Abstract
Clinical pharmacological research plays a vital role in cancer drug development. In recent years biomarkers studies have become integral to this process, specifically the use pharmacological biomarkers in the development of targeted therapies and their translation to clinical practice. In this overview, we discuss the validation of pharmacodynamics (PD) biomarkers and highlight the circulating tumor DNA as a promising cancer biomarker to illustrate how PD biomarkers can be powerful tools for guiding treatment strategies. We provide insights into PD biomarkers approaches for future development of novel therapies and their role in cancer medicine.
Introduction
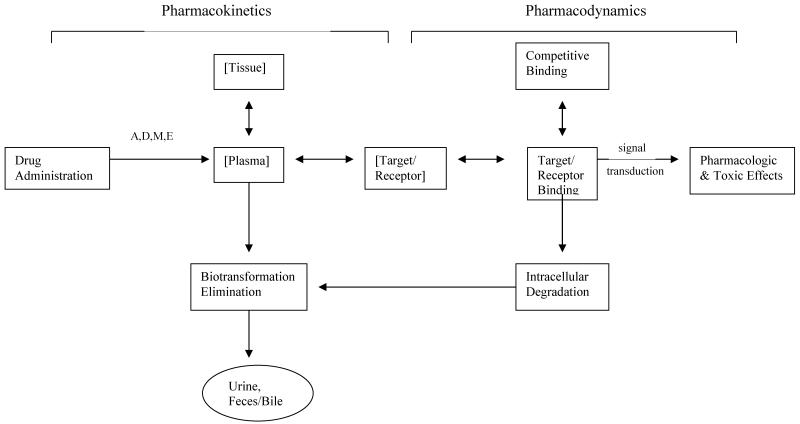
Clinical pharmacology is the science of drugs in the prevention, treatment and control of disease in humans. A comprehensive understanding of the principles of clinical pharmacology is essential for any clinician to deliver optimal therapeutics to individual patients. Clinical pharmacology is divided into three important disciplines that are inter-related: pharmacokinetics (PK - absorption, distribution, metabolism and elimination), pharmacogenetics (PG - genes that regulate pharmacokinetics, including drug metabolizing enzyme and drug transporter genes, and genes for pharmacological targets) and pharmacodynamics (PD - pharmacological effects manifested as a clinical response or adverse effect) (Figure 1) (1). It is the dependence of PD (drug action) on PK and PG that is a central theme in the six articles presented in this CCR FOCUS review (2-7). Our understanding of PD has greatly improved over the past two decades and most drug effects are the result of interactions with specific macromolecules or targets that induce a biochemical, physiological or molecular change. Hertz and McLeod (2) eloquently point out that although, in oncology, PD typically refers to a biochemical response that modulates oncogenic pathway in cancer cells, PD also applies to response (albeit most often an undesired response) in non-cancer cells. In this context, PD can be broken down into two types of pharmacological action: receptor or target pharmacology (agonist, competitive antagonist, enzyme inhibition, partial agonist, etc.) and non-receptor-mediated drug actions.
Figure 1.
Schematic representation of the processes determining drug disposition in the human body and the relationship of pharmacokinetics and pharmacodynamics to these processes. Reproduced from Beelen and Lewis (1) with kind permission from Springer Science + Business Media.
Whilst the theme of this CCR FOCUS edition is primarily PD biomarkers and their role in cancer medicine, it is important to recognize the comprehensive role that biomarkers now play in the management of the cancer problem, namely:
Predisposition biomarkers to predict the risk of developing cancer, e.g. germline mutations in tumor suppressor genes such as BRCA1 and BRCA2.
Screening biomarkers for early cancer detection, and hence more effective management, in the general or an at risk population, e.g. PSA.
Diagnostic biomarkers to define the exact type of tumor to be treated, e.g. cellular and molecular pathology.
Prognostic biomarkers for estimating the likely disease course and hence the most appropriate management strategy, e.g. radiological and pathological assessments.
Predictive biomarkers for selecting the most appropriate therapy, e.g. molecular target assessment to identify the appropriate targeted therapy (predictive biomarkers are also referred to as “theranostics” and “companion diagnostics”).
Pharmacological biomarkers, as discussed extensively in this CCR Focus edition, to demonstrate that active drug levels are achieved (PK biomarkers) and that these are associated with adequate drug-target interaction (proof-of-mechanism PD) and phenotypic effects (proof-of-concept PD) in the tumor. Furthermore, genetic factors can influence both PK and PD and these are determined by PG biomarkers.
Surrogate response biomarkers to detect clinical activity before volumetric changes in the tumor have occurred, e.g. changes in circulating tumor markers or functional imaging such as PET and MR.
Validation of PD biomarkers
Validation of a pharmacodynamic biomarker addresses whether the biomarker achieves its purpose in a carefully defined clinical setting and the population of interest. A critical distinction should be made between when a biomarker undergoes method validation versus clinical qualification. Analytical method validation is the process of assessing the assay, its performance characteristics, and the optimal conditions that will ensure the reproducibility and accuracy of the assay. Clinical qualification is the evidentiary process of linking a biomarker with biological processes and clinical endpoints (8), and is equivalent to clinical validation recently defined by Parkinson and colleagues (9). While “validation” and “qualification” have been used interchangeably in the literature, the distinction should be made to properly describe the particular phase the PD biomarker is transitioning through in the drug development process. The term “validation ”is usually reserved for analytical methods, and “qualification” for biomarker evaluation in relation to a clinical endpoint (8, 10). Both validation and qualification processes are intertwined and their integration guides biomarker development with the overriding principle of linking the biomarker with its intended use (11).
It is also important to point out that biomarker method validation is distinct from pharmacokinetic validation and routine laboratory validation. A “fit-for-purpose” approach for biomarker method development and validation is derived from the concept that assay validation should be tailored to meet the intended purpose of the biomarker study. Method validation should demonstrate the reliability of the assay for the intended application, with the rigor of the validation process increasing from the initial validation required for exploratory purposes to the more advanced validation that is needed to demonstrate the evidentiary status of the biomarker (11). Fit-for-purpose method validation is an umbrella terminology that is used to describe distinct stages of the validation process including pre-validation, exploratory and advanced method validation, and in-study method validation. Method validation is thus a continuous and iterative process of assay refinement with criteria that are driven by the application of the biomarkers with increasing rigor at each successive validation step, focusing on method robustness, cross-validation, and documentation control.
In 2001, the US FDA issued guidance for industry on “bio-analytical method validation” for assays to support PK studies that are specific for small-molecule drugs and which are not directly related to the validation of biomarker assays. As an update, in September 2013, the US FDA issued a revised draft guidance that includes biomarkers and diagnostics (12), and in order to ensure the development of validated analytical tests, the revised draft guidance established six fundamental parameters for the validation of a biomarker assay: accuracy, precision, selectivity, sensitivity, reproducibility, and stability. While the recommendations in this guidance pertain to the validation of assays to measure in vivo biomarker concentrations in biological matrices, such as blood or urine, the above parameters can be extended to any sample type or assay platform used in analytic method validation.
The concept of biomarker qualification has become integrated within the drug development process. The qualification of biomarkers as tools for efficient drug development originated from the US FDA Critical Path Initiative and the FDA’s Guidance for Industry on Pharmacogenomic Data Submissions (13). The guidance defined a valid biomarker as one that is measured in an analytical test system with well-established performance characteristics, and for which there is an established scientific framework or body of evidence that elucidates the physiologic, toxicologic, pharmacologic, or clinical significance of the test results. The classification of biomarkers is context-specific (14), and this context-specific concept resulted in the qualification process of biomarkers being defined as “…a conclusion that within the stated context of use, the results of assessment with a biomarker can be relied upon to adequately reflect a biological process, response, or event, and support use of the biomarker during drug development.” The term ‘context of use’ describes the setting(s) in which the biomarker is qualified, and boundaries within which the available data justify its use. In January 2014, the US FDA issued the latest guidance document on the qualification process for drug development tools (15). Biomarkers are measured using specific devices and biomarker qualification cannot be achieved without analytical and clinical validation of at least one device to measure the biomarker. It is essential that the assays used to measure the biomarker are analytically validated in a sequence of trials to generate the evidence to support the biomarker’s use in the given context of use. The qualification data incorporate consensus methods and evidence that associates biomarker measurements with sufficient clinical outcomes. Examples of the qualification of biomarkers for use in the development of oncology drugs can be best illustrated with the qualification of circulating tumor cells (16) and the qualification of imaging-based biomarkers (17), as measures of clinical benefit. Biomarker qualification is also observed in the co-development of biomarkers (in the form of “diagnostic” tests) and drugs with the use of these biomarkers being limited to the drug’s application (18). Co-development imposes the necessity to generate specific guidelines describing analytical test validation (sensitivity and specificity of the assays), clinical test validation (ability of the assays to detect and predict diseases), and clinical utility.
In the current CCR FOCUS edition, Kinders and colleagues (3) provide excellent examples of the challenges that can be encountered in the implementation of PD biomarkers in the evaluation of targeted therapies, using proof-of-mechanism and proof-of-concept PD biomarkers that are relevant to the development of poly(ADPribose)polymerase inhibitors as case examples. Importantly, the authors highlight the potential of using surrogate normal tissues, e.g. PBMCs and skin biopsies. However, the data derived from such studies, whilst of value in confirming that potentially effective drug levels are being achieved, are not a substitute for studies on tumor material, although the latter may not of course always be clinically accessible. As a consequence a number of laboratories have investigated the utility of circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) in biomarker studies.
Circulating tumor DNA as a biomarker
Cell-free fragments of DNA are shed into the bloodstream by cells undergoing apoptosis or necrosis and in patients a proportion of the fragmented DNA in circulation is derived directly from the tumor. While the circulating cell-free DNA, also known as circulating tumor DNA (ctDNA), may arise from tumor necrosis, apoptosis or active secretion, the exact mechanism remains unknown (19). Recent advances in sequencing technologies have enhanced the sensitivity and accuracy of DNA analysis, allowing for the genotyping of somatic genomic alterations in ctDNA. ctDNA contains genetic defects identical to those of the tumors themselves, enabling the detection of cancer-associated genetic alterations that include point mutations, rearrangements, amplifications, and aneuploidy.
In recent years, ctDNA has shown promise as a non-invasive cancer biomarker. The concept that circulating tumor DNA represents a sensitive biomarker of tumor burden, and hence has potential as both a prognostic and surrogate response biomarker, has come to fruition with the demonstration that ctDNA can relate to tumor staging and prognosis. Studies in solid tumors such as melanoma, ovarian, breast, and colon cancers have evaluated the potential utility of this approach to define tumor dynamics during therapy for patients with advanced disease (20-24). Specifically, detection of KRAS mutations in ctDNA can help with drug monitoring and thus early identification of patients who develop resistance to epidermal growth factor receptor (EGFR) blockade (19, 20). This potential role was further confirmed in two recent studies (25, 26) with Bettegowda et al. demonstrating the sensitivity of ctDNA for detection of clinically relevant KRAS gene mutations at 87.2% and its specificity was 99.2% (27). They also detected ctDNA across 14 tumor types that had not yet metastasized or released detectable circulating tumor cells, and found ctDNA at relatively high concentrations in the patients with metastatic cancer and at lower but detectable concentrations in those with localized cancers. Together, these findings suggest that ctDNA could be a reliable biomarker for early detection as well as for determining optimal treatment, and monitoring resistance.
The ability to detect and enumerate ctDNA offers broad clinical applications that have not been feasible with routine sequencing of tumor tissue or other circulating or imaging biomarker measurements. The relatively short half-life (approximately 2 hours) of ctDNA allows measurement of changes that take place over a short timeframe (hours), rather than months as seen with conventional volumetric measures of radiographic response or progression, making the measurement of ctDNA an ideal candidate marker of tumor dynamics (28). The high degree of specificity of ctDNA allows for interrogation of tumor-specific molecular alterations in the circulation as mutations found in ctDNA would be absent in matched normal DNA. Furthermore, in terms of sensitivity, ctDNA is abundant and readily detectable in most patients with advanced cancer; in cases with lower levels of ctDNA such as in early stage disease or minimal residual disease, detection may still be possible using advanced genomic methodologies. The analysis of ctDNA has considerable potential in the assessment of molecular heterogeneity, for monitoring tumor dynamics, identifying genetic determinants of therapy, and evaluating treatment response; as well as detecting acquired resistance and developing potential strategies to circumvent this (29). Thus, ctDNA is a broadly applicable, sensitive, and specific biomarker that can be a powerful tool for guiding treatment strategies in cancer. However, it will be necessary to develop standardized methodologies for ctDNA analyses and validation in large prospective clinical trials, prior to implementing this ‘liquid biopsy’ approach widely in the clinic.
PD biomarker approaches and issues addressed in this edition
Complementing and extending the potential of ctDNA as a biomarker in PD studies are investigations using circulating tumour cells (CTCs). The article by Yap et al. (4) in the current CCR Focus demonstrates the exciting progress that has been made in utilizing CTCs in PD studies, in addition to the extensively investigated role of CTCs as prognostic and surrogate response biomarkers. Correctly, the authors emphasize the two key issues of CTC heterogeneity and the potential for differences in PD readouts in CTCs versus those in the original solid tumor lesion. A particular issue in using CTCs for PD studies in that cells in the blood will be exposed to plasma drug concentrations, which may or may not be the same as drug levels achieved in solid tumors, emphasizing the more general point that PK and PD should not be considered in isolation, but as the two sides of the same pharmacological “coin”.
Although the direct measurement of tumour drug levels is extremely challenging, the article by van der Veldt and Lammertsma (5) demonstrates the potential of PET tracers in this context, as exemplified by the radiolabeled taxanes [18F]paclitaxel and [11C]docetaxel. These tracers provided significant insights into PK and bio-distribution models, enhancing predictive ability by addressing the question of whether or not the drug achieves active tumor levels. In addition, the authors reported that the induction of the ABCB1 transporter apparently reduced tumor uptake. These data, if confirmed, have tremendous clinical implications as accumulation was variable and associated with tumor perfusion, but not tumor size. Of concern was the observation that less than 1% of total drug administered accumulated the tumors, highlighting the importance of approaches designed to enhance drug delivery. Notwithstanding the low overall level of tumor drug uptake, higher tumor uptake was related to tumor response in patients with lung cancer. Furthermore, the enticing data presented on the interaction between bevacizumab and docetaxel was particularly impressive, where in patients with non-small cell lung cancer bevacizumab appeared to reduce both perfusion and the net influx rate of [11C]docetaxel, an effect that persisted for at least 4 days, suggesting that careful though should be given to the sequence of anti-angiogenic agent/chemotherapy drug combination regimens. Although this article demonstrates the potential of PET imaging in providing hard data on the distribution of drugs, the real value will come in showing target expression, and data are now emerging on both PK and PD.
The most successful class of targeted therapies to date in cancer medicine are the oncogenic kinase inhibitors or antagonists, either small molecule or antibody-based. These drugs are the “poster children” for personalized/stratified/precision medicine and provide unequivocal evidence that molecular insights into the disease can lead to significant improvements in outcomes. As the development of kinase inhibitors has evolved the critical role of predictive and PD biomarkers has become ever more clearly apparent, and Gainor and colleagues (6) provide an excellent overview of lessons learned and challenges still faced in lung cancer. Again, these authors emphasize the issue of accessing a hard-to-biopsy tumor for biomarker studies, and the potential of surrogate tissues, CTCs, ctDNA and imaging for PD studies in lung cancer.
Lastly, the interplay between GWAS and candidate SNP studies is well laid out in the reviews of Low et al. (7) and Hertz and McLeod (2), respectively. GWAS and candidate SNP studies are both necessary to fully understand the PG of a drug, and most PG candidate SNP approaches assume that the key drug metabolizing enzymes and transporters involved have been defined. There are now at least three commercially platforms available for studying drug metabolism and transporter genes. On the other hand, GWAS offers a hypothesis-free approach for generating insights into genetic determinates of PK and PD, as well as understanding of the mechanisms and pathways of underlying gene-phenotype interactions. Nonetheless, both approaches (GWAS and candidate SNP) have their limitations; in particular the failure to provide functional information. However, both approaches have strengths and the sequential use of GWAS to identify candidate SNPS that can then be translated into focused SNP panels, as illustrated in the article by Hertz and McLeod (2) is a viable route to clinical application. Co-ordination in the development of clinical PG biomarkers for routine use is critical and a number of national and international consortia have been established to do so.
Conclusions
As cancer becomes the primary cause of death from disease in an increasing number of developed countries, and the overall incidence of cancer increases with ageing populations, the need for more effective therapies becomes ever-more pressing. After many decades of investment in basic cancer research insights into cancer biology are now being translated into new treatments that offer real hope for both current and future generations of cancer patients. Importantly, in developing and optimizing these new therapies, the quality and intensity of science that led to their discovery must be sustained during their clinical evaluation, and biomarker studies are vehicle for so doing. Specifically, predictive, PK, proof-of-mechanism PD, proof-of-concept PD, and surrogate response biomarker studies are the tools that facilitate highly quality clinical pharmacological research, without which the early clinical development of targeted therapies as well as their routine use in cancer medicine will not be viable. The articles in this CCR FOCUS edition highlight both the considerable potential as well as many of the pitfalls of biomarker research. However, the significant progress made already gives confidence that, through integrated targeted drug and biomarker studies, progress seen in the chemotherapy of cancer will be maintained.
Acknowledgments
The authors are indebted to Cindy Chau for her assistance in writing this brief introduction, Lucia Allen and Corinne Mooney at the CCR Editorial Office for their unstinting and professional support in compiling this collection of articles, all the authors who contributed manuscripts in a timely and enthusiastic manner, and Dr. Susan Bates for providing the opportunity of covering this important topic in a CCR FOCUS edition.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Contributor Information
William Douglas Figg, Sr., Clinical Pharmacology Program in the Office of the Clinical Director, Molecular Pharmacology Section and Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, USA.
David R. Newell, Northern Institute for Cancer Research, Newcastle University, UK.
References
- 1.Beelen AP, Lewis LD. Clinical pharmacology overview. In: Figg WD, McLeod HL, editors. Handbook of Anticancer Pharmacokinetics and Pharmacodynamics. Springer; New York: 2004. pp. 111–27. [Google Scholar]
- 2.Hertz DL, McLeod HL. Using pharmacogene polymorphism panels to detect germline pharmacodynamic markers in oncology. Clin Cancer Res. 2014;20:xx–xx. doi: 10.1158/1078-0432.CCR-13-2780. [DOI] [PubMed] [Google Scholar]
- 3.Kinders R, Ferry-Galow K, Wang L, Srivastava AK, Ji JJ, Parchment RE. Implementation of validated pharmacodynamic assays in multiple laboratories: challenges, successes, and limitations. Clin Cancer Res. 2014;20:xx–xx. doi: 10.1158/1078-0432.CCR-14-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014;20:xx–xx. doi: 10.1158/1078-0432.CCR-13-2664. [DOI] [PubMed] [Google Scholar]
- 5.van der Veldt AAM, Lammertsma AA. In vivo imaging as a pharmacodynamic marker. Clin Cancer Res. 2014;20:xx–xx. doi: 10.1158/1078-0432.CCR-13-2666. [DOI] [PubMed] [Google Scholar]
- 6.Gainor JF, Longo DL, Chabner BA. Pharmacodynamic biomarkers: falling short of the mark? Clin Cancer Res. 2014;20:xx–xx. doi: 10.1158/1078-0432.CCR-13-3132. [DOI] [PubMed] [Google Scholar]
- 7.Low S-K, Takahashi A, Mushiroda T, Kubo M. Genome-wide association study: a useful tool to identify common genetic variants associated with drug toxicity and efficacy in cancer pharmacogenomics. Clin Cancer Res. 2014;20:xx–xx. doi: 10.1158/1078-0432.CCR-13-2755. [DOI] [PubMed] [Google Scholar]
- 8.Wagner JA. Overview of biomarkers and surrogate endpoints in drug development. Dis Markers. 2002;18:41–6. doi: 10.1155/2002/929274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkinson DR, McCormack RT, Keating SM, Gutman SI, Hamilton SR, Mansfield EA, et al. Evidence of clinical utility: an unmet need in molecular diagnostics for patients with cancer. Clin Cancer Res. 2014;20:1428–44. doi: 10.1158/1078-0432.CCR-13-2961. [DOI] [PubMed] [Google Scholar]
- 10.Biomarkers Definitios Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res. 2006;23:312–28. doi: 10.1007/s11095-005-9045-3. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration Guidance for industry: Bioanalytical method validation. Draft Guidance. 2013 [cited 2014 March]; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368107.pdf.
- 13.Campion S, Aubrecht J, Boekelheide K, Brewster DW, Vaidya VS, Anderson L, et al. The current status of biomarkers for predicting toxicity. Expert Opin Drug Metab Toxicol. 2013;9:1391–408. doi: 10.1517/17425255.2013.827170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration Guidance for industry: Pharmacogenomic data submissions. 2005 [cited 2014 March]; Available from: http://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126957.pdf.
- 15.US Food and Drug Administration Guidance for Industry and FDA Staff. Qualification Process for Drug Development Tools. 2014 [cited 2014 March]; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM230597.pdf.
- 16.King JD, Casavant BP, Lang JM. Rapid translation of circulating tumor cell biomarkers into clinical practice: technology development, clinical needs and regulatory requirements. Lab Chip. 2014;14:24–31. doi: 10.1039/c3lc50741f. [DOI] [PubMed] [Google Scholar]
- 17.Waterton JC, Pylkkanen L. Qualification of imaging biomarkers for oncology drug development. Eur J Cancer. 2012;48:409–15. doi: 10.1016/j.ejca.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 18.Goodsaid F, Frueh F. Process map proposal for the validation of genomic biomarkers. Pharmacogenomics. 2006;7:773–82. doi: 10.2217/14622416.7.5.773. [DOI] [PubMed] [Google Scholar]
- 19.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–65. [PubMed] [Google Scholar]
- 20.Bidard FC, Madic J, Mariani P, Piperno-Neumann S, Rampanou A, Servois V, et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int J Cancer. 2014;134:1207–13. doi: 10.1002/ijc.28436. [DOI] [PubMed] [Google Scholar]
- 21.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 22.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4:136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 24.Shinozaki M, O’Day SJ, Kitago M, Amersi F, Kuo C, Kim J, et al. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res. 2007;13:2068–74. doi: 10.1158/1078-0432.CCR-06-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz LA, Jr., Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–40. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–6. doi: 10.1038/nature11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misale S, Arena S, Lamba S, Siravegna G, Lallo A, Hobor S, et al. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6:224ra26. doi: 10.1126/scitranslmed.3007947. [DOI] [PubMed] [Google Scholar]
- 29.Diaz LA, Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–86. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]