Abstract
A 62-year-old man presented with a right-sided hemichorea-hemiballismus secondary to underlying non-ketotic hyperglycaemia. This condition is recognised to have a unique finding of unilateral basal ganglia lesion, which is hyperdense on CT and hyperintense on T1-weighted MRI. The clinical course of this condition is benign and has a good prognosis with early correction of the hyperglycaemia.
Background
Non-ketotic hyperglycaemia (NKH) in poorly controlled patients with diabetes presenting with hemichorea-hemiballismus is rare.1–5 It is often described to have a typical triad; unilateral involuntary movements, contralateral striatal abnormality on imaging and resolution of symptoms after correction of hyperglycaemia.3 6 Imaging plays an important role to separate this entity from other causes of movement disorders for rapid and correct initiation of treatment. We describe in this report, emphasising on the CT and MRI brain findings, the characteristic abnormalities in the basal ganglia while reviewing the relevant literatures.
Case presentation
A 62-year-old man with long-standing diabetes mellitus, hypertension and hypercholesterolaemia on medications was presented with a week history of progressive involuntary movement of the right-upper and lower limbs. His conscious level and speech was normal. There was no history of trauma, previous cerebrovascular accidents, thyroid disease or family history of movement disorders. He did not take any neuroleptic medications.
On examination, he was well oriented, normotensive and afebrile. At rest, he demonstrated quasipurposive writhing movements of the right-upper and lower limbs. The power and deep tendon reflexes of upper and lower limbs were normal. The examination of cranial nerves and cerebellar signs were unremarkable.
Investigations
The laboratory studies demonstrated high serum glucose (26 mmol/L) and glycated haemoglobin of 14%. There was no evidence of ketosis or other metabolic abnormalities.
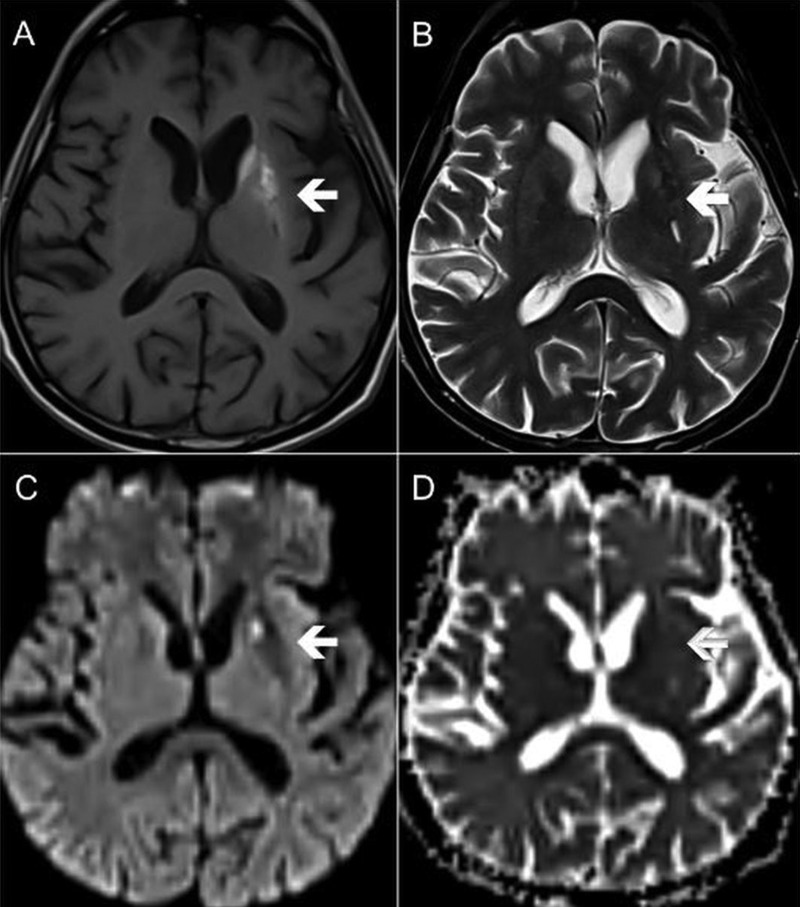
Cross-sectional imaging of the brain was performed to rule out basal ganglia pathology. A CT scan demonstrated unilateral faint hyperdensity of the left striatum (figure 1). MRI confirmed the left striatal abnormality, which was hyperintense in T1-weighted (T1W) images, hypointense in T2-weighted (T2W) images and non-diffusion restricted in diffusion-weighted images (figure 2). The ipsilateral internal capsule was preserved. There was no mass effect or perilesional oedema seen.
Figure 1.
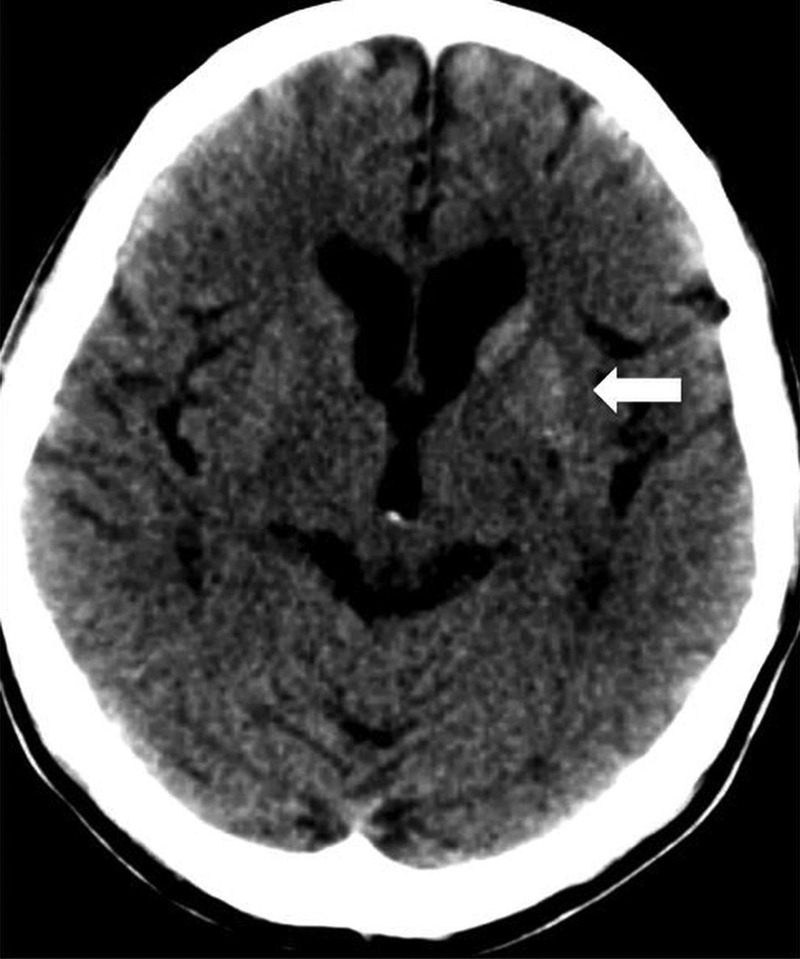
Axial image of the brain CT showing hyperdense left basal ganglia confined to the region of the left striatum (white arrow). Note that the internal capsule is spared.
Figure 2.
Axial MRI at the level of the basal ganglia. The affected left striatum (white arrows) showing high signal in (A) T1-weighted sequence, low signal in (B) T2-weighted sequence and absence of diffusion restriction in (C and D) diffusion-weighted sequence.
Differential diagnosis
The differential diagnosis of T1W hyperintense basal ganglia lesions includes deposition of paramagnetic substances namely iron, copper (Wilson's disease), melanin (malignant melanoma), manganese (chronic liver disease) and intracellular methaemoglobin in subacute haematoma.1 2 Other causes include chronic hepatic encephalopathy, postcardiac-arrest encephalopathy, hypoglycaemic coma and mild local ischaemia.4 In most of the metabolic causes however, bilateral involvement is expected. NKH on the other hand is known to be a unique entity presenting unilateral T1W hyperintensity. Differentiation from haemorrhage and tumour, which also present with unilateral abnormality, can be made as the hyperintense area on T1W in NKH conforms to the shape of basal ganglia sparing the internal capsule, with no perilesional oedema or mass effect.
Treatment
The glycaemic control was achieved with insulin injection, with no episodes of hypoglycaemia. The dosage of oral metformin was increased. Haloperidol 5 mg twice daily and sodium valproate 200 mg twice daily was also administered. There was marked improvement and finally resolution of the hemichorea.
Outcome and follow-up
There was no recurrence of hemichorea during the patient's stay in the hospital, and he was well when discharged.
Discussion
The main components of basal ganglia are striatum, globus pallidus, substantia nigra and subthalamic nucleus. The striatum is the largest component and is composed of caudate nucleus and putamen. These deep brain nuclei are involved in a variety of crucial brain functions, including voluntary motor control, action gating, movement timing and procedural learning relating to routine behaviours, among others. Hemichorea-hemiballismus refers to an involuntary, non-rhythmic movement disorder affecting one side of the body, most commonly caused by a vascular insult to the contralateral basal ganglia. Other causes include neoplasm, cerebral infection and systemic conditions such as thyrotoxicosis and systemic lupus erythematosus.3 5 7 NKH is known to be a rare cause of this movement disorder particularly in elderly Asian patients with diabetes,4 7–9 with the incidence reported to be higher in women compared to men.4 10
The acute presentation of a patient with diabetes due to abnormal blood glucose levels can manifest in many ways. Patients with hyperglycaemia can present with seizures, muscle weaknesses and hypotonia, pyramidal tract signs and hemichorea-hemiballismus. These clinical findings often mimic that of cerebrovascular accidents, and not uncommonly the patients are initially diagnosed with stroke. Thus in NKH, despite highly suggestive clinical findings, cross-sectional brain imaging, namely CT and MRI, is crucial to exclude other important causes for rapid and correct initiation of treatment. Delayed treatment in these conditions may cause potential significant morbidity and mortality.
The unique cross-sectional neuroimaging findings described in patients with NKH presenting with hemichorea-hemiballismus is unilateral basal ganglia hyperdensity on CT, contralateral to the symptomatic side. This characteristic basal ganglia lesion is hyperintense in T1W with no mass effect or perilesional oedema in the majority of reported cases in the literature.1–11 On T2W MRI, the signal changes are reported to be variable, with the lesions appearing either hypointense, isointense or hyperintense,1 7 with hypointensity predominating in many reports.8 10 11 The features of restricted diffusion7 8 10 and signal loss on gradient echo have also been reported.7 Oh et al4 in their meta-analysis found that putamen was involved in all cases, with no isolated lesion confined to the caudate nucleus and globus pallidus. The anterior limb of the internal capsule was spared in almost all of the cases. Ninety-eight per cent of the patients with hemichorea were found to have contralateral basal ganglia lesions on MRI, while 2% had ipsilateral abnormality. Expectedly, patients with bilateral chorea were found to have bilateral lesions and may be seen in a small proportion of cases. On follow-up MRI of these patients, most showed resolution of T1W basal ganglia hyperintensity along with improvement of chorea.10 11 The resolution of the striatal hyperintensity in the neuroimaging examinations is generally slower than the clinical course, with resolution of CT findings found to be earlier than those of MRI.11 Lin et al11 postulated that this is due to higher sensitivity of MRI in detection of the brain parenchymal changes compared to CT. Roy et al9 reported a low NAA/Cr on MR spectroscopy of their patients with NKH, suggesting neuronal dysfunction and pronounced energy depletion. This damage is due to either hypoperfusion or metabolic failure. Other studies on single, photon emission CT and PET reported on reduction of blood flow and metabolism in the contralateral striatum in NKH-related hemichorea-hemiballismus.4 9 12 However, hyperperfusion has also been described, particularly at the earlier stage of the disease.4 The conventional neuroimaging findings of the present case were compatible with the majority of the previous reported cases of NKH. The involvement was unilateral and the affected basal ganglia were hyperdense in CT and hyperintense in MR T1W imaging with no evidence of diffusion restriction.
The differentiation of vascular and NKH causes of hemichorea-hemiballismus is crucial owing to difference in treatment plan and prognosis. Patients with hemichorea-hemiballismus due to focal vascular lesions show a more gradual improvement or no improvement of symptoms with pharmacological therapy, while the patients with NKH as the underlying pathology usually show rapid improvement with correction of the hyperglycaemia.11
The pathophysiology leading to the peculiar changes of the basal ganglia being hyperdense on CT and hyperintense on T1W MRI in NKH is still debated with heterogeneity of the available data. Some authors proposed that the lesion might be due to vascular insult leading to ischaemic calcium deposition, as the clinical presentation is acute and the lesion corresponds to the territory of lenticulostriate artery.4 9 However, as follow-up imaging show resolution of the basal ganglia lesions with time, others proposed that petechial haemorrhage is a more likely cause of the abnormality.2 4 Mestre et al13 described postmortem neuropathological findings of the abnormal putamen which revealed astrocytic gliosis and extravascular haemosiderin deposits together with ferruginateous deposits on perforating vessels corresponded to striatum microhaemorrhage as the underlying pathological process. It was suggested that the microhaemorrhage was secondary to erythrocyte diapedesis due to hyperglycaemia-induced blood–brain barrier dysfunction.13 Other postulations include metabolic derangement which stimulated the reaction of astrocytes leading to an abundance of gemistocytes causing T1W shortening in the affected area,2 14 myelin damage or hyperviscosity 9 12 as the cause of abnormal signal. Shan et al,15 for instance, reported that a stereotactic biopsy of the affected putamen revealed a fragment of gliotic brain tissue with abundant gemistocytes with no apparent haemorrhage or infarction. The hemichorea-hemiballismus due to NKH is found to be more common in Asian descendants, suggesting an element of genetic predisposition of this condition.4 10
In summary, hemichorea-hemiballismus is a rare cause of NKH. It is recognised to have a unique finding of unilateral basal ganglia lesion, which is hyperdense on CT and hyperintense on T1W MRI, and our patient presented with these classic imaging features. The clinical course of this condition is benign with good prognosis with rapid institution of appropriate treatment.
Learning points.
Unilateral hemichorea-hemiballismus as a rare cause of non-ketotic hyperglycaemia (NKH) has a characteristic imaging finding of contralateral basal ganglia hyperdensity on CT and hyperintensity on T1-weighted MRI.
Cross-sectional brain imaging plays an important role in rapid and correct diagnosis of this condition, as its clinical course is benign with good prognosis, if the hyperglycaemia is duly corrected.
Changes conforming to the shape of the basal ganglia, sparing the internal capsule with no perilesional oedema or mass effect help to differentiate it from the hypertensive haemorrhage or tumour, which may also present as a unilateral abnormality of the basal ganglia.
Footnotes
Contributors: All the authors were involved in drafting, writing and revising of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hansford BG, Albert D, Yang E. Classic neuroimaging findings of nonketotic hyperglycemia on computed tomography and magnetic resonance imaging with absence of typical movement disorder symptoms (hemichorea-hemiballism). Radiol Case 2013;7:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Park SH, Hamm IS. Hemichorea-hemiballism with non-ketotic hyperglycemia in a chronic renal failure patient. J Korean Neurosurg Soc 2004;36:328–30 [Google Scholar]
- 3.Qi X, Yan Y-y, Gao Y, et al. Hemichorea associated with non-ketotic hyperglycaemia: a case report. Diabetes Res Clin Pract 2012;95:e1–3 [DOI] [PubMed] [Google Scholar]
- 4.Oh S-H, Lee K-Y, Im J-H, et al. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci 2002;200:57–62 [DOI] [PubMed] [Google Scholar]
- 5.Cheema H, Federman D, Kam A. Hemichorea–hemiballismus in non-ketotic hyperglycaemia. J Clin Neurosci 2011;18:293–4 [DOI] [PubMed] [Google Scholar]
- 6.El Otmani H, Moutaouakil F, Fadel H, et al. Chorea-ballismus in acute non-ketotic hyperglycaemia. Funct Neurol 2009;24:129–32 [PubMed] [Google Scholar]
- 7.Bathla G, Policeni B, Agarwal A. Neuroimaging in patients with abnormal blood glucose levels. AJNR Am J Neuroradiol Published Online First: 2 May 2013. doi:10.3174/ajnr.A3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wintermark M, Fischbein NJ, Mukherjee P, et al. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. Am J Neuroradiol 2004;25:975–6 [PMC free article] [PubMed] [Google Scholar]
- 9.Roy A, Raghunandan N, Sarma G, et al. A case series of hemichorea due to non ketotic-hyperglycemia with unique MRI brain finding. J Neurol Neurophysiol 2013;S11:006 [Google Scholar]
- 10.Yacoub HA. Abnormal magnetic tesonance imaging and hemichorea sssociated with non-ketotic hyperglycemia. J Neurol Res 2013;3:146–9 [Google Scholar]
- 11.Lin JJ, Lin GY, Shih C, et al. Presentation of striatal hyperintensity on T1-weighted MRI in patients with hemiballism-hemichorea caused by non-ketotic hyperglycemia: report of seven new cases and a review of literature. J Neurol 2001;248:750–5 [DOI] [PubMed] [Google Scholar]
- 12.Belcastro V, Pierguidi L, Tambasco N, et al. Decreased contralateral putamen [I]FP-CIT SPECT uptake in hyperglycemic hemichorea-hemiballismus. Eur Neurol 2011;65:307–8 [DOI] [PubMed] [Google Scholar]
- 13.Mestre TA, Ferreira JJ, Pimentel J. Putaminal petechial haemorrhage as the cause of non-ketotic hyperglycaemic chorea: a neuropathological case correlated with MRI findings. J Neurol Neurosurg Psychiatry 2007;78:549–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y, Wang G, Chen X, et al. Hemichorea in nonketotic hyperglycemia: putamenal and cerebellum lesion on MR imaging. World J Neurosci 2012;2: 138–40 [Google Scholar]
- 15.Shan DE, Ho DM, Chang C, et al. Hemichorea-hemiballism: an explanation for MR signal changes. Am J Neuroradiol 1998;19:863–70 [PMC free article] [PubMed] [Google Scholar]