Abstract
In vitro basophil responses have longed been used in mechanistic studies to help assess the human allergic diathesis, particularly during therapeutic intervention. Recent evidence points to the use of dendritic cells (DCs) in also being valuable in evaluating therapies aimed at lessening disease through immunomodulation. This review article therefore takes a look at some of the recent advances in old and new assays employing both basophils and DCs, with the added perception that the responses mediated by two cell types are insightful towards understanding immune cell mechanisms underlying allergic disease.
Keywords: basophil, dendritic cell, cytokine, mediators, human, methods
Introduction
Allergic diseases occur as a result of inappropriate immune responses to harmless antigens, and in one form or another, affect 25% of Americans in the United States with a disproportionate increase among children with asthma and food-related hypersensitivities. The mechanisms underlying the pathogenesis of chronic allergic disease, and allergic inflammation in general, are multifaceted, involving many immunological aspects that result from both genetic and environmental factors. For example, the proposed mechanisms underlying the Hygiene Hypothesis (Strachan, 2000) suggest that allergic disease results, in part, from insufficient stimulation of the Th1 and/or T regulatory (Treg) arm of the immune system by infectious agents such as viruses, bacteria, and parasites. As a consequence, this leads to an overactive Th2 arm and the production of allergen specific IgE that arms effector cells such as basophils and mast cells for the release of allergy-inducing inflammatory mediators (e.g. histamine, LTC4, Th2 cytokines). Consistent with the notion that such mechanisms exist, there is emerging evidence that certain pro-Th1 and/or anti-Th2 innate immune responses are, in fact, impaired in allergic disease. Therefore, this review will highlight such findings by focusing on laboratory assays that assess human immune cell function. And, while many cell types participate in allergic disease and thus could be included in this discussion, our focus will be limited to those cells central to our studies. In particular basophils, since these cells secrete three classes of mediators most expressed in allergic inflammation, namely vasoactive amines (histamine), leukotrienes (LTC4), and cytokines (IL-4/IL-13). Emphasis will also be given to our work done using immature blood dendritic cells, primarily plasmacytoid DC (pDC) but also myeloid DC (mDC). We will highlight those experiments showing how adaptive (FcεRI) and innate (TLR) immune receptors found on these cells counter-regulate one another’s functions. We will also discuss how DC subsets play an important role in regulating Th1/Th2 cytokine responses. Finally, we’ll describe different strategies in preparing these cells for the immunological methods discussed and how each is being used for mechanistic assays investigating current and novel approaches for therapeutic immunomodulation (Figure 1).
Figure 1. Different cell preparation strategies in conducting mechanistic studies involving blood basophil and DC responses.
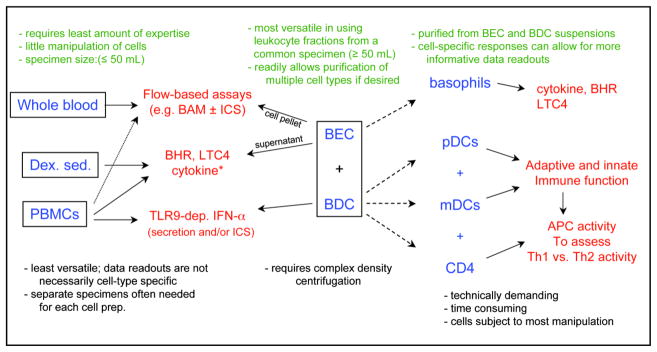
Shown are assays (red-type) routinely done in the authors’ laboratory using different cell preparations (blue-type). Key pros and cons of each are listed in green- and black- type, respectively. Dex. sed. (Dextran sedimentation); PBMC (peripheral blood mononuclear cells); BAM (basophil activation markers –CD63, CD203c); ICS (intracellular staining); BHR (basophil histamine release); BEC (basophil-enriched cell) and BDC (basophil-depleted cell) suspensions are prepared by double-density centrifugation (see text); *, denotes that concurrent measurements along with BHR and LTC4 are not generally possible using PBMC or with leukocytes prepared by Dex. sed.
Basophil Assays
Although basophils comprise only a small percentage of total leukocytes in the peripheral blood, these cells are regarded as critical effector cells of chronic allergic inflammation (Schroeder, 2009) and, more recently in mouse studies, as key orchestrators of Th2 immunity (Perrigoue et al., 2009; Sokol et al., 2009; Yoshimoto et al., 2009). Like mast cells, basophils express the high-affinity receptor for IgE (FcεRI) and are responsible for the anaphylactic release of mediators following allergen challenge. However, as the only cell in peripheral blood to release histamine in response to antigen, the accessibility of basophils has facilitated studies of allergen responses more than tissue-derived mast cells. The release of histamine from leukocyte suspensions challenged with antigen has long been used as an in vitro marker for immediate hypersensitivity. Decades ago, the percent of histamine released from the basophil in response to allergen (relative to total histamine content) was noted to correlate strongly with the severity of clinical symptoms experienced by individuals allergic to these allergens (Lichtenstein et al., 1967; Lichtenstein et al., 1968). For this reason, in vitro basophil histamine release has been used as a sensitive indicator of an individual’s allergic status, with the caveat that false positive results do occur and that a positive result is only meaningful to diagnose disease in the context of a supporting clinical history.
Multiple applications of the basophil histamine release assay have been developed over the years and are described in detail elsewhere (Schroeder and Kagey-Sobotka, 2002). Histamine release from normal donor leukocyte suspensions passively sensitized with IgE can be used to detect the presence of allergen-specific antibody in the sera (or plasma) of patients. This approach has the advantage over the more commonly used serologic assays that measure allergen-specific IgE antibody in that only biologically active IgE will elicit histamine release and only minute amounts of allergen are required for the assay. Moreover, this approach can be used to determine whether a patient has been sensitized to an uncommon allergen for which allergen-specific IgE testing is not available. In vitro basophil histamine release assays can also be used to check the quality of allergen preparations, which may be particularly important for immunotherapy studies where modified allergens can be tested for biological activity or cross-reactivity prior to in vivo human studies. The primary disadvantage of in vitro histamine release assays requiring passive sensitization includes the need for fresh leukocytes retrieved from nonallergic donors that are resilient enough to withstand the passive sensitization process (see below) and still retain responsiveness. Certain serum factors, such as IL-3, might also activate recipient basophils in a nonspecific way.
Quantification of mediators (histamine)
Several different approaches for measuring histamine have been developed, although automated fluorometry continues to be one of the most accurate, sensitive, reproducible, and rapid approaches. This technique briefly involves coupling of histamine with ophthalaldehyde (OPT) at a high pH to form a fluorescent product. The samples must be relatively free of protein, and therefore this approach cannot be used to measure histamine in whole blood or serum unless extensive acid precipitation and/or dialysis are first performed. Fluorometry is optimal for high-throughput analysis of samples prepared using buffers with low protein concentrations (for example, in vitro release of histamine from basophil or mast cell cultures) as well nasal or lung lavage fluids following experimental allergen challenge. Other methods to measure histamine, including competitive ELISAs, have been developed in recent years. These methods have the advantage of requiring relatively small sample volumes (as little as 0.05 ml), are not inhibited by the presence of protein, and can detect histamine in a variety of biological materials including cell culture supernatants, urine, and plasma. However, sensitivity, specificity and dynamic range of these assays can be limiting factors. ELISA kits for measuring histamine are now commercially available from several companies. Most recently, there is description of a flow-based assay (HistaFlow) to quantify “histamine release” at the single-cell level by using diamine oxidase-conjugated fluorochromes. Preliminary observations indicate that this method is useful in detecting both anaphylactic-type and piecemeal degranulation patterns (Ebo et al.).
Many variations on the protocol to perform in vitro histamine release have been developed over the years. Since basophils are the sole source of histamine in blood, these assays are possible without having to use pure basophil suspensions. Dextran sedimentation is often used to prepare washed leukocytes for histamine release since it involves little manipulation of the cells and is technically less difficult than other approaches, including those utilizing density centrifugation to enrich for basophils (see Figure 1). For this method, freshly drawn blood in EDTA is immediately and thoroughly mixed in a solution consisting of dextran, 0.1 M EDTA, and dextrose. The mixture is left undisturbed for 60–90 minutes at room temperature, and red blood cells settle more rapidly leaving a leukocyte-rich plasma. This fraction of the blood containing basophils is removed, and the leukocytes are washed in buffer several times to remove platelets. It is critical that the final wash be done in the absence of EDTA since histamine release requires calcium and residual EDTA can inhibit the reaction. The washed leukocytes are then added to reaction tubes and incubated at 37°C for 30–45 minutes. Although variable, on average 1 mL of blood per reaction tube will give total histamine levels of approximately 20ng. The total histamine content (often referred to as “completes”) is usually obtained by the lysis of cells in a duplicate set of reaction tubes using perchloric acid (1.6% final). However, it is important to note here that acid lysis can only be used when histamine is measured by fluorometry. Other detection assays (i.e. ELISA) cannot be used for assessing histamine in low pH samples. In this instance, the “completes” are commonly prepared by lysing cells with repeated freeze/thaw cycles. The amount of histamine released spontaneously is determined by incubating cells in buffer alone, and is generally less than 5% of the total histamine content. Although, it has been shown that basophils from up to 80% of children with food allergy (May, 1976; May and Remigio, 1982), and from some allergic asthmatic subjects (Marone et al., 1994), demonstrate high spontaneous histamine release (>10% of total). Reaction volumes are flexible and can range from 0.1 to 1.0 mL. Naturally, lower volumes are desired when test reagents are in short supply. At the end of the incubation, cells are centrifuged and the cell-free supernatants are removed for histamine measurement. If using automated fluorometry, then volumes are first adjusted to 1 mL, since this method of histamine detection requires 0.6–0.8 mL for sampling.
In some cases, histamine release assays can be used to detect the presence of an antigen-neutralizing blocking antibody in a subject’s serum, although this approach has been largely been replaced by radioimmunoassays (RIAs), radioenzymatic assays (REAs), and most recently by the facilitated allergen binding (FAB) assay, which also detects blocking antibody activity (Shamji et al., 2006). These types of assays are useful in the setting of allergen immunotherapy, which is often associated with a rise in antigen-specific IgG that can compete with IgE for binding to allergen. When using basophil histamine release as the readout, allergen (at a concentration that will elicit about 70% half-maximal histamine release) is pre-incubated (for up to 60 minutes) with serum from a patient undergoing immunotherapy, or with normal (type AB) serum as a control. Washed leukocytes from a donor whose basophils are known to release to the test allergen are then added to the serum, and histamine release is measured from the cell-free supernatants after incubation at 37°C as described above.
A modification of this protocol is more commonly used to measure the effectiveness of pharmacologic agents at inhibiting histamine release and other in vitro basophil responses. Here, washed leukocytes are mixed either with buffer alone, or several different dilutions of drug (typically 10−4 to 10−6 M as an initial screen) diluted in buffer. After pre-incubation for 10–15 minutes at 37°C, antigen, anti-IgE antibody, or other types of stimuli, are added for 45 minutes to induce histamine release. A recent example of this assay revealed surprising results demonstrating the importance of Bruton’s tyrosine kinase (Btk) in regulating FcεRI-dependent responses in human basophils (MacGlashan et al.).
As noted above, the presence of antigen-specific IgE in the serum or plasma of a patient can also be detected using an approach known as passive sensitization. Here, basophils from a nonallergic donor are incubated with serum/plasma from an allergic individual. Certain donor basophils (about 1 in 20 individuals) have sufficient numbers of unoccupied FcεRI sites that their basophils can be passively sensitized directly; however, often donor basophils must be treated with lactic acid first to remove endogenous IgE bound to the basophils (Pruzansky et al., 1983). This assay is typically done using mixed leukocyte preparations prepared by dextran sedimentation. Briefly, leukocytes are incubated up to several minutes in fresh lactic acid solution (pH 3.9) and then washed to remove unbound IgE. Passive sensitization is then done by incubating the cells at 37°C for 30 minutes with patient serum prepared in buffer containing 10U heparin/mL and 4mM EDTA, followed by antigen challenge. A positive result is defined as antigen-induced histamine release by basophils passively sensitized with patient serum but not by a control serum. Since IgE is heat labile, the inability of serum heated at 56°C for 45 minutes to confer reactivity would suggest that the reaction is IgE-dependent. In addition, it is now possible using omalizumab to definitively determine whether such reactions are IgE-dependent. When used in vitro during passive sensitization, this therapeutic agent blocks IgE from binding to its receptor. Thus, a positive result arising from passive sensitization can also be shown to be IgE-dependent if omalizumab suppresses the response (JTS, unpublished observations).
LTC4 Quantification
The release of histamine by basophils and mast cells following FcεRI crosslinking is closely followed by secretion of leukotriene C4 (LTC4), which is nearly complete approximately 30 minutes following activation. LTC4 is a lipid mediator that functions as a potent stimulus for smooth muscle contraction, and therefore is purported to be an important allergic mediator in inducing asthma symptoms. Indeed, studies have suggested leukotriene receptor antagonists may be efficacious in the treatment of allergic asthma. Like histamine, LTC4 can be used as a sensitive indicator of allergic status, and certain assays evaluating the inhibitory activity of 5-lipoxygenase inhibitors require measurement of LTC4 since these agents do not affect histamine release. The most common methods to measure LTC4 include competitive RIAs and competitive ELISA kits, which are now commercially available (Caymen Chemical, Ann Arbor, Michigan). Briefly, impure, enriched, or pure basophil suspensions can be used, although washed leukocytes prepared using dextran sedimentation will suffice. Both histamine and LTC4 can be measured from the same aliquot of cells, allowing LTC4 levels to be normalized to the amount of histamine released.
Cytokine Measurements
The cytokines IL-4 and IL-13 have long been recognized to play a critical role in the generation of Th2 immune responses, including IgE production, eosinophil migration, and the differentiation of Th2 lymphocytes. Basophils are now known to be the primary source of IL-4 (and IL-13) among leukocytes circulating in the blood or infiltrating allergic lesions (Schroeder, 2009). IL-4 and IL-13 are newly synthesized by basophils following IgE receptor crosslinking, and the concentration of allergen required to stimulate release of these cytokines is nearly 10-fold less than that required to induce release of histamine and LTC4. IL-4 secretion typically peaks 4–6 hours following activation, while IL-13 is first detectable at around 4 hours post-stimulation and peaks after 20 hours. It is now know believed that IL-13 secretion is more prolonged due to autocrine effects mediated by IL-3, which is also rapidly produced by basophils following IgE-dependent stimulation and is capable of directly activating these cells for IL-13 secretion (Schroeder et al., 2009). Certain secretagogues, such as C5a and N-formyl-methionyl-leucyl phenylalanine (FMLP), induce histamine release but do not commonly promote secretion of IL-4 and IL-13 from basophils, suggesting that cytokine secretion is more specific with IgE/FcεRI-mediated modes of basophil activation. Higher levels of IL-13 are produced by basophils from allergic donors compared to nonallergic donors following activation with non-IgE-dependent stimuli, such as IL-3, nerve growth factor, and TLR2 ligands (Sin et al., 2001; Bieneman et al., 2005). Spontaneous and/or increased IL-13 release has also been observed from circulating basophils of allergic donors following experimental allergen exposure in the nose and lung (Schroeder et al., ; Saini et al., 2004). Exactly why this happens is not fully understood, but the same allergen challenge protocols produce a concurrent decline in specific innate immune responses (IFN-α production by pDCs) –those that are known to suppress basophil IL-13 in vitro (Chen et al., 2003). No increased histamine is detected under these same conditions, suggesting production of IL-4 and IL-13 by basophils may exert novel functions independent of other basophil mediators, potentially in maintaining Th2 responses in the setting of chronic allergen exposure (Schroeder, ; Schroeder, 2009).
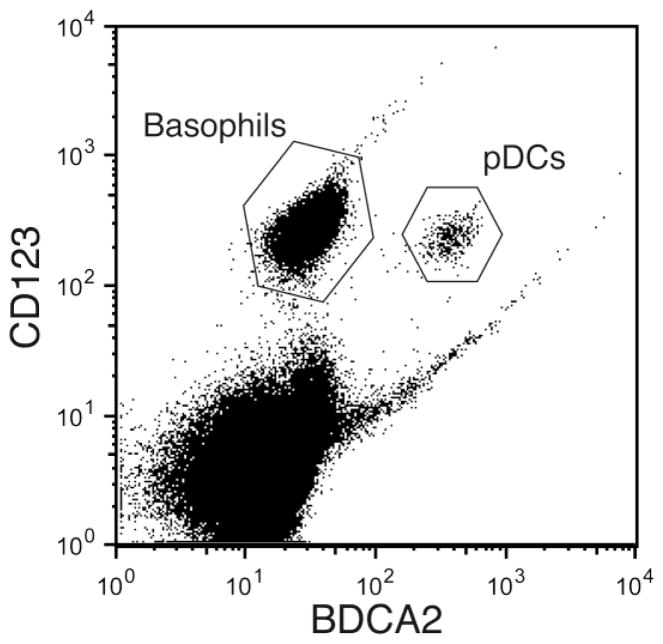
IL-4 and IL-13 protein levels are typically measured using an ELISA, and numerous commercially available kits are currently available for this purpose. Of course, variable results occur with different kits, since all come with their own set of standards. While ELISAs and multiplex kits can be used to measure levels of secreted protein, intracellular flow cytometry can be used to monitor cytokine generation at the single-cell level. For these experiments, cells are stimulated in the presence of an agent that blocks protein transport, such as brefeldin A, monesin, or a variety of commercially available proprietary agents that prevent cytokine secretion. The cells are then fixed, permeabilized, and stained with a flurochrome-conjugated antibody specific for the cytokine of interest. Using a multi-color staining protocol, this approach has been used to show that basophils are the predominant IL-4 and IL-13 producing cells among mixed leukocyte suspensions when stimulated by allergen (Devouassoux et al., 1999). In fact, we find that basophils are readily identifiable as IL-3 receptor (CD123) positive cells negative for blood dendritic cell antigen (BDCA)-2, also known as CD302 (a C-type lectin). Moreover, both basophils and pDCs are readily distinguished from one another using this staining approach (Figure 2), thus allowing flow-based assays that simultaneously detect these cells in PBMC suspensions for analyses of surface and intracellular biomarkers. Other markers such as CD203c have also proved useful in identifying basophils within mixed leukocyte suspensions using flow cytometry, but do not typically allow concurrent analyses of pDCs.
Figure 2. Use of flow cytometry to simultaneously identify basophils and plasmacytoid dendritic cells (pDCs) in a single sample.
Mixed leukocyte suspensions were surface stained with antibodies that recognize the IL-3 receptor (CD123) and blood dendritic cell antigen (BDCA-2). Basophils are identified as CD123+BDCA-2− cells, and pDCs as CD123+BDCA-2+ cells.
Washed leukocytes prepared from dextran sedimentation can be used to measure IL-4 secretion from basophils using the approach described above for histamine. However, ultrasensitive (Limit of Detection, LOD < 1pg/mL) IL-4 ELISAs are required, and the entire supernatant volume is generally needed, thereby preventing simultaneous measurement of histamine and IL-4 from the same sample. Alternatively, concurrent measurement of both mediators is possible, when using pure or basophil-enriched (>5% purity) suspensions (see Figure 1). Briefly, anticoagulated blood (in EDTA) is first centrifuged to obtain a buffy coat. Generally, 5mL of blood is required per reaction tube to measure both cytokine and mediator release from common cultures. The buffy coat interface is then diluted in buffer containing EDTA, and layered onto isotonic Percoll gradients consisting of 55% isotonic Percoll (d=1.072g/mL) underlaid with 61% (d=1.081g/mL) isotonic Percoll. The gradients are then centrifuged at ~700g for 20 minutes at room temperature (22–25 C). The cells halfway above the 55% Percoll interface are PBMC-like in consistency and can either be discarded or used in isolating other cell types (e.g. dendritic cells, T cells, B cells, monocytes); basophils are present in the lower fraction of the gradient consisting of the lower half of the 55% Percoll layer, the 61% interface, and the upper half of the 61% layer. This basophil-enriched cell (BEC) suspension is removed and washed twice in buffer containing EDTA with a final wash in EDTA-free buffer. The percentage of basophils in this suspension generally ranges from 5–50%, with significant variability among donors. Our decision to use the BEC suspension directly or to further purify basophils (e.g. with negative selection protocols) is often decided by the nature of the stimulus used in activating basophils. For example, BECs are suitable when using stimuli specifically targeting basophils (e.g. anti-IgE antibody). However, the use of pure suspensions should be considered if the stimuli being tested have a real probability of activating other cell types.
Basophil cytokine (and mediator) secretion is dependent on extracellular calcium. Thus, we have long used C-IMDM in culturing these cells (Schroeder et al., 1994). This medium consists of Iscove’s modified Dulbecco’s Medium, supplemented with 5% heat-inactivated (56 C for 30 minutes) fetal bovine serum, nonessential amino acids, gentamicin (10 μg/mL), with the pH adjusted to 7.2 – 7.4. IMDM contains ~1.4mM calcium, which is higher than those levels found in more popular media such as RPMI (0.3 mM Ca++). Approximately 100,000 to 200,000 basophils, as determined by Alcian blue staining (Gilbert and Ornstein, 1975), are cultured per well in a 96-well microtiter plate. Typically, cells are pre-warmed in media in a 37°C incubator (volume 0.125mL) for 15 minutes prior to adding the same volume of media with or without stimulus (e.g. allergen) that was also pre-incubated at 37°C. For cytokine secretion, cells are incubated for 4 hours at 37°C, and then centrifuged. Cell-free supernatants are then collected; a portion is used for histamine analysis and the remainder used for measuring IL-4 by ELISA. For histamine measurement, the upper 25–50μl of cell-free supernatant is carefully removed and added to buffer containing perchloric acid (1.6%), and incubated at 4°C overnight to allow precipitation of protein in the C-IMDM. Histamine is then measured by automated fluorometry or by other methods as described above. IL-4 protein is measured in the remaining supernatant by ELISA. Because the kinetics of IL-13 secretion from basophils is slower than that of IL-4, the cells must be incubated for 8–20 hours with stimulus, if measurement of this cytokine is desired.
Measurement of Activation Markers
In recent years, significant attention has focused on the use of basophil surface markers (e.g. CD63/CD203c) as surrogates for basophil activation, and their evaluation during various therapeutic interventions has been widely used (Gernez et al., ; Nopp et al., 2006; Shreffler, 2006; Foroughi et al., 2007). Expression of these markers is typically assessed using multi-color flow cytometry, by gating on CD203+ basophils or by using CD123+BDCA-2− staining (Figure 2). Basophil expression of CD69 has been followed as an indicator of prolonged IL-3 exposure, but exposure to other degranulation stimuli including FMLP and ionophores can also induce its expression. Expression of CD63 has been used as a marker for histamine release following allergen exposure since this protein is located in histamine-containing vesicles that fuse with the plasma membrane following IgE receptor cross-linking. Expression of the ectonucleotide pyrophosphatase/phosphodiesterase CD203c has been linked to “piecemeal” degranulation by basophils as opposed to “anaphylactic” degranulation that is associated with expression of CD63 (MacGlashan). Both types of degranulation result in histamine release and follow IgE receptor cross-linking, but are thought to be distinguished by the magnitude of the cytosolic calcium response that’s required as well as the activity of an unknown kinase. Recently, CD203c expression on basophils was reported increased during asthma exacerbations, but is declined following clinical improvement (Ono et al.). Resting levels of CD203c are also reportedly high on basophils analyzed from food allergic subjects, suggesting an ongoing activation of these cells in vivo. Interestingly, both omalizumab administration (or anti-IgE therapy) and sublingual/oral immunotherapy (or desensitization) protocols are reported to reduce this expression (Gernez et al., ; Keet et al., 2012). Decreased allergen-induced expression of CD63 has also been seen in the setting of many immunomodulatory-based trials. However, the specificity of these markers with respect to basophil degranulation has been questioned, and additional concerns relating to the kinetics of expression, the addition of IL-3, and the optimal marker to follow have been raised (Kleine-Tebbe et al., 2006). A broad range of allergen doses should be tested if expression of these markers is to be used for diagnostic testing with specific allergens, since the pattern and degree of basophil activation varies tremendously among donors. In addition, these assays are often performed using whole blood, and care must also be taken to assure that other cell types that also express these markers (such as adherent platelets) are not giving a false positive signal. Furthermore, agents used to lyse RBCS may also lead to nonspecific basophil activation. Finally, IL-3 is often co-administered to boost allergen-dependent responses, even though this cytokine itself is a potent activator of basophils and is directly capable of inducing the expression of these markers. Therefore, additional reaction tubes with and without IL-3 should be considered in order to control for effects mediated by this cytokine.
Other CD203c- and CD63- like markers are also reportedly found on basophils and whose expression increases following IgE-dependent (and IgE-independent) activation (Hennersdorf et al., 2005). These include CD13, CD107a, and CD164. Whether these novel basophil activation markers might be useful in evaluating efficacy during therapeutic intervention protocols remains to be investigated.
Dendritic Cells
DC-dependent T Cell Responses to Allergen
DCs are potent antigen presenting cells that capture, process, and present antigen to naïve T cells, and also respond to innate immune stimuli. In humans, two major types of immature DCs are found in the peripheral blood: Blood Dendritic Cell Antigen (BDCA)2+, BDCA4+, CD123hi, CD11c− plasmacytoid DCs (pDCs) and BDCA1+ BDCA3+ CD123lo CD11c+ myeloid DCs (mDCs). mDCs are also known as conventional DCs, and multiple subclasses have been defined. Both pDCs and mDCs can support Th1 or Th2 responses in vitro, depending on the cytokine milieu and innate immune triggers present in the local environment. Recent studies by our group have revealed a prominent role for DCs in modulating Th2 cytokine production by T cells, which could provide another valuable mechanistic endpoint to test during clinical trials of immune-based therapy (e.g. immunotherapy) (Frischmeyer-Guerrerio et al., ; Schroeder et al.). Both subtypes of DCs express the αγ2 variant of the high affinity receptor for IgE, FcεRI, which is thought to increase the efficiency of allergen presentation through a mechanism known as antigen focusing (Novak et al.). Expression of FcεRI on mDCs is at least 5-fold higher on mDCs than pDCs, yet 2-fold less than what is typically found on basophils (Schroeder et al., ; Foster et al., 2003). Although, there is some evidence suggesting that pDCs from allergic donors support Th2 cytokine production to a greater extent than mDCs when DC subsets are co-cultured with autologous CD4+ T cells in the presence of allergen (Farkas et al., 2004). Recently, we demonstrated that DC-CD4+ T cell co-cultures from children with food allergy (FA) spontaneously produced relatively large quantities of TH2 cytokines in the absence of allergen exposure (Frischmeyer-Guerrerio et al.). The amount of cytokine produced correlated with expression of FcεRI on the DC, and little to no cytokine was detectable when CD4+ T cells were cultured by themselves. These data suggest that DCs play a prominent role in directing Th2 cytokine production by T cells, and that the IgE receptor on the surface of these cells influences this activity. This conclusion is supported by our recent data that reductions in IL-5 and IL-13 produced in response to allergen was correlated with the degree of IgE neutralization and the decline in FcεRI expression on DCs following in vivo treatment with omalizumab (Schroeder et al.). However, DC-dependent T cell proliferation and cytokine production was not completely obliterated with omalizumab, suggesting that other mechanisms of antigen uptake and presentation (independent of IgE) contribute to the antigen presentation activities of these cells. However, IgE and DCs do appear to play a critical role in regulating Th2 cytokine production during the effector phases of allergic immune responses.
Immature DCs comprise <1% of leukocytes in the peripheral blood, but they are among the most potent antigen-presenting cells known. Co-cultures of DCs with autologous CD4+ T cells isolated from subjects undergoing a therapeutic intervention, such as treatment with omalizumab or allergen-specific immunotherapy, can provide unique insight into how these modalities modulate immune responses. Both pDCs and mDCs as well as CD4+ T cells can be sequentially isolated from the same peripheral blood sample at greater than 95% purity (Le et al., 2009). Blood is collected in EDTA and then subjected to double Percoll density centrifugation as described above. However, the upper fraction of cells (largely depleted of basophils) is collected and washed in buffer to remove platelets. A portion of these cells can be fixed at this point in 4% buffered paraformaldehyde for later analysis of DC maturation markers, FcεRI expression, etc. using flow cytometry. pDCs are first isolated from the remaining cells using positive selection by incubating the cells with BDCA4 immunomagnetic beads (Miltenyi Biotec). The cells are then applied over an LS column (Miltenyi).. Cells not retained in the column are collected by gravity filtration of buffer, and can be used for subsequent isolation of mDCs and T cells (see below). The cells retained on the column after extensive washing are highly enriched for pDCs, and these cells are then gently plunged off the column and subsequently cultured with T cells. We have found that a second pass of these cells through a smaller MS column (Miltenyi) can achieve higher pDC purity if necessary. For isolation of mDCS, cells from the BDCA4 flow-through are incubated with CD19 magnetic microbeads and biotinylated CD1c antibody. The cells are subsequently put over an LS column, and the flow-through is collected (CD19 positive cells, primarily B cells, are retained on the column and discarded). Cells from the CD19 flow-through are then incubated with anti-biotin microbeads and applied to an LS column. Cells retained on the washed column are highly enriched for mDCs, and are plunged off as described above for pDCs. Cells from the biotin flow-through are once again centrifuged, incubated with CD4 microbeads, and CD4+ T-cells are isolated by positive selection as described above. Dendritic cell subtypes are then individually cultured with CD4+ T cells at 1: 5 ratio (DC: T-cell) in Iscove modified Dulbecco medium supplemented with 5% serum (FCS, human AB, or autologous), nonessential amino acids, and gentamicin in the presence of allergen and/or other stimuli. Cultures are generally set up in 96-well round bottom plates, done in duplicate or triplicate, with allergen-driven proliferation and cytokine production being measured concurrently as readouts. Cell culture supernatants are harvested after 96 hours for measurement of cytokines using ELISA or multiplexing approaches as described above. At this point, an equal volume of fresh media (with allergen) is then added back to the cultures for an additional 48 hours of incubation. 3H-thymidine (1 μCi per well) is added 16 hours prior to harvesting of the cells onto glass fiber filters using a cell harvester. Incorporated radioactivity is then assessed using a liquid scintillation counter.
Innate Immune Responses
While DCs are important determinants of allergen-driven T-cell responses, these cell types are also well known for their role in directing innate immune responses. Both pDC and mDC subtypes express Toll Like Receptors (TLRs), which recognize broadly shared molecules expressed by pathogens such as bacteria and viruses. In recent years, several groups have suggested that DCs from allergic individuals may exhibit impaired anti-viral innate immune responses. pDCs, which express TLR7 and TLR9, have received the most attention in this regard. TLR9 recognizes unmethylated CpG sequences in DNA, which is a common feature of viral and bacterial genomes. TLR7 recognizes single-stranded RNA, which is common in certain viruses linked to respiratory diseases, including influenza and rhinovirus. Both TLR7 and -9 activation triggers a pro-inflammatory response characterized by production of high levels of Type I interferons, including IFN-β. We have shown that cross-linking of FcεRI on pDCs downregulated expression of TLR9 on pDCs, and inhibited the capacity of these cells to secrete IFN-α following stimulation of CpG (Schroeder et al., 2005; Schroeder et al., 2008). Gill and colleagues have likewise reported reductions in Type I IFN production induced by viruses targeting TLR7, following IgE/receptor cross-linking (Gill et al.). Collectively, these data suggest a mechanism by which pDCs can regulate both innate and adaptive immune responses. We have further shown that subjects which chronic allergic rhinitis had reduced capacity to secrete IFN-α upon activation with CpG compared to nonallergic individuals, while no difference in pDC frequency or expression of TLR9 on pDCs was observed (Tversky et al., 2008). Other groups have also shown impairment of innate immune responses by pDCs in patients with asthma (Gill et al., ; Bufe et al., 2002; Gehlhar et al., 2006). The ability of pDCs to modulate the innate and adaptive arms of the immune system may have important implications in strategies to treat allergic disease. The ability of IgE crosslinking on pDCs to suppress innate immune responses may suggest that strategies to deplete IgE, such as omalizumab, would restore the pro-Th1 capacity of these cells. Remarkably, we have preliminary evidence that allergen-specific immunotherapy may achieve the same outcome. Allergen immunotherapy was found to increase CpG-induced IFN-α production by pDCs from treated individuals 3–5 fold, such that levels approached those seen in non-allergic individuals (Tversky et al.). The exact mechanism(s) underlying this recovery in innate immune function remains elusive, but does suggest that incorporation of innate immune agonists into therapies for allergic disease may enhance their efficacy. In support of this theory, Creticos et al. found that patients with allergic rhinitis treated with a form of Amb a1, a major ragweed allergen, conjugated to a CpG oligonucleotide experienced long-term clinical benefit (Creticos et al., 2006). Additional studies will be needed to determine the efficacy of this approach compared to standard allergen immunotherapy, as well as its role in oral immunotherapy regimens such as those being studied to treat food allergy.
It is important to emphasize that the concept of innate immune function being impaired in allergic disease is evolving and therefore remains underdeveloped at this time. However, there which could eventually develop into routine mechanistic tests to monitor for immunomodulation during therapeutic intervention of allergic disease. In fact, Type I IFN responses are pDC-specific (Siegal et al., 1999), which provides rationale for conducting these assays using mixed leukocyte suspensions (e.g. PBMCs) rapidly prepared by density centrifugation. This is true even though pDCs typically represent <0.5% of PBMC suspensions. Type A oligodeoxynucleotides (ODNs) containing CpG motifs (ODN-CpG), such as ODN-2216, are particularly potent TLR9 agonists that induce high levels of IFN-α from pDCs, thus making it possible to quantify secreted levels of this cytokine by ELISA. In addition, several companies (e.g. TriLink Biotechnologies, San Diego; IBA Inc., Göttingen, Germany, Coley Pharmaceutical-Pfizer, Wellesley, MA) custom synthesize these agonists as ODNs containing phosphorothioated backbone linkages that significantly increase their stability in culture, compared to those with phosphodiester linkages. While ssRNA ODNs are also available as TLR7 agonists, most studies have instead made use of actual ssRNA viruses to assess these responses in pDCs. For example, influenza and rhinoviruses are two ssRNA viruses that will induce TLR7-dependent Type I IFN production in pDCs. However, with the evidence thus far indicating that both TLR7- and TLR9- dependent IFN-α responses are impaired in some allergic diseases (Gill et al., ; Schroeder et al., 2005; Tversky et al., 2008), then the general use of ODN-CpG as an agonist may simplify assays. For instance, maintaining and calibrating virus stocks is certain to be more tedious and laborious than keeping CpG-ODN on hand.
Actual assays are conducted in a manner similar to the basophil cytokine assays described above. Up to 2.5×106 PBMCs are added to culture wells of 96-well flat-bottom plates in a volume of 0.125 mL. After equilibrating to 37 C, 5% CO2, an equal volume of medium containing ODN-CpG (or stimulus of interest) is added, with the cultures incubated for 18–24h. Supernatants are harvested and measured for IFN-α using ELISA or multiplexing assays. Importantly, the use of PBMCs does not take into account that pDC frequencies might differ in a given subject over time and/or during treatment. Therefore, it is also necessary to determine pDC frequencies within the PBMC suspension using flow cytometry. Again, this is readily achieved by gating for BDCA-2+ cells or, if basophil analyses are also warranted, by a combination of CD123 and BDCA-2 staining (see Figure 2) (Frischmeyer-Guerrerio et al., ; Schroeder et al., ; Tversky et al.). Upon doing so, secreted cytokine levels (e.g. IFN-α) can then be normal to pDC frequencies. Certainly, there are other options one could take to assess the same (or additional) DC read-outs. These include: 1) purifying DC (pDCs and/or mDCs) as described above. This allows for a more direct determination of whether a particular DC subtype is secreting a given cytokine –including those (e.g. TNF-α, IL-6) potentially made by other cell types within a mixed leukocyte suspension. However, purifying these rare cells is both laborious and costly, and requires a significant amount of specimen (≥50 cc of blood); 2) the use of multi-color flow cytometry to detect intracellular cytokines within a specific DC subtype. This approach is quite versatile in requiring relatively few cells and in potentially determining cytokine production in multiple cell types. However, it lacks the quantitative power of determining the actual amount of cytokine produced, as achieved with ELISA and/or multiplex assays.
Concluding Remarks
Basophils respond to IgE-dependent stimuli (e.g. allergens) by secreting three classes of inflammatory mediators that are central to the pathogenesis of allergic disease, including vasoactive amines (histamine), cysteinyl leukotrienes (LTC4) and cytokines (IL-4/IL-13). As professional antigen-presenting cells (APCs) that constitutively express MHCII, DCs sample, capture and process peripheral antigens for presentation to T cells. In mediating this activity, DCs play an important role in both the priming of adaptive immune responses and in the activity of effector T cells recognizing self or innocuous antigens. Despite the rarity of basophils and DCs in human blood, there have been several technical advances during the past 20 years that now make it routine to study specific in vitro responses mediated by both cell types. As a consequence, these assays are providing important information regarding the allergic diethesis, while at the same time serving as valuable biomarkers in assessing clinical efficacy during therapeutic intervention.
Abbreviations
- DC
dendritic cell
- pDC
plasmacytoid dendritic cell
- mDC
myeloid dendritic cell
- FcεRI
high affinity IgE receptor
- TLR
Toll-Like Receptor
- RAST
radioallergosorbent test
- OPT
ophthalaldehyde
- RIAs
radioimmunoassays
- REAs
radioenzymatic assays
- FAB
facilitated allergen-binding
- Btk
Bruton’s tyrosine kinase
- BDCA
blood dendritic cell antigen
- BEC
basophil-enriched cell
- BDC
basophil-depleted cell
- CpG
cytosine – phosphate – guanine
- ODN
oligodeoxynucleotide
Footnotes
Supported in part by grants R21AI079853 (JTS), U19 AI070345-project 3 (JTS), and K23 AI091869 (PAF-G) from the National Institute of Allergy and Infectious Diseases, National Institutes Health, Bethesda, Maryland, as well as a Johns Hopkins Clinician Scientist Award (PAF-G) and ARTrust Faculty Development Award (PAF-G).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol. 2005;115:295–301. doi: 10.1016/j.jaci.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Bufe A, Gehlhar K, Grage-Griebenow E, Ernst M. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int Arch Allergy Immunol. 2002;127:82–8. doi: 10.1159/000048173. [DOI] [PubMed] [Google Scholar]
- Chen YH, Bieneman AP, Creticos PS, Chichester KL, Schroeder JT. IFN-alpha inhibits IL-3 priming of human basophil cytokine secretion but not leukotriene C4 and histamine release. J Allergy Clin Immunol. 2003;112:944–50. doi: 10.1016/j.jaci.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–55. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- Devouassoux G, Foster B, Scott LM, Metcalfe DD, Prussin C. Frequency and characterization of antigen-specific IL-4- and IL-13-producing basophils and T cells in peripheral blood of healthy and asthmatic subjects. J Allergy Clin Immunol. 1999;104:811–9. doi: 10.1016/s0091-6749(99)70292-7. [DOI] [PubMed] [Google Scholar]
- Ebo DG, Bridts CH, Mertens CH, Hagendorens MM, Stevens WJ, De Clerck LS. Analyzing histamine release by flow cytometry (HistaFlow): A novel instrument to study the degranulation patterns of basophils. J Immunol Methods. 2011 doi: 10.1016/j.jim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Farkas L, Kvale EO, Johansen FE, Jahnsen FL, Lund-Johansen F. Plasmacytoid dendritic cells activate allergen-specific TH2 memory cells: modulation by CpG oligodeoxynucleotides. J Allergy Clin Immunol. 2004;114:436–43. doi: 10.1016/j.jaci.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Foroughi S, Foster B, Kim N, Bernardino LB, Scott LM, Hamilton RG, Metcalfe DD, Mannon PJ, Prussin C. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120:594–601. doi: 10.1016/j.jaci.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcepsilonRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112:1132–8. doi: 10.1016/j.jaci.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Frischmeyer-Guerrerio PA, Guerrerio AL, Chichester KL, Bieneman AP, Hamilton RA, Wood RA, Schroeder JT. Dendritic cell and T cell responses in children with food allergy. Clin Exp Allergy. 2011;41:61–71. doi: 10.1111/j.1365-2222.2010.03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlhar K, Bilitewski C, Reinitz-Rademacher K, Rohde G, Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36:331–7. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- Gernez Y, Tirouvanziam R, Yu G, Ghosn EE, Reshamwala N, Nguyen T, Tsai M, Galli SJ, Herzenberg LA, Herzenberg LA, Nadeau KC. Basophil CD203c levels are increased at baseline and can be used to monitor omalizumab treatment in subjects with nut allergy. Int Arch Allergy Immunol. 2011;154:318–27. doi: 10.1159/000321824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert HS, Ornstein L. Basophil counting with a new staining method using alcian blue. Blood. 1975;46:279–86. [PubMed] [Google Scholar]
- Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennersdorf F, Florian S, Jakob A, Baumgartner K, Sonneck K, Nordheim A, Biedermann T, Valent P, Buhring HJ. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005;15:325–35. doi: 10.1038/sj.cr.7290301. [DOI] [PubMed] [Google Scholar]
- Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RA, Boden S, Steele P, Driggers S, AWB, Wood RA. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2011.10.023. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Tebbe J, Erdmann S, Knol EF, MacGlashan DW, Jr, Poulsen LK, Gibbs BF. Diagnostic tests based on human basophils: potentials, pitfalls and perspectives. Int Arch Allergy Immunol. 2006;141:79–90. doi: 10.1159/000094495. [DOI] [PubMed] [Google Scholar]
- Le T, Tversky J, Chichester KL, Bieneman AP, Huang SK, Wood RA, Schroeder JT. Interferons modulate Fc epsilon RI-dependent production of autoregulatory IL-10 by circulating human monocytoid dendritic cells. J Allergy Clin Immunol. 2009;123:217–23. doi: 10.1016/j.jaci.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein LM, Norman PS, Connell JT. Comparison between skin-sensitizing antibody titers and leukocyte sensitivity measurements as an index of the severity of ragweed hay fever. J Allergy. 1967;40:160–7. doi: 10.1016/0021-8707(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Lichtenstein LM, Norman PS, Winkenwerder WL. Clinical and in vitro studies on the role of immunotherapy in ragweed hay fever. Am J Med. 1968;44:514–24. doi: 10.1016/0002-9343(68)90052-1. [DOI] [PubMed] [Google Scholar]
- MacGlashan D., Jr Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2011;40:1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGlashan D, Jr, Honigberg LA, Smith A, Buggy J, Schroeder JT. Inhibition of IgE-mediated secretion from human basophils with a highly selective Bruton’s tyrosine kinase, Btk, inhibitor. Int Immunopharmacol. 2011;11:475–9. doi: 10.1016/j.intimp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone G, Spadaro G, Patella V, Genovese A. The clinical relevance of basophil releasability. J Allergy Clin Immunol. 1994;94:1293–303. doi: 10.1016/0091-6749(94)90345-x. [DOI] [PubMed] [Google Scholar]
- May CD. High spontaneous release of histamine in vitro from leukocytes of persons hypersensitive to food. J Allergy Clin Immunol. 1976;58:432–7. doi: 10.1016/0091-6749(76)90124-x. [DOI] [PubMed] [Google Scholar]
- May CD, Remigio L. Observations on high spontaneous release of histamine from leucocytes in vitro. Clin Allergy. 1982;12:229–41. doi: 10.1111/j.1365-2222.1982.tb02523.x. [DOI] [PubMed] [Google Scholar]
- Nopp A, Johansson SG, Ankerst J, Bylin G, Cardell LO, Gronneberg R, Irander K, Palmqvist M, Oman H. Basophil allergen threshold sensitivity: a useful approach to anti-IgE treatment efficacy evaluation. Allergy. 2006;61:298–302. doi: 10.1111/j.1398-9995.2006.00987.x. [DOI] [PubMed] [Google Scholar]
- Novak N, Bieber T, Peng WM. The immunoglobulin E-Toll-like receptor network. Int Arch Allergy Immunol. 2010;151:1–7. doi: 10.1159/000232565. [DOI] [PubMed] [Google Scholar]
- Ono E, Taniguchi M, Higashi N, Mita H, Kajiwara K, Yamaguchi H, Tatsuno S, Fukutomi Y, Tanimoto H, Sekiya K, Oshikata C, Tsuburai T, Tsurikisawa N, Otomo M, Maeda Y, Hasegawa M, Miyazaki E, Kumamoto T, Akiyama K. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol. 2011;125:483–489. e3. doi: 10.1016/j.jaci.2009.10.074. [DOI] [PubMed] [Google Scholar]
- Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzansky JJ, Grammer LC, Patterson R, Roberts M. Dissociation of IgE from receptors on human basophils. I. Enhanced passive sensitization for histamine release. J Immunol. 1983;131:1949–53. [PubMed] [Google Scholar]
- Saini S, Bloom DC, Bieneman A, Vasagar K, Togias A, Schroeder J. Systemic effects of allergen exposure on blood basophil IL-13 secretion and FcepsilonRIbeta. J Allergy Clin Immunol. 2004;114:768–74. doi: 10.1016/j.jaci.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Schroeder JT. Basophils: emerging roles in the pathogenesis of allergic disease. Immunol Rev. 2011;242:144–60. doi: 10.1111/j.1600-065X.2011.01023.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JT. Basophils beyond effector cells of allergic inflammation. Adv Immunol. 2009;101:123–61. doi: 10.1016/S0065-2776(08)01004-3. [DOI] [PubMed] [Google Scholar]
- Schroeder JT, Bieneman AP, Chichester KL, Breslin L, Xiao H, Liu MC. Pulmonary allergic responses augment interleukin-13 secretion by circulating basophils yet suppress interferon-alpha from plasmacytoid dendritic cells. Clin Exp Allergy. 2011;40:745–54. doi: 10.1111/j.1365-2222.2010.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JT, Bieneman AP, Chichester KL, Hamilton RG, Xiao H, Saini SS, Liu MC. Decreases in human dendritic cell-dependent T(H)2-like responses after acute in vivo IgE neutralization. J Allergy Clin Immunol. 2010;125:896–901. e6. doi: 10.1016/j.jaci.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, Liu MC. TLR9- and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175:5724–31. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- Schroeder JT, Chichester KL, Bieneman AP. Toll-like receptor 9 suppression in plasmacytoid dendritic cells after IgE-dependent activation is mediated by autocrine TNF-alpha. J Allergy Clin Immunol. 2008;121:486–91. doi: 10.1016/j.jaci.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Schroeder JT, Chichester KL, Bieneman AP. Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol. 2009;182:2432–8. doi: 10.4049/jimmunol.0801782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JT, Kagey-Sobotka A. Assay methods for measurement of mediators and markers of allergic inflammation. In: Rose NR, Hamilton RG, Detrick B, editors. Manual of Clinical Laboratory Immunology. 2002. pp. 899–909. [Google Scholar]
- Schroeder JT, MacGlashan DW, Jr, Kagey-Sobotka A, White JM, Lichtenstein LM. IgE-dependent IL-4 secretion by human basophils. The relationship between cytokine production and histamine release in mixed leukocyte cultures. J Immunol. 1994;153:1808–17. [PubMed] [Google Scholar]
- Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, Larche M, Durham SR, Francis JN. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317:71–9. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler WG. Evaluation of basophil activation in food allergy: present and future applications. Curr Opin Allergy Clin Immunol. 2006;6:226–33. doi: 10.1097/01.all.0000225165.83144.2f. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Sin AZ, Roche EM, Togias A, Lichtenstein LM, Schroeder JT. Nerve growth factor or IL-3 induces more IL-13 production from basophils of allergic subjects than from basophils of nonallergic subjects. J Allergy Clin Immunol. 2001;108:387–93. doi: 10.1067/mai.2001.117459. [DOI] [PubMed] [Google Scholar]
- Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55(Suppl 1):S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky JR, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Subcutaneous allergen immunotherapy restores human dendritic cell innate immune function. Clin Exp Allergy. 2010;40:94–102. doi: 10.1111/j.1365-2222.2009.03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky JR, Le TV, Bieneman AP, Chichester KL, Hamilton RG, Schroeder JT. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-alpha via Toll-like receptor 9. Clin Exp Allergy. 2008;38:781–8. doi: 10.1111/j.1365-2222.2008.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]