5-Taurinomethyluridine (τm5U) and 5-taurinomethyl-2-thiouridine (τm5s2U) are located at the wobble position of human mitochondrial (hmt) tRNALeu(UUR) and tRNALys, respectively, restricting decoding of the third codon position to A and G. Absence of modifications leads to severe mitochondrial dysfunctions (MELAS, MERRF). Site-specific chemical incorporation of τm5U and τm5s2U into 17-mers containing the sequence of anticodon arm is reported.
Keywords: human mitochondrial tRNA, modified ribonucleosides, 5-taurinomethyluridine, 5-taurinomethyl-2-thiouridine, phosphoramidite chemistry
Abstract
5-Taurinomethyluridine (τm5U) and 5-taurinomethyl-2-thiouridine (τm5s2U) are located at the wobble position of human mitochondrial (hmt) tRNALeu(UUR) and tRNALys, respectively. Both hypermodified units restrict decoding of the third codon letter to A and G. Pathogenic mutations in the genes encoding hmt-tRNALeu(UUR) and hmt-tRNALys are responsible for the loss of the discussed modifications and, as a consequence, for the occurrence of severe mitochondrial dysfunctions (MELAS, MERRF). Synthetic oligoribonucleotides bearing modified nucleosides are a versatile tool for studying mechanisms of genetic message translation and accompanying pathologies at nucleoside resolution. In this paper, we present site-specific chemical incorporation of τm5U and τm5s2U into 17-mers related to the sequence of the anticodon arms hmt-tRNALeu(UUR) and hmt-tRNALys, respectively employing phosphoramidite chemistry on CPG support. Selected protecting groups for the sulfonic acid (4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl) and the exoamine function (-C(O)CF3) are compatible with the blockage of the canonical monomeric units. The synthesis of τm5s2U-modified RNA fragment was performed under conditions eliminating the formation of side products of 2-thiocarbonyl group oxidation and/or oxidative desulphurization. The structure of the final oligomers was confirmed by mass spectroscopy and enzymatic cleavage data.
INTRODUCTION
Mitochondria have a largely autonomic system of genetic message expression. The human mitochondrial genome encodes 13 proteins responsible for oxidative phosphorylation process (OXPHOS), two rRNAs, and 22 tRNAs (Florentz et al. 2003; Sissler et al. 2008). Knowledge about the detailed structure of mitochondrial tRNAs (mt-tRNAs) is still limited (Suzuki et al. 2011b). Research using specific chemical and enzymatic structure probes (Sissler et al. 2008) has shown that the secondary (2D) and tertiary (3D) arrangements of mt-tRNAs more or less deviate from the classical cloverleaf and L-shaped organization characteristic of their cytosolic counterparts (Helm et al. 2000; Jühling et al. 2012; Wende et al. 2014). mt-tRNAs are also significantly less prone to post-transcriptional modifications than cytosolic tRNAs (Watanabe 2010).
5-Taurinomethyluridine (τm5U, 1, Fig. 1A) and 5-taurinomethyl-2-thiouridine (τm5s2U, 2, Fig. 1A) are present at the wobble position of human mitochondrial (hmt) tRNAs specific for Leu, Trp, and Lys, Glu, Gln, respectively (Kirino et al. 2004; Watanabe 2010). Like other xm5(s2)U-type wobble uridines, nucleosides 1, 2 decode A and G as the third codon letter (Kurata et al. 2008). The genes encoding hmt-tRNALeu and hmt-tRNALys are highly susceptible to point mutations. The most often occurring transitions A3243G, T3271C, and A8344G result in the absence of nucleosides 1, 2 (Suzuki et al. 2011a,b). Deficiency of the taurine-modified uridines 1, 2 is considered to be a key factor responsible for the severe mitochondrial diseases MELAS and MERRF (Suzuki et al. 2011a,b). In fact, the taurine residue linked to the atom C-5 by a methylene group has been found critical for G decoding (Kirino et al. 2004; Kurata et al. 2008), while the absence of both modifying functions (the C-5 substituent and the 2-thiocarbonyl group) results in a translational defect for the cognate codons UUA(G) and a disruption of protein biosynthesis (Suzuki et al. 2011a).
FIGURE 1.
(A) The structure of 5-taurinomethyluridine (τm5U, 1) and 5-taurinomethyl-2-thiouridine (τm5s2U, 2). (B) The sequence and secondary structure of the human mitochondrial tRNALeu(UUR) and tRNALys anticodon stem and loop (hmt-ASLLeu(UUR) and hmt-ASLLys) bearing τm5U and τm5s2U, respectively. The native sequence of hmt-ASLLeu(UUR) has pseudouridine (Ψ) at position 27, while in the sequence of hmt-ASLLys there are two pseudouridines (Ψ) at positions 27 and 28, and N-[(9-β-D-ribofuranosyl-9H-purin-6-yl)carbamoyl]-L-threonine (t6A) at position 37.
The abundance of mt-tRNAs is very poor as compared with their cytosolic homologues (Sissler et al. 2008) limiting their availability from biological material. hmt-tRNALeu(UUR) molecule devoid of τm5U (Kirino et al. 2004) as well as Escherichia coli tRNALeu(UUR) analog bearing only this one modified unit (Kurata et al. 2008) have been synthesized using the molecular surgery technique. These “chimeric” tRNAs have been used in bioassays investigating their binding to programmed ribosomes and as elements of a cell-free translation system (Kirino et al. 2004; Kurata et al. 2008). An RNA fragment recombination technique and the 5′,3′-bisphosphate of τm5U (pτm5Up) have been applied for the preparation of an analog of the hmt-tRNALeu anticodon arm sequence modified at the wobble position. To visualize the geometry of the codon-anticodon mini-helix, the construct was introduced into the A-site of Thermus thermophilus crystal 30S ribosomal subunit programmed with UUA(G) codon (Kurata et al. 2008). The 5′-half fragment of the molecule was observed to be dynamic, however, and the geometry of the wobble pair τm5U-G was not established in detail (Kurata et al. 2008).
Chemically synthesized modified tRNA fragments, and in particular anticodon stem–loop hairpins (ASLs), have been widely utilized in model studies on the influence of post-transcriptional modification on molecule conformation/3D structure dynamics in solution (Duram et al. 2005; Cantara et al. 2012) as well as on conformation in the solid state (Weixlbaumer et al. 2007; Cantara et al. 2013). Recently, similar model studies on the impact of modification (f5C) on the structure/dynamics of the anticodon arm domain have been extended to hmt-tRNA specific for Met (Lusic et al. 2008). Modified oligomers are also used as substrates for enzymatic ligation of RNA fragments to generate longer RNA constructs with site-specific location of the modified nucleoside(s) (Kurschat et al. 2005). In order to synthesize RNA oligomers including taurine-modified uridines 1, 2, appropriate protection for highly reactive functional groups, in particular the sulfonic acid residue, is required. An attempt to employ phenyl protection for the sulfonic acid residue of τm5U and τm5s2U phosphoramidites has been reported (Ogata and Wada 2006). However, their use in oligoribonucleotide solid-phase synthesis was limited to RNA dimers τm5(s2)UpU (Ogata and Wada 2008).
This paper presents a milligram scale chemical synthesis of τm5U and τm5s2U-modified oligoribonucleotides (phosphoramidite chemistry on solid support) related to the sequence of the hmt-tRNAs anticodon arm domain specific for Leu and Lys, respectively (Fig. 1B). The 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl group (neoO-dPS) was selected as the most useful protection for sulfonic acid residue of taurine derivatives.
RESULTS AND DISCUSSION
The incorporation of nucleosides 1 and 2 into the RNA sequences using phosphoramidite chemistry on CPG support requires such protection of the highly reactive aliphatic amine function as well as the sulfonic acid residue that would be compatible with the blockage of canonical monomeric units. The aliphatic amine function was protected with a base-labile trifluoroacetyl group, following the methodology developed previously for the incorporation of mnm5(s2)U into RNA oligomers (Malkiewicz and Sochacka 1983; Agris et al. 1995; Leszczynska et al. 2011, 2012).
The most appropriate method for the protection of alkyl or aryl sulfonic acids, including taurine, is their transformation into sulfonate esters (Roberts et al. 1997; Klán et al. 2002; Wrobel et al. 2002; Yan and Müller 2004; Avitabile et al. 2005; Seeberger et al. 2007; Hussain et al. 2008; Ali et al. 2009; Miller 2010). The masking of sulfonic acids as sulfonamides or ammonium salts has also been proposed (Klamann and Hofbauer 1953; Richman and Atkins 1974; Andrianov et al. 2004). The cleavage of most of the reported protecting groups employs, however, strongly acidic or basic conditions unsuitable for oligoribonucleotide synthesis. Substituted phenyl esters of taurine have been utilized for solid support synthesis of dimers with τm5(s2)U at the 5′ end (Ogata and Wada 2006, 2008). However, this blockage has proven too labile to be used in the synthesis of longer, hypermodified RNA sequences (G Leszczynska and A Malkiewicz, unpubl.). In search of alternative protection for the sulfonic acid function, model N-Boc taurine esters have been synthesized (Fig. 2) and their stability/cleavage has been examined under the typical reaction conditions of RNA synthesis on solid support.
FIGURE 2.
Protecting groups selected for sulfonic acid residue of taurine; TBDPS, tert-butyldiphenylsilyl; TBDMS, tert-butyldimethylsilyl; TMS, trimethylsilyl.
By analogy with the protection of the t6A carboxyl group (Boudou et al. 2000; Sundaram et al. 2000; Bajji and Davis 2002; Bajji et al. 2002; Eshete et al. 2007; Bilbille et al. 2009), variously substituted N-Boc taurine 2-phenylethyl esters (Fig. 2, 3a–3g) removable via the β-elimination process (10% DBU/MeCN) have been prepared (Leszczynska et al. 2013). Several attempts to prepare 2-(trimethylsilyl)ethyl ester (3j) which could be removed by treatment with fluoride anions have not been successful (data not shown). As an alternative, we have synthesized and tested 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl ester of N-Boc taurine (Fig. 2, 3h; Seeberger et al. 2007) and its analog, 4-(tert-butyldimethylsilanyloxy)-2,2-dimethylbutyl ester (3i).
The esters 3a–3i were stable in 8 M ethanolic ammonia (24 h, room temperature [rt]), offering a simple way for the simultaneous deblocking of base-labile protecting groups, e.g., 2-cyanoethyl, -tac, and the cleavage of oligomers from CPG support without the risk of amide formation. Among 2-arylethyl esters 3a–3g only 2-(p-nitrophenyl)ethyl (3a), 2-(p-trifluoromethylphenyl)ethyl (3b), and 2-(2,4,5-trifluorophenyl)ethyl (3g) esters of N-Boc taurine were found to be easily deprotected under β-elimination conditions (10% DBU/MeCN, 40 min, 45°C). Both p-substituted phenylethyl esters 3a, 3b were, however, significantly unstable also in the presence of other tertiary amines, e.g., Et3N or iPr2NEt, which excluded their use in the synthesis of τm5U and τm5s2U phosphoramidites. Examination of the stability of N-Boc taurine 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl ester (3h) as well as its analog, 4-(tert-butyldimethylsilanyloxy)-2,2-dimethylbutyl ester (3i), revealed their effective cleavage with a standard desilylating reagent, 1 M TBAF/NMP (24 h, rt) as well as 1 M TEAF/NMP (24 h, rt) and triethylamine trihydrofluoride (TEA•3HF/NMP, 24 h, rt). In contrast to neoO-dPS sulfonate ester 3h, ester 3i was unstable under detritylation conditions (3% TCA/DCM). The usefulness of neoO-dPS as a protecting group for sulfonic acid residue has been previously confirmed in the synthesis of variously N-substituted taurines (Seeberger et al. 2007). The removal of neoO-dPS was performed with a small excess of TBAF in THF via cleavage of the Si-O bond, and then spontaneous cyclization of the desilylated intermediate (Seeberger et al. 2007).
In summary, evaluation of the stability of various N-Boc taurine esters (Fig. 2) allowed for the selection of two protecting groups for the sulfonic acid residue, the 2-(2,4,5-trifluorophenyl)ethyl group and the 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl group. An attempt to introduce the 2-(2,4,5-trifluorophenyl)ethyl-protected τm5U and τm5s2U phosphoramidites into RNA sequences resulted, however, in very low yields of the target oligoribonucleotides (data not shown). The use of strong basic DBU solution required for the 2-(2,4,5-trifluorophenyl)ethyl deprotection caused a Michael-type addition of 2,4,5-trifluorostyrene to RNA and the loss of the 2-thiocarbonyl moiety in the case of τm5s2U-modified oligomer. The use of scavengers of 2,4,5-trifluorostyrene did not increase the yields of synthesis.
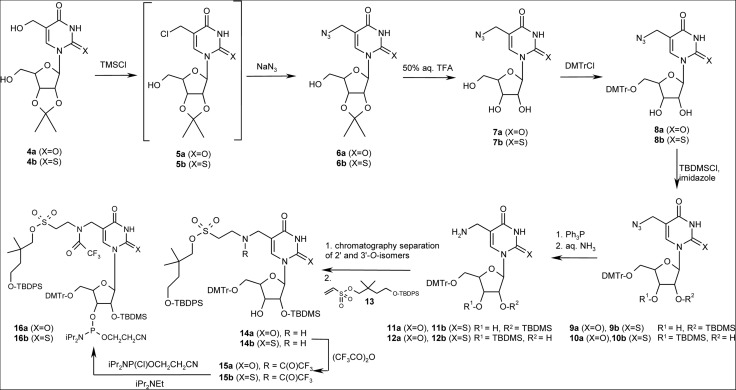
Finally, the 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl group (neoO-dPS) was effectively used for the synthesis of τm5(s2)U phosphoramidites 16a/16b (Fig. 3) and then for their incorporation into target RNA sequences.
FIGURE 3.
Chemical synthesis of τm5U and τm5s2U phosphoramidites. TMSCl, trimethylsilyl chloride; TFA, trifluoroacetic acid; DMTrCl, 4,4′-dimethoxytrityl chloride; TBDMSCl, tert-butyldimethylsilyl chloride; TBDPS, tert-butyldiphenylsilyl group.
Initially, the synthesis of 16a/16b involved the introduction of 5′-O-DMTr and 2′-O-TBDMS protecting groups after the installation of a taurine ester side chain at position C-5,1. However, the steric hindrance around the 5′-hydroxyl group, caused by the presence of a large substituent at the atom C-5, decreased the yield of dimethoxytritylation to 5% (data not shown). As an alternative strategy, a fully protected taurine skeleton was installed after 5′-O-DMTr and 2′-O-TBDMS protection of the sugar moiety (Fig. 3). 5-Hydroxymethyl-2′,3′-O-isopropylidene(-2-thio)uridine (4a/4b) was used for the preparation of 5-azidomethyl(-2-thio)uridine derivatives 6a/6b (Seio et al. 1998). The treatment of 4a/4b with an excess of trimethylsilyl chloride in 1,4-dioxane at 60°C gave 5-chloromethyl-2′,3′-O-isopropylidene(-2-thio)uridine 5a/5b, which without purification was reacted with an excess of sodium azide in DMF at 60°C. In comparison with the synthesis of 6a, a similar method of preparation of the 2-thio derivative 6b was considerably less effective (40% vs. 70%).
The 2′,3′-O-isopropylidene group was removed from 6a/6b by treatment with 50% aqueous (aq.) trifluoroacetic acid (Myerscough et al. 1992) to afford 7a/7b in 90% yield. Subsequently, incorporation of the 5′-O-DMTr, and then 2′(3′)-O-TBDMS, protecting groups was performed according to the standard procedures (Damha and Ogilvie 1993). A mixture of 2′ and 3′ TBDMS isomers (9a/9b and 10a/10b, respectively) was separated by column chromatography on silica gel only in the amount required for spectral analysis. The separation of 2′ and 3′ TBDMS isomers at this stage was not convenient because the alkaline conditions of the subsequent reaction led to spontaneous isomerization giving an equimolar mixture of 2′ and 3′ regiomers. Therefore, a mixture of azides 9a, 10a or their 2-thio analogous 9b, 10b was reduced to a suitable mixture of amines 11a, 12a or 11b, 12b, respectively, by treatment with triphenyl phosphine in anhydrous pyridine followed by 25% aq. ammonia (Seio et al. 1998). The resulting mixtures of regiomers 11a, 12a or 11b, 12b were effectively separated by flash chromatography. The storage of 3′ isomers 12a/12b in methanol for a longer period of time resulted in their partial isomerization, enabling improvement of the overall yield of 2′-TBDMS derivatives 11a/11b. Separated 5-aminomethyl(-2-thio)uridine 11a/11b was then used as a donor in a Michael-type addition to 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl ethenesulfonate (13) (Baxter et al. 2000; Ogata and Wada 2006; Seeberger et al. 2007). This strategy of synthesis of a fully protected taurine skeleton is more effective than procedures involving 5-chloromethyl- or 5-formyluridine as substrates (Leszczynska et al. 2013) and in the case of β-amino acids it should be considered as the method of choice. To exclude the isomerization of 2′-O-TBDMS 11a/11b to 3′-regiomers the reaction of ethenesulfonate 13 with 11a/11b requires an aprotic solvent. Consequently, an equimolar amount of amine 11a/11b and 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl ethenesulfonate (13) was mixed in DCM for 72 h at rt. The resulting material 14a/14b was purified by column chromatography in 70% yield.
Following the previously reported procedure (Malkiewicz et al. 1983), the amine function of 14a/14b was protected with trifluoroacetyl to afford 15a/15b in 80% yield. Phosphitylation of 15a/15b was performed with 2-cyanoethyl N,N-diisopropylaminochlorophosphoramidite under standard conditions (Damha and Ogilvie 1993) giving τm5(s2)U phosphoramidites 16a/16b in ∼85% yield.
Fully protected phosphoramidites 16a/16b were used for the synthesis of analogs of the anticodon arm domain of human mt-tRNALeu(UUR) and mt-tRNALys modified with τm5U and τm5s2U, respectively (Fig. 1B). The synthesis of oligomers was conducted manually on a 5-μmol scale using commercial tac-protected phosphoramidites of the canonical units and 5-(3,5-bis(trifluoromethyl)phenyl)-1H-tetrazole as the activator. The couplings were conducted in 8 molar excess of A, U, C, and G amidites for 8 min, while the modified units were used in 12 molar excess and coupled twice, each time using 6 molar excess of an amidite and 12-min coupling time. Coupling yields were in the range of 90%–95%.
It is generally known that the 2-thiocarbonyl group of 2-thiouridine derivatives reacts with various oxidizing reagents used in the oligoribonucleotide synthesis giving products of 2-thio → 2-oxo transformation and/or oxidative desulfurization (s2 → H2) (Sochacka 2001; Okamoto et al. 2006). In model studies (Leszczynska et al. 2011, 2012), a 0.02 M iodine solution (8 equiv, 2 min) in THF-H2O-pyridine (Okamoto et al. 2006) was selected as the most promising oxidizing agent, leading to very small amounts of side products of 2-thiocarbonyl group degradation. In contrast to diluted iodine solution, 0.25 M tBuOOH (8 equiv, 2 min) in toluene or acetonitrile gave considerable amounts of side products.
“Trityl-off” CPG-bound RNA was treated with Et3N in CH3CN, and then with 8 M ethanolic ammonia. The two-step deprotection procedure made it possible to avoid the reaction of heterobase residues with acrylonitrile generated during the deprotection of phosphate residue (Capaldi et al. 2003). For the simultaneous removal of the TBDMS and neoO-dPS protecting groups in τm5U-modified RNA, Et3N•3HF was effectively employed. An alternative use of 1 M Bu4NF gave the desired product, but the yield of the oligomer was drastically reduced. In the case of τm5s2U-modified RNA, desilylation was performed with several reagents. We found that the only effective condition for the complete removal of neoO-dPS and TBDMS protections without observable degradation of the 2-thiocarbonyl function was the treatment with 1 M Et4NF in NMP (24 h, rt). The use of Et3N•3HF caused a significant loss of the 2-thiocarbonyl function in the τm5s2U-modified oligomer. The excess of the desilylating reagent was deactivated by the addition of ethoxytrimethylsilane for Et3N•3HF or phosphate buffer for Et4NF. Crude products were purified by preparative IE-HPLC (Fig. 4A). The homogeneity and composition of synthetic oligoribonucleotides were verified by MALDI-TOF mass spectrometry (Supplemental Material) as well as RNA enzymatic digestion (Gehrke et al. 1982; Gehrke and Kuo 1989) to the expected mixture of nucleosides whose composition was tested by RP HPLC, and the data were compared with those recorded under identical conditions for modified nucleosides (Wada et al. 2002) as a reference (Fig. 4C,D).
FIGURE 4.
(A) Anion-exchange HPLC of crude, deprotected hmt-ASLLeu(UUR) (τm5U34) and hmt-ASLLys (τm5s2U34). (B) Analytical injection of desalted and lyophilized oligoribonucleotides. (C) HPLC nucleoside composition of mt-ASLs. (D) References of τm5U and τm5s2U injected in control experiments.
CONCLUSIONS
The 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl (neoO-dPS) protecting group has been adopted for solid supported synthesis of oligoribonucleotides (phosphoramidite chemistry) bearing taurine-modified wobble uridines 1, 2. NeoO-dPS blockage is compatible with the protection of commercially available canonical monomeric units resistant to treatment with 8 M ethanolic ammonia and removable under mild, neutral conditions with fluoride anions. The usefulness of the discussed methodology was verified by the site-specific insertion of nucleosides 1, 2 into the anticodon arm sequence of hmt-tRNALeu, Lys. The presented work enables effective chemical synthesis of hypermodified RNA sequences which can be used for model studies on the mechanism of decoding processes in mitochondria and their pathologies on a molecular level.
MATERIALS AND METHODS
NMR spectra were recorded on a Bruker Avance DPX 250 spectrometer at 250.0 (1H), 62.9 (13C), and 101.3 (31P) MHz or a Bruker Avance II Plus 700 spectrometer at 700.0 (1H) and 176.0 (13C) MHz. Chemical shifts are reported in ppm relative to TMS (internal standard) for 1H and 13C, and 85% phosphoric acid (external standard) for 31P. Chemical shifts are described as s (singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quartet), m (multiplet), and bs (broad singlet). Coupling constants (J) are reported in hertz. IR spectra were recorded on a Bruker FT-IR ALPHA spectrometer equipped with a platinum ATR QuickSnap module. High-resolution mass spectra were obtained from a Finnigan MAT 95 spectrometer (FAB ionization) and Maldi SYNAPT G2-S HDMS (ESI ionization). MALDI-TOF spectra were recorded on an Applied Biosystems Voyager-Elite mass spectrometer. Thin layer chromatography was done on Merck 60F254 coated plates, and Merck silica gel 60 (mesh 230–400) was used for column chromatography. HPLC was performed with a Waters chromatograph interfaced with a 996 spectral diode array detector.
5-Azidomethyl-2′,3′-O-isopropylidene(-2-thio)uridine (6a/6b)
Nucleoside 4a/4b (13.0 mmol, 1.0 equiv) was dissolved in 1,4-dioxane (128 mL), and then trimethylsilyl chloride (8 mL, 65 mmol, 5 equiv) was added. After being stirred for 4.5 h (4a)/7 h (4b) at 60°C, the mixture was cooled to rt, and anhydrous acetone (58 mL) was added. Stirring was continued for 1.5 h at rt. The mixture was then concentrated under reduced pressure and co-evaporated with anhydrous 1,4-dioxane. The resulting foam 5a/5b was dissolved in DMF (118 mL) and treated with NaN3 (5.1 g, 78.0 mmol, 6.0 equiv). The reaction mixture was stirred for 3 h at 60°C. NaCl precipitate was filtered off. The filtrate was concentrated under reduced pressure. The solid residue was dissolved in CH2Cl2/py (4:1, v/v; 58 mL) and washed with H2O (23 mL). The organic layer was dried over MgSO4, filtered, and concentrated under reduced pressure. Pyridine was removed by co-evaporation with anhydrous toluene. The resulting foam was purified by column chromatography.
Compound 6a was purified on a silica gel column with 5% MeOH in CHCl3 as eluent to obtain a white foam in 69% yield. Spectroscopic data were in agreement with those presented previously (Seio et al. 1998).
Compound 6b was purified on a silica gel column with 2% MeOH in CHCl3 as eluent to obtain a light yellow foam in 40% yield. TLC Rf = 0.52 (CHCl3/MeOH, 9:1 v/v); 1H NMR (700 MHz, CDCl3): δ 1.37 (s, 3H), 1.62 (s, 3H), 3.91 (dd, 1H, J = 2.80 Hz, J = 11.90 Hz), 4.06 (dd, 1H, J = 2.10 Hz, J = 11.90 Hz), 4.15 (q, 2H, J = 11.20 Hz), 4.35–4.36 (m, 1H), 4.77 (dd, 1H, J = 2.10 Hz, J = 5.60 Hz), 4.90 (q, 1H, J = 3.50 Hz), 6.84 (d, 1H, J = 2.80 Hz), 8.15 (s, 1H), 10.40 (s, 1H); 13C NMR (176 MHz, CDCl3): δ 26.49, 28.24, 48.20, 62.78, 80.19, 86.56, 87.60, 95.17, 115.04, 115.54, 140.28, 160.45, 176.16; IR (ATR): 2106 cm−1; HRMS (ESI): calcd for C13H17N5O5NaS [M + Na]+ 378.0848, found 378.0847.
5-Azidomethyl(-2-thio)uridine (7a/7b)
Nucleoside 6a/6b (4.5 mmol, 1 equiv) was dissolved in 50% aq. trifluoroacetic acid (13 mL). After being stirred for 1.5 h at rt, anhydrous toluene (18 mL) was added, and the mixture was concentrated under reduced pressure. The solid residue was co-evaporated with anhydrous toluene and purified by column chromatography.
Compound 7a was purified on a silica gel column with 12% MeOH in CHCl3 as eluent to obtain a white foam in 93% yield. TLC Rf = 0.18 (CHCl3/MeOH 9:1, v/v); 1H NMR (700 MHz, DMSO-d6): δ 3.61 (dd, 1H, J = 3.50 Hz, J = 11.90 Hz), 3.71 (dd, 1H, J = 3.50 Hz, J = 11.90 Hz), 3.91 (q, 1H, J = 3.50 Hz), 4.03 (t, 1H, J = 4.90 Hz), 4.09 (t, 1H, J = 4.90 Hz), 4.11 (s, 2H), 5.83 (d, 1H, J = 4.90 Hz), 8.13 (s, 1H); 13C NMR (176 MHz, DMSO-d6): δ 47.35, 61.10, 70.02, 73.89, 85.23, 88.36, 108.79, 140.53, 150.86, 163.25; IR (ATR): 2106 cm−1; HRMS (FAB−): calcd for C10H12N5O6 [M − H]− 298.0788, found 298.0788.
Compound 7b was purified on a silica gel column with 8% MeOH in CHCl3 as eluent to obtain a light yellow foam in 88% yield. TLC Rf = 0.28 (CHCl3/MeOH 9:1, v/v); 1H NMR (700 MHz, DMSO-d6): δ 3.60–3.62 (m, 1H), 3.74–3.76 (m, 1H), 3.90–3.92 (m, 1H), 3.98 (q, 1H, J = 5.60 Hz), 4.05–4.06 (m, 3H), 5.08 (d, 1H, J = 5.60 Hz), 5.29 (t, 1H, J = 4.90 Hz), 5.43 (d, 1H, J = 4.90 Hz), 6.50 (d, 1H, J = 3.50 Hz), 8.36 (s, 1H), 12.81 (s, 1H); 13C NMR (176 MHz, DMSO-d6): δ 47.43, 60.12, 69.11, 74.98, 85.02, 93.28, 113.68, 140.30, 160.11, 176.32; IR (ATR): 2101 cm−1; HRMS (ESI):calcd for C10H13N5O5NaS [M + Na]+ 338.0535, found 338.0524.
5-Azidomethyl-5′-O-(4,4′-dimethoxytrityl)(-2-thio)uridine (8a/8b)
Nucleoside 7a/7b (3.5 mmol, 1 equiv) was dissolved in anhydrous pyridine (25 mL). 4,4′-Dimethoxytrityl chloride (1.4 g, 4.2 mmol, 1.2 equiv) was added, and the mixture was stirred at rt for 24 h. The reaction was quenched with H2O (25 mL). The resulting solution was extracted with CHCl3 (3 × 50 mL). The combined organic layers were washed with water (2 × 15 mL), dried over MgSO4, filtered and concentrated under reduced pressure. The crude product 8a/8b was purified by column chromatography.
Compound 8a was isolated by flash chromatography using 2% MeOH in CHCl3 as eluent to obtain a white foam in 45% yield. TLC Rf = 0.57 (CHCl3/MeOH 9:1, v/v); 1H NMR (700 MHz, acetone-d6): δ 3.41 (dd, 1H, J = 2.80 Hz, J = 11.20 Hz), 3.45–3.47 (m, 2H), 3.69 (d, 1H, J = 14.00 Hz), 3.79 (s, 6H), 4.15–4.16 (m, 1H), 4.43–4.45 (m, 2H), 5.97 (d, 1H, J = 4.90 Hz), 6.90–7.50 (m, 13H), 7.87 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ 46.83, 54.67, 63.31, 70.50, 74.43, 83.58, 86.63, 89.39, 109.03, 113.19, 126.94, 127.89, 128.21, 130.13, 130.15, 135.52, 135.77, 139.42, 144.90, 150.34, 158.90, 158.91, 162.52; IR (ATR): 2089 cm−1; HRMS (FAB−): calcd for C31H30N5O8 [M − H]− 600.2094, found 600.2095.
Compound 8b was isolated by flash chromatography using 1% MeOH in CHCl3 as eluent to obtain a yellow foam in 74% yield. TLC Rf = 0.63 (CHCl3/MeOH 9:1, v/v); 1H NMR (700 MHz, acetone-d6): δ 3.30 (d, 1H, J = 14.00 Hz), 3.49 (dd, 1H, J = 2.10 Hz, J = 10.50 Hz), 3.54 (dd, 1H, J = 3.50 Hz, J = 10.50 Hz), 3.65 (d, 1H, J = 14.00 Hz), 3.80 (s, 6H), 4.22–4.24 (m, 1H), 4.32 (d, 1H, J = 7.00 Hz), 4.45–4.47 (m, 1H), 4.48–4.51 (m, 1H), 4.81 (d, 1H, J = 4.90 Hz), 6.65 (d, 1H, J = 2.80 Hz), 6.92–7.51 (m, 13H), 8.01 (s, 1H), 11.46 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ 46.73, 54.66, 62.44, 69.59, 75.06, 83.43, 86.66, 93.87, 113.22, 113.74, 127.00, 127.95, 128.23, 130.15, 130.18, 135.39, 135.68, 138.99, 144.79, 158.94, 158.96, 159.27, 176.30; IR (ATR): 2097 cm−1; HRMS (ESI): calcd for C31H31N5O7NaS [M + Na]+ 640.1842, found 640.1842.
5-Azidomethyl-2′-O-(tert-butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)(-2-thio)uridine (9a/9b) and 5-azidomethyl-3′-O-(tert-butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)(-2-thio)uridine (10a/10b)
The 5′-DMT nucleoside 8a/8b (1.7 mmol, 1.0 equiv) was dissolved in anhydrous pyridine (17 mL), then imidazole (0.34 g, 5.0 mmol, 3 equiv) and tert-butyldimethylsilyl chloride (0.31 g, 2.1 mmol, 1.2 equiv) were added. After being stirred for 24 h at rt, the reaction was quenched with H2O (20 mL). The resulting solution was extracted with CHCl3 (3 × 35 mL). The combined organic layers were washed with water (35 mL), dried over MgSO4, and the solvent was removed under reduced pressure. An equimolar mixture of 2′- and 3′-TBDMS isomers was purified by column chromatography and separated only in an amount sufficient for spectral analysis.
Mixture of 2′- and 3′-TBDMS isomers (9a, 10a) was purified on a silica gel column with 3% acetone in DCM as eluent to obtain a white foam in 82% yield. Compound 9a: TLC Rf = 0.69 (DCM/acetone 9:1, v/v); 1H NMR (700 MHz, acetone-d6): δ 0.17 (s, 3H), 0.18 (s, 3H), 0.94 (s, 9H), 3.41 (d, 1H, J = 14.00 Hz), 3.44 (dd, 1H, J = 2.8 Hz, J = 10.5 Hz), 3.47 (dd, 1H, J = 3.50 Hz, J = 10.50 Hz), 3.64 (d, 1H J = 13.30 Hz), 3.80 (s, 6H), 3.89 (d, 1H, J = 5.60 Hz), 4.17–4.19 (m, 1H), 4.38–4.40 (m, 1H), 4.53 (t, 1H, J = 4.90 Hz), 5.99 (d, 1H, J = 4.90 Hz), 6.91–7.50 (m, 13H), 7.91 (s, 1H), 10.33 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ −5.44, 17.87, 25.29, 46.75, 54.68, 63.22, 70.76, 76.11, 83.54, 86.78, 88.88, 109.19, 113.22, 127.02, 127.94, 128.17, 130.14, 135.40, 135.64, 139.05, 144.86, 150.24, 158.95, 158.97, 162.39; IR (ATR): 2100 cm−1; HRMS (FAB−): calcd for C37H44N5O8Si [M − H]− 714.2959, found 714.2944. Compound 10a: TLC Rf = 0.48 (DCM/acetone 9:1, v/v); 1H NMR (700 MHz, (CD3)2CO): δ 0.05 (s, 3H), 0.12 (s, 3H), 0.87 (s, 9H), 3.37 (dd, 1H, J = 3.50 Hz, J = 10.50 Hz), 3.49–3.52 (m, 2H), 3.71 (d, 1H, J = 13.30 Hz), 3.77 (s, 1H), 3.80 (s, 6H), 4.13–4.14 (m, 1H), 4.40–4.42 (m, 1H), 4.51 (t, 1H, J = 4.90 Hz), 5.96 (d, 1H, J = 4.90 Hz), 6.91–7.50 (m, 13H), 7.91 (s, 1H), 10.29 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ −4.61, −4.26, 18.81, 26.29, 47.83, 55.64, 63.91, 72.78, 75.31, 84.84, 87.71, 90.59, 109.98, 114.14, 114.16, 127.96, 128.85, 129.19, 131.11, 131.13, 136.42, 136.55, 140.32, 145.69, 151.25, 159.92, 163.43; IR (ATR): 2101 cm−1.
Mixture of 2′- and 3′-TBDMS isomers (9b, 10b) was purified on a silica gel column with CHCl3 as eluent to obtain a white foam in 86% yield. Compound 9b: TLC Rf = 0.53 (CHCl3/MeOH 99:1, v/v); 1H NMR (700 MHz, acetone-d6): δ 0.18 (s, 3H), 0.19 (s, 3H), 0.95 (s, 9H), 3.32 (d, 1H, J = 14.00 Hz), 3.49 (1H, dd, J = 11.20 Hz, J = 2.10 Hz), 3.52 (1H, dd, J = 11.20 Hz, J = 3.50 Hz), 3.61 (d, 1H, J = 14.00 Hz), 3.81 (s, 6H), 4.12 (d, 1H, J = 5.60 Hz), 4.25–4.27 (m, 1H), 4.42 (q, 1H, J = 4.90 Hz), 4.56 (t, 1H, J = 4.9 Hz), 6.78 (d, 1H, J = 3.50 Hz), 6.92–7.50 (m, 13H), 8.01 (s, 1H), 11.50 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ −5.18, −5.15, 17.99, 25.43, 46.60, 54.67, 62.87, 70.43, 76.85, 83.54, 86.85, 93.15, 113.24, 114.11, 127.08, 127.97, 128.21, 130.18, 135.28, 135.52, 139.00, 144.75, 158.98, 159.01, 159.06, 176.60; IR (ATR): 2095 cm−1; HRMS (ESI): calcd for C37H45N5O7NaSiS [M + Na]+ 754.2707, found 754.2704. Compound 10b: TLC Rf = 0.37 (CHCl3/MeOH 99:1, v/v); 1H NMR (700 MHz, acetone-d6): δ 0.02 (s, 3H), 0.10 (s, 3H), 0.83 (s, 9H), 3.31 (d, 1H, J = 12.60 Hz), 3.41 (dd, 1H, J = 11.20 Hz, J = 3.50 Hz), 3.60 (d, 1H, J = 14.00 Hz), 3.64 (dd, 1H, J = 10.50 Hz, J = 2.10 Hz), 3.81 (s, 6H), 4.11 (d, 1H, J = 4.90 Hz), 4.23–4.25 (m, 1H), 4.44–4.46 (m, 1H), 4.54–4.56 (m, 1H), 6.66 (d, 1H, J= 2.10 Hz), 6.92–7.50 (m, 13H), 8.12 (s, 1H), 11.45 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ −5.63, −5.20, 17.73, 25.25, 46.74, 54.69, 62.04, 70.73, 75.01, 83.48, 86.78, 94.12, 113.19, 113.22, 113.69, 127.14, 127.93, 128.36, 130.25, 130.27, 135.29, 135.38, 138.92, 144.52, 159.05, 159.28, 176.24; IR (ATR): 2096 cm−1.
5-Aminomethyl-2′-O-(tert-butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)(-2-thio)uridine (11a/11b)
A mixture of 2′- and 3′-TBDMS isomers 9a, 10a/9b, 10b (1.36 mmol, 1.0 equiv) was dissolved in anhydrous pyridine (8.1 mL), and Ph3P (0.64 g, 2.5 mmol, 1.8 equiv) was added. After being stirred for 24 h at rt, 25% NH4OH (8.1 mL) was added. The solution was stirred for 1 h at rt and then extracted with CHCl3 (3 × 30 mL). The combined organic layers were dried over MgSO4 and the solvent was removed under reduced pressure. The solid residue was co-evaporated with anhydrous toluene and purified by column chromatography. 2D COSY NMR experiments were used to confirm the identity of 2′ isomers from the correlation of H3′ with 3′OH. To obtain additional quantities of the 2′ TBDMS isomer 11a/11b, the 3′ isomer 12a/12b was isomerized to an equimolar mixture of 2′ and 3′ isomers by stirring in methanol.
Compound 11a was purified on a silica gel column with 4% MeOH in DCM as eluent to obtain a white foam in 65% yield. TLC Rf = 0.52 (CHCl3/MeOH 9:1, v/v); 1H NMR (700 MHz, DMSO-d6): δ 0.04 (s, 3H), 0.06 (s, 3H), 0.85 (s, 9H), 2.99 (d, 1H, J = 14.00 Hz), 3.08 (d, 1H, J = 14.00 Hz), 3.24 (d, 2H, J = 3.50 Hz), 3.75 (s, 6H), 3.97–3.99 (m, 1H), 4.01–4.02 (m, 1H), 4.25 (t, 1H, J= 4.90 Hz), 5.07 (d, 1H, J = 5.60 Hz), 5.85 (d, 1H, J = 4.90 Hz), 6.90–7.41 (m, 13H), 7.55 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ −5.46, −5.44, 17.88, 25.30, 46.58, 54.66, 63.74, 70.81, 75.77, 83.38, 86.47, 88.34, 113.14, 114.02, 126.75, 127.84, 128.12, 130.13, 130.16, 131.79, 131.84, 135.72, 136.79, 145.12, 150.48, 158.79, 162.77; HRMS (ESI): calcd for C37H48N3O8Si [M + H]+ 690.3211, found 690.3204.
Compounds 11b was purified on a silica gel column with 1% MeOH in CHCl3 as eluent to obtain a white foam in 60% yield. TLC Rf = 0.46 (CHCl3/MeOH 9:1, v/v); 1H NMR (250 MHz, DMSO-d6): δ 0.05 (s, 3H), 0.07 (s, 3H), 0.86 (s, 9H), 2.99 (q, 2H, J = 14.50 Hz), 3.23–3.44 (m, 2H), 3.73 (s, 6H), 3.96–4.01 (m, 1H), 4.05–4.10 (m, 1H), 4.22–4.26 (m, 1H), 5.18 (d, 1H, J = 5.50 Hz), 6.67 (d, 1H, J = 3.80 Hz), 6.88–7.42 (m, 13H), 7.63 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ −4.26, 18.91, 26.38, 47.44, 55.57, 64.62, 71.54, 77.75, 84.32, 87.41, 93.95, 114.05, 114.06, 119.88, 127.69, 128.77, 129.03, 131.04, 136.52, 136.61, 138.14, 145.96, 159.69, 159.71, 160.44, 176.86; HRMS (ESI): calcd for C37H48N3O7SiS [M + H]+ 706.2982, found 706.2973.
2′-O-(tert-butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)-N-[(1-β-D-ribofuranosyl-1H-(-2-thio)pyrimidin-5-yl)methyl]taurine 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl ester (14a/14b)
Nucleoside 11a/11b (0.56 mmol, 1.0 equiv) was dissolved in DCM (1.8 mL) and cooled in an ice bath. Then, 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl ethenesulfonate (13, 0.25 g, 0.56 mmol, 1.0 equiv) was dissolved in DCM (275 μL) and added dropwise (total synthesis of ester 13 was previously described by Seeberger et al. 2007). The reaction mixture was stirred at rt for 72 h and then concentrated under reduced pressure. The resulting material was purified by column chromatography to obtain the pure compound 14a/14b.
Compound 14a was purified on a silica gel column with 1% MeOH in CHCl3 as eluent to obtain a white foam in 70% yield. TLC Rf = 0.45 (CHCl3/MeOH 98:2, v/v); 1H NMR (700 MHz, C6D6): δ 0.27 (s, 3H), 0.38 (s, 3H), 0.92 (s, 6H), 1.03 (s, 9H), 1.30 (s, 9H), 1.64 (t, 2H, J = 7.00 Hz), 2.88–2.92 (m, 4H), 3.01 (d, 1H, J = 13.30 Hz), 3.33 (d, 1H, J = 14.00 Hz), 3.53–3.54 (m, 6H), 3.71–3.73 (m, 2H), 3.83 (t, 2H, J = 7.00 Hz), 3.91 (s, 2H), 4.25–4.26 (m, 1H), 4.45 (t, 1H, J = 4.90 Hz), 4.54 (t, 1H, J = 4.20 Hz), 6.29 (d, 1H, J = 3.50 Hz), 6.96–7.91 (m, 23H), 8.00 (s, 1H); 13C NMR (176 MHz, C6D6): δ −5.24, −4.57, 18.00, 19.13, 23.99, 25.65, 26.90, 33.56, 40.94, 43.20, 45.62, 49.82, 54.73, 54.75, 60.55, 63.14, 70.75, 76.28, 77.37, 83.68, 87.10, 89.15, 112.93, 113.59, 113.61, 127.29, 128.19, 128.49, 129.85, 130.47, 130.49, 133.89, 135.65, 135.68, 135.79, 137.08, 145.12, 150.56, 159.19, 163.63; HRMS (ESI): calcd for for C61H82N3O12Si2S [M + H]+1136.5158, found 1136.5187.
Compound 14b was purified on a silica gel column with 1% MeOH in CHCl3 as eluent to obtain a white foam in 65% yield. TLC Rf = 0.70 (CHCl3/MeOH 98:2, v/v); 1H NMR (700 MHz, acetone-d6): δ 0.17 (s, 3H), 0.19 (s, 3H), 0.95 (s, 9H), 0.96 (s, 6H), 1.05 (s, 9H), 1.66 (t, 2H, J = 7.00 Hz), 2.73 (t, 2H, J = 7.00 Hz), 2.79 (d, 1H, 14.00 Hz), 3.05–3.09 (m, 2H), 3.16 (d, 1H, J = 14.00 Hz), 3.49 (d, 2H, 2.80 Hz), 3.79 (s, 6H), 3.81 (t, 2H, J = 7.00 Hz), 3.92 (s, 2H), 4.04 (bs, 1H), 4.24–4.25 (m, 1H), 4.35–4.36 (m, 1H), 4.55 (t, 1H, J = 4.20 Hz), 6.86 (d, 1H, J = 4.20 Hz), 6.92–7.72 (m, 23H), 7.94 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ −4.28, −4.18, 18.90, 19.67, 24.54, 24.55, 26.36, 27.29, 34.45, 41.70, 43.92, 46.03, 50.28, 55.64, 61.30, 64.08, 71.56, 77.70, 78.66, 84.54, 87.65, 93.71, 114.19, 118,79, 128.70, 128.91, 129.10, 130.66, 131.14, 134.49, 136.29, 136.34, 136.50, 138.21, 145.84, 159.84, 159.87, 160.51, 177.11; HRMS (ESI): calcd for C61H82N3O11Si2S2 [M + H]+ 1152.4929, found 1152.4932.
2′-O-(tert-butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)-N-[(1-β-D-ribofuranosyl-1H-(-2-thio)pyrimidin-5-yl)methyl]-N-(trifluoroacetyl)taurine 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl ester (15a/15b)
Nucleoside 14a/14b (0.52 mmol, 1.0 equiv) was dissolved in anhydrous pyridine (11 mL), cooled in an ice bath, and trifluoroacetic anhydride (211 µL, 1.56 mmol, 3.0 equiv) was added dropwise. The mixture was stirred at rt for 2 h. The reaction was quenched with 5% aq. NaHCO3 (30 mL). The resulting solution was extracted with CHCl3 (3 × 40 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated under reduced pressure. Pyridine was removed by co-evaporation with anhydrous toluene, and the resulting foam was purified by column chromatography affording products 15a/15b as rotamers about the –NC(O)CF3 amide bond (two chemical shifts were observed for some 1H and 13C NMR resonances; secondary shifts in 13C NMR spectra are given in parentheses).
Compound 15a was purified on a silica gel column with 2% MeOH in CHCl3 as eluent to obtain a white foam in 81% yield. TLC Rf = 0.51 (CHCl3/MeOH 98:2, v/v); 1H NMR (700 MHz, C6D6): δ 0.30 (s, 2.4H), 0.36 (s, 0.6H), 0.37 (s, 2.4H), 0.48 (s, 0.6H), 1.01 (s, 6H), 1.06 (s, 7.2H), 1.10 (s, 1.8H), 1.34 (s, 1.8H), 1.35 (s, 7.2H), 1.64 (t, 0.4H, J = 7.00 Hz), 1.71 (t, 1.6H, J = 7.00 Hz), 3.22–3.28 (m, 1H), 3.31–3.35 (m, 1H), 3.56–3.57 (m, 6H), 3.66 (dd, 0.8H, J = 11.20 Hz, J = 4.20 Hz), 3.71 (dd, 0.2H, J = 11.20 Hz, J = 4.20 Hz), 3.80 (d, 1H, J = 14.00 Hz), 3.84–3.87 (m, 1H), 3.90 (t, 2H, J = 7.00 Hz), 3.93 (d, 1H, J = 14.00 Hz), 4.09–4.13 (m, 2H), 4.20–4.24 (m, 1H), 4.35–4.40 (m, 2H), 4.46–4.48 (m, 1H), 4.56–4.59 (m, 1H), 6.20 (d, 0.2H, J = 2.80 Hz), 6.22 (d, 0.8H, J = 4.20 Hz), 7.00–7.96 (m, 23H), 8.34 (s, 1H); 13C NMR (176 MHz, acetone-d6): δ −3.21 (−3.18), −3.10 (−3.04), 20.22 (20.27), 21.11, 25.93, 25.95, 27.66 (27.69), 28.71 (28.73), 35.97, 43.17 (43.20), 43.82, 45.73, 47.24, 50.09, 56.98, 62.73, 65.85 (66.01), 72.67 (72.78), 78.19 (78.28), 80.55 (80.95), 85.34 (85.88), 88.77 (88.81), 91.46, 110.28, 115.49, 118.68 (q, J = 287.23 Hz), 129.10, 130.20, 130.60, 132.12, 132.58, 135.93, 137.79, 138.34, 144.22, 147.35, 152.35, 158.72 (q, J = 35.73 Hz), 161.12, 165.67; HRMS (ESI): calcd for C63H80N3O13F3NaSi2S [M + Na]+ 1254.4800, found 1254.4789.
Compound 15b was purified on a silica gel column with 2% MeOH in CHCl3 as eluent to obtain a white foam in 80% yield. TLC Rf = 0.46 (CHCl3/MeOH 95:5, v/v); 1H NMR (700 MHz, acetone-d6): δ 0.15 (s, 2.4 H), 0.17 (s, 3H), 0.21 (s, 0.6H), 0.94 (s, 7.2H), 0.95 (s, 1.8H), 0.99 (s, 1.2H), 1.01 (s, 4.8H), 1.04 (s, 1.8H), 1.05 (s, 7.2H), 1.68 (t, 0.4H, J = 7.00 Hz), 1.70 (t, 1.6H, J = 7.00 Hz), 3.47 (dd, 0.8H, J = 11.20 Hz, J = 2.10 Hz), 3.50 (dd, 0.2H, J = 11.20 Hz, J = 2.10 Hz), 3.54 (dd, 0.2H, 11.20 Hz, J = 4.90 Hz), 3.62 (dd, 0.8H, J = 11.20 Hz, J = 5.60 Hz), 3.66–3.72 (m, 2H), 3.78–3.79 (m, 6H), 3.81–3.85 (m, 2H), 3.92 (s, 2H), 3.99–4.02 (m, 1H), 4.05–4.12 (m, 3H), 4.15–4.19 (m, 1H), 4.27–4.31 (m, 1H), 4.41–4.46 (m, 1H), 6.66 (d, 0.2H, J = 2.10 Hz), 6.69 (d, 0.8H, J = 2.80 Hz), 6.84–7.72 (m, 23H), 7.89 (s, 0.2H), 8.02 (s, 0.8H), 11.52 (s, 0.8H), 11.55 (s, 0.2H); 13C NMR (176 MHz, acetone-d6): δ −2.83, −2.78 (−2.61), 20.38 (20.45), 21.13, 25.95, 25.97, 27.86 (27.90), 28.73, 35.98, 43.20 (44.01), 45.93, 47.37 (48.15), 50.06, 56.97, 57.02, 62.74, 65.72 (66.13), 72.39 (72.60), 79.09 (79.20), 80.56 (81.04), 85.29 (85.88), 88.88 (88.92), 95.88 96.69, 115.03 (115.10), 115.52 (q, J = 288.64 Hz), 129.16 (129.40), 129.78 (129.84), 130.15–130.26 (m), 130.68, 131.54, 132.13, 132.60–132.68 (m), 135.93, 137.80, 138.22, 138.33, 142.43 (142.70), 144.20, 147.21 (147.48), 158.81 (q, J = 35.20 Hz), 162.47, 178.34 (178.51); HRMS (ESI): calcd for C63H80N3O12F3NaSi2S2 [M + Na]+ 1270.4572, found 1270.4581.
2′-O-(tert-butyldimethylsilyl)-5′-O-(4,4′-dimethoxytrityl)-N-[(1-β-D-ribofuranosyl-1H-(-2-thio)pyrimidin-5-yl)methyl]-N-(trifluoroacetyl)taurine 4-(tert-butyldiphenylsilanyloxy)-2,2-dimethylbutyl ester 3′-(cyanoethyl N,N-diisopropylphosphoramidite) (16a/16b)
The 5′-DMTr, 2′-TBDMS nucleoside 15a/15b (0.15 mmol, 1.0 equiv) was dissolved in anhydrous DCM (928 μL) under Ar atmosphere, then diisopropylethylamine (102 μL, 0.59 mmol, 4.0 equiv) and 2-cyanoethyl N,N-diisopropylaminochlorophosphoramidite (65 μL, 0.30 mmol, 2.0 equiv) were added. The reaction mixture was stirred at rt for 3 h. The solution was diluted with DCM (12 mL) and washed with 5% aq. NaHCO3 (8 mL) and water (2 × 8 mL). The organic layer was dried over MgSO4 and the solvent was removed under reduced pressure. The crude product 16a/16b was purified by flash chromatography using petroleum ether:ethyl acetate (2:1 v/v). The material exists as a mixture of stereoisomers about phosphorus, wherein each is a rotamer about the –NC(O)CF3 amide bond and two or more chemical shifts are observed for some of the 1H and 31P NMR resonances.
Compound 16a was isolated as a white foam in 83% yield. TLC Rf = 0.38 (benzene, DCM, Et3N 7:2:1 v/v/v); 1H NMR (700 MHz, C6D6): δ 0.17–0.27 (m, 6H), 0.97–1.02 (m, 9H), 1.07–1.12 (m, 18H), 1.22–1.26 (m, 9H), 1.75–1.80 (m, 2H), 3.43–3.58 (m, 2H), 3.62–3.80 (m, 6H), 3.85–3.96 (m, 9H), 4.00–4.16 (m, 5H), 4.19–4.29 (m, 3H), 4.35–4.46 (m, 1H), 4.52–4.65 (m, 1H), 5.94–6.09 (m, 1H), 6.95–7.02 (m, 3H), 7.30–7.52 (m, 16H), 7.77–7.90 (m, 4H), 8.02–8.09 (m, 1H), 10.43 (s, 1H); 31P NMR (101.25 Hz, C6D6): δ 149.62, 150.18, 150.61, 150.81; HRMS (ESI): calcd for C72H97N5O14F3NaSi2SP [M + Na]+ 1454.5879, found 1454.5863.
Compound 16b was isolated as a light yellow foam in 92% yield. TLC Rf = 0.70 (benzene, DCM, Et3N 7:2:1 v/v/v); 1H NMR (250 MHz,C6D6): δ 0.20–0.30 (m, 6H), 0.98–1.03 (m, 9H), 1.05–1.12 (m, 18H), 1.18–1.31 (m, 9H), 1.74–1.80 (m, 2H), 3.54–3.81 (m, 7H), 3.86–3.95 (m, 9H), 3.97–4.06 (m, 2H), 4.08–4.11 (m, 2H), 4.14–4.17 (m, 3H), 4.20–4.29 (m, 2H), 4.43–4.54 (m, 1H), 4.57–4.66 (m, 1H), 6.90–7.04 (m, 4H), 7.24-.67 (m, 16H), 7.77–7.81 (m, 4H), 8.11–8.17 (m, 1H), 11.63 (s, 1H); 31P NMR (101.25 Hz, C6D6): δ 149.80, 150.75, 150.95, 151.24; HRMS (ESI): calcd for C72H97N5O13F3NaSi2S2P [M + Na]+ 1470.5650, found 1454.5636.
Oligonucleotide synthesis
Oligoribonucleotides were synthesized manually on a 5-µmol scale using slightly modified Sproat's procedure (Sproat 2005). Commercially available monomeric units A, C, U, and G were protected with DMTr and TBDMS on the 5′- and 2′-hydroxy functions, respectively, and the exocyclic amine functions of A, C, and G were masked with 4-tert-butylphenoxyacetyl (tac) (Proligo). Typical rA(tac)-succinyl-CPG (Proligo) support and 0.1 M acetonitrile solutions of monomeric units were used. A, U, C, and G amidites were coupled in 8 molar excess for 8 min in the presence of Activator 42 (0.25 M solution of 5-(3,5-bis(trifluoromethyl)phenyl)-1H-tetrazole in CH3CN), while modified units were used in 12 molar excess and coupled twice, each time using 6 molar excess of amidite and 12 min coupling time. Capping was performed with tac anhydride (Fast protection Cap A:Cap B 1:1.1 v/v) for 2 min. A 0.02 M iodine solution in THF-H2O-pyridine (90.5:0.45:9.05 v/v/v; 8 equiv) was used as an oxidizing agent for 2 min for each oxidation step.
RNA deprotection and purification
The “trityl-off” CPG-bound RNA was transferred from the column to a screw cap glass vial, and 6.5 mL of Et3N/CH3CN 1:1 v/v was added. The solution was stirred for 25 min, and then the solvent was removed. The support-bound RNA was washed with acetonitrile, dried in vacuum for 30 min, and treated with 8.5 mL of 8 M ethanolic ammonia at rt for 8 h. The supernatant was removed and the support was washed with an additional 3 mL of anhydrous ethanol. The combined washings were evaporated on a Speed-Vac concentrator.
Desilylation of hmt-ASLLeu(UUR)(τm5U34) was conducted with Et3N•3HF/NMP (1:1, v/v, 2 mL) at rt for 24 h. The reaction was quenched by addition of ethoxytrimethylsilane (4 mL), and crude RNA was precipitated using t-butyl methyl ether (10 mL). RNA was collected by centrifugation, washed with t-butyl methyl ether (2 × 10 mL), and purified using IE-HPLC.
hmt-ASLLys(τm5s2U34) was dissolved in 5 mL of 1 M TEAF in NMP and stirred. Desilylation was conducted at rt for 24 h and then quenched by the addition of 0.05 M Na2HPO4–NaH2PO4 buffer solution (pH 7.6). Crude RNA was desalted on a column packed with Sephadex G-25 (elution with 20% aqueous ethanol), monitored by UV detection at 260 nm. The RNA-containing eluate was lyophilized and then purified.
Fully deprotected RNAs were purified by anion-exchange HPLC on a Waters AP-2 column packed with TSK SuperQ-5PW resin. For elution, we used a linear gradient of NaBr (50–650 mM) in sterile 20 mM Na2HPO4–NaH2PO4 buffer solution (pH 7.5), containing EDTA (50 µM) and 10% CH3CN; flow 9 mL/min. Fractions containing the desired product were collected, concentrated, and desalted on a column packed with Sephadex G-25. The desalted RNAs were lyophilized to obtain 120 OD260 of hmt-ASLLeu(UUR)(τm5U34) and 100 OD260 of hmt-ASLLys(τm5s2U34). The oligomers were analyzed by MALDI-TOF (Supplemental Material).
RNA enzymatic digestion and HPLC analysis of nucleoside composition
The nucleoside composition of oligoribonucleotides was confirmed by enzymatic hydrolysis of RNAs to nucleosides using nuclease P1 and alkaline phosphatase (Gehrke et al. 1982; Gehrke and Kuo 1989). The resulting nucleoside mixtures were analyzed with a C18 column (ODS2, 4.6 mm × 250 mm), with a linear gradient of buffer A (10 mM KH2PO4; pH 5.3) and buffer B (20% methanol in 10 mM KH2PO4; pH 5.1) with a flow of 0.75 mL/min. As shown in Figure 4C, HPLC elutions monitored at 264 nm indicate that hmt-ASLLeu(UUR) and hmt-ASLLeu contain τm5U (9.51 min) and τm5s2U (18.04 min), respectively. The peaks were compared with reference samples of the modified units in separate control experiments (Fig. 4D).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
This study was supported by grant 1306/B/H03/2011/40 from the National Science Centre, Poland (to G.L.).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.044412.114.
REFERENCES
- Agris PF, Malkiewicz A, Kraszewski A, Everett K, Nawrot B, Sochacka E, Jankowska J, Guenther R 1995. Site-selected introduction of modified purine and pyrimidine ribonucleosides into RNA by automated phosphoramidite chemistry. Biochimie 77: 125–134 [DOI] [PubMed] [Google Scholar]
- Ali AM, Hill B, Taylor SD 2009. Trichloroethyl group as a protecting group for sulfonates and its application to the synthesis of a disulfonate analog of the tyrosine sulfated PSGL-143–50 peptide. J Org Chem 74: 3583–3586 [DOI] [PubMed] [Google Scholar]
- Andrianov AK, Marin A, Chen J, Sargent J, Corbett N 2004. Novel route to sulfonated polyphosphazenes: single-step synthesis using “noncovalent protection” of sulfonic acid functionality. Macromolecules 37: 4075–4080 [Google Scholar]
- Avitabile BG, Smith CA, Judd DB 2005. Pentafluorophenyl sulfonate ester as a protecting group for the preparation of biaryl- and heterobiaryl sulfonate esters. Org Lett 7: 843–846 [DOI] [PubMed] [Google Scholar]
- Bajji AC, Davis DR 2002. Synthesis of the tRNALys,3 anticodon stem–loop domain containing the hipermodified ms2t6A nucleoside. J Org Chem 67: 5352–5358 [DOI] [PubMed] [Google Scholar]
- Bajji AC, Sundaram M, Myszka DG, Davis DR 2002. An RNA complex of the HIV-1 A-loop and tRNALys,3 is stabilized by nucleoside modifications. J Am Chem Soc 124: 14302–14303 [DOI] [PubMed] [Google Scholar]
- Baxter NJ, Rigoreau LJM, Laws AP, Page MI 2000. Reactivity and mechanism in the hydrolysis of β-sultams. J Am Chem Soc 122: 3375–3385 [Google Scholar]
- Bilbille Y, Vendeix FAP, Guenther R, Malkiewicz A, Ariza X, Vilarrasa J, Agris PF 2009. The structure of the human tRNALys3 anticodon bound to the HIV genome is stabilized by modified nucleosides and adjacent mismatch base pairs. Nucleic Acids Res 37: 3342–3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudou V, Langridge J, Van Aerschot A, Hendix C, Millar A, Weiss P, Herdewijn P 2000. Synthesis of the anticodon hairpin tRNAfMet containing N-{[9-(β-D-ribofuranosyl)-9H-purin-6-yl]carbamoyl}-L-threonine (=N6-{{[(1S,2R)-1-Carboxy-2-hydroxypropyl]amino}carbonyl} adenosine, t6A). Helv Chim Acta 83: 152–161 [Google Scholar]
- Cantara WA, Bilbille Y, Kim J, Kaiser R, Leszczynska G, Malkiewicz A, Agris PF 2012. Modifications modulate anticodon loop dynamics and codon recognision of E.coli tRNAArg1,2. J Mol Biol 416: 579–597 [DOI] [PubMed] [Google Scholar]
- Cantara WA, Murphy FV IV, Demirci H, Agris PF 2013. Expanded use of sense codons is regulated by modified cytidines in tRNA. Proc Natl Acad Sci 110: 10964–10969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DC, Gaus H, Krotz AH, Arnold J, Carty RL, Moore MN, Scozzari AN, Lowery K, Cole DL, Ravikumar VT 2003. Synthesis of high-quality antisense drugs. Addition of acrylonitrile to phosphorothioate oligonucleotides: adduct characterization and avoidance. Org Process Res Dev 7: 832–838 [Google Scholar]
- Damha MJ, Ogilvie KK 1993. Oligoribonucleotide synthesis. The silyl-phosphoramidite method. Methods Mol Biol 20: 81–114 [DOI] [PubMed] [Google Scholar]
- Duram PC, Bajji AC, Sundaram M, Kumar RK, Davis DR 2005. Structural effects of hypermodified nucleosides in the Escherichia coli and human tRNALys anticodon loop: the effect of nucleosides s2U, mcm5U, mcm5s2U, mnm5s2U, t6A and ms2t6A. Biochemistry 44: 8078–8089 [DOI] [PubMed] [Google Scholar]
- Eshete M, Marchbank MT, Deutscher SL, Sproat B, Leszczynska G, Malkiewicz A, Agris PF 2007. Specificity of phage display selected peptides for modified anticodon stem and loop domains of tRNA. Protein J 26: 61–73 [DOI] [PubMed] [Google Scholar]
- Florentz C, Sohm B, Tryoen-Tóth P, Pütz J, Sissler M 2003. Human mitochondrial tRNAs in health and disease. Cell Mol Life Sci 60: 1356–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke CW, Kuo KC 1989. Ribonucleoside analysis by reversed-phase high-performance liquid chromatography. J Chromatogr 471: 3–36 [DOI] [PubMed] [Google Scholar]
- Gehrke CW, Kuo KC, McCune RA, Gerhardt KO 1982. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J Chromatogr B Biomed Sci Appl 230: 297–308 [PubMed] [Google Scholar]
- Helm M, Brulé H, Friede D, Giegé R, Pütz J, Florentz C 2000. Search for characteristic structural features of mammalian mitochondrial tRNAs. RNA 6: 1356–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M, Ahmed V, Hill B, Ahmed Z, Taylor SD 2008. A re-examination of the difluoromethylenesulfonic acid group as a phosphotyrosine mimic for PTP1B inhibition. Bioorg Med Chem 16: 6764–6777 [DOI] [PubMed] [Google Scholar]
- Jühling E, Pütz J, Florentz C, Stadler PF 2012. Armless mitochondrial tRNAs in Enoplea (Nematoda). RNA Biol 9: 1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y, Yasukawa T, Ohta S, Akira S, Ishihara K, Watanabe K, Suzuki T 2004. Codon-specific translational defect caused by a wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc Natl Acad Sci 101: 15070–15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamann D, Hofbauer G 1953. Reduktive Spaltungen von Sulfamiden. Chem Ber 86: 1246–1252 [Google Scholar]
- Klán P, Pelliccioli AP, Pospíšil T, Wirz J 2002. 2,5-dimethylphenacyl esters: a photoremovable protecting group for phosphates and sulfonic acids. Photochem Photobiol Sci 1: 920–923 [DOI] [PubMed] [Google Scholar]
- Kurata S, Weixlbaumer A, Ohtsuki T, Shimazaki T, Wada T, Kirino Y, Takai K, Watanabe K, Ramakrishnan V, Suzuki T 2008. Modified uridines with C5-methylene substituents at the first position of the tRBA anticodon stabilize U·G wobble pairing during decoding. J Biol Chem 283: 18801–18811 [DOI] [PubMed] [Google Scholar]
- Kurschat WC, Muller J, Wombacher R, Helm M 2005. Optimizing splinted ligation of highly structured small RNAs. RNA 11: 1909–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynska G, Pie¸ta J, Sproat B, Malkiewicz A 2011. Chemical synthesis of an RNA sequence containing 2-thiocytidine (s2C): the DY647 labelled anticodon stem and loop sequence of Staphylococcus aureus tRNAArg (s2C32, mnm5U34, t6A37). Tetrahedron Lett 52: 4443–4447 [Google Scholar]
- Leszczynska G, Pie¸ta J, Leonczak P, Tomaszewska A, Malkiewicz A 2012. Site-specific incorporation of 5-methylaminomethyl-2-thiouridine and 2-thiouridine(s) into RNA sequences. Tetrahedron Lett 53: 1214–1217 [Google Scholar]
- Leszczynska G, Leonczak P, Dziergowska A, Malkiewicz A 2013. mt-tRNA components: synthesis of (2-thio)uridines modified with blocked glycine/taurine moieties at C-5,1. Nucleosides Nucleotides Nucleic Acids 32: 599–616 [DOI] [PubMed] [Google Scholar]
- Lusic H, Gustilo EM, Vendeix FA, Kaiser R, Delaney MO, Graham WD, Moye VA, Cantara WA, Agris PF, Deiters A 2008. Synthesis and investigation of the 5-formylcytidine, modified anticodon stem and loop of the human mitochondrial tRNAMet. Nucleic Acids Res 36: 6548–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkiewicz A, Sochacka E 1983. The protected derivatives of 5-methylaminomethyl-2-thiouridine and 5-carbomethoxymethyl-2-thiouridine as components for the oligonucleotide synthesis. Tetrahedron Lett 24: 5387–5390 [Google Scholar]
- Miller SC 2010. Profiling sulfonate ester stability: identification of complementary protecting groups for sulfonates. J Org Chem 75: 4632–4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerscough PM, Fairbanks AJ, Jones AH, Bruce I, Choi SS, Fleet GWJ, Al-Daher SS, di Bello IC, Winchester B 1992. Inhibition of α-mannosidases by seven carbon sugars: synthesis of some seven carbon analogues of mannofuranose. Tetrahedron 48: 10177–10190 [Google Scholar]
- Ogata T, Wada T 2006. Chemical synthesis of RNA including 5-taurinomethyluridine. Nucleic Acids Symp Ser 50: 9–10 [DOI] [PubMed] [Google Scholar]
- Ogata T, Wada T 2008. Chemical synthesis of RNA including 5-taurinomethyluridine and 5-taurinomethyl-2-thiouridine. Nucleic Acids Symp Ser 52: 323–324 [DOI] [PubMed] [Google Scholar]
- Okamoto I, Seio K, Sekine M 2006. Improved synthesis of oligonucleotides containing 2-thiouridine derivatives by use of diluted iodine solution. Tetrahedron Lett 47: 583–585 [Google Scholar]
- Richman JE, Atkins TJ 1974. Nitogen analogs of crown ethers. J Am Chem Soc 96: 2268–2270 [Google Scholar]
- Roberts JC, Gao H, Gopalsamy A, Kongsjahju A, Patch RJ 1997. Neopentyl ester protecting groups for arylsulfonic acids. Tetrahedron Lett 38: 355–358 [Google Scholar]
- Seeberger S, Griffin RJ, Hardcastle IR, Golding BT 2007. A new strategy for the synthesis of taurine derivatives using the ‘safety-catch’ principle for the protection of sulfonic acids. Org Biomol Chem 5: 132–138 [DOI] [PubMed] [Google Scholar]
- Seio K, Wada T, Sakamoto K, Yokoyama S, Sekine M 1998. Chemical synthesis and properties of conformationally fixed diuridine monophosphates as building blocks of the RNA turn motif. J Org Chem 63: 1429–1443 [Google Scholar]
- Sissler M, Lorber B, Messmer M, Schaller A, Pütz J, Florentz C 2008. Handling mammalian mitochondrial tRNAs and aminoacyl-tRNA synthetases for functional and structural characterization. Methods 44: 176–189 [DOI] [PubMed] [Google Scholar]
- Sochacka E 2001. Efficient assessment of modified nucleoside stability under conditions of automated oligonucleotide synthesis: characterization of the oxidation and oxidative desulfurization of 2-thiouridine. Nucleosides Nucleotides Nucleic Acids 20: 1871–1879 [DOI] [PubMed] [Google Scholar]
- Sproat BS 2005. RNA synthesis using 2′-O-(tert-butyldimethylsilyl) protection. Methods Mol Biol 288: 17–31 [DOI] [PubMed] [Google Scholar]
- Sundaram M, Crain PF, Davis DR 2000. Synthesis and characterization of the native anticodon domain of E. coli tRNALys: simultaneous incorporation of modified nucleosides mnmn5s2U, t6A, and pseudouridine using phosphoramidite chemistry. J Org Chem 65: 5609–5614 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nagao A, Suzuki T 2011a. Human mitochondrial diseases caused by lack of taurine modification in mitochondrial tRNAs. WIREs RNA 2: 376–386 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nagao A, Suzuki T 2011b. Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases. Annu Rev Genet 45: 299–329 [DOI] [PubMed] [Google Scholar]
- Wada T, Shimazaki T, Nakagawa S, Otuki T, Kurata S, Suzuki T, Watanabe K, Saigo K 2002. Chemical synthesis of novel taurine-containing uridine derivatives. Nucleic Acids Res Suppl 2: 11–12 [DOI] [PubMed] [Google Scholar]
- Watanabe K 2010. Unique feature of animal mitochondrial translation systems. The non-universal genetic code, unusual features of the translational apparatus and their relevance to human mitochondrial diseases. Proc Jpn Acad Ser B Phys Biol Sci 86: 11–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weixlbaumer A, Murphy FV IV, Dziergowska A, Malkiewicz A, Vendeix FAP, Agris PF, Ramakrishnan V 2007. Mechanism for expanding the decoding capacity of transfer RNAs by modification of uridines. Nat Struct Mol Biol 14: 498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende S, Platzer EG, Jühling F, Pütz J, Florentz C, Stadler PF, Mörl M 2014. Biological evidence for the world's smallest tRNAs. Biochimie 100: 151–158 [DOI] [PubMed] [Google Scholar]
- Wrobel J, Rogers J, Green D, Kao W 2002. Use of an isopropyl ester moiety as a sulfonic acid protecting group in a greatly improved synthesis of an arylsulfonic acid-based follicle stimulating hormone antagonist. Synth Commun 32: 2695–2704 [Google Scholar]
- Yan L, Müller CE 2004. Preparation, properties, reactions, and adenosine receptor affinities of sulfophenylxanthine nitrophenyl esters: toward the development of sulfonic acid prodrugs with peroral bioavailability. J Med Chem 47: 1031–1043 [DOI] [PubMed] [Google Scholar]