Abstract
The authors present an unusual case of a 51-year-old man who developed relatively mild non-specific symptoms following intravesical BCG instillation for superficial transitional cell carcinoma of the bladder, with radiological investigations demonstrating typical features of miliary tuberculosis (TB). Transbronchial biopsy showed small foci of poorly formed granuloma suggestive of Mycobacterium infection. The patient's respiratory symptoms only became apparent 7 days after discharge having had 4 weeks of unremarkable inpatient stay where he remained clinically well. Prompt anti-TB treatment resulted in a remarkable improvement in his symptoms and radiological appearance, supporting the diagnosis of disseminated Mycobacterium bovis infection. This case highlights the importance of recognising miliary M bovis as a potential complication in patients receiving BCG immunotherapy, and that the disease course can be subclinical with delayed onset of symptoms.
Background
BCG is a well-established treatment for superficial transitional cell carcinoma (TCC) of the bladder.1 The efficacy of BCG immunotherapy has been extensively studied, which has demonstrated that its use after transurethral resection of bladder tumour (TURBT) is associated with increased length of disease progression-free survival.2–5 Furthermore, meta-analyses and prospective trials have shown that the use of intravesical BCG following TURBT is superior to TURBT alone or TURBT plus intravesical chemotherapy in delaying time to first recurrence.6–8
BCG immunotherapy is relatively well tolerated. Common side effects include dysuria, frequency, haematuria and flu-like symptoms, which are usually mild and self-limiting. Serious adverse events of intravesical BCG are rare. In a review of 2602 patients, less than 5% of the patients experienced major adverse events, which include granulomatous prostatitis, epididymo-orchitis, hepatitis, pneumonitis and BCG sepsis.9 These patients required a minimum of 3 months treatment with isoniazid and rifampicin depending on their severity.10 In disseminated BCG sepsis, the patient typically presents with high fever, haemodynamic instability and mental deterioration, which can be followed by multiorgan failure and disseminated intravascular coagulopathy. BCG sepsis carries high mortality and it has been reported that approximately one death occurs for every 12 500 patients receiving BCG as cancer treatment.9
The BCG infection following intravesical instillation of BCG can be classified into early and late onset disease. Early manifestation of the disease results from systemic infection by relatively low virulent Mycobacterium bovis in an immunocompetent host. Patients usually present within 3 months with fever and generalised symptoms, often accompanied by liver and lung involvement.11 In contrast, late onset disease is due to reactivation of infection after successful immunological control of early dissemination.12 In these circumstances, patients typically present after 12 months with localised disease involving genitourinary tract, the vascular tree, vertebral bones, retroperitoneal soft tissues and the chest wall.11
The disseminated infection manifesting as miliary pattern nodules in lung parenchyma is rarely reported in the literature.13–19 In these cases, patients presented with various systemic and respiratory symptoms, such as fever, shortness of breath, night sweats, cough, often associated with hypoxaemia and occasionally severe acute respiratory failure.19
We report an interesting case of a patient with miliary M bovis infection following BCG immunotherapy for bladder TCC who presented with atypical symptoms, remaining clinically well for a long duration before requiring antimicrobials, despite having demonstrated extensive bilateral miliary nodules in CT on his initial presentation.
Case presentation
A 51-year-old man, who was previously fit and well, was diagnosed with pTa G2 papillary TCC of the bladder 15 months prior to admission to our institution. After TURBT, the patient received four cycles of intravesical BCG without any problems. Few days after the fifth cycle, the patient developed a generalised weakness, vomiting and prominent anosmia. Working as a chef in a restaurant, anosmia caused significant disturbance in his work performance, and this led him to come to the hospital. On taking further history, he also admitted that he had lost about 5 kg of weight over 2 months. The patient was otherwise well, and denied any fever, night sweats, chest pain, shortness of breath and cough. Other than bladder cancer he had no significant medical history. He was normally fully independent with an active lifestyle.
On admission, his temperature was 36.7°C, blood pressure was 127 over 84 mm Hg with pulse rate of 88 bpm with regular rhythm. Oxygen saturation was 98% in room air with respiratory rate of 12 breaths/min. His cardiovascular, respiratory, abdominal examinations were unremarkable. Except for objective loss of smell, cranial and peripheral nervous system examination was normal.
Investigations
Results of the laboratory test revealed mildly raised C reactive protein 25.4 mg/L (<8 mg/L), total white cell count 8.1×109/L (4–11×109/L) and platelet count 302×109/L (150–440×109/L). Bone profile showed albumin level of 25 g/L (35–50 g/L) and adjusted calcium level of 2.18 mmol/L (2.05–2.60 mmol/L). Liver function test, coagulation screen, renal function and electrolytes were normal. His ECG showed normal sinus rhythm. However, chest radiograph showed bilaterally diffuse increased shadowing consistent with mild pulmonary oedema (figure 1).
Figure 1.
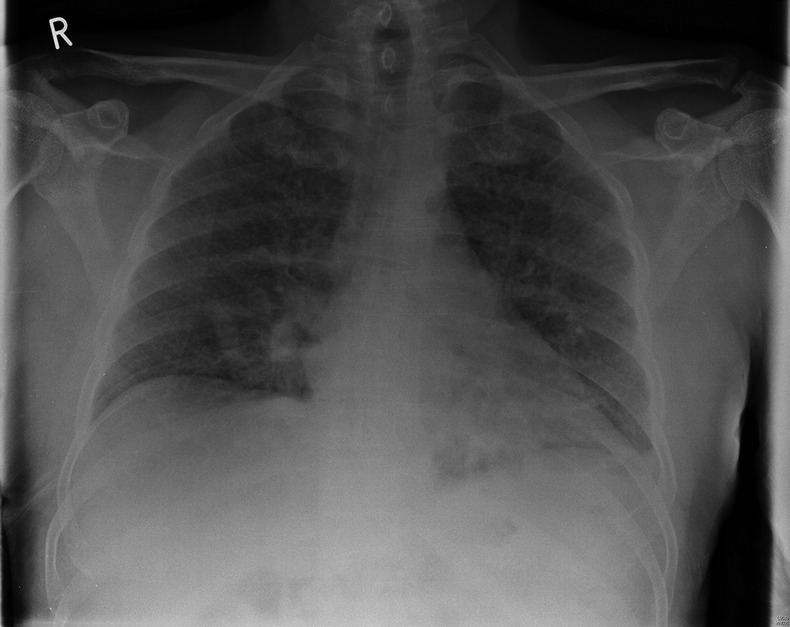
The patient's initial chest radiograph on admission to the hospital: showing bilaterally diffuse increased shadowing consistent with mild pulmonary oedema.
The patient was admitted to the general medical ward for investigation of vomiting, weight loss and abnormal chest radiograph. He underwent upper gastrointestinal endoscopy, colonoscopy and abdominal ultrasound scan, all of which showed no abnormalities. Duodenal and terminal ileum biopsy showed no abnormal cells and coeliac screen was negative. Following this, the patient had CT of the chest, abdomen and pelvis, which did not demonstrate any intra-abdominal or pelvic abnormalities. However, the chest CT showed multiple bilateral miliary nodules of random distribution, associated with bilateral hilar, subcarinal, paratracheal and prevascular lymphadenopathy (figures 2–4) with bilateral lower lobe atelectasis.
Figure 2.
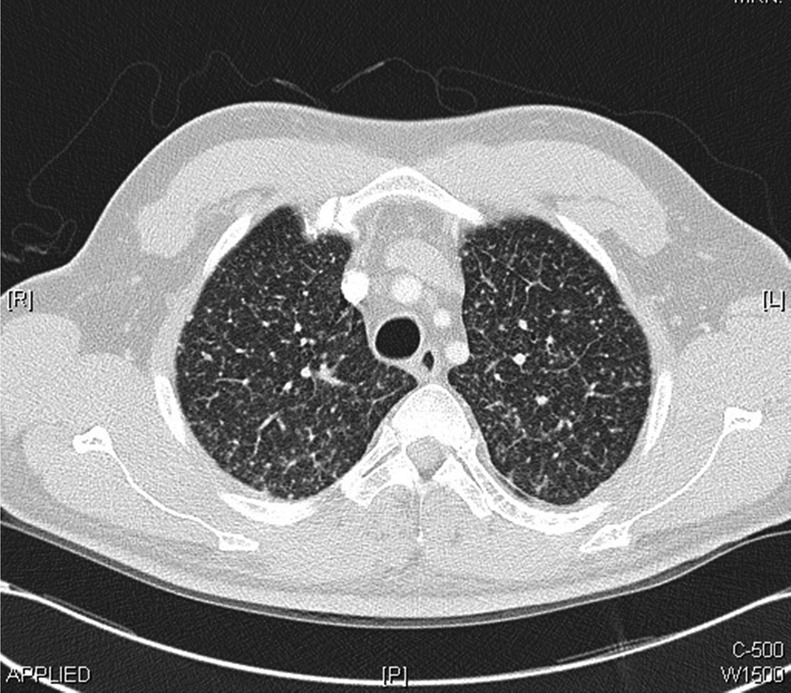
Chest CT scans performed within the first week of admission demonstrating bilateral miliary nodules of random distribution with bilateral hilar, subcarinal, paratracheal and prevascular lymphadenopathy.
Figure 3.
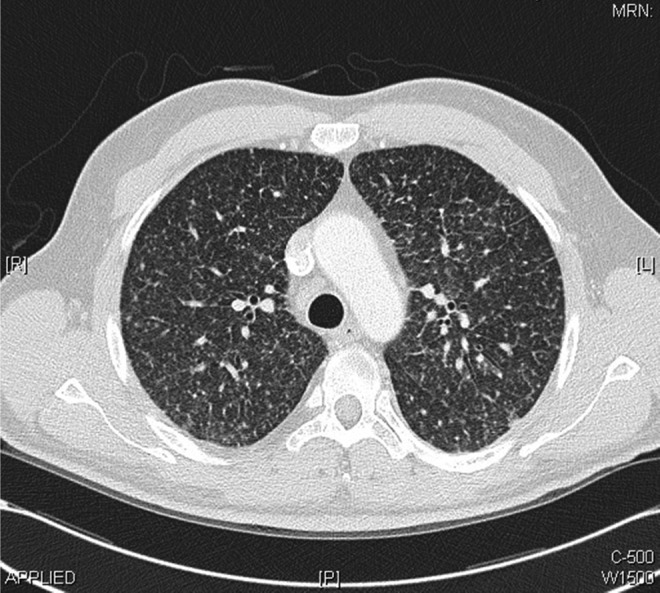
Chest CT scans performed within the first week of admission demonstrating bilateral miliary nodules of random distribution with bilateral hilar, subcarinal, paratracheal and prevascular lymphadenopathy.
Figure 4.
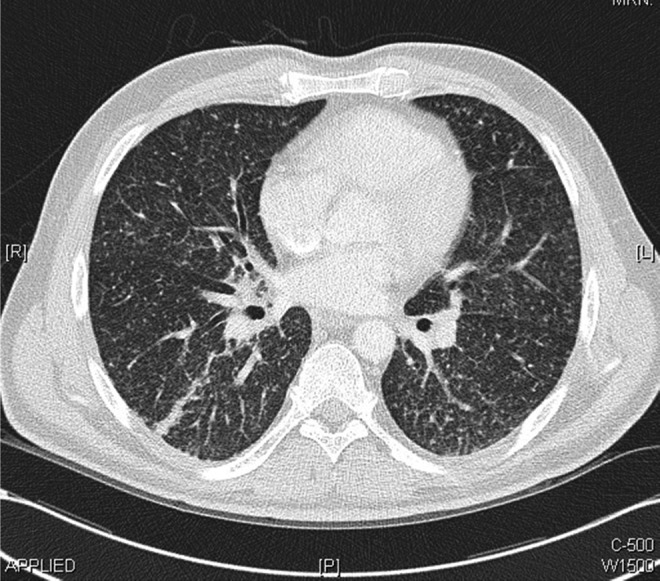
Chest CT scans performed within the first week of admission demonstrating bilateral miliary nodules of random distribution with bilateral hilar, subcarinal, paratracheal and prevascular lymphadenopathy.
Differential diagnosis
The main differential diagnoses considered were miliary tuberculosis (TB), lung metastasis and sarcoidosis. Further laboratory investigations included serum ACE and calcium, which were both within normal ranges. The blood, urine and sputum cultures were all negative. Immunological screenings for antinuclear antibodies, antineutrophil cytoplasmic antibodies, antiextractable nuclear antigens, antimitochondrial antibodies, antismooth muscle antibodies, liver/kidney microsomal antibodies and antigastric parietal cells were all negative.
The patient then underwent bronchoscopy. Direct microscopy showed no apparent acid-alcohol fast bacilli and cultures were negative at 8 weeks for transbronchial biopsy and bronchial washing. However, biopsy showed lung parenchyma with mild interstitial fibrosis and chronic inflammation, with an area of small foci that had features suggestive of poorly formed granulomata (figures 5 and 6) indicating likely Mycobacterium infection.
Figure 5.
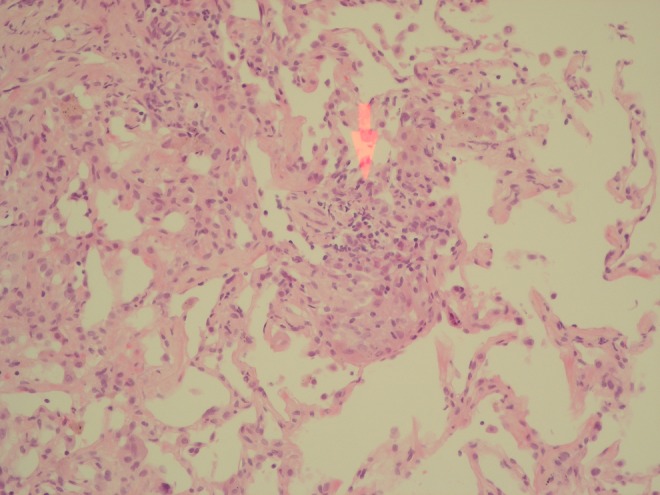
Histological images of transbronchial biopsy showing a lung parenchyma with mild interstitial fibrosis and chronic inflammation, with an area of small foci that has features suggestive of poorly formed granulomata (areas highlighted by red arrows).
Figure 6.
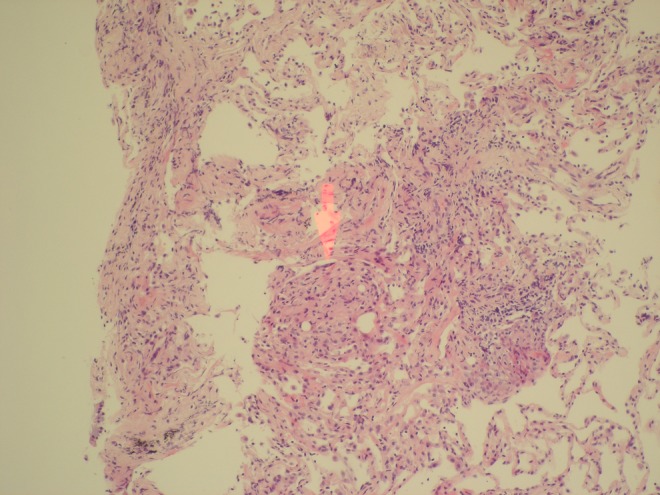
Histological images of transbronchial biopsy showing a lung parenchyma with mild interstitial fibrosis and chronic inflammation, with an area of small foci that has features suggestive of poorly formed granulomata (areas highlighted by red arrows).
Treatment
The patient remained well throughout his 4 weeks as an inpatient without requiring antimicrobials. He did not develop any fever, night sweats or respiratory symptoms. However, he still reported persistent anosmia, and for this reason he subsequently underwent MRI of the brain with gadolinium contrast, to look for any evidence of central nervous system involvement. This showed multiloculated T2-weighted hyperintense lesions within the skull base at the petrous apices bilaterally, but there were no other abnormal findings. Lumbar puncture was performed to rule out leptomeningeal disease, but cerebrospinal fluid culture did not demonstrate any organisms. White cell count was less than 1/mm3.
Since the patient did not develop any further symptoms, he was discharged with rifampicin, isoniazid and ethambutol as an empirical anti-TB regime with infectious disease follow-up.
Outcome and follow-up
Unfortunately, he was readmitted 3 days after discharge reporting overwhelming nausea and vomiting. It was felt that this was due to the side effects of the TB therapy. Therefore, it was stopped temporarily with an aim to reintroduce the regime in a week's time as he remained well otherwise.
The patient was reviewed in clinic in 7 days time. Unfortunately, he had developed dyspnoea and described that he was now breathless on minimal exertion, although he remained systemically well. Anti-TB medications were therefore reintroduced, and within a week he was free of dyspnoea and vomiting. He had a repeat chest CT, which showed considerable improvement in appearance with disappearance of many of the tiny intrapulmonary nodules and resolution of the thickened interlobular septa which was seen in the previous scan (figures 7–9).
Figures 7.
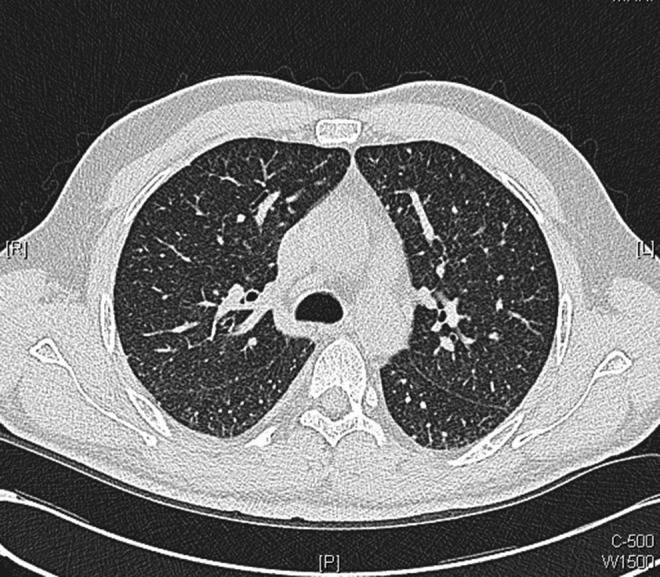
Axial images from repeat chest CT scans performed 10 days after initiation of antituberculosis medications: showing considerable improvement in appearance with disappearance of many of the tiny intrapulmonary nodules with resolution of thickened interlobular septa.
Figure 8.
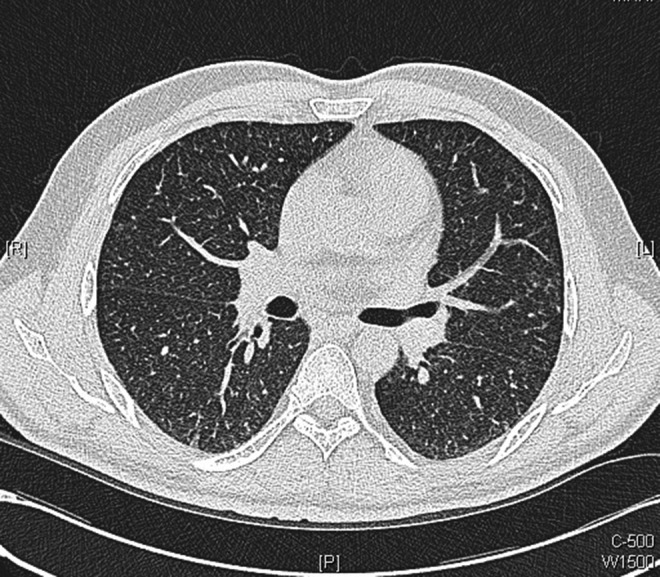
Axial images from repeat chest CT scans performed 10 days after initiation of antituberculosis medications: showing considerable improvement in appearance with disappearance of many of the tiny intrapulmonary nodules with resolution of thickened interlobular septa.
Figure 9.
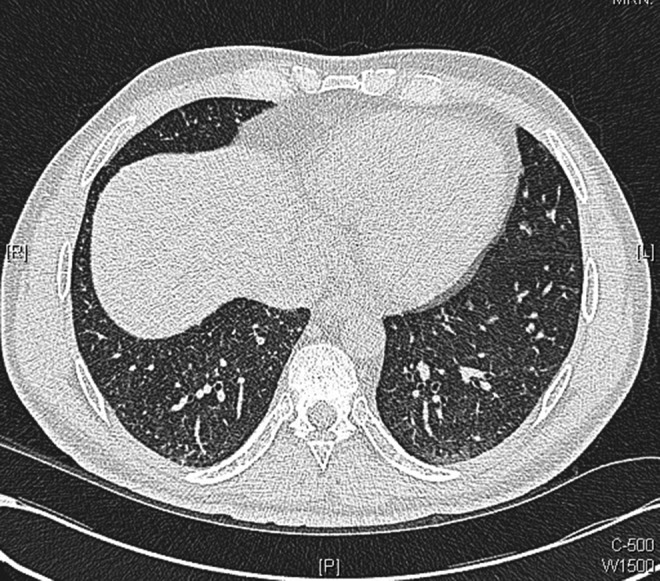
Axial images from repeat chest CT scans performed 10 days after initiation of antituberculosis medications: showing considerable improvement in appearance with disappearance of many of the tiny intrapulmonary nodules with resolution of thickened interlobular septa.
After 6 weeks, the patient was completely free of any symptoms, and described that his sensation of smell had returned. Ethambutol was stopped at this point and the regime was changed to isoniazid and rifampicin only. Finally, he was reviewed again in 2 months time and his repeat CT showed further improvement with resolution of many of the smaller intrapulmonary nodules (figures 10–13). He had regained almost all of his original weight and now he is back to his fully active lifestyle.
Figure 10.
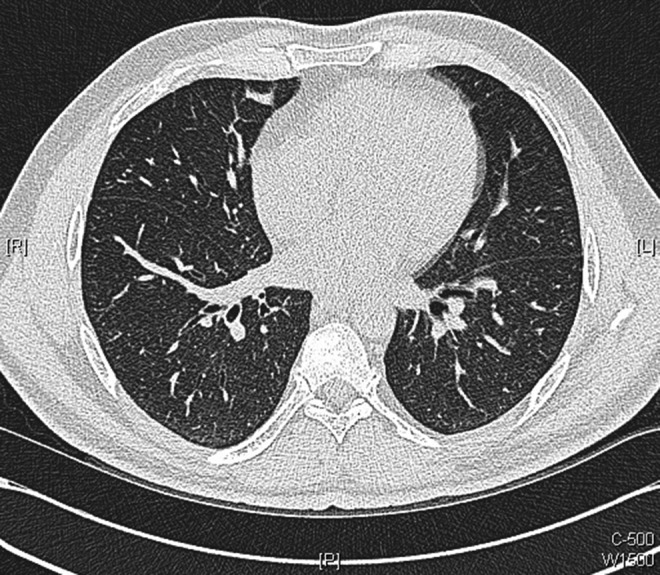
Axial images of repeat chest CT scans performed 10 weeks after initiation of antituberculosis medications: showing further improvement with resolution of many of the smaller intrapulmonary nodules.
Figure 11.
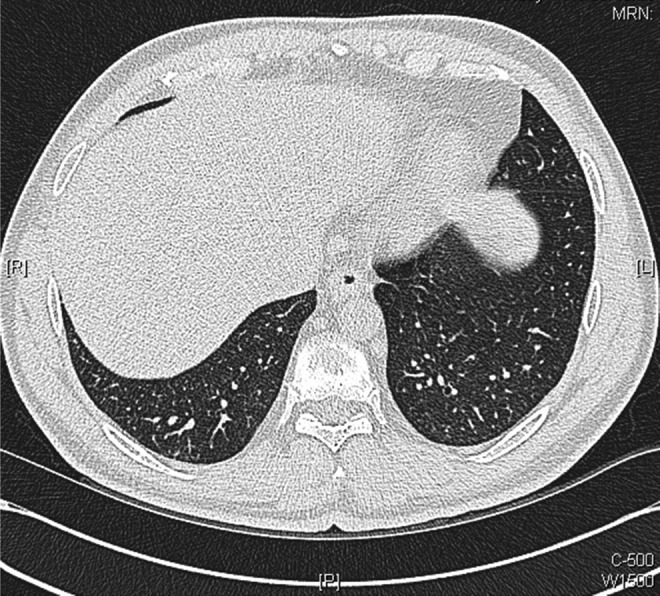
Axial images of repeat chest CT scans performed 10 weeks after initiation of antituberculosis medications: showing further improvement with resolution of many of the smaller intrapulmonary nodules.
Figure 12.
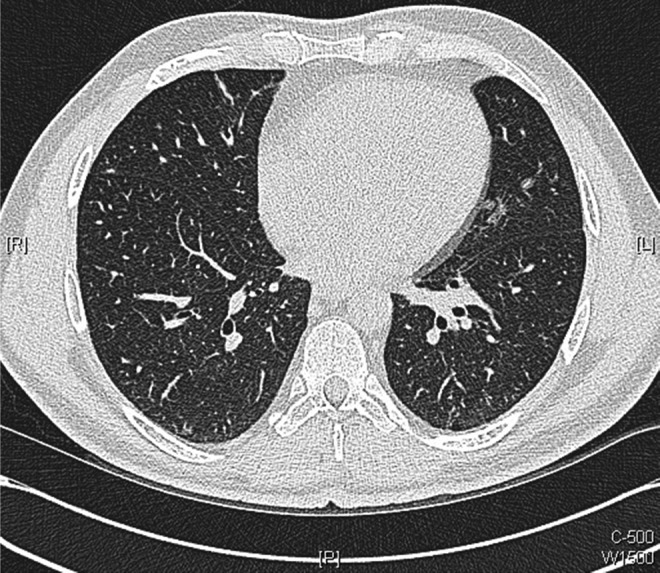
Axial images of repeat chest CT scans performed 10 weeks after initiation of antituberculosis medications: showing further improvement with resolution of many of the smaller intrapulmonary nodules.
Figure 13.
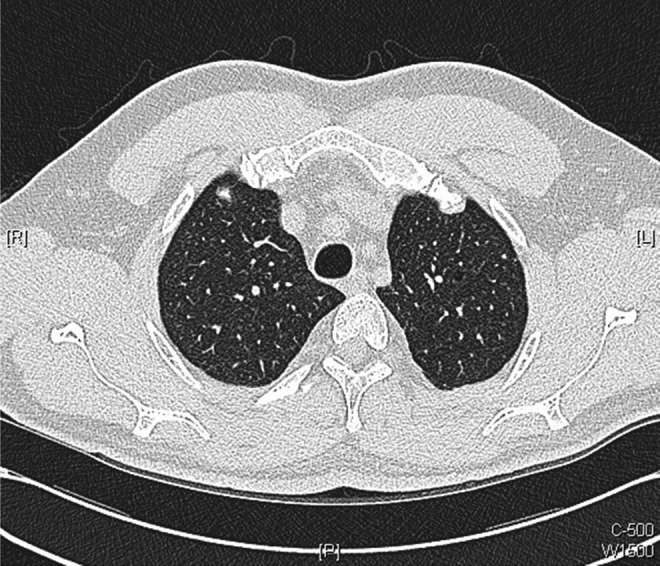
Axial images of repeat chest CT scans performed 10 weeks after initiation of antituberculosis medications: showing further improvement with resolution of many of the smaller intrapulmonary nodules.
Discussion
BCG is a live-attenuated vaccine that has the ability to stimulate immune response, promoting an antitumour environment.20 The efficacy of prophylactic intravesical BCG instillation following TURBT in decreasing recurrence, and its superiority compared with intravesical chemotherapy has been demonstrated in meta-analyses21 and prospective randomised controlled trials.5 7 8 Consequently, it is currently the first choice adjuvant therapy in the treatment of non-muscle invasive bladder cancer.20
The exact mechanism of its action for its efficacy in preventing recurrence of bladder cancer is not fully understood. However, several in vitro murine studies suggest that BCG binds to the urothelium via fibronectin attachment protein,22 23 which triggers innate immunity to produce proinflammatory cytokines. This leads to the recruitment of neutrophils, macrophages and dendritic cells, which release second group of cytokines and chemokines that activate CD4, CD8 and natural killer cells resulting in cell-mediated cytotoxicity, which destroys tumour cells.24–30
Intravesical BCG instillation is thought to be safe and well tolerated by the majority of patients; however, it has been reported to cause localised complications including dysuria, haematuria and increased urinary frequency. Rarely, it leads to disseminated systemic complications such as pneumonitis, hepatitis and sepsis.9 11 31 32 Important risk factors for systemic complications have been suggested, which include traumatic catheterisation and recurrent cystitis that results in increased systemic BCG absorption.9
Nadasy et al have previously reported four patients who had disseminated M bovis infection following intravesical BCG. All of these patients had constitutional symptoms such as cough, shortness of breath and fever, of which one underwent traumatic catheterisation. Only one patient out of the four had positive acid-fast bacilli with all four patients shown to have necrotising granulomas on transbronchial biopsy. Three of the patients were treated with a combination of anti-TB medications, isoniazid being used on all of the patients. Interestingly however, one of the patients recovered from the complications without any form of treatment.16
Other authors reported patients with similar presenting features including fever, dyspnoea, weight loss, night sweats or productive cough with radiological evidence of diffuse miliary pattern. Transbronchial biopsy results were somewhat varied, such as acid-fast rod bacterium,33 granulomas with central necrosis13 and non-caseating granulomas.14 15 34 In all cases however, patients were successfully treated with anti-TB regime, supporting the diagnosis of infectious aetiology. M bovis is usually susceptible to most of the anti-TB drugs such as isoniazid, rifampicin and ethambutol. However, the treatment regime for these patients should not include pyrazinamide or cycloserine as M bovis is highly resistant to these drugs.35
Interestingly, some authors suggested that miliary pattern pulmonary manifestations are possibly secondary to hypersensitivity reaction rather than disseminated BCG infection. This is supported by observations that some patients achieved clinical remission with corticosteroids only, without any anti-TB treatments.36 37 The pathogenesis for pulmonary manifestation following intravesical BCG is still a subject of debate until now.
In our case, it can be argued that the patient's symptoms were due to hypersensitivity reaction rather than disseminated BCG. Negative culture results from transbronchial biopsy and bronchial washing as well as absence of acid-fast bacilli on direct microscopy certainly favour hypersensitivity rather than infective aetiology. However, the presence of poorly formed granuloma in transbronchial biopsy, supported by the fact that the course of anti-TB treatment had led to remarkable clinical and radiological improvement supports disseminated BCG infection as more likely diagnosis in this case. It should be noted that inability to identify M bovis in acid-fast stain and culture is not entirely unexpected, as this is affected by many factors, such as number of organisms present, the tissue handling and culture techniques.17 Furthermore, infection with low virulence organisms such as M bovis in an immunocompetent host may result in well-formed granulomas in which organisms cannot be easily detected.11
The patient presented in this report had several atypical features. First, his symptoms were non-specific with the main symptom being anosmia. Second, he remained clinically well throughout the initial 4 weeks of inpatient stay without any treatment. The anti-TB treatment was only started on discharge and he subsequently developed respiratory symptoms on discontinuing the medications due to side effects. The long period of a relatively asymptomatic phase may indicate that this condition can have a long duration of subclinical course before more serious symptoms develop. There is no clear explanation as to what could have caused anosmia, although response to anti-TB medications may suggest that it was part of the post BCG instillation and disseminated mycobacterial process.
In conclusion, we report an infrequent complication of intravesical BCG instillation presenting as disseminated miliary Mycobacterium infection. Although findings from case reports should be interpreted with caution, this case highlights the importance of recognising disseminated mycobacterial infection as a potential complication in any patient receiving intravesical BCG instillation; non-specific symptoms should not be overlooked as these may have a relatively long subclinical phase.
Learning points.
Disseminated BCG infection should be recognised as a potential complication in patients receiving intravesical BCG for bladder tumour, which can lead to localised and systemic symptoms that can mimic active tuberculosis (TB) infection.
Disseminated BCG infection can present subclinically with non-specific symptoms and may have a long duration of relatively asymptomatic phase.
Recognised risk factors for systemic absorption of BCG include traumatic catheterisation and recurrent cystitis.
There is contradicting evidence as to whether systematic manifestations of complication of BCG instillation are secondary to haematogenous spread of Mycobacterium or hypersensitivity reaction.
Treatment options include rifampicin, isoniazid and corticosteroids (if severe and not responding to anti-TB regime). Mycobacterium bovis is highly resistant to pyrazinamide; therefore, it should not be included in the treatment regime.
Acknowledgments
The authors would like to acknowledge Dr James Evans, Dr Faisal Majid and Dr Jennifer Turner who were involved in looking after this patient and guided them through the process of preparing this manuscript.
Footnotes
Contributors: C-HRC was a house officer looking after the patient presented. He collected necessary clinical data and prepared the manuscript. SOL was a medical student at the Imperial College when the patient was admitted, and helped C-HRC with data collection and manuscript preparation. GS was the consultant physician looking after this patient during this patient’s admission and guided the other two authors in writing this manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 1976;116:180–3 [DOI] [PubMed] [Google Scholar]
- 2.Chade DC, Shariat SF, Dalbagni G. Intravesical therapy for urothelial carcinoma of the urinary bladder: a critical review. Int Braz J Urol 0000;35:640–50. discussion 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamm DL. Efficacy and safety of bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis 2000;31(Suppl 3):S86–90 [DOI] [PubMed] [Google Scholar]
- 4.Lamm DL. Comparison of BCG with other intravesical agents. Urology 1991;37(5 Suppl):30–2 [DOI] [PubMed] [Google Scholar]
- 5.Krege S, Giani G, Meyer R, et al. A randomized multicenter trial of adjuvant therapy in superficial bladder cancer: transurethral resection only versus transurethral resection plus mitomycin C versus transurethral resection plus bacillus Calmette-Guerin. Participating clinics . J Urol 1996;156:962–6 [DOI] [PubMed] [Google Scholar]
- 6.Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int 2001;88:209–16 [DOI] [PubMed] [Google Scholar]
- 7.Pawinski A, Sylvester R, Kurth KH, et al. A combined analysis of European Organization for Research and Treatment of Cancer, and Medical Research Council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council Working Party on Superficial Bladder Cancer. J Urol 1996;156:1934–40 discussion 1940–1. [PubMed] [Google Scholar]
- 8.Witjes JA, v d Meijden AP, Collette L, et al. Long-term follow-up of an EORTC randomized prospective trial comparing intravesical bacille Calmette-Guérin-RIVM and mitomycin C in superficial bladder cancer. EORTC GU Group and the Dutch South East Cooperative Urological Group. European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Collaborative Group. Urology 1998;52:403–10 [DOI] [PubMed] [Google Scholar]
- 9.Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol 1992;147:596–600 [DOI] [PubMed] [Google Scholar]
- 10.Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am 1992;19:565–72 [PubMed] [Google Scholar]
- 11.Gonzalez OY, Musher DM, Brar I, et al. Spectrum of bacille Calmette-Guérin (BCG) infection after intravesical BCG immunotherapy. Clin Infect Diss 2003;36:140–8 [DOI] [PubMed] [Google Scholar]
- 12.Izes JK, Bihrle W, Thomas CB. Corticosteroid-associated fatal mycobacterial sepsis occurring 3 years after instillation of intravesical bacillus Calmette-Guerin. J Urol 1993;150(5 Pt 1):1498–500 [DOI] [PubMed] [Google Scholar]
- 13.Skoutelis A, Melekos MD, Bassaris HJ. Miliary tuberculosis secondary to transurethral bacillus Calmette-Guerin administration for superficial transitional cell carcinoma of the bladder. J Natl Cancer Inst 1990;82:1219–20 [DOI] [PubMed] [Google Scholar]
- 14.Rabe J, Neff KW, Lehmann KJ, et al. Miliary tuberculosis after intravesical bacille Calmette-Guérin immunotherapy for carcinoma of the bladder. AJR Am J Roentgenol 1999;172:748–50 [DOI] [PubMed] [Google Scholar]
- 15.Palayew M, Briedis D, Libman M, et al. Disseminated infection after intravesical BCG immunotherapy. Detection of organisms in pulmonary tissue. Chest 1993;104:307–9 [DOI] [PubMed] [Google Scholar]
- 16.Nadasy KA, Patel RS, Emmett M, et al. Four cases of disseminated Mycobacterium bovis infection following intravesical BCG instillation for treatment of bladder carcinoma. South Med J 2008;101:91–5 [DOI] [PubMed] [Google Scholar]
- 17.McParland C, Cotton DJ, Gowda KS, et al. Miliary Mycobacterium bovis induced by intravesical bacille Calmette-Guérin immunotherapy. Am Rev Respir Dis 1992;146(5 Pt 1):1330–3 [DOI] [PubMed] [Google Scholar]
- 18.Iantorno R, Nicolai M, Storto ML, et al. Miliary tuberculosis of the lung in a patient treated with bacillus Calmette-Guerin for superficial bladder cancer. J Urol 1998;159:1639–40 [DOI] [PubMed] [Google Scholar]
- 19.Del Castillo Duran Y, Santos Bodí F, Castander Serentill D, et al. [Tuberculosis miliar in a patient treated with intravesical instillations of bacillus Calmette-Guérin]. Med Intensiva 2006;30:116–19 [DOI] [PubMed] [Google Scholar]
- 20.Lokeshwar VB, Merseburger AS, Hautmann SH. Bladder tumors: molecular aspects and clinical management (Google eBook). Springer; 2011:466 [Google Scholar]
- 21.Shelley MD, Wilt TJ, Court J, et al. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int 2004;93:485–90 [DOI] [PubMed] [Google Scholar]
- 22.Kavoussi LR, Brown EJ, Ritchey JK, et al. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest 1990;85:62–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao W, Schorey JS, Bong-Mastek M, et al. Role of a bacillus Calmette-Guérin fibronectin attachment protein in BCG-induced antitumor activity. Int J Cancer 2000;86:83–8 [DOI] [PubMed] [Google Scholar]
- 24.Prescott S, James K, Hargreave TB, et al. Radio-immunoassay detection of interferon-gamma in urine after intravesical Evans BCG therapy. J Urol 1990;144:1248–51 [DOI] [PubMed] [Google Scholar]
- 25.Böhle A, Nowc C, Ulmer AJ, et al. Elevations of cytokines interleukin-1, interleukin-2 and tumor necrosis factor in the urine of patients after intravesical bacillus Calmette-Guerin immunotherapy. J Urol 1990;144:59–64 [DOI] [PubMed] [Google Scholar]
- 26.De Boer EC, De Jong WH, Steerenberg PA, et al. Induction of urinary interleukin-1 (IL-1), IL-2, IL-6, and tumour necrosis factor during intravesical immunotherapy with bacillus Calmette-Guérin in superficial bladder cancer. Cancer Immunol Immunother 1992;34:306–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson AM, Alexandroff AB, McIntyre M, et al. Induction of ICAM 1 expression on bladder tumours by BCG immunotherapy. J Clin Pathol 1994;47:309–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson AM, Alexandrov AB, Prescott S, et al. Role of adhesion molecules in lymphokine-activated killer cell killing of bladder cancer cells: further evidence for a third ligand for leucocyte function-associated antigen-1. Immunology 1992;76:286–91 [PMC free article] [PubMed] [Google Scholar]
- 29.Lattime EC, Gomella LG, McCue PA. Murine bladder carcinoma cells present antigen to BCG-specific CD4+ T-cells. Cancer Res 1992;52:4286–90 [PubMed] [Google Scholar]
- 30.Prescott S, Jackson AM, Hawkyard SJ, et al. Mechanisms of action of intravesical bacille Calmette-Guérin: local immune mechanisms. Clin Infect Dis 2000;31 (Suppl 3):S91–3 [DOI] [PubMed] [Google Scholar]
- 31.Koga H, Kuroda M, Kudo S, et al. Adverse drug reactions of intravesical bacillus Calmette-Guerin instillation and risk factors of the development of adverse drug reactions in superficial cancer and carcinoma in situ of the bladder. Int J Urol 2005;12:145–51 [DOI] [PubMed] [Google Scholar]
- 32.Elkabani M, Greene JN, Vincent AL, et al. Disseminated Mycobacterium bovis after intravesicular bacillus calmette-Gu rin treatments for bladder cancer. Cancer Control 2000;7:476–81 [DOI] [PubMed] [Google Scholar]
- 33.Frickmann H, Jungblut S, Hanke P, et al. [Tuberculosis induced by bacillus Calmette-Guerin immuno-prophylaxis — case study]. Pneumologie 2004;58:773–6 [Article in German]. [DOI] [PubMed] [Google Scholar]
- 34.Gupta RC, Lavengood R, Smith JP. Miliary tuberculosis due to intravesical bacillus Calmette-Guerin therapy. Chest 1988;94:1296–8 [DOI] [PubMed] [Google Scholar]
- 35.Durek C, Rüsch-Gerdes S, Jocham D, et al. Sensitivity of BCG to modern antibiotics. Eur Urol 2000;37 (Suppl 1):21–5 [DOI] [PubMed] [Google Scholar]
- 36.Molina JM, Rabian C, D'Agay MF, et al. Hypersensitivity systemic reaction following intravesical bacillus Calmette-Guerin: successful treatment with steroids. J Urol 1992;147:695–7 [DOI] [PubMed] [Google Scholar]
- 37.Reinert KU, Sybrecht GW. T helper cell alveolitis after bacillus Calmette-Guerin immunotherapy for superficial bladder tumor. J Urol 1994;151:1634–7 [DOI] [PubMed] [Google Scholar]


