Abstract
Purkinje cells in the brain region known as the cerebellum act by inhibiting their target neurons. A paper in this issue provides an explanation for how this inhibition might be used to control the timing of action potentials. But experts are not equally convinced about the functional relevance of this finding.
Time for action
JAVIER F. MEDINA
Person and Raman1 identify the conditions that allow Purkinje cells to control the timing of spikes (action potentials) in their target cerebellar nuclear neurons with millisecond precision. This is very exciting news for several reasons.
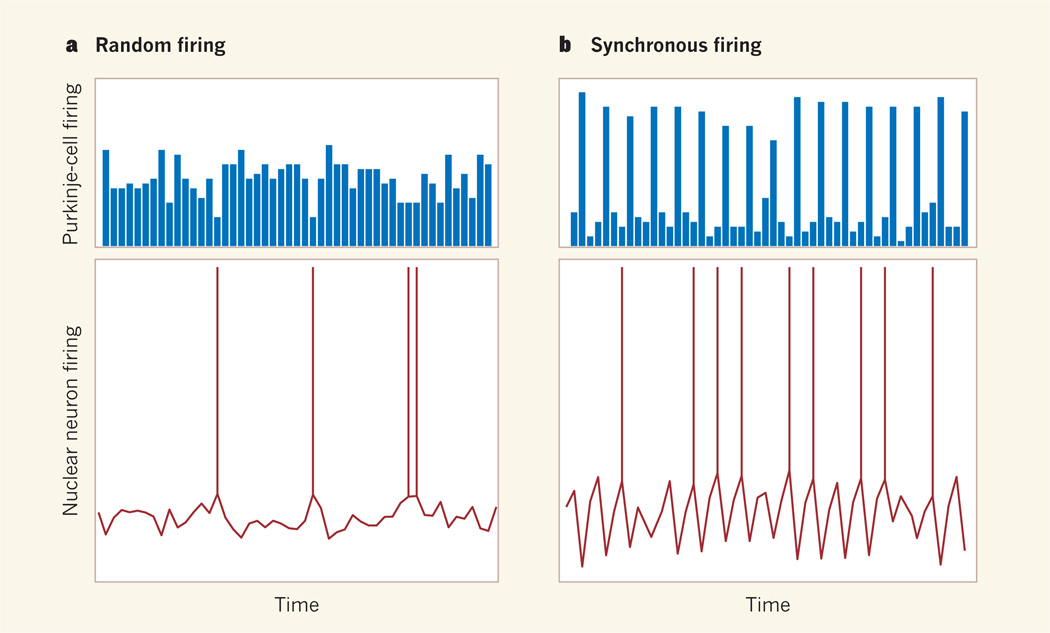
From a purely computational standpoint, the paper reveals a time-locking mechanism (Fig. 1) for neurons receiving inhibitory input from groups of cells that fire action potentials at high and irregular rates. This mechanism, which may be applicable to other inhibitory circuits in the brain, stands in stark contrast to the precise regulation of spike timing in another brain region — the cerebral cortex — by synchronous inputs that depolarize the neuronal membrane. It relies on several idiosyncratic features of cerebellar nuclear neurons that were previously unknown, including a high intrinsic firing rate, ultra-fast inhibitory synapses (neuronal connections) and, as also suggested by others2, some degree of synchronous activity in the Purkinje-cell input.
Figure 1. Decoding Purkinje-cell inputs.
a, Purkinje cells of the cerebellum send inhibitory inputs (blue bars) to their target nuclear neurons. Consequently, nuclear neurons get little chance to fire action potentials (red lines). b, Person and Raman1 report that if two or more inhibitory inputs from Purkinje cells are synchronized (longer bars), it creates more opportunities for nuclear neurons to fire action potentials in the gaps between such ‘bundles’ of inhibitory inputs, entraining their spiking. (This graphic is not an exact representation of the authors’ data.)
The findings are equally intriguing for our understanding of how the brain controls movement, especially given that a time-locking mechanism seems perfectly suited to contribute to one of the most notable functions ascribed to the cerebellum — coordinating muscles with utmost accuracy and temporal precision. But does the cerebellum really use time-locking to control movement? After all, years of research have shown that much of the information sent to motor areas from the cerebellum is conveyed by a rate code3 — a modulation in the firing frequency of neurons in which the precise timing of the spikes is irrelevant because the rate is averaged over tens to hundreds of milliseconds.
Clearly, modulation of firing frequency has a crucial role in cerebellar processing. But before discarding time-locking of nuclear neurons as a computationally elegant, but otherwise functionally irrelevant, mechanism, let’s remember that codes based on the timing or the rate of spikes are not mutually exclusive. Previous work has demonstrated that spikes in Purkinje cells less than 100 micrometres apart can be precisely synchronized4,5, and that the level of synchrony is dynamically regulated4. So, at least in theory3, this would allow inputs from different groups of Purkinje cells to become synchronized for brief periods of time, and, when a high degree of temporal precision is required (for instance, at the beginning or end of a movement), to control the exact timing of spikes in nuclear neurons. To control other aspects of movement, such as amplitude or speed, the cerebellum might switch Purkinje cells back to the asynchronous mode and modulate their firing frequency up or down, thus regulating the firing rate of nuclear neurons.
As with every landmark paper, several questions remain. For example, in addition to inhibitory synapses from Purkinje cells, nuclear neurons receive excitatory inputs from mossy and climbing fibres of neurons in other parts of the brain. How does the cerebellum process such opposing signals to achieve its goals? Examining the patterns of excitatory and inhibitory inputs in vivo, and understanding how they are integrated in nuclear neurons, should provide an answer.
Another question arises from work6 showing that cerebellar nuclear neurons can be classified into different functional groups according to not only the proteins they express or their particular anatomical connectivities, but also their electrical properties. Do these cell groups also differ in the way they process the inputs they receive?
Finally, Person and Raman’s findings reveal what nuclear neurons can do, but not what they actually do. Does synchronization of Purkinje cells result in the spiking activity of nuclear neurons during normal cerebellar processing? The answer may come from whole-cell recordings in awake animals to check whether the membrane-voltage signature of individual nuclear cells in vivo is consistent with a spike-generating mechanism based on membrane repolarization after synchronous inhibition. As for the question of whether time-locking is part of the cerebellar code used for motor control, it will be necessary to record from many nuclear cells simultaneously, and to examine the timing and synchronization of their spikes relative to movement when the animal is active.
Sync or sink?
KAMRAN KHODAKHAH
Elucidating the principles by which neurons and neuronal circuits ‘compute’ remains a cardinal objective in neuroscience. Through an ingenious set of experiments, Person and Raman1 describe one such computational principle: how a (nuclear) neuron decodes the temporal structure of its synchronous (Purkinje cell) inhibitory inputs while ignoring the asynchronous ones. The question is how relevant this temporal decoding mechanism is to cerebellar processing.
Person and Raman demonstrate in vitro that synchronous activity of even two Purkinje-cell inputs is sufficient to modestly entrain (time-lock) the activity of their target nuclear neuron. They posit that temporal decoding is the mechanism by which nuclear neurons encode information in vivo. But a confounding limitation of such temporal decoding is that the probability of firing entrained spikes is reduced so much that nuclear neurons cannot rate-code — that is, the persistent activity of the asynchronous inputs reduces the probability of a nuclear neuron firing entrained spikes to such an extent that it can no longer encode the firing rate of its synchronous inputs in its own firing rate1. This is problematic, because it has been known3,7,8 for more than 40 years that the activity rates of individual nuclear and Purkinje neurons correlate with movement and thereby with each other.
Moreover, to date, no in vivo data have refuted the assertion that the firing rate of a nuclear neuron is inversely related to the average firing rate of its synaptically connected Purkinje cells9. Therefore, even if several Purkinje cells could be perfectly synchronized in vivo, it is doubtful that, as implied1, nuclear neurons process and encode movement-related information through temporal decoding alone.
It is quite possible that some sort of temporal decoding occurs intermittently in vivo, for example during brief conditions when very precise timing of muscle movement is needed. However, the study1 suggests that it often takes several spikes from synchronously active Purkinje cells (requiring tens to hundreds of milliseconds) to entrain a nuclear neuron. It is therefore questionable whether a rate-coding nuclear neuron can rapidly switch modes and transiently time-lock to a few synchronous Purkinje-cell spikes.
Because of the severely diminished probability of firing of entrained spikes, the authors often had to artificially depolarize the nuclear neurons to promote spiking. But what depolarizing inputs could permit temporal decoding in vivo without diminishing time-locking? A neuromodulator molecule may partially provide sustained depolarization free of synaptic ‘noise’, although there is no evidence for this premise. The most likely depolarizing source is the excitatory inputs from mossy and climbing fibres. These inputs powerfully modulate the firing of Purkinje cells in vivo, and their collateral synaptic inputs to nuclear neurons should also depolarize the latter. But the strength of these inputs changes with movement, and they will also generate synaptic noise. In vivo, these inputs are therefore likely to significantly diminish the precision of the modest time-locked spiking seen in vitro1.
Linking in vitro and in vivo studies is always challenging, and in this case it is unclear whether nuclear neurons can temporally decode synchronous activity of a small number of Purkinje cells in vivo. With strong electrical stimulation of the cerebellar cortex, Person and Raman1 could evoke time-locked responses. But estimating the number of activated Purkinje cells in these experiments is challenging, and electrical stimulation does not have any specificity for activating cell types or axonal processes of neurons. A more rigorous test of temporal decoding using the technique of optogenetics will allow selective, synchronous activation of a few Purkinje cells in vivo, while eliminating complications that arise from the unavoidable electrical activation of mossy and climbing fibres.
Lastly, even if a small number of synchronized Purkinje cells could modestly promote entrainment of nuclear neurons in vivo, complete synchronous activity of even two Purkinje cells is improbable. When examined pairwise in vivo during brief periods of high synchrony, the correlation between the firing of two Purkinje cells is of the order of a few per cent10,11 — tenfold lower than the 100% synchrony assumed here1. To what extent nuclear neurons can meaningfully entrain when a few Purkinje cells are only partially synchronized remains to be established.
The new computational principle described by Person and Raman unveils a powerful tool in the brain’s information-processing tool kit. Future experiments will undoubtedly identify the circuits in which this tool is used, and may outline the conditions under which it contributes to motor coordination by the cerebellum.
THE PAPER IN BRIEF.
Purkinje cells regulate the entire output of the cerebellar cortex — a brain area responsible for generating precise movement.
They do so by sending inhibitory signals to nuclei (groups of neurons) lying deep within the cerebellum.
So, while Purkinje cells are firing, the downstream nuclear neurons should be inhibited.
However, the firing rate of Purkinje cells and nuclear neurons do not always vary inversely.
Person and Raman1 (page 502) propose that the answer to this conundrum lies in synchrony of firing.
Through in vitro experiments, they find that, as expected, random firing of Purkinje neurons powerfully inhibits nuclear cells.
But when a small number of Purkinje cells fire synchronously, nuclear neurons fire action potentials that are ‘time-locked’ to the gaps between the inhibitory Purkinje inputs (Fig. 1).
Contributor Information
Javier F. Medina, Department of Psychology, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6241, USA. jmed@sas.upenn.edu
Kamran Khodakhah, Dominick P. Purpura Department of Neuroscience, Albert Einstein College of Medicine, Bronx, New York 10461, USA. k.khodakhah@einstein.yu.edu.
References
- 1.Person AL, Raman IM. Nature. 2012;481:502–505. doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauck V, Jaeger D. J. Neurosci. 2000;20:3006–3016. doi: 10.1523/JNEUROSCI.20-08-03006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Zeeuw CI, et al. Nature Rev. Neurosci. 2011;12:327–344. doi: 10.1038/nrn3011. [DOI] [PubMed] [Google Scholar]
- 4.Bell CC, Grimm RJ. J. Neurophysiol. 1969;32:1044–1055. doi: 10.1152/jn.1969.32.6.1044. [DOI] [PubMed] [Google Scholar]
- 5.Ebner TJ, Bloedel JR. J. Neurophysiol. 1981;45:948–961. doi: 10.1152/jn.1981.45.5.948. [DOI] [PubMed] [Google Scholar]
- 6.Uusisaari M, Knöpfel T. Cerebellum. 2011;10:637–646. doi: 10.1007/s12311-010-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thach WT. J. Neurophysiol. 1968;31:785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DM, Edgley SA. J. Physiol. 1984;351:411–432. doi: 10.1113/jphysiol.1984.sp015253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDevitt CJ, Ebner TJ, Bloedel JR. Brain Res. 1987;425:1–13. doi: 10.1016/0006-8993(87)90477-x. [DOI] [PubMed] [Google Scholar]
- 10.Wise AK, Cerminara NL, Marple-Horvat DE, Apps R. J. Physiol. 2010;588:2373–2390. doi: 10.1113/jphysiol.2010.189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heck DH, Thach WT, Keating JG. Proc. Natl Acad. Sci. USA. 2007;104:7658–7663. doi: 10.1073/pnas.0609966104. [DOI] [PMC free article] [PubMed] [Google Scholar]