Abstract
Background:
Dexmedetomidine is an alpha 2 adrenergic agonist, prolongs analgesia when used in neuraxial and peripheral nerve blocks. We studied the effect of addition of dexmedetomidine to bupivacaine to perform transversus abdominis plane (TAP) block.
Materials and Methods:
A total of 50 patients scheduled for abdominal hysterectomy were divided into two equal groups in a randomized double-blinded way. Group B patients (n = 25) received TAP block with 20 ml of 0.25% bupivacaine and 2 ml of normal saline while Group BD (n = 25) received 0.5 mcg/kg (2 ml) of dexmedetomidine and 20 ml of 0.25% bupivacaine bilaterally. Time for first analgesic administration, totally used doses of morphine, pain scores, hemodynamic data and side-effects were recorded.
Results:
Demographic and operative characteristics were comparable between the two groups. The time for the first analgesic dose was longer in Group BD than Group B (470 vs. 280 min, P < 0.001) and the total doses of used morphine were less among Group BD patients in comparison to those in Group B (19 vs. 29 mg/24 h, P < 0.001). Visual analog scores were significantly lower in Group BD in the first 8 h post-operatively when compared with Group B, both at rest and on coughing (P < 0.001). In Group BD, lower heart rate was noticed 60 min from the induction time and continued for the first 4 h post-operatively (P < 0.001).
Conclusions:
The addition of dexmedetomidine to bupivacaine in TAP block achieves better local anesthesia and provides better pain control post-operatively without any major side-effects.
Keywords: Bupivacaine, dexmedetomidine, pain, transversus abdominis plane block
INTRODUCTION
Inadequate control of post-operative pain leads to several unwanted adverse events ranging from patients’ discomfort, prolonged immobilization to thromboembolic phenomenon and pulmonary complications.[1] Analgesic multimodalities were recommended to relieve the post-operative pain.[2] Opioids although provide satisfactory analgesia, they are associated with unwanted side-effects.[3] Epidural analgesia is the commonly used regional technique to alleviate pain after abdominal and gynecological surgeries although it might be contraindicated in some patients.[4] Transversus abdominis plane (TAP) block is a novel type of peripheral nerve block that involves innervations of the anterolateral abdominal wall derived from T6-L1.[5] It provides adequate post-operative pain relieve following the various abdominal surgeries.[6,7,8,9] Unfortunately, TAP block duration is limited to effect of administered local anesthetics (LA). The use of an infusion catheter to administer LA is an option to prolong the block's duration.[10,11] Recently, adjuvant medications were added to LA to prolong the effect of TAP block.[12] Dexmedetomidine is a selective alpha 2 (α2) adrenergic agonist with both analgesic and sedative properties.[13] Its use with bupivacaine either epidurally or intrathecally associated with prolongation of the LA effect.[14,15,16,17,18] In a prospective, double-blinded, randomized study, we try to assess the analgesic effect of adding dexmedetomidine to bupivacaine on TAP block for patients undergoing abdominal hysterectomy through laparotomy.
MATERIALS AND METHODS
After approval from the Research and Ethics Committee, 60 patients were screened for eligibility to participate in the study. Written informed consent was obtained from 50 patients over the age of 18 years, American Society of Anaesthesiologists’ physical class I or II patients and scheduled for abdominal hysterectomy through pfannenstiel incision.
Exclusion criteria were patient refusal, patients with a history of cardiac, respiratory, renal or hepatic failure, coagulation disorders, local infection at the site of block, psychological disorders, allergy to study medications and chronic use of pain medications or adrenoreceptors agonists or antagonists. During the pre-operative anesthetic assessment of patients, visual analog scale (VAS) for pain assessment from 0 to 10 cm, with 0 meaning no pain and 10 meaning the worst pain imaginable was explained to patients and the use of intravenous-patient controlled analgesia (IV-PCA) for post-operative pain control was described to patients as well. IV Midazolam 0.03 mg/kg was administered 15 min before induction of general anesthesia. Patients were monitored by non-invasive blood pressure, heart rate (HR), pulsoximetry, temperature and bispectral index (BIS). General anesthesia was standardized for all patients in both groups. Fentanyl 2 mcg/kg, propofol 2 mg/kg was intravenously administered and cisatracurium 0.1 mg/kg was given to facilitate tracheal intubation. Endotracheal tube size 7.0 mm was used to intubate the trachea. Lungs were ventilated by pressure controlled mode to maintain normocapnia and sevoflurane/O2 /air mixture was administered to keep BIS values between 40 and 60. All surgical interventions were performed by the same surgical team.
Randomization was performed using a computer generated program to allocate patients to various study groups using the method of random number. The pharmacy prepared the study medications according to list of study numbers. In Group B (n = 25); patients were received TAP block on each side using 22 ml of study medication, which consisted of 20 ml of bupivacaine 0.25% and 2 ml of normal saline. While Group BD (n = 25) patients were received TAP block on each side with 22 ml, in which dexmedetomidine 0.5 mcg/kg was dissolved in 2 ml of normal saline and added to 20 ml of bupivacaine 0.25%.
Following skin preparation, TAP blocks were performed by one of the investigators under dynamic ultrasound guidance (M-Turbo, Sonosite Inc., Bothell, WA, USA). Broadband linear array ultrasound probe was placed in the axial plane across the mid-axillary line midway between costal margin and iliac crest. Following identification of the three different layers of the abdominal wall, block needle (22-G, 90 mm SonoPlex Stim cannula, Pajunk® GmbH, Geisingen, Germany) was inserted in plane until its tip was located in between the internal oblique and transverses abdominis muscles. After careful aspiration injection of study medication was performed and hypoechoic layer was detected on ultrasound.
Fentanyl 1 mcg/kg IV was administered for any intra-operative increase in the HR or mean arterial pressure (MAP) above 20% of baseline. After completion of the surgical procedure, patients’ tracheas were extubated after reversal of neuromuscular blockade effect. Patients were transferred to post-anesthesia care unit, (PACU) and IV-PCA was commenced with morphine (1 mg bolus, lock out time interval of 10 min and 4-h limit of 0.25 mg/kg without baseline infusion). IV-PCA was continued for 24 h post-operatively.
Throughout the procedure HR, MAP, end-tidal sevoflurane (ET sev) vol% and BIS values were recorded at 5, 10, 15, 30, 60 min. Furthermore, the number of administered fentanyl doses as a rescue medicine was documented.
In the PACU: Time to first analgesia request where recorded from the completion of TAP block to first given morphine dose. VAS was used to assess post-operative pain (VAS; where 0 = no pain and 10 = worst imaginable pain) during rest and on coughing.
Number of used PCA boluses of morphine at 0-4 h, 4-8 h, 8-12 h, 12-18 h, 18-24 h was reported and the total consumption of morphine (mg) in 24 h was calculated.
Nausea and vomiting were recorded using a categorical scoring system (0 = none, 1 = nausea, 2 = retching, 3 = vomiting). IV Metoclopramide 10 mg bolus was offered for any patient with a score 31. Inverted observer assessment of alertness/sedation (OAA/S) scale where: 1 = awake and 5 = asleep and unarousable was used to assess sedation level in the post-operative period.
In PACU and in first 24 h post-operatively, MAP, HR, VAS (at rest and on coughing), nausea and vomiting, sedation score (OAA/S) were recorded on admission to PACU, 1, 4, 8, 12, 18, 24 h post-operatively by an observer who was unaware of the study protocol.
Statistical analysis
The required sample size was calculated using G*Power© software version 3.1.0 (Institute of Experimental Psychology, Heinrich Heine University, Dusseldorf, Germany). The primary outcome measure was time for first analgesic request while secondary measures were VAS pain assessment scores and total analgesic consumption. Depending upon our practice, it was observed that the addition of dexmedetomidine to LA solutions for TAP block associated with a large effect size (d) of 0.8 in regard to the outcome measures. Therefore, it was estimated that a sample size of 25 patients in each study group would achieve a power of 80% to detect an effect size of 0.8 in the outcome measures of interest, assuming a type I error of 0.05. Statistical analysis was carried out on a personal computer using Medcalc© for Windows© v.12 statistical package (MedCalc Software, Mariakerke, Belgium). Normality of a numerical data distribution was tested using the Kolmogorov-Smirnov test. Normally distributed numerical data were presented as mean (standard deviation) and between-groups differences were compared using the independent-samples Student t-test. Skewed data were presented as median (interquartile range) and differences between the two groups were compared non-parametrically using the Mann Whitney U test. Categorical data were presented as ratio and inter-group comparison was performed using the Pearson Chi-square test or Fisher's exact test if >20% of cells in a contingency table had expected count <5.
Reported P values are two-tailed. P < 0.05 was considered as statistically significant.
RESULTS
Demographic data and operative characteristics in the two groups were comparable [Table 1]. None of the patients in both groups requested extra-doses of intra-operative fentanyl. Post-operatively, the time for first analgesic dose was longer in Group BD than Group B [470 vs. 280 min, P < 0.001; Figure 1] and the total doses of used morphine in the first 24 h were less among patients in Group BD when compared with those in Group B [19 vs. 29 mg, P < 0.001; Figure 2]. Figure 3 presented the longer time to the first PCA bolus and the less frequent use of PCA boluses among Group BD patients in comparison with Group B patients. VAS was significantly reduced in all post-operative points for the first 8 h in Group BD when compared with Group B, both at rest and on coughing [P < 0.001, Figure 4a and b]. Table 2 shows HR readings among both groups in the intra-operative and post-operative period. In Group BD lower HR was noticed 60 min from the starting time and continued until 4 h post-operatively (P < 0.001). Changes in MAP, BIS, or ET sev readings were not statistically significant in both groups. 11 cases of Group B complained from nausea (score 1) and three patients in Group BD. In both groups, the incidence of sedation, nausea and vomiting and the use of anti-emetic medications were not statistically significant except for the first post-operative hour where Group BD patients were more sedated than those in Group B [P < 0.005, [Table 3].
Table 1.
Demographic data and operative characteristics in the two study groups

Figure 1.

Time to first analgesic (TFA) in both groups in minutes. Boxplot of TFA. Box represents interquartile range. Horizontal line across box represents the median. Error bars represent 5th and 95th percentiles
Figure 2.

The cumulative doses of morphine consumed by the patient in 24-h. Box represent interquartile range. Horizontal line across box represents the median. Error bars represent 5th and 95th percentiles and markers lying beyond these limits represent outliers
Figure 3.
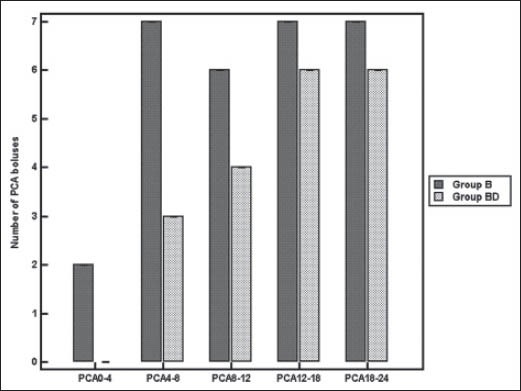
The median values of patient controlled analgesia boluses used by patients in both groups. Error bars represent interquartile range
Figure 4a.

Median values of visual analog scores for pain assessment during the rest in both groups. Error bars represent interquartile range
Figure 4b.

Median values of visual analog scores for pain assessment on coughing in both groups. Error bars represent interquartile range
Table 2.
Changing in Heart rate intra-operativly and during postoperative period among both groups

Table 3.
Postoperative sedation scores, incidence of nausea and vomiting and the need for anti-emetic medication in the two study groups

DISCUSSION
The major finding of this study is that addition of dexmedetomidine to bupivacaine in TAP block provides prolonged post-operative analgesia and better pain control than LA alone. The duration of LA was longer, VAS was lower and the need for rescue morphine doses was less when dexmedetomidine was added to bupivacaine. Brummett et al. have reported that perineural administration of high-dose dexmedetomidine in combination with bupivacaine enhanced LA blockade in rats without inducing neurotoxicity.[19] Many studies have found that the addition of dexmedetomidine to LA in central neuraxial blocks and in peripheral nerve blockades in human was a safe and effective way to potentiate the LA effect and reduce the required analgesics.[14,15,16,17,18,20,21,22,23] On the other hand, Ozalp et al. have compared dexmedetomidine -ropivacaine mixture to ropivacaine alone in patient controlled interscalene analgesia and they reported similar pain scores in both groups without any advantageous effect of dexemedetomidine.[24] Literatures’ review did not reveal any study describe the addition of dexemedetomidine to bupivacaine for TAP block. TAP block is an expanding regional anesthesia technique that provides good analgesia to the skin and musculature of the anterior abdominal wall in patients undergoing various abdominal surgeries.[6,7,8,9] McDonnell et al. in their study contributed the prolonged effect of ropivacaine TAP block to the relatively poorly vascularised TAP resulting in a slower rate of drug clearance.[6] In our study, the addition of dexmedetomidine to bupivacaine in TAP block led to further prolongation of analgesia, less requirement of rescue morphine and lower VAS pain scores. Similar to our finding, many investigators reported that the addition of dexmedetomidine to different types of LA agents in various types of peripheral nerve blocks resulted in prolongation of analgesic effect.[20,21,22,23] On the other hand, Masuki et al. suggested that dexmedetomidine induces vasoconstriction through an action on α2 adrenoceptors in the human forearm[25] and the later might contribute to the longer duration of action. Other investigators have supported a third mechanism of action through α2 adrenoceptors agonist effect rather than vasoconstriction. They contributed that to the direct effect on the peripheral nerve activity.[26] Whatever the mechanism of dexmedetomidine's action, it seems that it potentiates the LA effect and prolongs the analgesic duration.
Dexmedetomidine might associate with some side-effect such as hypotension, bradycardia and sedation particularly at higher doses.[14] In our study, we noticed a significant fall in the HR 60 min following the administration of dexmedetomidine opposite to the control group. This effect persisted for 4 h, but without any hemodynamic instability. The decrease in pulse rate might be related to the post-synaptic activation of central α2 adrenoceptors, leading to decreased sympathetic activity and slower HR.[27,28] Similar to HR increased sedation was noticed in the first post-operative hour among Group BD patients. None of our patients required treatment for the low HR or sedation. The low dose of dexmedetomidine used in our study might be the reason behind the minor adverse events. Further studies are needed to determine the safe-effective dose of dexmedetomidine and to assess the risk of perineural administration of dexmedetomidine among bigger patients’ sample.
One limitation of this study is lack of proper assessment of success rate of TAP block procedure as it was performed following the induction of general anesthesia, but we depend upon the skills of the investigators and the use of ultrasonography guided block for proper placement of blocking needle. A second limitation is the inability to assess dexmedetomidine plasma concentration among study patients to determine whether its action was related to systemic absorption or pure local effect.
CONCLUSION
The addition of dexmedetomidine to bupivacaine in TAP block achieves better local anesthesia conditions and provides better pain control post-operatively without any major side-effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Ganai S, Lee KF, Merrill A, Lee MH, Bellantonio S, Brennan M, et al. Adverse outcomes of geriatric patients undergoing abdominal surgery who are at high risk for delirium. Arch Surg. 2007;142:1072–8. doi: 10.1001/archsurg.142.11.1072. [DOI] [PubMed] [Google Scholar]
- 2.Elia N, Lysakowski C, Tramèr MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103:1296–304. doi: 10.1097/00000542-200512000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Lo Y, Chia YY, Liu K, Ko NH. Morphine sparing with droperidol in patient-controlled analgesia. J Clin Anesth. 2005;17:271–5. doi: 10.1016/j.jclinane.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Christie IW, McCabe S. Major complications of epidural analgesia after surgery: Results of a six-year survey. Anesthesia. 2007;62:335–41. doi: 10.1111/j.1365-2044.2007.04992.x. [DOI] [PubMed] [Google Scholar]
- 5.Rozen WM, Tran TM, Ashton MW, Barrington MJ, Ivanusic JJ, Taylor GI. Refining the course of the thoracolumbar nerves: A new understanding of the innervation of the anterior abdominal wall. Clin Anat. 2008;21:325–33. doi: 10.1002/ca.20621. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH, et al. The analgesic efficacy of transversus abdominis plane block after cesarean delivery: A randomized controlled trial. Anesth Analg. 2008;106:186–91. doi: 10.1213/01.ane.0000290294.64090.f3. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell BD, McDonnell JG, McShane AJ. The transversus abdominis plane (TAP) block in open retropubic prostatectomy. Reg Anesth Pain Med. 2006;31:91. doi: 10.1016/j.rapm.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 8.McDonnell JG, O’Donnell B, Curley G, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: A prospective randomized controlled trial. Anesth Analg. 2007;104:193–7. doi: 10.1213/01.ane.0000250223.49963.0f. [DOI] [PubMed] [Google Scholar]
- 9.Carney J, McDonnell JG, Ochana A, Bhinder R, Laffey JG. The transversus abdominis plane block provides effective postoperative analgesia in patients undergoing total abdominal hysterectomy. Anesth Analg. 2008;107:2056–60. doi: 10.1213/ane.0b013e3181871313. [DOI] [PubMed] [Google Scholar]
- 10.Hebbard P, Fujiwara Y, Shibata Y, Royse C. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35:616–7. [PubMed] [Google Scholar]
- 11.Bjerregaard N, Nikolajsen L, Bendtsen TF, Rasmussen BS. Transversus abdominis plane catheter bolus analgesia after major abdominal surgery. Anesthesiol Res Pract. 2012;596536:1–5. doi: 10.1155/2012/596536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammar AS, Mahmoud KM. Effect of adding dexamethasone to bupivacaine on transversus abdominis plane block for abdominal hysterectomy: A prospective randomized controlled trial. Saudi J Anaesth. 2012;6:229–33. doi: 10.4103/1658-354X.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care. 2001;7:221–6. doi: 10.1097/00075198-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 15.Jain D, Khan RM, Kumar D, Kumar N. Perioperative effect of epidural dexmedetomidine with intrathecal bupivacaine on haemodynamic parameters and quality of analgesia. South Afr J Anaesth Analg. 2012;18:105–9. [Google Scholar]
- 16.Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A Comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27:339–43. doi: 10.4103/0970-9185.83678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 18.Elhakim M, Abdelhamid D, Abdelfattach H, Magdy H, Elsayed A, Elshafei M. Effect of epidural dexmedetomidine on intraoperative awareness and post-operative pain after one-lung ventilation. Acta Anaesthesiol Scand. 2010;54:703–9. doi: 10.1111/j.1399-6576.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 19.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 21.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 22.Marhofer D, Kettner SC, Marhofer S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2012;15:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 23.Ammar AS, Mahmoud KM. Ultrasound-guided single injection infraclavicular brachial plexus block using bupivacaine alone or combined with dexmedetomidine for pain control in upper limb surgery: A prospective randomized controlled trial. Saudi J Anaesth. 2012;6:109–14. doi: 10.4103/1658-354X.97021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozalp G, Tuncel G, Savli S, Celik A, Doger C, Kaya M, et al. The analgesic efficacy of dexmedetomidine added to ropivacaine patient controlled interscalene analgesia via the posterior approach. J Anaesth. 2006;21:409–12. [Google Scholar]
- 25.Masuki S, Dinenno FA, Joyner MJ, Eisenach JH. Selective alpha2-adrenergic properties of dexmedetomidine over clonidine in the human forearm. J Appl Physiol. 2005;99:587–92. doi: 10.1152/japplphysiol.00147.2005. [DOI] [PubMed] [Google Scholar]
- 26.Eledjam JJ, Deschodt J, Viel EJ, Lubrano JF, Charavel P, d’Athis F, et al. Brachial plexus block with bupivacaine: Effects of added alpha-adrenergic agonists: Comparison between clonidine and epinephrine. Can J Anaesth. 1991;38:870–5. doi: 10.1007/BF03036962. [DOI] [PubMed] [Google Scholar]
- 27.Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70. doi: 10.1097/00000542-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Fukushima K, Nishini Y, Mori K, Takeda J. The effect of epidurally administered dexmedetomidine on central and peripheral nervous system in man. Anesth Analg. 1997;84:S292. [Google Scholar]


