ferro A, Schneider K, Vega RR, Caballero AE. Improving food purchasing selection among low-income Spanish-speaking Latinos. Am J Prev Med. 2013;44(suppl 3):S267–S273. doi: 10.1016/j.amepre.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 54.National Forum for Heart Disease and Stroke Prevention. http://www.nationalforum.org
- 55.Frieden TR, Berwick DM. The ‘Million Hearts’ initiative – preventing heart attacks and strokes. N Engl J Med. 2011;365:e27. doi: 10.1056/NEJMp1110421. [DOI] [PubMed] [Google Scholar]
- 56.HealthCare.gov Overview: proposed rule for health insurance market reforms. 2012. http://www.healthcare.gov/news/factsheets/2012/11/market-reforms11202012a.html (accessed June 16, 2013).