Abstract
Scedosporium prolificans are opportunistic moulds that can cause mycetoma following penetrating injuries. This fungus is more virulent than other species and treatment options are limited. Here we describe the first known case in the UK of S. prolificans osteomyelitis, in a 4 year old following penetrating injury. Successful outcome with limb salvage and foot function is achieved after repeated surgical debridement, and combination chemotherapy with voriconazole/terbinafine.
Keywords: Scedosporium prolificans, Fungal osteomyelitis, Mycetoma, Voriconazole, Terbinafine
1. Introduction
Scedosporium prolificans is a ubiquitous fungi present in the soil, water and potted plants [1]. Since the first description of S. prolificans as an agent of human disease, several cases have been reported from across the developed world, in both immunocompetent and immunocompromised patients. Though septic arthritis and osteomyelitis after penetrating injury are recognised in immunocompetent patients [2–4], there are no cases reported from the United Kingdom. Here we report a case of osteomyelitis caused by S. prolificans in a 4-year-old immunocompetent child treated with surgery and combination antifungal agents.
2. Case
A previously well 4-year-old boy presented to the Accident & Emergency Department (day 0) accompanied by his mother in June 2013 with painful left ankle. There was a history of penetrating injury by a thorn 2 weeks previously. The thorn had been removed promptly by the parents. The child had been born in United Kingdom to Welsh father and Vietnamese mother who settled in UK. The family spent 6 weeks in Vietnam between February and March 2013 and apart from a diarrhoeal illness had been well during that time.
On examination, child was alert, apyrexial but walking with a limp. The medial malleolus was swollen and tender and the ankle joint was warm but there was no restriction of movement. A small healing wound from the thorn injury was noted on the midsole but there was no suggestion of any foreign body. Initial plain X-ray of the left foot was unremarkable (Fig. 1). He was sent home and reviewed 2 days later with little change in his condition. Mild swelling was noticed distal to medial malleolus which was tender but he remained apyrexial. Initial blood tests showed a white cell count of 12.8×109/L (normal limits) and CRP of 26 g/L (mildly raised). He was sent home on analgesic treatment with a plan to review in the orthopaedic clinic the following week.
Fig. 1.
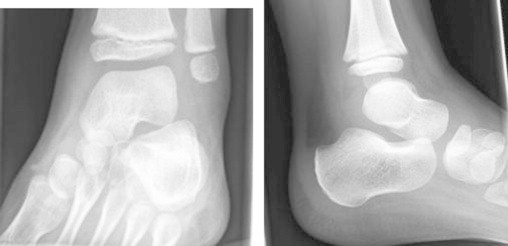
AP and lateral views of the foot – no significant abnormality seen.
Two days later, his mother brought him back to hospital with increased pain and swelling of the left ankle. An ultrasound scan of the foot showed synovial thickening and increased vascularity around the talonavicular joint, most marked laterally, suggesting septic arthritis. An ultrasound guided aspiration of the joint and blood cultures were taken and he was started on co-amoxiclav. All cultures were negative on microscopy and culture.
After 5 days of co-amoxiclav, the CRP continued to rise peaking at 150 g/L. Clinically he remained in pain and unable to weight bear. An MRI scan (day 9) of left foot (Fig. 2) showed a thick effusion and synovial proliferation in the talonavicular and anterior subtalar joints, marrow oedema in the medial aspect of the talar head but no evidence of any intraosseous collection.
Fig. 2.
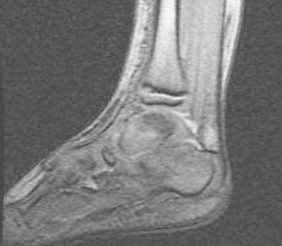
MRI of the foot showing effusion and synovial proliferation in the talonavicular and anterior subtalar joints.
A washout of the subtalar and talonavicular joints was performed the same day and washout fluids and an interoperative swab were sent for culture. Culture from the swab grew a mycelial fungus after 3 days incubation although other specimens remained negative. Initial presumptive identification based on microscopic appearance suggested Scedosporium spp.
The orthopaedic team was advised to repeat the washout and send multiple tissue and fluid samples to exclude contamination (day 14). Antifungal treatment was not started at this stage but he was commenced on flucoxacillin and fucidic acid empirically. Synovial tissue and pus samples taken at the second washout again grew a mycelial mould and presumptive identification was modified to Exophiala species based on microscopic appearance and colonial morphology. Isolates were sent to the reference laboratory for further identification and susceptibility testing. Itraconazole with a loading dose of 10 mg/kg/day followed by maintenance dose 5 mg/kg/day in 2 divided doses was commenced on day 19.
Six days after starting itraconazole (day 25) the patient deteriorated clinically with pus oozing from the wound. A further washout was undertaken and samples taken at this stage again grew a mould.
Reports from the reference laboratory received day 28 identified the mould as S. prolificans resistant to amphotericin B, itraconazole, posaconazole, terbinafine; intermediate susceptibility to miconazole and susceptible to voriconazole. Itraconazole was discontinued and the patient was commenced on voriconazole and terbinafine.
Repeat MRI scan on day 29 (Fig. 3) showed extensive fluid and synovial thickening within the talonavicular and calcaneocuboid joints associated with significant periarticular oedema. Also, there is complete loss of articular cartilage at these two joints associated with erosions at the calcaneocuboid joint and inferior medial aspect of the talus. He was taken to theatre for radical debridement involving washout followed by synovectomy and removal of the navicular bone and 50% of the talar head articular surface; large erosions in the cuboid and calcaneum were curetted to healthy bone. Samples taken during debridement were sent for routine and mycobacterial cultures. All samples were negative at this stage. Full immunological review was undertaken and no defects were detected.
Fig. 3.
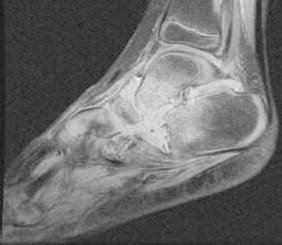
Extensive synovial thickening within the talonavicular and calcaneocuboid joints associated with complete loss of articular cartilage at these two joints.
Alternate day washouts and theatre review were performed and a vacuum drain was inserted. Intravenous flucloxacillin and fusidic acid were discontinued. All further cultures were negative for all organisms. No further progression of the bony destruction was seen and the boy improved clinically. He underwent weekly change of vacuum dressings in theatre until the cavity was small enough to be treated comfortably in the outpatient clinic.
He was sent home 6 weeks after initial diagnosis on voriconazole and terbinafine with weekly review in clinic. At 3 months he had healed both lateral and medial wounds and was weight bearing in a functional boot. Whilst clinical progress is good, minor radiological abnormalities persist. (Fig. 4) At 4 months finger nail discoloration and skin rash were noted and were presumed to be related to terbinafine. The plan was to continue long-term antifungal treatment with therapeutic drug monitoring of voriconazole for at least 6 months. However, therapy was discontinued after 5 months due to distressing hallucinations attributed to voriconazole. Voriconazole level at that time was 1.6 mg/L which was under therapeutic level. At 6 months he was mobile and pain free with near full range of movement. He is under regular clinical follow up to ensure no reactivation of the infection.
Fig. 4.
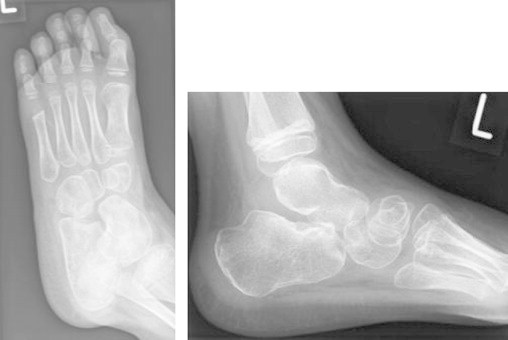
AP and lateral views of the foot – 2 months post radical debridement showing good healing of calcaneum and talar bone although minor radiological abnormalities persist.
3. Discussion
Scedosporium species are opportunist moulds that can cause a variety of medical problems ranging from colonisation and hypersensitivity in cystic fibrosis patients, neurological infection following near-drowning incidents and disseminated disease in immunocompromised individuals [1,5]. However they were first described as causative agents of mycetoma following penetrating injuries resulting in swelling bony destruction and sinus formation [3,6,7]. The two main species comprise the more common Scedosporium apiospermum (the asexual anamorph of Pseudallescheria boydii) and S. prolificans (previously considered as two species Scedosporium inflatum and Lomentospora prolificans) [1].
Scedosporium species are ubiquitous in natural vegetation and can be found in water, soil and potted plants. S. apiospermum is widely distributed in temperate regions, but S. prolificans seems to be restricted to the northern Spain and Australia [8] and infections are rare in the UK.
Diagnosis is made by recovery of the organism from culture of infected sites but identification of S. prolificans can be can be difficult and is based on specific morphologic characteristics such as swollen, flask-shaped conidiophores (Fig. 5).
Fig. 5.
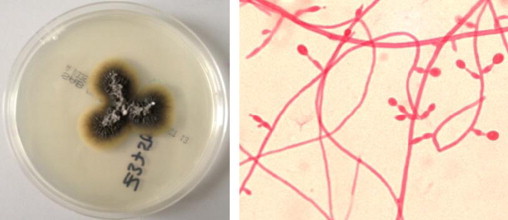
S. prolificans – growth on the SAB plate and typical microscopic appearance showing inflated conidiophores (Lactofuchsin stain, 40×).
The first known case of isolation of S. prolificans in the UK was reported in respiratory samples of AIDS patient in 1994 [9]. Other cases in have been in severely immunocompromised patients [10].
S. prolificans is more virulent than other species and is resistant to most antifungal agents and treatment options are limited. It is generally resistant to amphotericin B, flucytosine, azoles and triazoles, and echinocandins [11] although some in vitro activity to voriconazole can often be demonstrated [12] and synergy with terbinafine is reported [13–15]. Extensive debridement is essential to bring infections under control and amputation is often required.
Our case presented as a localised osteoarticular infection after a minor injury but diagnosis was delayed by the sub-acute presentation and difficulties in accurate identification of the causative fungus which led to starting the patient on itraconazole 100 mg bd. Increasing the itraconazole dose to 200 mg bd was a therapeutic option but based on the susceptibility information, a switch to voriconazole with terbinafine was considered preferable. Radical debridement and combination therapy with voriconazole and terbinafine was not instituted until more than 4 weeks after the initial injury. Therapeutic drug monitoring of voriconazole is essential and levels were maintained in the therapeutic range despite which he developed adverse event leading to premature discontinuation [16]. The limb has been salvaged and function of the foot is good with full range of movement.
Improvements in rapid identification of moulds are coming about through the introduction of MALDI-TOF mass spectrometry techniques [17]. However, accuracy of identification is dependent on representation of the mould in stringent comparative databases [18]. For rare moulds such as S. prolificans, these are in the early stages of development.
Although this case is extremely rare in the UK, increased global travel and the emergence of new fungal pathogens [19] means that increased awareness of unusual infections is required for early recognition and treatment by multidisciplinary approach [20].
Conflict of interest statement
There are none.
Acknowledgements
We acknowledge the work of Public Health England (PHE) Mycology Reference Laboratory in identifying the fungus.
References
- 1.Cortez K.J., Roilides E., Quiroz-Telles F., Meletiadis J., Antachopoulos C., Knudsen T. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21(1):157–197. doi: 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Vidal C., Cabellos C., Ayats J., Font F., Ferran E., Fernandez-Viladrich P. Fungal postoperative spondylodiscitis due to Scedosporium prolificans. Spine J. 2009;9(9):e1–e7. doi: 10.1016/j.spinee.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Kesson A.M., Bellemore M.C., O’Mara T.J., Ellis D.H., Sorrell T.C. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with a novel agent, hexadecylphospocholine (miltefosine), in combination with terbinafine and voriconazole: a case report. Clin Infect Dis. 2009;48(9):1257–1261. doi: 10.1086/597772. [DOI] [PubMed] [Google Scholar]
- 4.Studahl M., Backteman T., Stalhammar F., Chryssanthou E., Petrini B. Bone and joint infection after traumatic implantation of Scedosporium prolificans treated with voriconazole and surgery. Acta Paediatr. 2003;92(8):980–982. doi: 10.1080/08035250310004595. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach W.J., Perfect J.R. Scedosporium species infections and treatments. J Chemother. 2003;15:16–27. doi: 10.1179/joc.2003.15.Supplement-2.16. [DOI] [PubMed] [Google Scholar]
- 6.Dalton P.A., Munckhof W.J., Walters D.W. Scedosporium prolificans: an uncommon cause of septic arthritis. ANZ J Surg. 2006;76(7):661–663. doi: 10.1111/j.1445-2197.2006.03796.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinbach W.J., Schell W.A., Miller J.L., Perfect J.R. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J Clin Microbiol. 2003;41(8):3981–3985. doi: 10.1128/JCM.41.8.3981-3985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Tudela J.L., Berenguer J., Guarro J., Kantarcioglu A.S., Horre R., De Hoog G.S. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med Mycol. 2009;47(4):359–370. doi: 10.1080/13693780802524506. [DOI] [PubMed] [Google Scholar]
- 9.Hopwood V., Evans E.G.V., Matthews J., Denning D.W. Scedosporium prolificans, a multiresistant fungus, from a UK aids patient. J Infect. 1995;30(2):153–155. doi: 10.1016/s0163-4453(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 10.Gosbell I.B., Morris M.L., Gallo J.H., Weeks K.A., Neville S.A., Rogers A.H. Clinical, pathologic and epidemiologic features of infection with Scedosporium prolificans: four cases and review. Clin Microbiol Infect. 1999;5(11):672–686. [Google Scholar]
- 11.Cuenca-Estrella M., Ruiz-Díez B., Martínez-Suárez J.V., Monzón A., Rodríguez-Tudela J.L. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother. 1999;43(1):149–151. doi: 10.1093/jac/43.1.149. [DOI] [PubMed] [Google Scholar]
- 12.Troke P., Aguirrebengoa K., Arteaga C., Ellis D., Heath C.H., Lutsar I. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother. 2008;52(5):1743–1750. doi: 10.1128/AAC.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meletiadis J., Mouton J.W., Meis J., Verweij P.E. Combination chemotherapy for the treatment of invasive infections by Scedosporium prolificans. Clin Microbiol Infect. 2000;6(6):336–337. doi: 10.1046/j.1469-0691.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 14.Meletiadis J., Mouton J.W., Meis J., Verweij P.E. in vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedospotium prolificans isolates. Antimicrob Agents Chemother. 2003;47(1):106–117. doi: 10.1128/AAC.47.1.106-117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meletiadis J., Meis J., Mouton J.W., Rodriquez-Tudela J.L., Donnelly J.P., Verweij P.E. in vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob Agents Chemother. 2002;46(1):62–68. doi: 10.1128/AAC.46.1.62-68.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruth Ashbee H, Barnes Rosemary A., Johnson Elizabeth M., Richardson Malcolm D., Gorton Rebecca, Hope William W. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2013 doi: 10.1093/jac/dkt508. (dkt508v1–dkt508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posterard B., De Carolis E., Vella A., Sanguinetti M. MALDI-TOF mass spectrometry in the clinical mycology laboratory: identification of fungi and beyond. Expert Rev Proteomics. 2013;10(2):151–164. doi: 10.1586/epr.13.8. [DOI] [PubMed] [Google Scholar]
- 18.Lau A.F., Drake S.K., Calhoun L.B., Henderson C.M., Zelazny A.M. Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013;51(3):828–834. doi: 10.1128/JCM.02852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritter J.M., Muehlenbachs A., Blau D.M., Paddock C.D., Shieh W.-J., Drew C.P. Exserohilum infections associated with contaminated steroid injections: a clinicopathologic review of 40 cases. Am J Pathol. 2013;183(3):881–892. doi: 10.1016/j.ajpath.2013.05.007. (Epub 2013 June) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cetrulo C.L., Jr, Barone A.A.L., Jordan K., Chang D.S., Louie K., Buntic R.F. A multi-disciplinary approach to the management of fungal osteomyelitis: current concepts in post-traumatic lower extremity reconstruction: a case report. Microsurgery. 2012;32(2):144–147. doi: 10.1002/micr.20956. [DOI] [PubMed] [Google Scholar]


