Abstract
GLUT1, the primary glucose transport protein in human erythrocytes [red blood cells (RBCs)], also transports oxidized vitamin C [dehydroascorbic acid (DHA)]. A recent study suggests that RBC GLUT1 transports DHA as its primary substrate and that only a subpopulation of GLUT1 transports sugars. This conclusion is based on measurements of cellular glucose and DHA equilibrium spaces, rather than steady-state transport rates. We have characterized RBC transport of DHA and 3-O-methylglucose (3-OMG), a transported, nonmetabolizable sugar. Steady-state 3-OMG and DHA uptake in the absence of intracellular substrate are characterized by similar Vmax (0.16 ± 0.01 and 0.13 ± 0.02 mmol·l−1·min−1, respectively) and apparent Km (1.4 ± 0.2 and 1.6 ± 0.7 mM, respectively). 3-OMG and DHA compete for uptake, with Ki(app) of 0.7 ± 0.4 and 1.1 ± 0.1 mM, respectively. Uptake measurements using RBC inside-out-membrane vesicles demonstrate that 3-OMG and DHA compete at the cytoplasmic surface of the membrane, with Ki(app) of 0.7 ± 0.1 and 0.6 ± 0.1 mM, respectively. Intracellular 3-OMG stimulates unidirectional uptake of 3-OMG and DHA. These findings indicate that DHA and 3-OMG bind at mutually exclusive sites at exo- and endofacial surfaces of GLUT1 and are transported via the same GLUT1 complex.
Keywords: GLUT1, erythrocyte, dehydroascorbic acid transport, glucose transport
vitamin C is an essential micronutrient that plays critical roles in a number of biological processes, including the biosynthesis of collagen and the neurotransmitter norepinephrine (19, 20). In addition to acting as a critical enzyme cofactor, vitamin C also serves as a major, water-soluble antioxidant, with the reduced form, ascorbic acid (AA), donating two electrons to form dehydroascorbic acid (DHA). Unlike the majority of other vertebrates, humans and several other mammals have lost the ability to biosynthesize AA because of a mutation in the gene encoding gulonolactone oxidase (8). As a result, the proper ingestion, processing, and delivery of dietary vitamin C are essential to maintain adequate supply to the various tissues of the body. Chief among these tissues is the brain, where vitamin C is present in millimolar concentrations and plays vital roles ranging from neurotransmitter synthesis and regulation to detoxification of reactive oxygen species (28). High demand for AA, therefore, necessitates efficient AA transport from circulating blood, across the blood-brain barrier, and into the brain.
Vitamin C is transported into human cells via two classes of transport proteins: sodium-dependent vitamin C transporters 1 and 2 are responsible for the bulk of AA transport in a wide variety of tissues, while DHA transport is mediated by the cytochalasin B (CCB)-inhibitable, class I glucose transporters GLUT1, GLUT3, and GLUT4 (29). Among these, GLUT1 is ubiquitously expressed and serves as the primary glucose transporter in blood-tissue barriers and the erythrocytes [red blood cells (RBCs)] of the circulatory system. Recently, Montel-Hagen et al. (25) suggested that the main role for GLUT1 expressed in mature human RBCs is transport of DHA, rather than glucose. It has been proposed that RBC GLUT1 interacts with the protein stomatin, which regulates GLUT1, through an unknown mechanism, such that glucose is unable to compete with DHA for transport (25, 26) and 3-O-methylglucose (3-OMG) transport is inhibited (33). These results were unexpected, because this substrate discrimination was not observed in previous studies with human RBCs (18, 29).
In an attempt to resolve this discrepancy, we have undertaken an analysis of 3-OMG and DHA transport by GLUT1 in mature RBCs isolated from whole human blood. The glucose analog 3-OMG was used throughout this study, because it is not phosphorylated by hexokinase and, thus, allows for unambiguous determination of GLUT1 activity without complications arising from subsequent metabolism of substrate. We describe initial rates of 3-OMG and DHA transport by RBCs and the relative affinities of endo- and exofacial transport sites for these substrates. We have also evaluated the ability of these substrates to coparticipate in accelerated exchange uptake via the same transporter complex. A clear understanding of how GLUT1 binds and transports DHA relative to glucose is crucial for comprehending how the interplay between the two substrates affects distribution and recycling of AA in health and disease.
MATERIALS AND METHODS
Materials
[3H]3-OMG and l-[1-14C]AA were purchased from American Radiolabeled Chemicals (St. Louis, MO). AA, DHA, 3-OMG, ascorbate oxidase, CCB, [3H]CCB, d-[3H]glucose, and type IX trypsin were purchased from Sigma Aldrich (St. Louis, MO). Custom-synthesized rabbit anti-GLUT1 antibody was raised against amino acids 480–492 and produced by New England Peptide. Mouse anti-stomatin antibody (GARP50) was a generous gift from Dr. Rainer Prohaska (University of Vienna).
Methods
RBC, ghost, and inside-out vesicle preparation.
Deidentified, whole human blood, stored in citrate-phosphate-dextrose, was purchased from Biological Specialty (Colmar, PA). RBCs were washed three times with ice-cold RBC wash buffer [150 mM KCl, 5 mM HEPES (pH 7.4), and 0.5 mM EDTA] and centrifuged at 3,000 g at 4°C for 5 min to remove serum and buffy coat. For zero-trans uptake measurements, RBCs were depleted of intracellular sugar by dilution with 40 volumes of RBC wash buffer and incubation at room temperature for 1 h.
Ghosts were prepared as previously described (13). Briefly, 1 volume of packed, washed RBCs was resuspended with 30 volumes of ice-cold RBC lysis buffer [10 mM Tris·HCl (pH 7.2) and 4 mM EDTA] and incubated on ice for 10 min. Membranes were pelleted at 34,000 g at 4°C for 15 min and washed three times with ice-cold RBC lysis buffer. Ghost membranes were resuspended with 10 volumes of RBC wash buffer and incubated at 37°C for 1 h to reseal. Resealed ghosts were collected at 16,000 g at 4°C for 10 min and resuspended with an equal volume of RBC wash buffer.
Inside-out vesicles (IOVs) were prepared as previously described (4). Briefly, lysed and washed ghost membranes were resuspended with 10 volumes of vesiculation medium [10 mM Tris·HCl and 4 mM EDTA (pH 7.5)] and incubated at 37°C for 1 h. Membranes were then pelleted as described above for ghosts, resuspended with an equal volume of RBC wash buffer, and passed five times through a 27-gauge needle. All washed RBCs, ghosts, and IOVs were prepared fresh prior to each transport experiment. Membrane orientation of ghosts and IOVs was evaluated by incubation of vesicles at 37°C for 3 h in the absence or presence of 2:1 membrane protein-trypsin. Vesicles were then washed three times with RBC wash buffer and incubated with 0.4 μM [3H]CCB in the presence or absence of 40 μM unlabeled CCB at 4°C for 1 h. Nonspecific binding of CCB to actin was inhibited by inclusion of 10 μM unlabeled CCB in the binding reactions. Total and unbound samples were assayed in duplicate for the presence of the radiolabel by liquid scintillation counting (Beckman Coulter).
Transport measurements.
Buffers and tubes were chilled to ice temperature prior to the start of all uptake measurements, unless otherwise indicated. AA was converted to DHA by addition of ascorbate oxidase (10 U/ml) and incubation at room temperature for 30 min. The absence of residual AA was determined using a ferric-reducing ascorbate assay kit (Biovision, Milpitas, CA). Each uptake reaction was performed in triplicate with 20 μl of a 50% suspension of RBCs, ghosts, or IOVs. Reactions were initiated by addition of 100 μl of uptake solution containing 1 μCi/ml of [3H]3-OMG and/or l-[1-14C]DHA and the indicated concentrations of unlabeled substrates. Reactions were stopped by addition of 1 ml of ice-cold stop solution containing 10 μM CCB and 100 μM phloretin in RBC wash buffer. Stopped cells were pelleted at 16,000 g for 1 min and washed once with stop solution. Cell pellets were then lysed in 0.5 ml of 3% perchloric acid and centrifuged at 16,000 g for 1 min, and 200 μl of the clear supernatant were assayed in duplicate for retained label by simultaneous dual-isotope liquid scintillation counting (13).
Immunoblotting.
Samples of an equal amount of membranes were prepared by addition of one volume of 2× Laemmli sample buffer (Sigma). These samples were run on 4–12% Bis-Tris acrylamide gels (Invitrogen), transferred to polyvinylidene difluoride membranes, and blocked for 1 h with 5% bovine serum albumin in 20 mM Tris base (pH 7.6), 135 mM NaCl, and 0.2% Tween 20 (TBST). Blots were incubated with a rabbit anti-GLUT1 or mouse anti-stomatin (GARP50) primary antibody in TBST at room temperature for 1 h, washed with TBST, and then incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit or anti-mouse; Jackson Laboratories). After additional washing, blots were incubated with chemiluminescence visualizer (Pierce) and developed using an imaging instrument (LAS-4000, Fuji).
Kinetic analysis.
Data analysis was performed by nonlinear regression using GraphPad Prism version 6 (La Jolla, CA). Substrate dose-response data were fit to the expression
| (1) |
where Vmax and Km are Vmax and Km for substrate (S) transport, respectively, and k describes nonspecific substrate transport or binding.
Competitive inhibition was evaluated by fitting competition dose-response data to Eq. 2
| (2) |
where Y0 is the amount of uptake in the absence of the competing substrate I and YΔ is the maximum amount of transport depressed by saturating levels of competing substrate, Y0 − YΔ is nonspecific substrate uptake or binding, and
| (3) |
where Ki is the intrinsic dissociation constant for competing substrate binding to the transporter.
When Vmax and Km for substrate transport are known, an alternative, more explicit analysis is available using the expression
| (4) |
where v′/v is the ratio of inhibited to uninhibited transport at any given competing substrate concentration ([I]).
Fractional equilibration of intracellular water with extracellular substrate is described by
| (5a) |
where Si and So represent intra- and extracellular substrate, respectively, C is the ratio of accessible cell water to total cell water, t is time, and k is the first-order rate constant (Vmax/Km) for transport when [S] << Km. If rapid and slow phases of equilibration are observed (14), uptake is described by
| (5b) |
where C1 and C2 describe the fast and slow equilibration compartments, respectively (C1 + C2 = 100% free water content of the cell) and k1 and k2 are the corresponding rate constants. When intracellular substrate is metabolized to a nontransportable form, fractional equilibration of intracellular water with extracellular radioisotope is described by
| (5c) |
where C is the ratio of accessible cell water to total cell water, t is time, k is the first-order rate constant (Vmax/Km) for transport when [S] << Km, and k0 is the zero-order rate constant describing substrate conversion to a nontransportable form.
RESULTS
Specificity and Time Course of DHA Transport
[14C]DHA uptake into substrate (d-glucose)-depleted RBCs was measured over 30 s at ice temperature. DHA transport is robust (≥50% as fast as transport of the nonmetabolizable glucose analog 3-OMG) and is sensitive to the glucose transporter inhibitor CCB (Fig. 1A). Uptake of the [14C]DHA precursor AA is much slower than uptake of DHA. Indeed, uptake of AA-derived radioisotope requires pretreatment of AA with ascorbate oxidase. The residual uptake of [14C]AA is CCB-sensitive and is inhibited by unlabeled DHA, but not by AA, indicating the presence of a small fraction of oxidized substrate in the radiolabeled stock AA and confirming the previously described absence of robust AA uptake in RBCs (21).
Fig. 1.
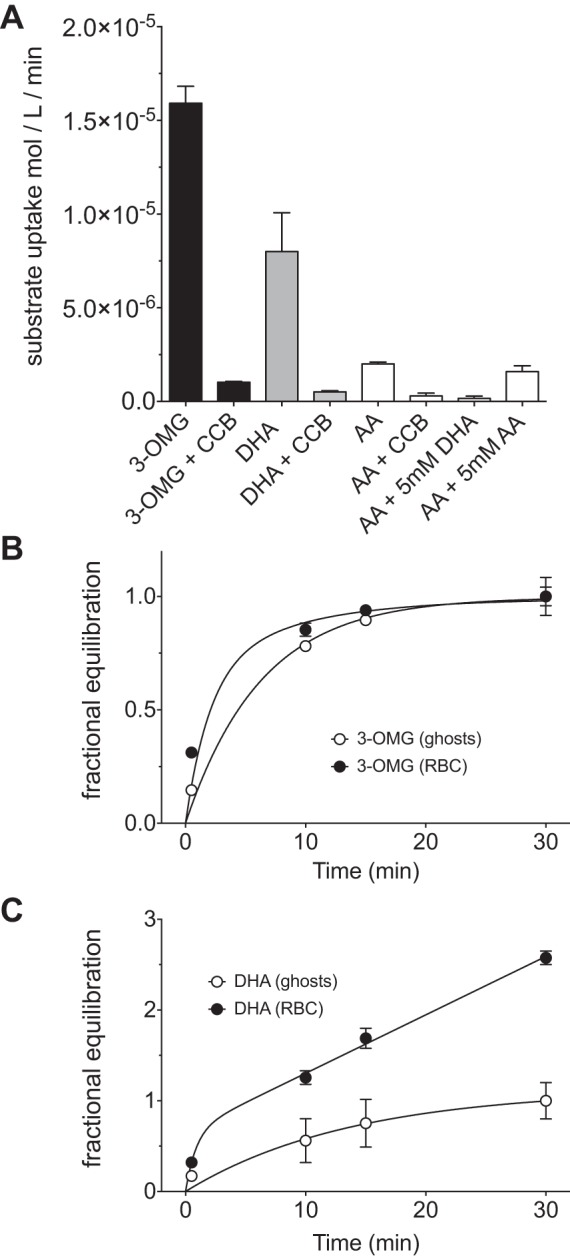
Time course and specificity of dehydroascorbic acid (DHA) transport in red blood cells (RBCs) and ghosts. A: uptake of 100 μM 3-O-methylglucose (3-OMG) or DHA into RBCs is sensitive to cytochalasin B (CCB). Ascorbic acid (AA) transport is also sensitive to CCB and is inhibited by unlabeled DHA, indicating that the radiolabeled AA contains residual, oxidized AA. Measurements were made at ice temperature for 30 s. B: zero-trans time courses of RBC and ghost equilibration with 100 μM 3-OMG. Curves drawn through the points were computed by nonlinear regression with the assumption of 1 accessible intracellular water compartment in ghosts (Eq. 5a) or 2 compartments in RBCs (Eq. 5b). Fits are as follows: k = 0.16 ± 0.03 min−1, equilibrated space = 0.99 ± 0.06 of total cell water for ghosts; k1 = 0.5 ± 0.2 min−1, C1 = 0.66 ± 0.09 of total cell water, k2 = 0.12 ± 0.06 min−1, C1 = 0.33 ± 0.04 of total cell water for RBCs. C: time course of 100 μM DHA uptake by RBCs and ghosts. Curves drawn through the data were computed by nonlinear regression with the assumption that, in ghosts, DHA equilibrates with 1 accessible intracellular water compartment (Eq. 5a) but that, in RBCs, DHA uptake is the sum of 2 processes: the simple, equilibrative process described for ghosts and a metabolic component in which DHA is converted to AA and is no longer a substrate for transport (Eq. 5c). Fits are as follows: k = 1.15 ± 0.49 min−1, k0 = 0.06 ± 0.01 min−1, equilibrated space = 0.66 ± 0.11 of total cell water for RBCs; k = 0.07 ± 0.03 min−1, equilibrated space = 1.12 ± 0.21 of total cell water for ghosts. Values are means ± SE of 3 independent experiments, each performed in triplicate.
3-OMG is not a hexokinase substrate and, upon transport into the cell, simply equilibrates with RBC water. This behavior is unaffected by the removal of intracellular contents when RBCs are lysed and resealed to form ghosts (Fig. 1B). In contrast, DHA is rapidly reduced back to AA following transport into RBCs. As AA is not a GLUT1 substrate, it becomes trapped in the cell, where it accumulates (22). This results in a near-linear time course of uptake into RBCs at early time points. In RBC ghosts that have been depleted of intracellular contents, DHA is no longer enzymatically reduced and shows an equilibration profile similar to that of 3-OMG (Fig. 1C).
Kinetics and Competitive Inhibition of DHA Transport
A recent study suggests that the primary role of RBC GLUT1 is the transport and distribution of DHA, rather than glucose (25). However, to unambiguously evaluate the relative kinetics of DHA and glucose transport, measurements must be made across a range of substrate concentrations. Importantly, these measurements must also be made at early time points, i.e., well before equilibration of cytoplasmic water with extracellular substrate, thereby ensuring that initial rates of GLUT1-mediated substrate uptake, rather than equilibrium cytoplasmic substrate space, are measured. Under these conditions, we observe saturable DHA and 3-OMG uptake into DHA- and 3-OMG-free RBCs (Fig. 2A). Apparent Km [Km(app)] for 3-OMG and DHA uptake are 1.4 ± 0.2 and 1.6 ± 0.7 mM, respectively (Table 1, zero-trans). DHA and 3-OMG are characterized by similar maximum rates of uptake (0.15 mmol·l−1·min−1).
Fig. 2.
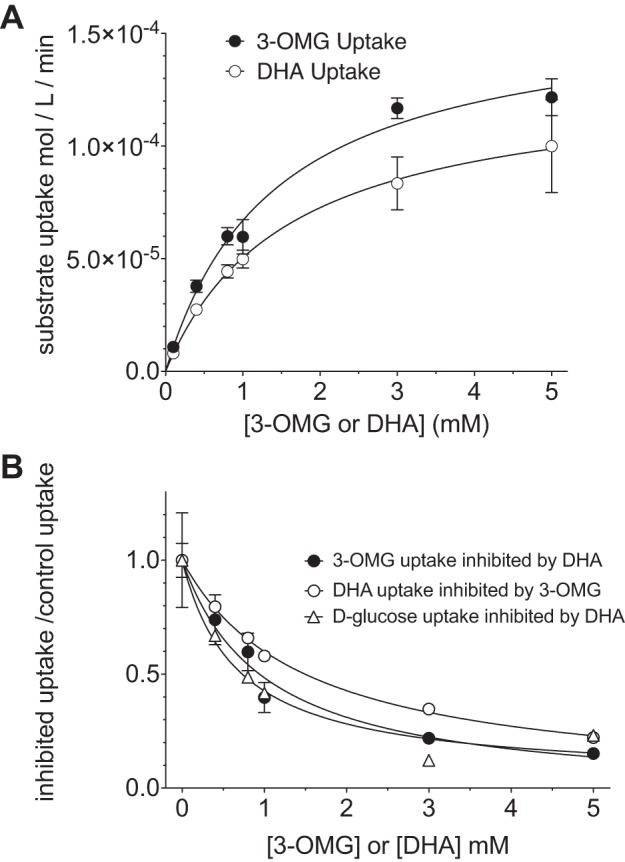
3-OMG and DHA transport in RBCs are characterized by similar affinity and velocity constants and are mutually competitive. A: concentration dependence of 3-OMG and DHA uptake in substrate-depleted RBCs (zero-trans uptake). Curves drawn though the points were computed by nonlinear regression using Eq. 1. Fits are as follows: Vmax = 161 ± 10 μmol·l−1·min−1, Km = 1.4 ± 0.2 mM, k = 0, R2 = 0.93 for 3-OMG; Vmax = 130 ± 21 μmol·l−1·min−1, Km = 1.6 ± 0.6 mM, k = 0, R2 = 0.71 for DHA. B: competition between 3-OMG, d-glucose, and DHA for net uptake in substrate-depleted RBCs. Uptake of radiolabeled 3-OMG, d-glucose, or DHA was measured in the presence of increasing concentrations of unlabeled DHA or 3-OMG, respectively. Curves were computed by nonlinear regression using Eqs. 2 and 4, yielding identical results. Apparent Ki [Ki(app)] obtained from application of Eq. 2 was adjusted for competition by 100 μM transported substrate by use of Eq. 3 and Km values obtained in Fig. 2A. Application of Eq. 4 requires direct substitution of Vmax and Km obtained in Fig. 2A. Results are as follows: Ki = 0.95 ± 0.22, k = 0, R2 = 0.98 for 3-OMG uptake inhibition by DHA; Ki = 0.63 ± 0.17, k = 0.02 ± 0.05, R2 = 0.97 for d-glucose uptake inhibition by DHA; Ki = 1.4 ± 0.10, k = 0, R2 = 0.99 for DHA uptake inhibition by 3-OMG. Uptake was measured for 30 s at ice temperature. Values are means ± SE of 3 independent experiments, each carried out in triplicate.
Table 1.
Kinetic parameters of 3-OMG and DHA uptake into substrate-free or 3-OMG-loaded RBCs
| Transported Substrate | Condition | Km, mM | Vmax, mmol·l−1·min−1 | Ki, mM |
|---|---|---|---|---|
| 3-OMG | Zero-trans uptake | 1.4 ± 0.2 | 0.16 ± 0.01 | |
| 3-OMG | Infinite-trans uptake | 1.8 ± 0.6 | 1.43 ± 0.14 | |
| DHA | Zero-trans uptake | 1.6 ± 0.6 | 0.13 ± 0.02 | |
| DHA | Infinite-trans uptake | 1.3 ± 0.4 | 0.73 ± 0.09 | |
| d-Glucose | DHA inhibition of RBC; Zero-trans d-glucose uptake | 0.6 ± 0.2 | ||
| 3-OMG | DHA inhibition of RBC; Zero-trans 3-OMG uptake | 1.0 ± 0.2 | ||
| DHA | 3-OMG inhibition of RBC; Zero-trans DHA uptake | 1.4 ± 0.1 | ||
| 3-OMG | 3-OMG inhibition of IOV; Zero-trans 3-OMG uptake | 0.9 ± 0.4 | ||
| 3-OMG | DHA inhibition of IOV; Zero-trans 3-OMG uptake | 1.0 ± 0.7 |
Values are means ± SE of a global fit (using Eqs. 2 and 4) to 3 separate experiments. Kinetic parameters were extracted from Figs. 2, 3, and 5. Uptake into cells lacking (zero-trans uptake) or containing (infinite-trans uptake) 40 mM 3-O-methylglucose (3-OMG) was measured, or competition studies were undertaken in which dehydroascorbic acid (DHA) was used to inhibit zero-trans 3-OMG uptake in red blood cells (RBCs) or inside-out vesicles (IOVs), 3-OMG was used to inhibit DHA uptake in RBCs, or 3-OMG was used to inhibit 3-OMG uptake in IOVs. Ki values were obtained from competition dose-response analyses (Figs. 2B and 5).
In addition to reduced uptake relative to DHA, it has been suggested that glucose is unable to compete with DHA for uptake into mature RBCs (25). This finding contrasts with previous evidence for competition between the two substrates. The proposed explanation for this discrepancy is the use of supraphysiological concentrations of unlabeled glucose (>5 mM) in studies demonstrating competition (18). We tested this by measuring the uptake of 3-OMG or DHA at 4°C and 30 s and in the presence of increasing concentrations of the opposing substrate (DHA or 3-OMG, respectively) over a range of concentrations from 0.1 to 5 mM, well within the physiological range for glucose. Reciprocal competitive inhibition is observed between the two substrates, with apparent Ki values of 0.7 ± 0.4 mM for DHA inhibition of 3-OMG uptake and 1.1 ± 0.1 mM for 3-OMG inhibition of DHA uptake (Fig. 2B, Table 1). To ensure that the competition is not sugar analog-specific, we also evaluated whether d-glucose and DHA compete for transport. DHA inhibits GLUT1-mediated d-glucose transport, with a Ki of 0.6 ± 0.2 mM (Fig. 2B, Table 1).
Accelerated Heteroexchange Transport of DHA by 3-OMG
GLUT1-mediated sugar uptake is characterized by a phenomenon called “trans-acceleration” (24, 32). When cells are sugar-free, the rate of unidirectional sugar uptake is low. When cells are preloaded with sufficient sugar to partially or fully saturate the GLUT1 endofacial sugar-binding site, the rate of unidirectional sugar uptake is markedly stimulated. The extent of this stimulation, or trans-acceleration, depends on the extent of saturation of the endofacial site and temperature. This behavior distinguishes many carriers from channels (32), and while different theoretical models have been proposed to explain this behavior (1, 15), this phenomenon indicates that substrate uptake and exit are coupled through the same transporter complex, a process termed exchange transport (13, 24, 32). We therefore asked whether DHA and sugar movements are also coupled on the same transporter molecule or, as has been suggested (25), some GLUT1 proteins transport sugars while others transport DHA. Washed RBCs were preequilibrated with 40 mM unlabeled 3-OMG (sufficient sugar to achieve 97% saturation of the internal site; see below). The uptake of labeled DHA or 3-OMG by these cells was then measured at 30 s and 4°C. The presence of intracellular 3-OMG did not appreciably affect the affinity of GLUT1 for either substrate [Km(app) for exchange uptake = 1.8 ± 0.4 and 1.3 ± 0.4 mM for 3-OMG and DHA, respectively; Fig. 3A, Table 1]. In contrast, intracellular 3-OMG increases Vmax for transport of each substrate by 5- to 10-fold (Vmax = 1.43 and 0.73 mmol·l−1·min−1 for 3-OMG and DHA, respectively; Fig. 3A; Table 1). Uptake at a subsaturating extracellular concentration of either substrate (100 μM) is increased approximately fivefold by 40 mM intracellular 3-OMG (Fig. 3B).
Fig. 3.
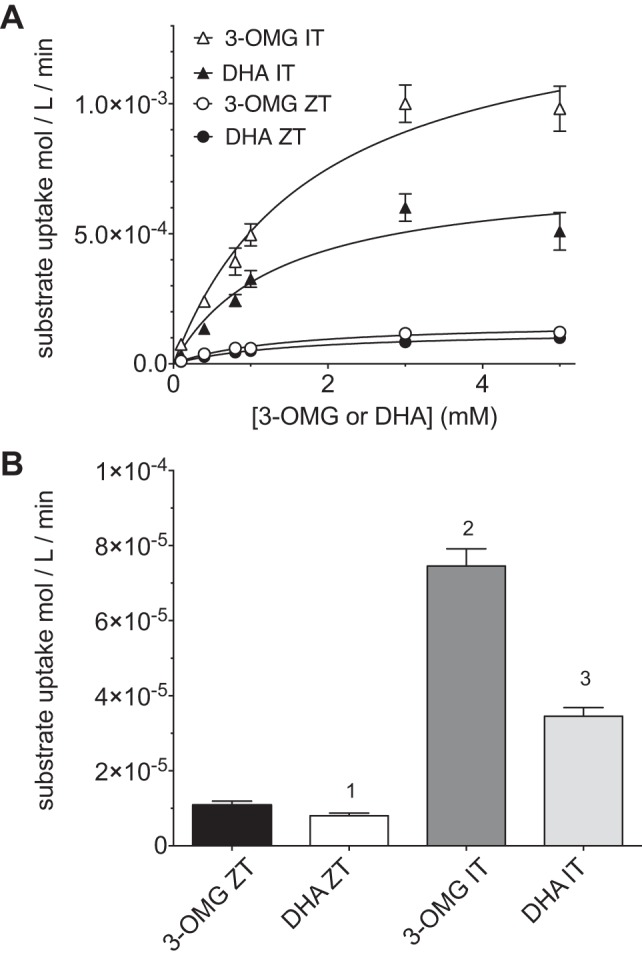
Intracellular 3-OMG accelerates uptake of 3-OMG and DHA. A: RBC zero-trans and 40 mM-trans uptake dose responses to 3-OMG and DHA. Uptake of labeled 3-OMG or DHA with increasing concentrations the same unlabeled substrate into substrate-free RBCs (ZT) or cells equilibrated with 40 mM unlabeled 3-OMG (IT) was measured. Curves were computed by nonlinear regression using Eq. 1. Results are as follows: Vmax = 1,432 ± 142 μmol·l−1·min−1, Km = 1.8 ± 0.64 mM, k = 0, R2 = 0.90 for 3-OMG IT; Vmax = 733 ± 85 μmol·l−1·min−1, Km = 1.3 ± 0.4 mM, k = 0, R2 = 0.78 for DHA IT; see Fig. 2A legend for 3-OMG ZT and DHA-ZT. B: uptake of 100 μM 3-OMG or DHA under zero-trans and infinite-trans (cells contain 40 mM 3-OMG) conditions. One-way ANOVA indicates no significant difference (α level = 0.05) between 3-OMG ZT and DHA ZT uptake (1), 3-OMG IT uptake is significantly greater (α level = 0.001) than 3-OMG ZT, DHA ZT, and DHA IT uptake (2), and DHA IT uptake is significantly greater (α level = 0.001) than 3-OMG ZT and DHA ZT uptake (3). Uptake was measured for 30 s at ice temperature. Values are means ± SE of 3 independent experiments, each performed in triplicate.
Competition for Intracellular Binding and Efflux
Steck and colleagues (31) devised a method for inverting ghost membranes prior to resealing. The resulting IOVs allow easy access to the inner surface of the membrane and direct measurement of intracellular binding and transport by GLUT1. CCB is a membrane-permeable GLUT1 inhibitor that binds at a GLUT1 endofacial binding site (34). CCB also binds to the peripheral membrane protein actin, but this binding is specifically, competitively abolished by inclusion of 10 μM cytochalasin D in our binding solutions (11). CCB binding to IOVs and resealed ghosts is indistinguishable (Fig. 4A, “control”). Trypsin is not membrane-permeable; thus GLUT1 cytoplasmic CCB binding sites are protease-accessible in IOVs, but not in RBC ghosts. Trypsin treatment of ghosts does not affect CCB binding, while IOV digestion reduces binding to 30% of the undigested control (Fig. 4A, “control” and “trypsin”). This reduction of CCB binding is similar to that produced by inclusion of a 100-fold molar excess of unlabeled CCB in the binding reaction (Fig. 4A, “unlabeled CCB”). Furthermore, trypsin treatment of sealed ghosts produces GLUT1 digestion products previously described for exofacial cleavage of GLUT1 and using a GLUT1 COOH-terminal peptide-directed antibody for GLUT1 detection (6) (Fig. 4B). In contrast, digestion of IOVs results in cleavage of the GLUT1 COOH terminus at lysine 477, thereby eliminating detectable antigen. These results confirm that the IOVs are, indeed, inside-out. Importantly, the three washing steps involved in preparing ghosts and IOVs do not affect the relative levels of GLUT1 and the integral membrane protein stomatin (a protein proposed to interact with GLUT1 to convert it from a glucose to a DHA-preferring transporter) (25) (Fig. 4C). Therefore, any direct contribution of this protein to GLUT1 function and regulation is unlikely to be lost. We then evaluated the ability of unlabeled DHA to compete with labeled 3-OMG for uptake into IOVs under the conditions described in Fig. 3 legend. These dose-response experiments show that transport of labeled 3-OMG into IOVs is GLUT1-mediated (CCB-sensitive) and is competitively inhibited with unlabeled 3-OMG or DHA with equal efficacy (Fig. 5). Ki values for 3-OMG and DHA inhibition of 3-OMG uptake in IOVs are 0.9 ± 0.4 and 1.0 ± 0.7 mM, respectively (Table 1).
Fig. 4.
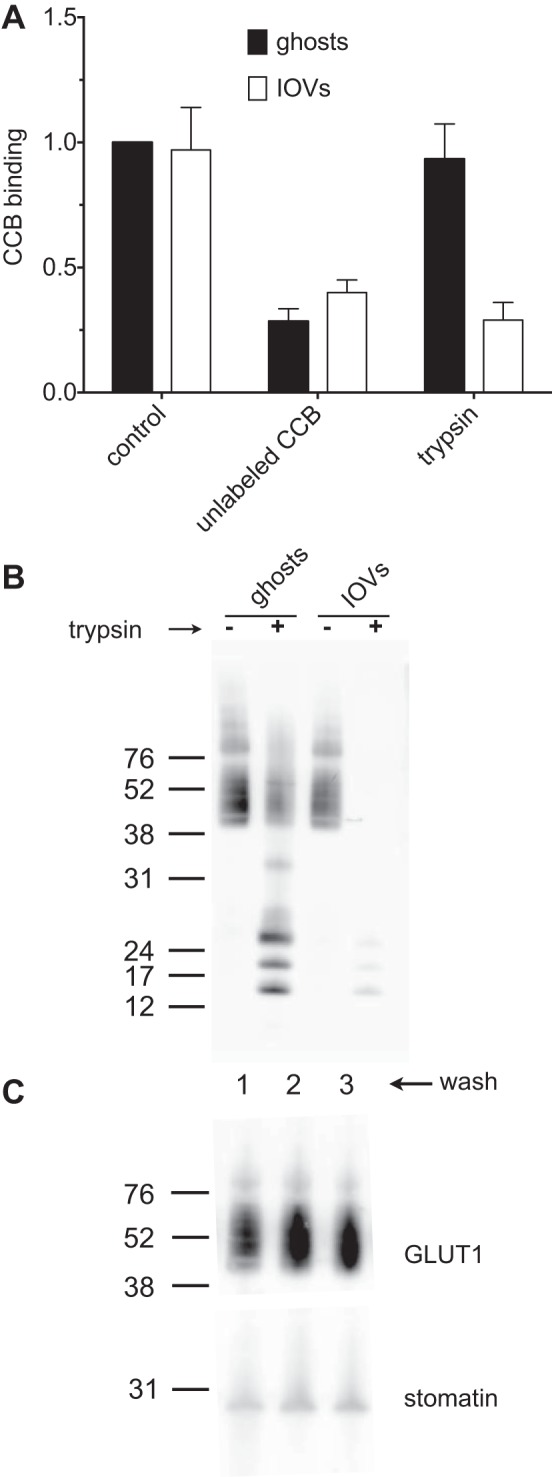
Characterization of RBC ghost and inside-out vesicle (IOV) membrane orientation and stomatin content. A: [3H]CCB binding to IOVs and ghosts is inhibited by unlabeled CCB, but the inside-out orientation of IOVs renders the GLUT1 CCB binding site sensitive to digestion by trypsin. [3H]CCB binding to ghosts or IOVs was evaluated in the presence or absence of a 100-fold molar excess of unlabeled CCB or following trypsin digestion of membranes for 3 h at 37°C. All binding is expressed relative to binding measured in control ghost membranes. Values are means ± SE of 3 experiments. B: Western blot of ghosts and IOVs treated with trypsin for 3 h at 37°C and probed with a GLUT1 COOH-terminal peptide-directed antibody. Lanes 1 and 2 contain ghosts that were treated with (+) or without (−) trypsin. Lanes 3 and 4 contain IOVs treated with (+) or without (−) trypsin. Mobility of molecular standards (kDa) is shown to the left of lane 1. C: ghost preparations do not become depleted of membrane stomatin. Ghost membranes were subjected to up to 3 successive washes with RBC lysis buffer and probed with antibodies against GLUT1 and stomatin.
Fig. 5.
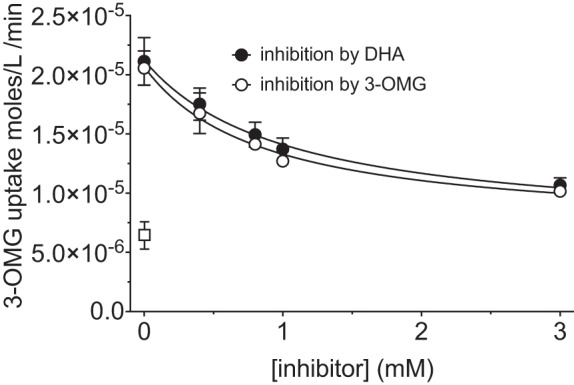
Mutual competition for 3-OMG and DHA uptake in IOVs. Uptake of radiolabeled 3-OMG was measured in the presence of increasing concentrations of unlabeled 3-OMG or DHA. Curves drawn through the points were computed by nonlinear regression using Eq. 2. Ki(app) was not adjusted for competition by 100 μM [3H]3-OMG. Results are as follows: Ki = 1.0 ± 0.7, R2 = 0.67 for DHA inhibition of 3-OMG uptake; Ki = 0.9 ± 0.4, R2 = 0.85 for 3-OMG inhibition of 3-OMG uptake. Uptake was measured for 30 s at ice temperature. Values are means ± SE of 3 independent experiments, each performed in triplicate. Background levels of uptake (leakage) were determined by inclusion of a control reaction containing 10 μM CCB (□). One-way ANOVA demonstrates that inhibition of [3H]3-OMG uptake by unlabeled 3-OMG is significant at >0.4 mM 3-OMG (P values for uptake at 0.4, 0.8, 1, and 3 mM unlabeled 3-OMG compared with uptake in the absence of unlabeled 3-OMG are 0.083, 0.0048, 0.0012, and 0.0001, respectively). Inhibition of [3H]3-OMG uptake by DHA is significant at >0.4 mM DHA (P values for uptake at 0.4, 0.8, 1, and 3 mM unlabeled DHA compared with uptake in the absence of unlabeled DHA are 0.227, 0.021, 0.006, and 0.002, respectively). Uptake of [3H]3-OMG when the concentrations of unlabeled 3-OMG and DHA are the same is not significantly different (P > 0.99 in all instances).
Effect of Time and Temperature on Competition Measurements
A previous study has indicated that uptake of DHA into mature human RBCs is not inhibited by 5 mM unlabeled glucose (25). The methodology described by Montel-Hagen et al. (25) suggests that unlabeled glucose was added to RBCs at room temperature for an unspecified period prior to initiation of uptake determinations (also at room temperature) by addition of radiolabeled substrate. Since RBC sugar transport is ∼500 times faster at room temperature than at ice temperature (16), this protocol could result in partial to full equilibration of RBC water (and, thus, partial saturation of the GLUT1 endofacial sugar-binding site) with glucose prior to measurement of substrate uptake. A less ambiguous approach would have been to include the competing sugar only in the uptake solutions containing radiolabeled substrate. To compare these different approaches, unlabeled sugar was added to the uptake solution (as was done throughout the experiments of present study) or to the RBCs prior to uptake [as was done in the previous study (25)]. When uptake is measured over a 10-min interval and at room temperature (as described by Monte-Hagen et al.), labeled 3-OMG has ample time to completely equilibrate; therefore, no effect of 5 mM glucose is observed, regardless of when the competing substrate is added (Fig. 6, “10 min 24°C”). However, transport over 2 s at 24°C produces ∼40% equilibration of cell water with radiolabel, and the presence of unlabeled glucose has a clear, competitive effect. Importantly, this effect is only observed when the competing sugar is present in the uptake solution (Fig. 6, “2 sec 24°C”). If added to the cells prior to the 2-s measurement, allowing the unlabeled sugar time to equilibrate, stimulation of labeled 3-OMG uptake is observed. This stimulation is due to the higher rate of transport under exchange conditions than zero-trans conditions (no intracellular sugar). When the system is chilled to ice temperature, a 30-s uptake measurement allows only ∼30% equilibration of cell water with radiolabel, and competing sugar in the uptake solution reduces labeled 3-OMG uptake to background levels, displaying the full effect of competition on binding and transport (Fig. 6, “30 sec 4°C”).
Fig. 6.
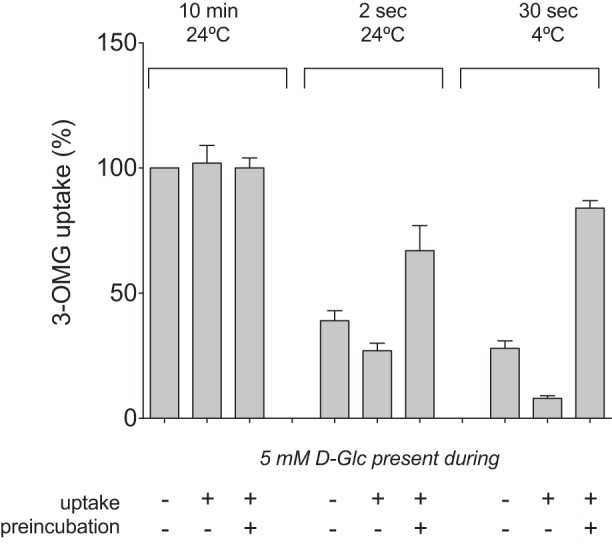
Competitive inhibition of 3-OMG uptake by unlabeled glucose (d-Glc) is observed only when initial rates of 3-OMG uptake are measured. Uptake of labeled 3-OMG was measured for the indicated times and at the indicated temperatures. Unlabeled d-glucose (5 mM) was omitted from or included in the uptake medium (uptake). Unlabeled d-glucose (5 mM) was also absent or included in the cell suspension for 5 min prior to initiation of uptake measurements (preincubation). Values are means ± SE of 3 independent experiments, each performed in triplicate and expressed relative to uptake measured over 10 min at 24°C in the absence of d-glucose.
DISCUSSION
Determinations of Michaelis and velocity constants for substrate transport require careful measurements under well-defined conditions (15). In cells expressing high levels of a transporter of interest, this problem is compounded by very high rates of substrate transport. In this study, we compare the binding and transport of DHA and the glucose analog 3-OMG in human RBCs at 30 s and ice temperature across a range of physiological glucose concentrations. DHA and 3-OMG transport are characterized by Michaelis-Menten kinetics, with indistinguishable Km and Vmax parameters for substrate uptake into substrate-free cells. These results strongly suggest that the same GLUT1 transporter complexes are involved in the uptake of 3-OMG and DHA, since differentially regulated transporter pools might be expected to have unique kinetic parameters. Further evidence supporting DHA and 3-OMG transport by a common pool of GLUT1 transporters is the reciprocal competitive inhibition between the two substrates. This competition is observed at intra- and extracellular binding surfaces across a range of concentrations at or below the normal glucose concentration in circulating blood. Ki calculated for competitive inhibition by each substrate is nearly identical to the respective Km for transport, suggesting that the two bind at identical, overlapping or mutually exclusive sites on the same transporter.
While competition for binding between DHA and 3-OMG is evident, it is conceivable that one substrate could compete with another for binding without actually being transported. For example, maltose competitively inhibits 3-OMG uptake by RBCs but, being a disaccharide, is not transported (9). We addressed this possibility by evaluating the ability of intracellular 3-OMG to accelerate the rate of uptake of labeled DHA. Our observation that efflux of 3-OMG from RBCs increases the rate of DHA import indicates that efflux of one substrate is coupled to import of the second substrate via the same GLUT1 transport complex.
Previously described evidence for substrate discrimination by human RBC GLUT1 (25) may be attributed to differences in the conditions and techniques employed to measure uptake. Unambiguous evaluation of competition between 3-OMG and DHA for GLUT1 binding and transport requires the measurement of initial rates of transport. The very high GLUT1 content of RBC membranes (2, 30) means that exposure of RBCs to GLUT1 substrates prior to initiation of transport measurements can result in equilibration of cell water with extracellular substrate. The length of time required for equilibration depends on the amount of substrate added and the temperature at which measurements are made. For example, RBCs equilibrate micromolar concentrations of glucose or DHA within ∼4 s at room temperature (5, 16, 18). If longer incubation intervals are used to measure transport rates, the amount of labeled substrate that is retained by RBCs is a function of cell water space (equilibrated volume) and the extent to which the substrate is metabolically trapped (e.g., glucose or 2-deoxy-d-glucose phosphorylation by hexokinase or DHA reduction), each being independent of rates of GLUT1 binding and transport. In RBCs under such conditions, these subsequent reactions become rate-limiting for overall accumulation of labeled substrate. This may have contributed to the previous discrepant results (25), since glucose phosphorylation and DHA reduction would not compete with one another. Measurements are further complicated when unlabeled glucose is introduced to the cells prior to uptake, rather than in the uptake solution itself. Because RBC equilibration with sugars at room temperature is rapid, glucose exposure even seconds prior to initiation of uptake measurements can result in glucose loading and consequent trans-acceleration of labeled substrate uptake by intracellular unlabeled sugar.
Taken together, the findings of this study support a model in which uptake of glucose and DHA by human RBCs occurs via the same GLUT1 complexes and proceeds through the same general mechanism. These results argue against the presence of a stomatin-regulated pool of GLUT1 that preferentially transports DHA, rather than glucose (25).
The ability of RBCs to effectively take up DHA and convert it back to AA prevents the irreversible breakdown of DHA to 2,3-diketogulonic acid and loss of the vital micronutrient. The extremely rapid rate of RBC GLUT1-mediated transport at physiological temperature renders any suggested advantage arising from the loss of competition between glucose and DHA for GLUT1-mediated transport (25) unnecessary for effective AA recycling from imported DHA (23). Glucose transport rates at 37°C (16) indicate that competition between sugar and DHA for transport at that temperature will cause an insignificant reduction of RBC equilibration with extracellular substrate during the time (27) required to transit the cerebral microvasculature (RBC equilibration with 5 mM glucose over 2 s = 0.89 vs. 0.89 in the presence of 100 μM DHA; RBC equilibration with 100 μM DHA over 2 s = 0.96 vs. 0.89 in the presence of 5 mM glucose). Indeed, we observe rapid and complete equilibration of a trace concentration of 3-OMG (16 nM) in the presence of 5 mM unlabeled glucose at room temperature (Fig. 6), confirming that, even in the face of physiological concentrations of competing glucose and the absence of a preference for DHA, RBCs would be effective DHA transporters.
In addition to RBCs, GLUT1 is also the sole transporter for oxidized vitamin C at the blood-brain endothelial barrier and in astrocytes (10). Sodium-dependent ascorbate transport is absent in endothelial cells and astrocytes but is present in neurons and at the choroid plexus (10). Therefore, GLUT1-mediated DHA transport at the blood-brain barrier has been investigated as a therapeutic mechanism for rapid delivery of vitamin C to the brain following ischemic stroke (3, 12, 17). While studies in mice and rats suggest that DHA administration can serve a protective role, similar studies in primates have been less conclusive (7, 17). Indeed, several important questions regarding cerebral AA-DHA cycling remain unanswered. Is intracellular DHA reduced to AA in endothelial cells, or is it transported across the blood-brain barrier as DHA? What are the relative contributions of secondary active ascorbate transport at the choroid plexus and passive, GLUT1-mediated DHA transport at the blood-brain barrier to overall brain vitamin C levels? Does GLUT1-mediated DHA transport in astrocytes recycle neurally derived DHA to AA? The answers to these questions await further study.
GRANTS
This work was supported, in whole or in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-36081 and DK-44888.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.S. and A.C. are responsible for conception and design of the research; J.M.S. performed the experiments; J.M.S. and A.C. analyzed the data; J.M.S. interpreted the results of the experiments; J.M.S. and A.C. prepared the figures; J.M.S. drafted the manuscript; J.M.S. and A.C. edited and revised the manuscript; J.M.S. and A.C. approved the final version of the manuscript.
REFERENCES
- 1.Baker GF, Naftalin RJ. Evidence of multiple operational affinities for d-glucose inside the human erythrocyte membrane. Biochim Biophys Acta 550: 474–484, 1979 [DOI] [PubMed] [Google Scholar]
- 2.Baldwin SA, Baldwin JM, Gorga FR, Lienhard GE. Purification of the cytochalasin B binding component of the human erythrocyte monosaccharide transport system. Biochim Biophys Acta 552: 183–188, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Bemeur C, Ste-Marie L, Desjardins P, Vachon L, Butterworth RF, Hazell AS, Montgomery J. Dehydroascorbic acid normalizes several markers of oxidative stress and inflammation in acute hyperglycemic focal cerebral ischemia in the rat. Neurochem Int 46: 399–407, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Carruthers A, Melchior DL. Asymmetric or symmetric? Cytosolic modulation of human erythrocyte hexose transfer. Biochim Biophys Acta 728: 254–266, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Carruthers A. Facilitated diffusion of glucose. Physiol Rev 70: 1135–1176, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Coderre PE, Cloherty EK, Zottola RJ, Carruthers A. Rapid substrate translocation by the multisubunit, erythroid glucose transporter requires subunit associations but not cooperative ligand binding. Biochemistry 34: 9762–9773, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Ducruet AF, Mack WJ, Mocco J, Hoh DJ, Coon AL, D'Ambrosio AL, Winfree CJ, Pinsky DJ, Connolly ES. Preclinical evaluation of postischemic dehydroascorbic acid administration in a large-animal stroke model. Transl Stroke Res 2: 399–403, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Ha MN, Graham FL, D'Souza CK, Muller WJ, Igdoura SA, Schellhorn HE. Functional rescue of vitamin C synthesis deficiency in human cells using adenoviral-based expression of murine l-gulono-γ-lactone oxidase. Genomics 83: 482–492, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Hamill S, Cloherty EK, Carruthers A. The human erythrocyte sugar transporter presents two sugar import sites. Biochemistry 38: 16974–16983, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med 46: 719–730, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helgerson AL, Carruthers A. Equilibrium ligand binding to the human erythrocyte sugar transporter. Evidence for two sugar-binding sites per carrier. J Biol Chem 262: 5464–5475, 1987 [PubMed] [Google Scholar]
- 12.Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, Fox WD, Israel RJ, Boyd TA, Golde DW, Connolly ES. Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc Natl Acad Sci USA 98: 11720–11724, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitch JM, Carruthers A. ATP-dependent sugar transport complexity in human erythrocytes. Am J Physiol Cell Physiol 292: C974–C986, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitch JM, Carruthers A. α- and β-Monosaccharide transport in human erythrocytes. Am J Physiol Cell Physiol 296: C151–C161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieb WR, Stein WD. Testing and characterizing the simple carrier. Biochim Biophys Acta 373: 178–196, 1974 [DOI] [PubMed] [Google Scholar]
- 16.Lowe AG, Walmsley AR. The kinetics of glucose transport in human red blood cells. Biochim Biophys Acta 857: 146–154, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Mack WJ, Mocco J, Ducruet AF, Laufer I, King RG, Zhang Y, Guo W, Pinsky DJ, Connolly ES. A cerebroprotective dose of intravenous citrate/sorbitol-stabilized dehydroascorbic acid is correlated with increased cerebral ascorbic acid and inhibited lipid peroxidation after murine reperfused stroke. Neurosurgery 59: 383–388, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Mann GV, Newton P. The membrane transport of ascorbic acid. Ann NY Acad Sci 258: 243–252, 1975 [DOI] [PubMed] [Google Scholar]
- 19.May JM. Vitamin C transport and its role in the central nervous system. Subcell Biochem 56: 85–103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May JM, Qu ZC, Nazarewicz R, Dikalov S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res Bull 90: 35–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May JM, Qu ZC, Whitesell RR, Cobb CE. Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes. Biochemistry 34: 12721–12728, 1995 [DOI] [PubMed] [Google Scholar]
- 22.May JM, Qu ZC, Whitesell RR, Cobb CE. Ascorbate recycling in human erythrocytes: role of GSH in reducing dehydroascorbate. Free Radic Biol Med 20: 543–551, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Mendiratta S, Qu ZC, May JM. Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic Biol Med 24: 789–797, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Miller DM. The kinetics of selective biological transport. II. Equations for induced uphill transport of sugars in human erythrocytes. Biophys J 5: 417–423, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, Delaunay J, Sitbon M, Taylor N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell 132: 1039–1048, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Montel-Hagen A, Sitbon M, Taylor N. Erythroid glucose transporters. Curr Opin Hematol 16: 165–172, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Puls I, Becker G, Maurer M, Mullges W. Cerebral arteriovenous transit time (CTT): a sonographic assessment of cerebral microcirculation using ultrasound contrast agents. Ultrasound Med Biol 25: 503–507, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci 23: 209–216, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J Biol Chem 272: 18982–18989, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Sogin DC, Hinkle PC, Singer SJ. Characterization of the glucose transporter from human erythrocytes. J Supramol Struct 8: 447–453, 1978 [DOI] [PubMed] [Google Scholar]
- 31.Steck TL, Weinstein RS, Straus JH, Wallach DF. Inside-out red cell membrane vesicles: preparation and purification. Science 168: 255–257, 1970 [DOI] [PubMed] [Google Scholar]
- 32.Stein WD. Transport and Diffusion Across Cell Membranes. New York: Academic, 1986 [Google Scholar]
- 33.Zhang JZ, Abbud W, Prohaska R, Ismail-Beigi F. Overexpression of stomatin depresses GLUT-1 glucose transporter activity. Am J Physiol Cell Physiol 280: C1277–C1283, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Zoccoli MA, Baldwin SA, Lienhard GE. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem 253: 6923–6930, 1978 [PubMed] [Google Scholar]


