Abstract
Background:
Oxidative stress has repeatedly been implicated as the leading cause of several disease conditions.
Aim:
This study was designed to investigate the effects of nicotine administration on serum antioxidant levels in male albino rats.
Materials and Methods:
Forty male rats (150-180 g) were divided into five groups and treated orally for 30 days. Group I (control) received 0.2 ml/kg normal saline and Groups II and III received 0.5 and 1.0 mg/kg body weight (BW) of nicotine, respectively for 30 days. The fourth and fifth groups were administered with 0.5 and 1.0 mg/kg BW of nicotine for 30 days, but were left untreated for another 30 days. Serum was assayed for nitric oxide (NO), lipid peroxidation, and antioxidant enzyme.
Results:
The levels of superoxide dismutase (SOD) and catalase (CAT) were significantly decreased (P < 0.05) in 0.5 and 1.0 mg/kg nicotine treated groups. Glutathione peroxidase (GPx) and glutathione reductase (GR) were significantly decreased (P < 0.05), while NO and malondialdehyde (MDA) were significantly increased (P < 0.05) in 1.0 mg/kg treated group when compared with the control.
Conclusion:
The present study shows that nicotine administration is associated with decreased serum antioxidant and increase lipid peroxidation ameliorated by nicotine withdrawal in male rat.
Keywords: Antioxidant, Lipid peroxidation, Nicotine, Rats
Introduction
Nicotine can be consumed in different forms but more often than not, it is consumed as smoked tobacco. Cigarette smoking has become a very common occurrence and a serious health and economic issue in most societies. It is documented that tobacco usage, according to the World Health Organisation (WHO), is associated with approximately one-third of the world's population older than 15 years of age.[1]
Increased oxidative stress results from excess generation of reactive chemical species called free radicals from a number of sources and/or from decreased enzymic and nonenzymic antioxidant defences.[2] Free radicals and other reactive species have been implicated in the progression of not less than hundred different diseases,[3] thus the interest in free radicals and oxidative stress has grown in recent times.
Nicotine has been documented to alter the oxidant and antioxidant balance in rat lymphocyte in a dose- and time-dependent manner[4] and it also alters lipid peroxidation and antioxidant enzyme in plasma and ovaries of female rats.[5] However, no study has investigated the effect of nicotine on serum oxidant and antioxidant balance in male rats.
The present study was therefore designed to investigate the effects of nicotine on antioxidant profile in the serum of male albino rats during treatment and withdrawal periods.
Materials and Methods
Nicotine preparation
Nicotine hydrogen tartrate with product number 26140 (95% Nicotine) was purchased from BDH Chemical Ltd, Poole, England. The Nicotine dosage prepared in normal saline for each group of animals was delivered at 0.5 and 1.0 mg/kg body weight (BW). The working solutions were stored in foil-wrapped glass bottle at 4°C for no longer than 10 days.
Animal treatment
Forty male albino rats weighing between 150 and 180 g were used for the study. They were housed in well-ventilated cages maintained at 25 ± 2°C, on a 12-h light-dark cycle. The rats were fed on standard rat chow and tap water without restriction. They were acclimatized for 2 weeks before the commencement of experiments. Procedures involving animals and their care were performed in accordance with the guidelines of the Institution Animal Ethics Committee and the National Institutes of Health (NIH) for the care and use of animals.
The rats were assigned randomly to one of the five experimental groups as follows with eight rats per group: Group I, which served as the control and received 0.2 ml/kg normal saline, Groups II and III received 0.5 and 1.0 mg/kg BW of nicotine, respectively for 30 days. The fourth and fifth groups were administered with 0.5 and 1.0 mg/kg BW of nicotine for 30 days, respectively, but were left untreated for another 30 days. The latter two groups served as the recovery groups.
Blood sample collection
Blood was collected from each animal via the retro-orbital sinus with 70 μl heparinized capillary tube[6] and put into plain sample bottle for lipid peroxidation and antioxidant analysis.
Lipid peroxidation and anti-oxidant assay
Serum malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) were assayed using standard laboratory methods described in previous studies.[7,8]
Determination of NO level
For the estimation of NO production, biochemical assessment of stable NO oxidative metabolites, nitrite, and nitrate was performed. Assessment of nitrite and nitrate levels was based on the Griess method.[9] Data in this study presents the sum of nitrite and nitrate levels, which are NO metabolites.
Statistical analysis
Analysis of data was conducted with Statistical Package for Social Sciences (SPSS) software (version 13.0; SPSS Inc, IL, USA) for Windows. Results were expressed as the mean ± standard error of the mean (SEM). Comparisons among groups that were more than two were performed using one-way analysis of variance (ANOVA).
Results
Effect of nicotine on serum GPx level
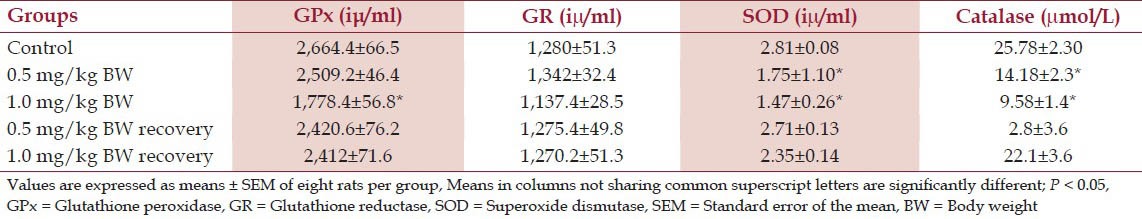
Rats treated with 1.0 mg/kg BW had a significant decrease (P < 0.05) in the mean serum glutathione peroxide when compared with the control group. The recovery groups and 0.5 mg/kg BW treated group had an insignificant decrease (P > 0.05) in the mean serum GPx level when compared with the control as shown in [Table 1].
Table 1.
Serum antioxidant profile of experimental rats treated with nicotine
Effect of nicotine on serum GR level
The results showed an insignificant increase (P < 0.05) in the mean serum GR level in 0.5 mg/kg BW, while 1.0 mg/kg BW group had an insignificant decrease when compared with the control.
The recovery groups both showed an insignificant decrease in the mean serum GR level when values were compared with the control group as shown in Table 1.
Effect of nicotine on serum SOD level
The results showed that there was a significant decrease (P < 0.05) in the mean serum SOD level that received both 0.5 and 1.0 mg/kg BW nicotine daily when compared with their control counterpart though, these effects were dose dependent.
However, the recovery groups showed an insignificant decrease (P > 0.05) in the mean serum SOD level when compared with the control group as shown in Table 1.
Effect of nicotine on serum CAT
The results showed that there was a significant decrease (P < 0.05) in the mean CAT level of rats that received 0.5 and 1.0 mg/kg BW nicotine daily for 4 weeks when compared with their control. However, their recovery groups showed an insignificant decrease (P > 0.05) in their mean serum CAT level when compared with the control as shown in Table 1.
Effect of nicotine on serum nitric oxide (NO)
The results showed that there was a significant decrease (P < 0.05) in the mean NO level of rats that received 1.0 mg/kg BW nicotine daily for 4 weeks when compared with their control. However, 0.5 mg/kg BW and their recovery groups showed an insignificant decrease (P > 0.05) in their mean NO level when compared with the control as shown in [Figure 1].
Figure 1.
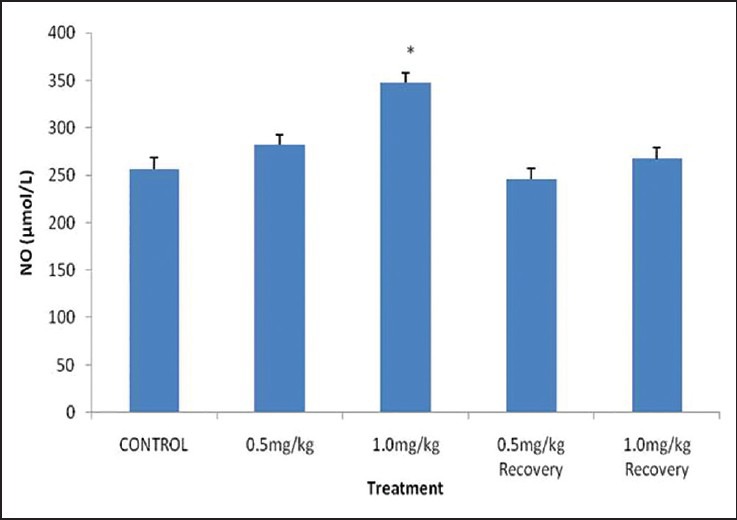
Serum nitric oxide level in male rats treated with nicotine. Values are expressed as mean ± standard error of the mean (SEM) of eight rats. *P < 0.05 vs control
Effect of nicotine on serum MDA level
Results showed that there was an insignificant and significant increase (P < 0.05) in the mean serum MDA level of 0.5 and 1.0 mg/kg BW, respectively when compared with their control counterpart.
However, there was no significant (P < 0.05) change in the mean serum MDA level of the recovery groups when values were compared with the control group as shown in [Figure 2].
Figure 2.
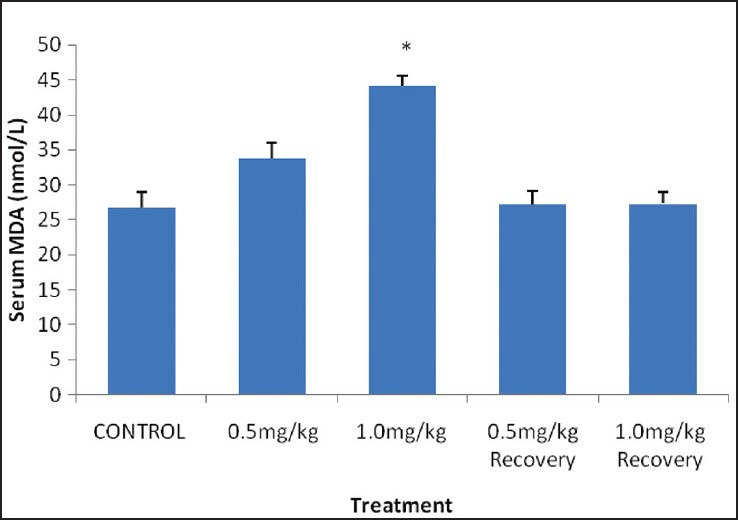
Serum malondialdehyde (MDA) level in male rats treated with nicotine. Values are expressed as mean ± SEM of eight rats. *P < 0.05 vs control
Discussion
The present data showed that nicotine administration produced marked oxidative impact as shown by the significant increase in serum lipid peroxidation and NO level as well as a significant decrease in antioxidants. This might suggest an inhibitory action of nicotine on antioxidant enzyme in serum.
GPx is reported to have a broad protective spectrum. The suppression of the activity of GPx leads to its downregulation in serum of rats treated with nicotine.
In this study, nicotine did not affect GR activity in the serum in vivo when compared with the control group. This study also suggests that the activity of SOD which catalyzes the dismutation of superoxide (O2−) radical to hydrogen peroxide (H2O2)[10] was downregulated in the serum in both the low and high treated group, although these effects were dose dependent. The observed decrease in serum SOD activity may be a consequence of decreased de novo synthesis of enzyme proteins or oxidative inactivation of enzyme protein.
The inhibition of serum SOD activity might also be attributed to either hyperglycemia as reported by Sharpe et al., who found that glucose-induced oxidative stress in different tissues and/or due to loss of enzyme cofactors namely copper and zinc.[11] In addition, previous studies have also indicated an increase in glucose level with nicotine administration.[12]
Administration of nicotine significantly decreased CAT activity in the serum. Decreased CAT activity in serum after exposure to nicotine is indicative of an inefficient elimination of toxic H2O2 by GPx in tissues. This study also demonstrated that nicotine administration is associated with increase NO level in serum. This observation is in consonance with previous study.[13] Nicotine treated rats showed an elevation in MDA level when compared with the control group. An increased MDA concentration might be a consequence of decreased production of antioxidants in the nicotine treated rats’ tissues, thereby shifting the delicate balance in favor of reactive oxygen species. Lipid peroxidation of unsaturated fatty acids is a frequently used indicator of increased oxidative stress and subsequent oxidative damage.[14]
The recovery groups of GPx, GR, SOD, CAT, NO, and MDA showed comparable values with the control indicating that the effect of nicotine on antioxidant enzyme could be ameliorated by nicotine cessation.
In conclusion, the results obtained from this present study show that nicotine reduces serum antioxidant level in male rats leading to an increase in lipid peroxidation. The data also indicate that nicotine withdrawal for a particular period of time could ameliorate the observed effects. However, further investigations are still needed to confirm whether chronic administration of nicotine will have similar effect during administration and withdrawal.
Acknowledgment
The authors are grateful to the Tertiary Education Trust Fund (TETFUND) Nigeria for funding this research.
Footnotes
Source of Support: The authors are grateful to the Tertiary Education Trust Fund (TETFUND) Nigeria for funding this research
Conflict of Interest: None declared.
References
- 1.Mohammed AG, Mohammed A, Behrooz M. In vitro inhibition of human sperm creatinine kinase by Nicotine, cotinine and cadmium as a mechanism in smoker men infertility. Int J Fertil Steril. 2008;2:125–30. [Google Scholar]
- 2.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JM. Oxford: Oxford University Press; 1999. Free radicals in medicine and biology. [Google Scholar]
- 4.Das S, Chakraborty SP, Roy S, Roy S. Nicotine induced pro-oxidant and antioxidant imbalance in rats lymphocytes: In vivo dose and time dependent approach. Toxicol Mech Methods. 2012;22:711–20. doi: 10.3109/15376516.2012.718812. [DOI] [PubMed] [Google Scholar]
- 5.Chattopadhyay K, Chattopadhyay BD. Effect of nicotine on lipid profile, peroxidation and antioxidant enzyme in female rats with restricted dietary protein. Indian J Med Res. 2008;127:571–6. [PubMed] [Google Scholar]
- 6.Ezzai SD. Effect of intrathecal morphine on blood glucose, glucagons and tissue glucagons in rats: Comparison with the effect of xanthan gum on blood glucose. J Pharm Belg. 1996;51:195–9. [PubMed] [Google Scholar]
- 7.Adewole SO, Ojewole JA. Protective effects of Annona muricata Linn.(annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2008;6:30–41. doi: 10.4314/ajtcam.v6i1.57071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khatibi F, Yaghoubi AR, Rahbani NM. Study of antioxidant enzymes, lipid peroxidation, lipid profile and immunologic factor in coronary artery disease in East Azarbijan. Int J Med Biomed Res. 2012;1:147–52. [Google Scholar]
- 9.Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–3. [PubMed] [Google Scholar]
- 10.McCord JM, Keele BB, Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: The physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971;68:1024–7. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe P, Liuk W, Yue K, McMaster D, Catherwood MA, McGinty AM, et al. Glucose-induced oxidative stress in vascular contractile cells: Comparison of aortic smooth muscle cells and retinal pericytes. Diabetes. 1998;47:801–9. doi: 10.2337/diabetes.47.5.801. [DOI] [PubMed] [Google Scholar]
- 12.Alada RA. Effects of calcium channel blockers on nicotine-induced hyperglycaemia in the rat. Afr J Med Med Sci. 2001;30:57–9. [PubMed] [Google Scholar]
- 13.Swami S, Suryakar AN, Katkam RV, Kumbar KM. Absorption of nicotine induces oxidative stress among bidi workers. Indian J Public Health. 2006;50:231–5. [PubMed] [Google Scholar]
- 14.Khatibi F, Yaghoubi AR, Rahbani NM. Study of antioxidant enzymes, lipid peroxidation, lipid profile and immunologic factor in coronary artery disease in East Azarbijan. Int J Med Biomed Res. 2012;1:147–52. [Google Scholar]


