Abstract
Lymphedema (LE) is a morbid disease characterized by chronic limb swelling and adipose deposition. Although it is clear that lymphatic injury is necessary for this pathology, the mechanisms that underlie lymphedema remain unknown. IL-6 is a known regulator of adipose homeostasis in obesity and has been shown to be increased in primary and secondary models of lymphedema. Therefore, the purpose of this study was to determine the role of IL-6 in adipose deposition in lymphedema. The expression of IL-6 was analyzed in clinical tissue specimens and serum from patients with or without LE, as well as in two mouse models of lymphatic injury. In addition, we analyzed IL-6 expression/adipose deposition in mice deficient in CD4+ cells (CD4KO) or IL-6 expression (IL-6KO) or mice treated with a small molecule inhibitor of IL-6 or CD4 depleting antibodies to determine how IL-6 expression is regulated and the effect of changes in IL-6 expression on adipose deposition after lymphatic injury. Patients with LE and mice treated with lymphatic excision of the tail had significantly elevated tissue and serum expression of IL-6 and its downstream mediator. The expression of IL-6 was associated with adipose deposition and CD4+ inflammation and was markedly decreased in CD4KO mice. Loss of IL-6 function resulted in significantly increased adipose deposition after tail lymphatic injury. Our findings suggest that IL-6 is increased as a result of adipose deposition and CD4+ cell inflammation in lymphedema. In addition, our study suggests that IL-6 expression in lymphedema acts to limit adipose accumulation.
Keywords: IL-6, adipose, lymphedema, inflammation, serum levels
lymphedema is a debilitating disease that occurs as a result of lymphatic injury, obstruction, infection, or developmental anomaly. Abnormal lymphatic function in patients who suffer from lymphedema initially leads to accumulation of protein rich interstitial fluid that is treated with massage and compression garments. However, in its late stages, lymphedema results in pathologic fibroadipose tissue deposition, making the disease less likely to respond to these treatments. Understanding the mechanisms that regulate adipose deposition in lymphedema is therefore important for development of novel treatment strategies. Furthermore, the parallels between adipose tissue deposition in lymphedema and obesity are intriguing and suggest that lymphedema, in its late stages, may represent a form of regional obesity. This is important because the incidence of obesity and its associated morbidity are rapidly increasing in Western countries. Therefore, a better understanding of how the lymphatic system and obesity are related is crucial and has wide scientific relevance.
Previous studies have suggested that even minor lymphatic injury or dysfunction can promote adipose tissue deposition. For example, Harvey and colleagues (17) reported that mice with a heterozygous inactivating mutation of the Prox-1 gene not only have lymphatic developmental abnormalities but also become obese as adults as compared with their wild-type littermate controls. Using a surgical mouse model of axillary lymphadenectomy in which the draining lymph nodes of the forelimb are removed, our group has shown that even this relatively minor injury increases the expression of adipose differentiation genes such as CEPB-α and Ppar-γ (2) as well as early inflammatory cascades in the forelimb subcutaneous tissues (39). By comparing tissues harvested from mice that had undergone axillary lymph node dissection with a more severe model of lymphatic injury in the mouse tail, we have also shown that the degree of adipose deposition is related to the severity of lymphatic obstruction and tissue inflammation (6). The association between inflammation and adipose deposition is important and supported by our previous studies demonstrating that depletion of T cells or blockade of T-helper type 2 (Th2) cell inflammation potently inhibits adipose tissue deposition in the mouse-tail model (6, 40). However, although it is evident that inflammatory changes are necessary for adipose deposition in lymphedema, it remains unclear which inflammatory pathways regulate adipose homeostasis in lymphedematous tissues.
Several lines of evidence suggest that IL-6 may play a role in the regulation of adipose homeostasis in lymphedema. For example, the expression of IL-6 has been shown to correlate with adipose tissue depots in obese patients accounting for as much as 15–30% of circulating IL-6 levels (14, 26). Furthermore, recombinant human IL-6 administration has been shown to increase lipolysis and fatty acid oxidation in human subjects as well as in vitro adipose cultures (19, 23, 29, 34). In addition, previous studies have shown that the expression of IL-6 is increased in both primary and surgical models of lymphedema (20, 28). Finally, a recent study has shown that the concentration of IL-6 is higher in lymph fluid as compared with subcutaneous fat, suggesting that the lymphatics play a role in transport or clearance of inflammatory cytokines (38).
The purpose of the current study was to determine the role of IL-6 in lymphedema-mediated adipose deposition. Using clinical biopsy specimens, serum from patients with or without lymphedema, and tissues from mouse models of lymphatic injury, we show that the expression of IL-6 is increased in the serum and of lymphedematous specimens tissues. We show that elevated expression of IL-6 correlates with adipose deposition and inflammation, that CD4+ cell inflammation is necessary for these processes, and that loss of IL-6 function significantly increases adipose deposition after lymphatic injury.
METHODS
Human lymphedema tissue and serum samples.
Matched tissue biopsies were obtained from the normal and lymphedematous upper extremity of patients with post-surgical lymphedema (grades I–III; see below) by Professor Waldemar Olszewski at the Polish Academy of Science. Full-thickness (5 mm) skin biopsies were fixed, paraffin-embedded, and sectioned at a thickness of 5 μm using our previously described methods (40). The Institutional Review Boards (IRB) of the Polish Academy of Science and Memorial Sloan-Kettering Cancer Center (MSKCC) approved all studies.
To confirm our histologic studies, serum from 26 patients with post-mastectomy lymphedema (grade I–III) and 20 patients with a history of breast cancer but without lymphedema were obtained from the Stanford Center for Lymphatic and Venous Disorders under IRB-approved protocols. Serum IL-6 was measured using a multiplex bead-based immunoassay (Luminex, Austin, TX) and quantified as median fluorescent intensity. Patient demographics including body mass index (BMI) and lymphedema grade (I–III) were collected. Lymphedema grading was established using the International Society of Lymphedema classification scheme (ISL; Table 1) (18).
Table 1.
International Society of Lymphedema classification
| Stage | Clinical Presentation |
|---|---|
| I | Pitting edema that resolves with elevation |
| II | Pitting edema that does not subside with elevation alone |
| III | Nonpitting edema with overlying skin changes |
Animals and mouse models.
All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at MSKCC. Female C57BL/6J, IL-6 deficient (IL-6KO; B6.129S2-IL-6tm1Kopf/J), and CD4 knockout [CD4KO; CBY.129S2 (B6)-Cd4tm1mak/J] mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were maintained in a temperature and light-controlled environment.
We used two models of lymphatic injury to study the effects of varying degrees of adipose deposition on IL-6 expression. We used our previously described axillary lymph node dissection (ALND) model to study the effects of lymphatic injury without significant adipose deposition (40). In this model, the axillary lymph nodes are completely excised through a 1-cm skin incision. Control animals are treated with an axillary skin incision without lymph node excision. To study the effects of more significant lymphatic injury and adipose deposition, we used a well-described mouse tail model of lymphedema (3, 4, 6, 40). In this model, the superficial and deep lymphatic system of the midportion of the tail are disrupted by excising a 2-mm portion of the skin and microsurgically ligating the deep lymphatic channels that run along the lateral tail veins. We have previously shown that this intervention results in sustained lymphatic fluid stasis lasting at least 6 wk in the distal region of the tail with resultant inflammation, adipose deposition, and fibrosis that is histologically similar to clinical lymphedema (3, 4, 6, 40). Control animals were treated with an incision in the same region of the tail without deep lymphatic ligation. We have shown that this treatment results in only mild tail edema that resolves completely by 6 wk postoperatively (11).
CD4 depletion experiments.
Adult female C57B6 mice underwent tail skin and lymphatic ablation as outlined above, allowed to recover for 2 wk, and then randomly assigned to experimental or control groups (n = 8–10 animals/group). Mice in the experimental group received CD4 monoclonal neutralizing antibodies via an intraperitoneal route at a concentration of 10 μg/g every 5 days for a total of 4 wk as previously described (40). Control animals received nonspecific isotype control antibodies at the same time, dose, and route. Depletion was confirmed using flow cytometric analysis of splenic cell populations in the control animals versus the experimental animals at the time of euthanization.
STAT inhibition.
To study the effects of IL-6 blockade on adipose deposition, we used a well-described small molecule inhibitor of JAK1/2 (AZD1480; Astra-Zeneca, Wilmington, DE), the primary signaling pathway of IL-6 (27). The JAK1/2 inhibitor was administered at a dose of 60 mg/kg (in 0.5% hydroxypropyl methylcellulose/0.1% Tween 80) by daily oral gavage, for 6 wk beginning in the immediate postoperative period. Control animals were treated with vehicle gavage.
Histology.
Cross-sectional tissues from the forelimb just above the wrist or the distal region of the tail, 1.5 centimeters distal to the zone of lymphatic injury in mice, were harvested 6 wk after surgery, fixed in 4% paraformaldehyde, decalcified in buffered EDTA (Sigma Aldrich, St. Louis, MO), and paraffin embedded. Human lymphedema specimens were obtained and processed as stated above. Sections were cut at a thickness of 5 μm, and immunohistochemical staining was performed using monoclonal or polyclonal antibodies to identify leukocytes (CD45; Rat monoclonal No. 30-F11; R&D Systems, Minneapolis, MN), CD4+ cells (goat polyclonal No. AF552; R&D), IL-6+ cells (rabbit polyclonal No. ab6672; Abcam, Cambridge, MA), and phosphorylated STAT-3+ (pSTAT3) cells (rabbit polyclonal, No. NB100-92644; Novus Biologicals, Littleton, CO). Negative control sections were incubated with isotype control antibodies or secondary antibody alone. Specificity was confirmed using single-stained sections and negative controls. Images were captured (Mirax Scanner; Carl Zeiss, Munich, Germany) and analyzed by counting the number of positive cells per high-powered fields (Panoramic Viewer; 3DHISTECH, Budapest, Hungary). Cell counts were performed on randomly chosen high-powered fields (40×) of the various tissue sections from a minimum of 4–6 human or animal sections per group and 4 to 5 high-powered fields/subject by two blinded reviewers.
Subcutaneous fat thickness was measured using hematoxylin and eosin-stained sections using ImageJ software in standardized sections at a magnification of 2.5× by two blinded reviewers (40). We used standardized regions located at each quadrant of the tail spanning the distance between the maximum height of the tail musculature and the level of the reticular dermis to analyze the adipose deposition in the tail.
ELISA.
Protein lysate and serum were harvested and quantified using the Bradford method (Bio-Rad, Hercules, CA; n = 6–8 per group). Serum and tissue IL-6 levels were quantified in 40 μg of total protein using a mouse ELISA according to the manufacturer's directions (eBioscience, San Diego, CA).
Statistical analysis.
The Student's t-test was used to compare differences between two groups, whereas comparison of multiple groups was performed using ANOVA with post hoc tests to compare differences between individual groups. Pearson's coefficient was used to determine the correlation between groups. Analysis of clinical lymphedema samples was performed using the Wilcoxon matched pair t-test. Data are presented as means ± SD unless otherwise noted, with P < 0.05 considered significant.
RESULTS
Patients with lymphedema have increased expression of IL-6 both locally and systemically.
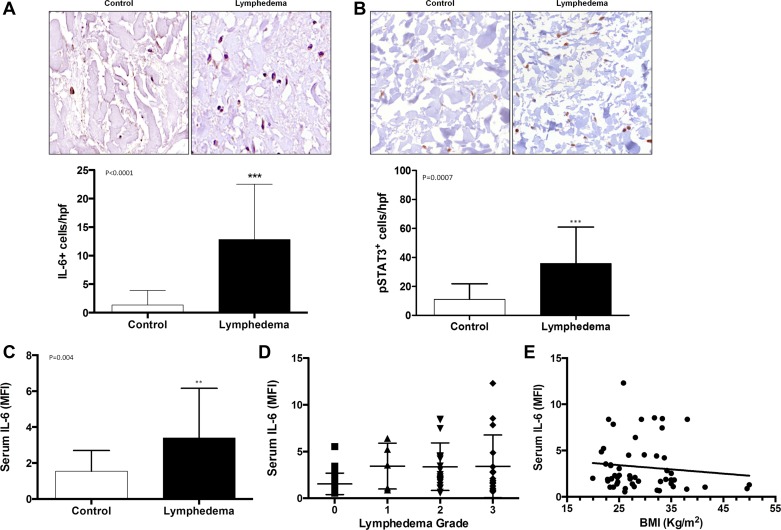
Using immunohistochemical localization, we found that the expression of IL-6 (IL-6+ cells/high powered field) was significantly increased in lymphedematous tissues as compared with the matched control tissues (Fig. 1A). Furthermore, lymphedematous tissues also had increased numbers of cells that expressed pSTAT-3, an intracellular downstream mediator of IL-6 receptor activation, as compared with matched control tissues (Fig. 1B). Analysis of serum IL-6 levels supported our immunohistochemical findings and demonstrated that patients with lymphedema have significantly increased levels of serum IL-6 as compared with control patients who did not have lymphedema (Fig. 1C). However, we did not find a correlation between lymphedema grade and serum IL-6 levels (Fig. 1D). In addition, we were somewhat surprised to find no correlation between serum IL-6 and BMI (Fig. 1E). However, this finding may be related to the fact that the majority of women in our study had a BMI that was less than 30 (i.e., overweight but not obese).
Fig. 1.
Patients with lymphedema have increased expression of IL-6 both locally and systemically. A: representative high power (40×) photomicrographs and quantification of IL-6+cells/high-powered fields (HPF) in matched clinical lymphedema and control specimens. B: representative high power (40×) photomicrographs and quantification of pSTAT3+cells/HPF in matched clinical lymphedema and control specimens. C: quantification of serum IL-6 expression in patients with and without lymphedema [MFI (median fluorescent intensity)]. D: correlation of serum IL-6 and lymphedema grade. E: correlation of serum IL-6 and body mass index (BMI; in kg/m2).
Expression of IL-6 correlates with adipose deposition after lymphatic injury.
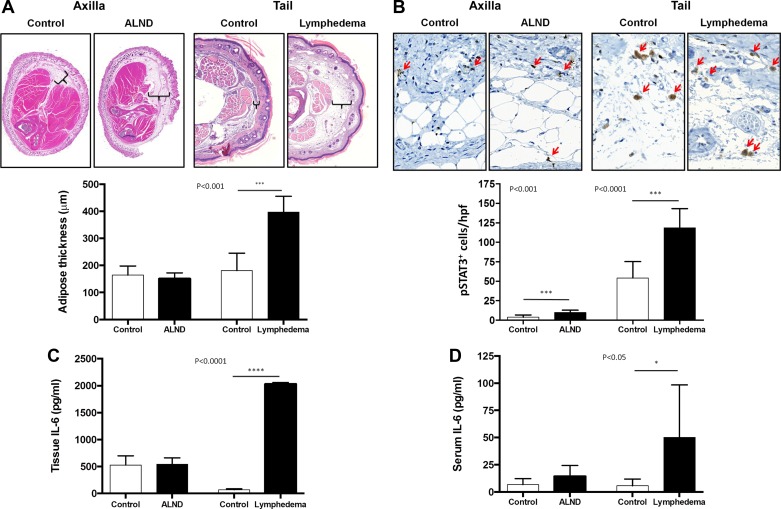
As expected, histological analysis of cross-sections obtained from the forelimb or tails in our mouse models demonstrated significant adipose deposition only in mice that underwent tail skin and lymphatic excision (lymphedema; Fig. 2A). Immunohistochemical analysis of these tissues demonstrated a minor, although significant increase in the number of pSTAT-3+ cells in the forelimb tissues of animals treated with an ALND. In contrast, analysis of tail tissues demonstrated a more than twofold increase in the number of pSTAT3+cells/HPF as compared with controls (Fig. 2B). Consistent with our pSTAT3 analysis showing massive increases in IL-6 expression only in the tail model, we found no significant differences in tissue IL-6 concentrations in protein harvested from the forelimb of ALND or control animals (Fig. 2C). In contrast, tissues harvested from lymphedematous tails had a nearly 20-fold increase in IL-6 levels as compared with controls. These changes, similar to our clinical findings, correlated with increased serum levels of IL-6 in animals with tail lymphedema but not animals treated with ALND (Fig. 2D). Taken together, these findings suggest that the expression of IL-6 in lymphedema is associated with adipose deposition.
Fig. 2.
Expression of IL-6 correlates with adipose deposition after lymphatic injury. A: representative hematoxylin and eosin (H&E) low power (2.5×) cross-sectional photomicrographs and quantification of fat thickness in the forelimb of mouse control/axillary lymph node dissection (ALND) and tail control/tail lymphedema specimens 6 wk after surgery. Brackets indicate subcutaneous adipose tissue thickness. B: representative high power (40×) photomicrographs and quantification of pSTAT3+cells/HPF in the forelimb of mouse control/ALND and tail control/lymphedema specimens. C and D: ELISA quantification of tissue IL-6 (C) and serum IL-6 (D) in both the axillary dissection model and tail lymphedema model with their respective controls.
Chronic inflammation is associated with adipose deposition and IL-6 expression.
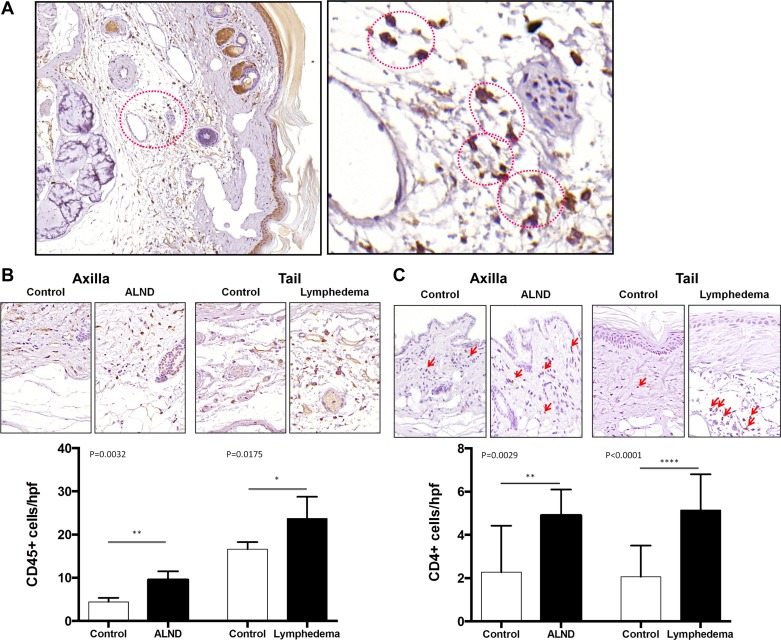
We have previously shown that tail lymphedema and ALND result in inflammation (40). To determine whether this inflammatory reaction was spatially associated with adipose deposition, we stained cross sections of the forelimb and tail tissues with CD45, a pan leukocyte marker (Fig. 3, A and B). Consistent with our previous studies, we found that the number of CD45+ cells/HPF was significantly increased in animals treated with ALND or tail skin/lymphatic excision as compared with controls. More importantly, we found that inflammatory cells were localized to the regions of adipose tissues particularly in the tail lymphedema model. Interestingly, we consistently found that CD45+ cells were clustered around adipocytes, reminiscent of crown-like structures recently described in inflamed adipose tissues of obese patients (10). Similarly, consistent with our previous studies, we found that the number of CD4+ cells was also significantly increased in animals that had either ALND or tail skin/lymphatic excision (Fig. 3C). Interestingly, these cells were localized to both the dermal tissues as well as the subcutaneous adipose layer.
Fig. 3.
Chronic inflammation is associated with adipose deposition and IL-6 expression. A: representative low (10×) and high power (40×) photomicrographs of crown-like structures in the adipose tissue of the murine tails following lymphatic ablation. Red circles denote the dead adipocyte and surrounding CD45+ cells. B: representative high power (40×) photomicrographs and quantification of CD45+ cells/HPF in the forelimb of mouse control/ALND and tail control/lymphedema specimens 6 wk after surgery. C: representative high power (40×) photomicrographs and quantification of CD4+ cells/HPF in the forelimb of mouse control/ALND and tail control/lymphedema specimens 6 wk after surgery.
CD4+ T lymphocyte inflammation is necessary for adipose deposition and IL-6 expression.
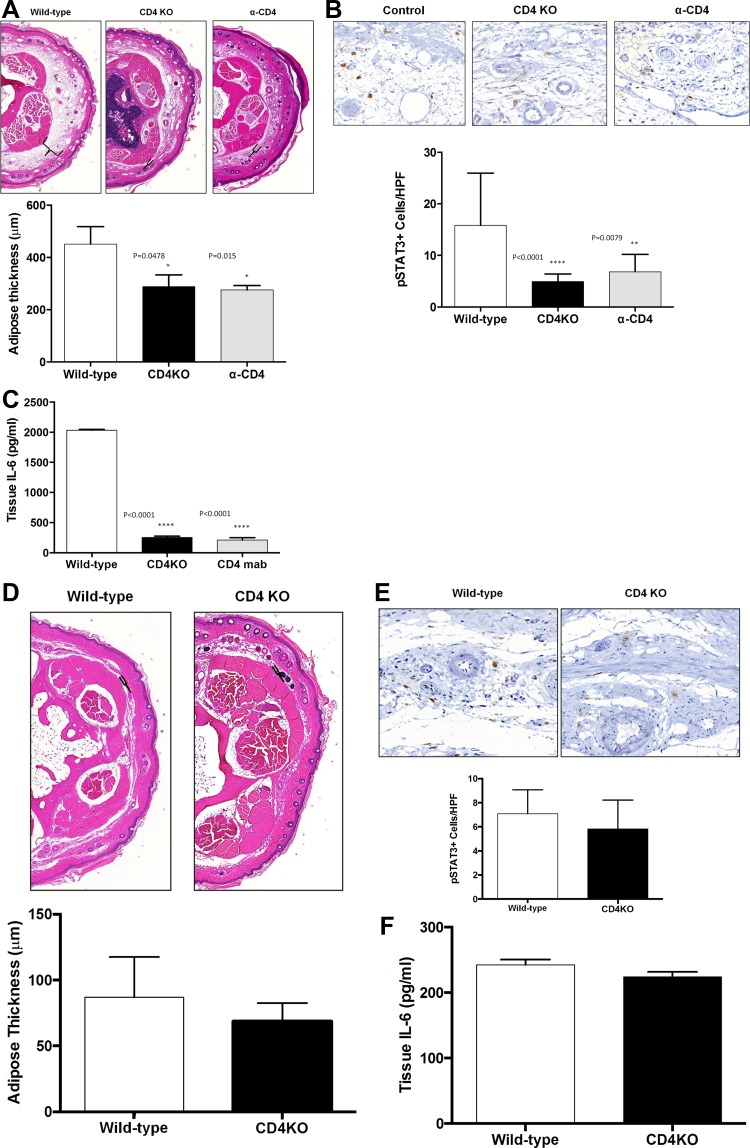
We have previously demonstrated that mice that lack CD4+ T cells (CD4 knockout mice; CD4KO) do not develop lymphedema following ligation of their tail lymphatics (6). To test the hypothesis that adipose deposition and chronic CD4+ cell inflammation are necessary for IL-6 expression, we analyzed tissues from wild-type, CD4KO mice, or mice treated with CD4 depleting monoclonal antibodies (α-CD4) subsequently treated with tail skin and lymphatic excision. Consistent with our previous reports, we found that CD4KO mice and α-CD4 mice had significantly less adipose tissue deposition in the tail after tail skin/lymphatic excision (Fig. 4A). This decreased tail adipose tissue deposition correlated with significantly decreased numbers of pSTAT-3+ cells and tissue IL-6 expression in both CD4KO mice and α-CD4 mice (Fig. 4, B and C). Preoperative tail adipose thickness, pSTAT3 positive cell counts, and tissue IL-6 levels are no different between CD4KO mice and their wild-type counterparts (Fig. 4, D–F). Taken together, these findings suggest that both adipose deposition and chronic inflammation are associated with IL-6 expression in lymphedema.
Fig. 4.
CD4+ T lymphocyte inflammation is necessary for adipose deposition and IL-6 expression. A: representative low-power (5×) cross-sectional photomicrographs and quantification of adipose tissue thickness in tails of wild-type, CD4KO, and CD4 depleted (α-CD4) mice 6 wk after tail skin/lymphatic excision. Brackets indicate subcutaneous adipose tissue thickness. B: representative high power (40×) cross-sectional photomicrographs and quantification of pSTAT3+cells/HPF in tails of wild-type, CD4KO, and α-CD4 animals 6 wk after tail skin/lymphatic excision. C: ELISA quantification of tissue IL-6 in wild-type, CD4KO, and α-CD4 tail tissues 6 wk after skin/lymphatic excision. D: representative low-power (5×) cross-sectional photomicrographs and quantification of adipose tissue thickness in tails of wild-type and CD4KO mice preoperatively. E: representative high power (40×) cross-sectional photomicrographs and quantification of pSTAT3+ cells/HPF in tails of wild-type and CD4KO mice preoperatively. F: ELISA quantification of tissue IL-6 in wild-type and CD4KO mice preoperatively.
Loss of IL-6 function results in increased adipose deposition.
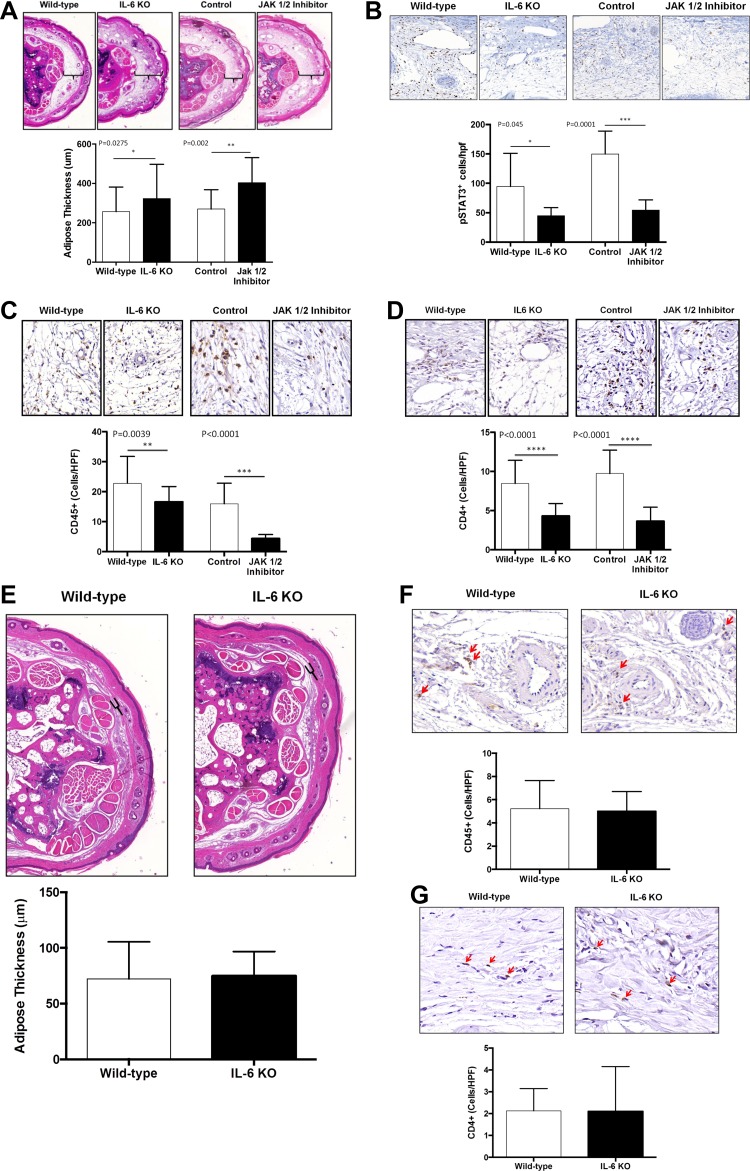
To determine how increased IL-6 expression regulates adipose deposition and inflammation, we performed tail skin and lymphatic excision surgeries in wild-type and IL-6 knockout mice (IL-6-KO) and analyzed these outcomes 6 wk after surgery. In addition, to confirm our knockout studies we analyzed the effect of a small molecule inhibitor of JAK 1/2, the principal signaling pathway for IL-6, as compared with animals treated with a vehicle control. Preoperative tail adipose thickness, CD45, and CD4 cell counts were found to be no different between IL-6 KO mice and their wild-type counterparts (Fig. 5, E–G).
Fig. 5.
Loss of IL-6 function results in increased adipose deposition. A: representative low power (5×) cross-sectional photomicrographs and quantification of adipose tissue thickness in tail tissues of wild-type, IL-6KO, and JAK1/2 inhibitor treated mice and their respective controls 6 wk following tail lymphatic ablation. Brackets indicate subcutaneous adipose tissue thickness. B–D: representative high power (40×) cross-sectional photomicrographs and quantification of pSTAT3+cells/HFP (B), CD45+cells/HPF (C), and CD4+cells/HPF (D) in wild-type, IL-6KO, and JAK1/2 inhibitor treated mice and their respective controls 6 wk after surgery. E: representative low power (5×) cross-sectional photomicrographs and quantification of adipose tissue thickness in tail tissues of wild-type and IL-6KO mice preoperatively. F and G: representative high power (40×) cross-sectional photomicrographs and quantification of CD45+ cells/HPF (F), and CD4+ cells/HPF (G) in tails of wild-type and IL-6KO preoperatively.
Interestingly, we found that loss of IL-6 function either in knockout mice or by inhibition of JAK1/2 activation resulted in significantly increased subcutaneous adipose deposition in mice treated with tail skin/lymphatic excision (Fig. 5A). Not surprisingly, we found that IL-6 KO mice and mice treated with JAK1/2 inhibitor also had decreased numbers of pSTAT3+ cells/HPF as compared with controls confirming that IL-6 function is decreased in these animals (Fig. 5B). Furthermore, we found that loss of IL-6 function in both IL-6 KOs and JAK1/2-treated animals resulted in decreased adipose associated tissue inflammation (defined by infiltration of leukocytes and presence of crown-like structures) of leukocytes in general (CD45+) and of CD4+ cells in particular (Fig. 5, C and D). Taken together, these findings suggest that increased IL-6 expression may act to decrease adipose deposition and resultant adipose tissue inflammation in lymphedema.
DISCUSSION
Using human lymphedema tissue specimens as well as serum from patients with and without lymphedema, we found that the expression of IL-6 and its downstream pathways are increased in lymphedematous tissues as well as the peripheral serum. This finding is important and suggests that IL-6 may be a useful serum marker that may aid in the diagnosis or treatment of lymphedema. For example, serial analysis of serum IL-6 levels may be used to follow a patient over time as means of following response to surgical or medical treatments for lymphedema. This is important since current means of following lymphedema patients rely almost exclusively on volume or circumference measurements. These measurements are often cumbersome to perform and fraught with technical difficulties, making them less reliable for following lymphedema. Thus the addition of a reliable and quantifiable physiologic measurement, such as IL-6 measurements, may provide a more objective means of following response to treatments in lymphedema patients. Future studies will be required to validate this hypothesis.
Interestingly, despite the fact that serum IL-6 levels were increased in patients with lymphedema, we found no correlation with the clinical degree of lymphedema. Although this finding may simply reflect a lack of statistical power in our study to discern minor differences between groups, it more likely represents difficulties in clinical staging of lymphedema. In fact, although several clinical staging systems have been developed for lymphedema, the differences between various grades of lymphedema are somewhat subjective and do not take into account changes in adipose deposition or chronicity of lymphedema. Furthermore, clinical staging systems for lymphedema do not take into account BMI or other indexes of body weight/composition in general. Thus grade II lymphedema in a thin patient may actually be much more significant pathologically than the same grade in an obese patient. Use of staging systems that incorporate physiologic changes such as lymphatic function and adipose deposition may therefore be a more accurate means of categorizing lymphedema as compared with commonly used methods of limb measurements or subjective changes in tissue turgor.
Previous studies have shown that there appears to be a correlation between obesity and IL-6 levels (8, 30). However, we observed no correlation between serum IL-6 and BMI in our current study. This observation may be related to the confounding effect of lymphedema in our patient population (i.e., a thin patient with lymphedema may have a disproportionate increase in serum IL-6 levels). Alternatively, it is possible that the population of patients in our study were overall thinner than previous reports. This is supported by the fact that the average BMI for our obese patients was 28 kg/m2, whereas studies demonstrating a correlation between BMI and IL-6 levels studied obese patients with an average BMI greater than 35 kg/m2 (8, 30).
The clinical study of lymphedema in humans is difficult given that its temporal onset, rate of progression, and severity is variable. To circumvent these difficulties, we used two different mouse models to study the effects of variable degrees of lymphatic injury on IL-6 expression. We have previously shown that ALND in mice results in significant but subtle increases in arm volume peaking at 3 wk and returning to normal by 6 wk (24, 39). In this model, similar to clinical findings after lymphadenectomy, the ipsilateral limb is essentially normal in appearance by 6 wk. However, despite this relatively normal appearance we have shown that ALND results in sustained tissue inflammation. Therefore, the ALND model enables us to study the effects of lymph stasis without significant adipose deposition. In contrast, the mouse tail model used in this study enables us to analyze the effects of more profound lymphatic injury and adipose deposition, since we and others have previously shown that this treatment results in substantial subcutaneous adipose deposition and that this process is chronic lasting as long as 12 wk postop (4, 11, 32, 33). Using a direct comparison of these models, we found that IL-6 expression, activation of its downstream mediator, and serum changes in IL-6 concentration occur to a more significant degree in the tail model as compared with the ALND model. This finding is important since it suggests that the degree of lymphatic injury is a critical regulator of IL-6 expression. This hypothesis is supported by previous studies demonstrating that adipose tissues are a major source of circulating IL-6 levels (14, 26) and that IL-6 functions as an adipolytic cytokine regulating circulating levels of fatty acids (19, 22, 23, 29, 34, 36).
A classic histologic hallmark of lymphedema is the presence of inflammation. We have previously demonstrated that CD4+ cells play a significant role in the pathology of lymphedema including fibrosis and lymphatic dysfunction (6, 40). Not surprisingly, we found that both ALND and tail skin/lymphatic excision resulted in chronic inflammatory reactions with increased numbers of CD45+ and CD4+ cells in the subcutaneous and dermal tissues. More interestingly, we found that the inflammatory cells were in close physical proximity to the adipose tissues, suggesting that inflammation and adipose deposition are related. This relationship is likely bidirectional: inflammation promoting adipose tissue deposition and adipose tissue deposition promoting inflammation. This hypothesis is supported by previous studies demonstrating critical roles for inflammatory reactions in adipose deposition and pathology of obesity (21, 25). For example, studies have demonstrated that inflammation is associated with both visceral and hepatic adipose deposition and NF-κβ activation (21). In addition, this hypothesis is supported by numerous studies demonstrating that adipose tissues in obesity promote inflammatory reactions leading to metabolic syndrome, insulin sensitivity, and other pathology (9, 12, 15, 16, 31, 37).
It has been well established that the main sources of IL-6 are inflammatory cells including macrophages and, under certain circumstances, T lymphocytes (1). Our study suggests that chronic inflammation is also a critical regulator of IL-6 expression in lymphedematous tissues since we found that loss of CD4+ cell function resulted in significantly decreased IL-6 expression and activation of its downstream mediators. Although this finding was not unanticipated, we were somewhat surprised when we found that loss of IL-6 function or inhibition of IL-6 function using a small molecule inhibitor resulted in increased adipose deposition and decreased inflammation. This finding suggests that IL-6 in lymphedema acts to regulate and decrease adipose deposition and may also contribute to chronic inflammation. This hypothesis is in line with previous studies demonstrating that IL-6 is a pleotropic cytokine with a prominent role in mediating the inflammatory response (1). In addition, this hypothesis is supported by recent studies that have shown that IL-6 has adipolytic functions playing a homeostatic role in adipocyte physiology (13, 35). For example, Wallenius et al. found that mice that lacked IL-6 went on to develop adult onset obesity and that supplementation with IL-6 partially reversed this outcome (35). Similarly, Franckhauser et al. demonstrated that overexpression of IL-6 lead to significant weight loss and decreased overall body fat composition and reduced metabolic derangements (13). Taken together, our findings suggest that increased IL-6 expression in lymphedema may represent a compensatory or homeostatic response aiming to decrease adipose deposition in response to chronic inflammation and lymphatic dysfunction.
In conclusion, our study demonstrates that patients with lymphedema have a significant increase in serum and tissue IL-6 levels. This increase is mirrored by our findings in the mouse tail model. In addition, our mouse model findings suggest that chronic CD4+ cell inflammation acts either directly or indirectly to regulate IL-6 expression. Taken as a whole, these findings suggest that IL-6 is a key regulator of lymphedema associated adipose deposition whose role appears to be to maintain adipose homeostasis in the lymphedematous tissues.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL111130-02 (to B. J. Mehrara) and a Plastic Surgery Educational Foundation (PSEF) Pilot Research Grant (to D. A. Cuzzone), T32 grant (to D. A. Cuzzone), T32 grant (to E. Weitman), and PSEF grant (to E. Weitman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.A.C., E.W., J.F.B., W.L.O., S.G.R., and B.J.M. conception and design of research; D.A.C., N.J.A., S.G., I.L.S., J.G., W.J., J.T., W.L.O., and S.G.R. performed experiments; D.A.C., N.J.A., S.G., I.L.S., J.G., W.J., J.T., and S.G.R. analyzed data; D.A.C., E.W., N.J.A., S.G., I.L.S., J.G., W.J., J.T., and B.J.M. interpreted results of experiments; D.A.C., E.W., N.J.A., S.G., I.L.S., J.G., W.J., and B.J.M. prepared figures; D.A.C., E.W., and B.J.M. drafted manuscript; D.A.C., E.W., N.J.A., S.G., I.L.S., J.G., W.J., J.T., J.F.B., W.L.O., S.G.R., and B.J.M. edited and revised manuscript; D.A.C., E.W., J.F.B., W.L.O., S.G.R., and B.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mesruh Turkekul for expert technical and histologic assistance. We also thank the Molecular Cytology Core at MSKCC for assistance with both histology and tissue imaging.
REFERENCES
- 1.Akira D, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol 54: 1–78, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part II expression of adipose differentiation genes. Plast Reconstr Surg 129: 838–847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avraham T, Clavin NW, Daluvoy SV, Fernandez J, Soares MA, Cordeiro AP, Mehrara BJ. Fibrosis is a key inhibitor of lymphatic regeneration. Plast Reconstr Surg 124: 438–450, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade Of transforming growth factor-β1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177: 3202–3214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, Bromberg J, Mehrara BJ. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J 27: 1114–1126, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 85: 3338–3342, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bastard JP, Maachi M, Lagathu C, Kim Mj, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17: 4–12, 2006 [PubMed] [Google Scholar]
- 10.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46: 2347–2355, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295: H2113–H2127, 2008 [DOI] [PubMed] [Google Scholar]
- 12.De Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem 54: 945–955, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Franckhauser S, Elias I, Rotter Sopasakis V, Ferre T, Nagaev I, Andersson CX, Agudo J, Ruberte J, Bosch F, Smith U. Overexpression of IL-6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia 51: 1306–1316, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83: 847–850, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Gil A, Maria Aguilera C, Gil-Campos M, Canete R. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Br J Nutr 98, Suppl 1: s121–s126, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 29: 4467–4483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 37: 1072–1081, 2005 [DOI] [PubMed] [Google Scholar]
- 18.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the international society of lymphology. Lymphology 46: 1–11, 2013 [PubMed] [Google Scholar]
- 19.Ji C, Chen X, Gao C, Jiao L, Wang J, Xu G, Fu H, Guo X, Zhao Y. IL-6 induces lipolysis and mitochondrial dysfunction, but does not affect insulin-mediated glucose transport in 3t3-l1 adipocytes. J Bioenerg Biomembr 43: 367–375, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Karlsen TV, Karkkainen MJ, Alitalo K, Wiig H. Transcapillary fluid balance consequences of missing initial lymphatics studied in a mouse model of primary lymphoedema. J Physiol 574: 583–596, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lé ka, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, Beale E, Xie C, Greenberg AS, Allayee H, Goran MI. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes 60: 2802–2809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyngso D, Simonsen L, Bulow J. Interleukin-6 production in human subcutaneous abdominal adipose tissue: the effect of exercise. J Physiol 543: 373–378, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyngso D, Simonsen L, Bulow J. Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol 543: 379–386, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehrara BJ, Avraham T, Soares M, Fernandez JG, Yan A, Zampell JC, Andrade VP, Cordeiro AP, Sorrento CM. P21cip/WAF is a key regulator of long-term radiation damage in mesenchyme-derived tissues. FASEB J 24: 4877–4888, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Mei M, Zhao L, Li Q, Chen Y, Huang A, Varghese Z, Moorhead JF, Zhang S, Powis SH, Li Q, Ruan XZ. Inflammatory stress exacerbates ectopic lipid deposition in C57BL/6J mice. Lipids Health Dis 10: 110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 82: 4196–4200, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev 22: 83–89, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Olszewski WL, Jamal S, Lukomska B, Manokaran G, Grzelak I. Immune proteins in peripheral tissue fluid-lymph in patients with filarial lymphedema of the lower limbs. Lymphology 25: 166–171, 1992 [PubMed] [Google Scholar]
- 29.Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA, Pedersen BK. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am J Physiol Endocrinol Metab 288: E155–E162, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Roytblat L, Rachinsky M, Fisher A, Greemberg L, Shapira Y, Douvdevani A, Gelman S. Raised interleukin-6 levels in obese patients. Obes Res 8: 673–675, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J 276: 5738–5746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-c upregulation, and the protective role of MMP-9. Microvasc Res 72: 161–171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabibiazar R, Cheung L, Han J, Swanson J, Beilhack A, An A, Dadras SS, Rockson N, Joshi S, Wagner R, Rockson SG. Inflammatory manifestations of experimental lymphatic insufficiency. PLos Med 3: e254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 88: 3005–3010, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Wolsk E, Mygind H, Grondahl TS, Pedersen BK, Van Hall G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am J Physiol Endocrinol Metab 299: E832–E840, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 33: 54–66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaleska M, Olszewski WL, Durlik M, Miller NE. Signaling proteins are represented in tissue fluid/lymph from soft tissues of normal human legs at concentrations different from serum. Lymphat Res Biol 11: 203–210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zampell JC, Yan A, Avraham T, Andrade V, Malliaris S, Aschen S, Rockson SG, Mehrara BJ. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol 300: C1107–C1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zampell JC, Yan A, Elhadad S, Avraham T, Weitman E, Mehrara BJ. CD4+ cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLos One 7: e49940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]