Abstract
The role of NADPH oxidase (Nox) in both the promotion and impairment of compensatory collateral growth remains controversial because the specific Nox and reactive oxygen species involved are unclear. The aim of this study was to identify the primary Nox and reactive oxygen species associated with early stage compensatory collateral growth in young, healthy animals. Ligation of the feed arteries that form primary collateral pathways in rat mesentery and mouse hindlimb was used to assess the role of Nox during collateral growth. Changes in mesenteric collateral artery Nox mRNA expression determined by real-time PCR at 1, 3, and 7 days relative to same-animal control arteries suggested a role for Nox subunits Nox2 and p47phox. Administration of apocynin or Nox2ds-tat suppressed collateral growth in both rat and mouse models, suggesting the Nox2/p47phox interaction was involved. Functional significance of p47phox expression was assessed by evaluation of collateral growth in rats administered p47phox small interfering RNA and in p47phox−/− mice. Diameter measurements of collateral mesenteric and gracilis arteries at 7 and 14 days, respectively, indicated no significant collateral growth compared with control rats or C57BL/6 mice. Chronic polyethylene glycol-conjugated catalase administration significantly suppressed collateral development in rats and mice, implying a requirement for H2O2. Taken together, these results suggest that Nox2, modulated at least in part by p47phox, mediates early stage compensatory collateral development via a process dependent upon peroxide generation. These results have important implications for the use of antioxidants and the development of therapies for peripheral arterial disease.
Keywords: collateral artery, hydrogen peroxide, NADPH oxidase, Nox2, p47phox
flow-mediated outward remodeling constitutes a major, natural compensation to arterial occlusions such as those occurring in peripheral vascular disease. This compensation relies on the development of preexisting bypass arteries into collateral pathways by a process of luminal enlargement [see Ziegler et al. (78) for review]. The outward remodeling that occurs as part of the collateral growth process is dependent on chronically increased flow and shear stress, and the process halts when shear stress has been restored to initial levels (23, 30, 65). While collateral growth may largely compensate for peripheral arterial occlusions due to injury or disease in some cases (14, 27), risk factors associated with vascular dysfunction and oxidant stress, such as aging, diabetes, and hypertension, may impair this process in humans and animals (1, 2, 26, 33, 48, 62, 64). Recent studies have implicated oxidative stress, possibly due to NADPH oxidase (Nox)-derived reactive oxygen species (ROS), as a primary mechanism responsible for impaired vascular compensation to arterial occlusion (4, 45, 46), and these same studies demonstrated that administration of an antioxidant reversed the impairment. The results of such studies suggest that the use of antioxidant therapy to reduce excess ROS may be effective as primary or adjuvant means to promote vascular compensation to arterial occlusion. However, other studies performed with conduit or resistance vessels in the absence of ROS-associated risk factors have demonstrated that ROS are required for flow-mediated remodeling (3, 7, 8). Thus additional studies are needed to better understand the origin and roles of ROS and Nox in both the promotion and impairment of flow-mediated remodeling and collateral development.
The primary source of ROS in the vasculature is thought to be the family of Nox enzymes that includes Nox1, -2, -4, and -5 and their associated regulatory subunits such as p22phox, p47phox, p40phox, and p67phox [see Lassegue et al. (39) for review]. Nox1, -2, and -4 are present in rodent vasculature, whereas Nox5 is present only in humans. The contribution of Nox activation to oxidant stress in the context of pathological remodeling has been extensively studied (39), but less is known about the role and regulation of Nox during physiological remodeling, especially in the smaller arteries that form collateral pathways. Early evidence indicated a flow-mediated mechanism for free radical release from large artery endothelium (40), and more recent work has shown that Nox may be activated by increased shear stress (19). While evidence exists from several studies for Nox-mediated redox control of altered peripheral tissue perfusion in response to arterial ligation (20, 59, 69), these studies do not directly evaluate compensatory enlargement of the small feed arteries that form the primary collaterals. The limited data available on the function of Nox in flow-mediated compensatory remodeling indicate either that Nox2 plays a role in mediating the luminal expansion (3) or suggest a role for p47phox but not Nox2 (7, 17). However, the influence on the results of these studies due to differences in flow/shear level, artery type, or compensation in whole animal models with genetic deletion are not known.
Although superoxide is the primary, immediate product of most Nox activity, subsequent dismutation generates hydrogen peroxide, which is longer-lived and thus may diffuse further into the vasculature. Excess peroxide is known to be a damaging ROS that participates in the development of vascular pathology (32, 73) and to inhibit physiological processes (20, 25), but physiological concentrations have been shown to facilitate vascular processes such as capillary growth or angiogenesis (55, 70) and arterial dilatation (31). Although increased flow has been shown to stimulate peroxide production in coronary arterioles of young rats (31), its role in collateral growth is unclear.
The objective of the current study was to determine the identity of the primary Nox associated with early stage collateral growth in young, healthy animals. A second objective was to determine if peroxide had a role in compensatory collateral development. To address these issues, we used both rat mesenteric and mouse hindlimb femoral artery ligation models that allowed diameter measurements in conjunction with molecular and pharmacological approaches, including Nox subunit ablation, small interfering RNA (siRNA), and pharmacological inhibition. Taken together, the results indicate important roles for Nox2 and hydrogen peroxide in compensatory collateral development in young, healthy animals.
METHODS
Animals and groups.
All procedures performed in this study were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. Young male Wistar-Kyoto (WKY) rats were obtained from Charles River (Wilmington, MA), acclimated for a minimum of 3 days before use and studied at 2 to 3 mo of age. Male C57BL/6 and p47phox−/− mice were obtained from Jackson Laboratories (Bar Harbor, ME) and studied at 4.5–6 mo of age. Apocynin (acetovanillone) was obtained from Fisher Scientific (Pittsburg, PA), Nox2ds-tat (gp91ds-tat) and scramble peptides were custom synthesized by EZbiolabs (Westfield, IN) according to Rey et al. (53), and polyethylene glycol-conjugated catalase (PEG-CAT) was from Sigma-Aldrich (St. Louis, MO). Apocynin (3.0 mM; ∼60 mg·kg−1·day−1) was administered in drinking water 3 days before model creation and continued until final experimentation when vessel diameters were obtained. The Nox2ds-tat peptide (rats, 1.0 mg·kg−1·day−1; and mice, 10 mg·kg−1·day−1) and PEG-CAT (10,000 U·kg−1·day−1) were delivered via Alzet osmotic pumps (Durect, Cupertino, CA: rat, 2ML1; and mouse, 1002). The pumps were aseptically implanted in a subcutaneous pocket in the nape of the neck at the time of arterial ligation. Concentrations/doses of agents were derived from earlier studies that demonstrated effectiveness in reducing oxidative stress (41, 49, 53, 57), normalizing arterial ROS and nitric oxide (NO) concentrations (76, 77), affecting arterial remodeling (3, 45, 46), or suppressing mesenteric artery mRNA expression (72). The p47phox siRNA (ON-Target Plus SmartPool duplex; Thermo Scientific/Dharmacon, Pittsburg, PA) was administered via tail vein using the hydrodynamic method as previously described (66). Briefly, ∼50 μg (230 μg/kg) of p47phox siRNA or SmartPool universal siRNA control (Dharmacon) was reconstituted with RNase-free water and diluted to a final volume of 6.0 ml with TransIT in vivo transfection agent (Mirus Bio, Madison, WI). The siRNA was injected 1 day before collateral model creation, with a second injection of ∼25 μg at day 4, and final diameter measurements were made on day 7 as described below.
Model of rat mesenteric collateral growth and its assessment.
Experiments used a well-characterized model of mesenteric artery collateral growth as previously described (46, 65). This model uses sequential ligation of mesenteric arteries to create a flow-dependent collateral pathway and allows comparison of high-flow collateral arteries to in-animal control arteries that have not experienced increased flow. Briefly, a laparotomy was performed on an anesthetized WKY rat, and the terminal ileum was exteriorized into a heated tissue support chamber. The bowel and mesentery were immersed in phosphate-buffered saline or covered with plastic wrap at all times to prevent tissue desiccation. Several sequential ileal arteries were ligated such that a region of bowel containing ∼45 microvascular perfusion units was dependent upon collateral arteries for perfusion [see Miller et al. (46) for model illustration]. Digital images of the two primary collateral and two same-animal control arteries were acquired under conditions of maximal dilation (0.1 mM adenosine and 0.01 mM sodium nitroprusside) with a dissecting microscope (Leica MZ 9.5; Leica Microsystems, Buffalo Grove, IL) and camera (Spot Insight 4 Firewire; SPOT Imaging Solutions, Sterling Heights, MI). The bowel was returned to the abdominal cavity and the incision closed. After 7 days, the laparotomy and acquisition of digital images was repeated [see Miller et al. (46) for example images]. From these images, inner arterial diameter was determined by measuring the red cell column (mean of 3 measurements/artery) with image analysis software (ImageJ, National Institutes of Health) and the percent change in diameter was calculated.
Model of mouse hindlimb collateral growth and its assessment.
A model of focal arterial occlusion was created in C57BL/6 and p47phox−/− mice as previously described (17) by ligating the distal superficial femoral artery, as this is the most common site of occlusion in patients with peripheral arterial disease (44a, 71). Ligation at this site increases flow through the gracilis arteries, which form the primary collateral pathways in this model (9, 17). To perform the ligation, an ∼7-mm incision was made in the skin above the origin of the saphenous artery under 2.5% inhaled isoflurane anesthesia via aseptic technique. The femoral vein and nerve were gently teased away from the artery, and a ligation was made using 6-0 sterile silk suture placed proximal to the trifurcation of the femoral, but distal to the origin of the superficial epigastric artery [see diagram in supplemental Fig. S1 in DiStasi et al. (17)]. Following ligation, 5-0 sterile absorbable suture was used to close the skin. Throughout the procedure, great care was taken to keep vessels and tissues moist and to minimize trauma to adjacent tissue in an attempt to minimize local inflammation. At 14 days postligation, the animal was anesthetized and then perfused at physiological pressure with 10 ml of the same dilator cocktail as used for the rat model above, followed by 10 ml of 4% Zn-formalin via anterograde cannulation of the infrarenal abdominal aorta with polyethylene-50 tubing. To allow visualization and subsequent diameter measurements of the gracilis collaterals, ∼0.1 ml of Microfil vascular casting compound (Flow Tech, Carver, MA) was advanced through the aortic cannula into both hindlimbs until retrograde filling of the saphenous artery distal to the ligation was apparent (17). The carcasses were enveloped in plastic wrap and stored at 4°C overnight to allow for complete curing of the casting agent. The midzone (42) of the gracilis collaterals as well as the same-animal contralateral controls were then carefully dissected, in situ digital images of the control and collateral vessels were obtained at matching locations in both limbs [see (17) for example images], and vessel diameters were determined with image analysis software (ImageJ).
Quantitative RT-PCR.
Relative differences in mesenteric artery Nox subunit mRNA expression were determined using reverse transcription real-time quantitative PCR as previously described (45). Briefly, RNA was isolated from control and collateral mesenteric arteries perfused with cold phosphate-buffered saline followed by RNAlater (Ambion, Austin, TX). Following perfusion, the jejunum, ileum, and cecum with intact mesenteric vasculature were excised and placed on a prechilled silicone elastomer-coated petri dish for dissection. Control and collateral arteries extending from the superior mesenteric artery to the marginal artery near the bowel wall were isolated from the mesenteric neurovascular bundle. Before excision, each isolated artery was carefully cleaned under a dissecting microscope to remove adventitial tissue to limit cellular sources of Nox primarily to the media and intima. If not processed immediately, arteries were preserved in RNAlater at −20°C before RNA isolation. Tissues were disrupted using a bead homogenizer (FastPrep System; QBIOgene, Carlsbad, CA) followed by total RNA purification using an RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA), which included on-column DNase digestion. RNA sample concentration was determined using A260/A280 (NanoDrop ND1000 Spectrophotometer; NanoDrop Products, Wilmington, DE), and RNA integrity was verified by analysis with either an Agilent 2100 Bioanalyzer (RNA 6000 Nano Chip Kit; Agilent, Santa Clara, CA) or Experion automated electrophoresis station (RNA HighSens chips; Bio-Rad, Hercules, CA). Aliquots of purified total RNA (0.3 μg) were enzymatically treated to remove potential contaminating genomic DNA (DNAfree Ambion) and then reverse transcribed using Ready-To-Go You-Prime First-Strand Beads (GE Healthcare/Amersham Biosciences, Piscataway, NJ) with random decamer priming. For PCR, aliquots of cDNA (5.0 μl, 1:50 dilution) were combined with primers and probes for Nox1, Nox2, Nox4 (PrimeTime qPCR Assays; Integrated DNA Technologies, Coralville, IA), and p47phox or hexokinase endogenous control (TaqMan Gene Expression Assays; Applied Biosystems, Foster City, CA) in the presence of a PCR master mix (FastStart Universal Probe Master Mix; Roche Applied Science, Indianapolis, IN). Reactions were run in triplicate on an Applied Biosystems 7500 Real-Time PCR System using relative quantification (ΔΔ threshold cycle) with dual-labeled (FAM/MGB) probes as the product detection method. Standard two-step 7500 PCR cycling conditions (40 cycles) were used. Differences in PCR product yields between groups were determined by comparing the fold differences between target mRNAs after normalization to hexokinase. We have found hexokinase to be invariant between control and collateral arteries, and its expression level closely matches that of the Nox subunits studied (S. J. Miller; unpublished observation).
Statistics.
Statistical analyses were done by one- or two-way repeated-measures ANOVA (SigmaPlot 12.0). When significance between groups was detected, multiple pairwise comparisons were performed with the Holm-Sidak method. Data are presented as group means ± SE. The criterion for significance was P < 0.05.
RESULTS
Assessment of temporal Nox expression.
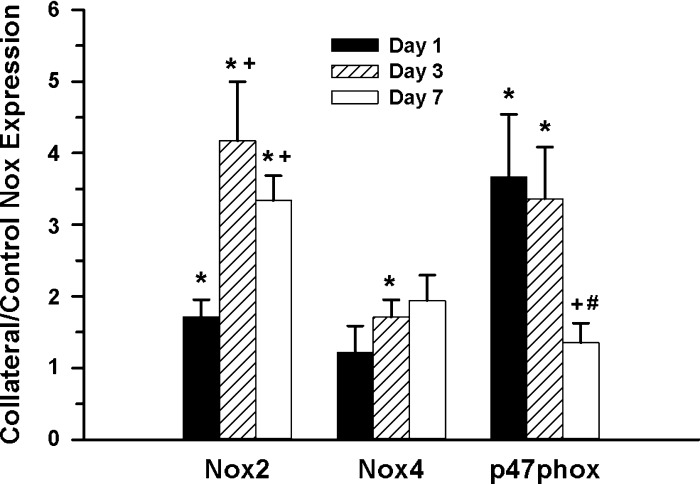
To assess the potential involvement of Nox subunits during collateral remodeling, collateral artery mRNA expression of Nox1, -2, -4, and p47phox relative to control arteries was determined at 1, 3, and 7 days postligation. These time points were chosen to focus on the intervals before and during the most rapid phase of collateral growth in the rat mesenteric model (63). As shown in Fig. 1, various temporal expression patterns were observed for the different Nox components. Collateral mRNA expression was significantly increased for Nox2 at all three time points, with the greatest changes at 3 and 7 days. In contrast, p47phox expression was significantly increased at both 1 and 3 days, but by 7 days was normalized to control values. Nox4 expression was significantly elevated only at 3 days, but to a lesser extent than either Nox2 or p47phox. Although detectable, Nox1 expression was too low to quantify using the same conditions as for the other Nox subunits. Taken together, the results indicated a potential role for selected Nox components in early stage collateral development.
Fig. 1.
Temporal expression of NADPH oxidase (Nox) mRNA in rat mesenteric collateral artery. Changes in collateral artery Nox mRNA subunit expression at 1, 3, and 7 days were determined compared with same animal control arteries and expressed as a collateral-to-control ratio (*, +, #: P < 0.05 vs. control, 1 day, 3 day, respectively; n = 4–6).
Nox2/p47phox interaction and collateral growth.
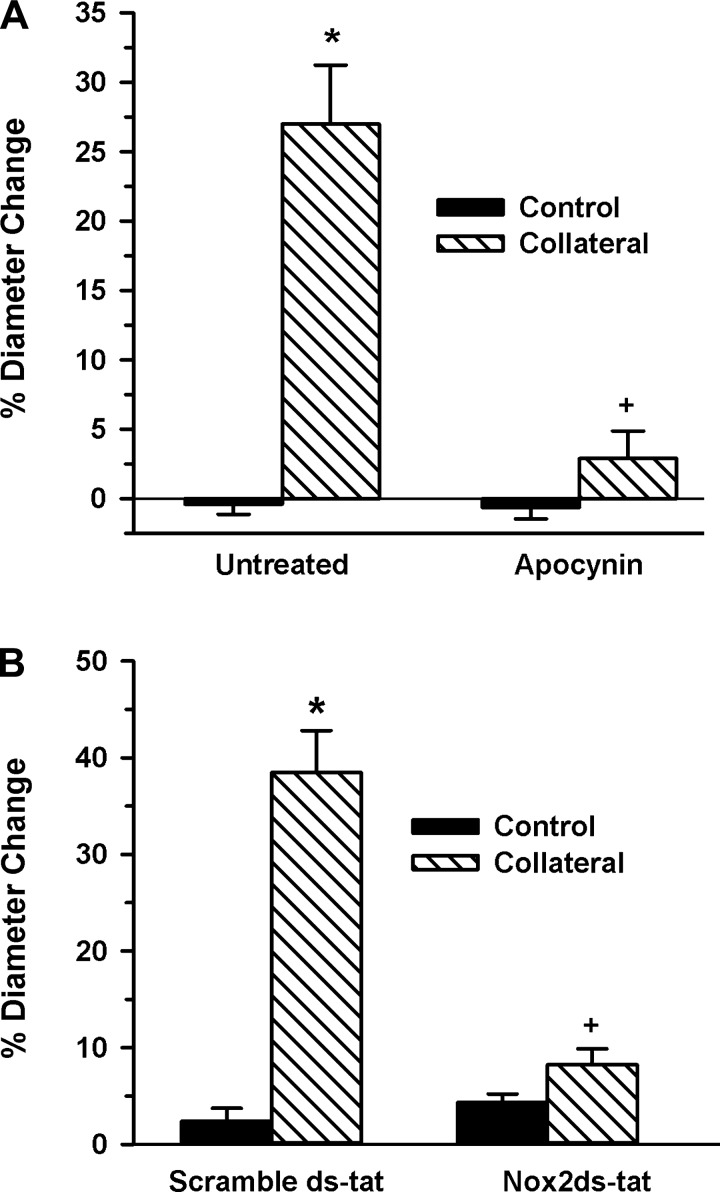
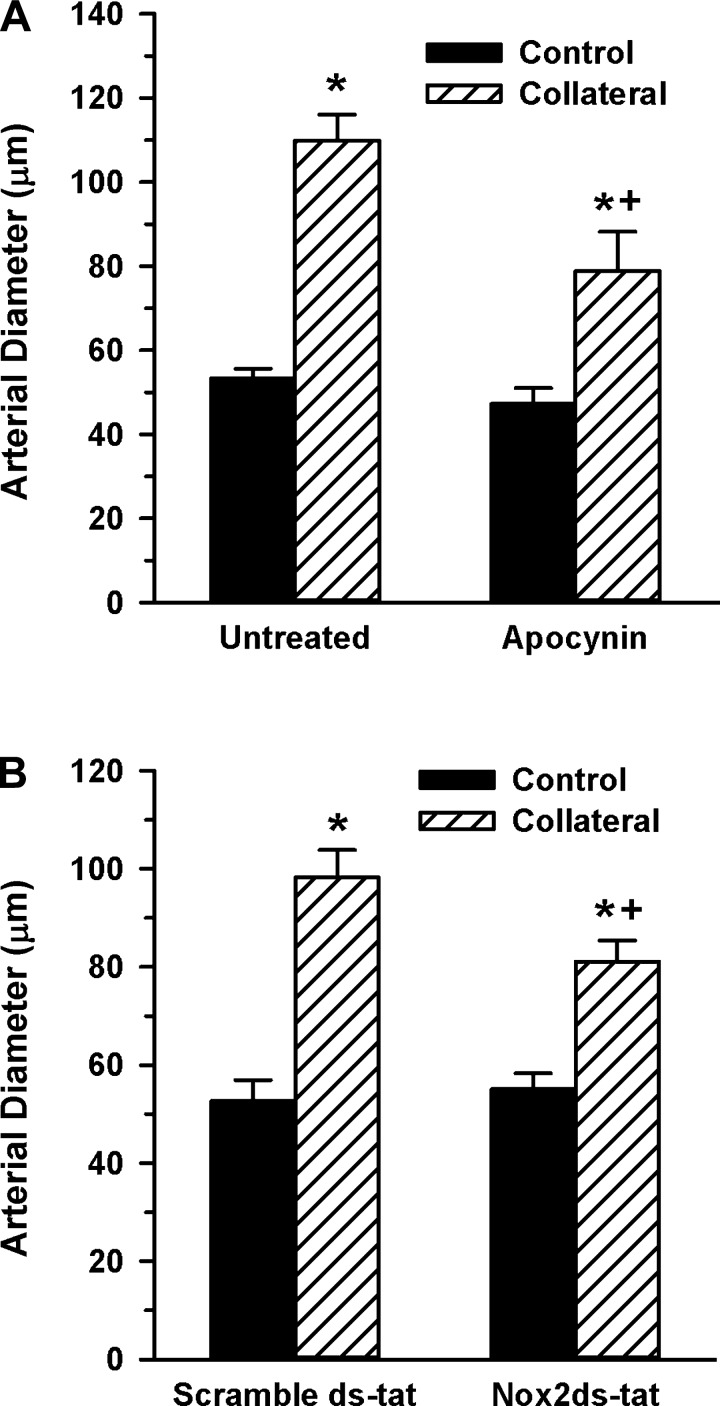
The increased expression of Nox2 and p47phox in the initial period of collateral growth (1–3 days) and subsequent decreased p47phox expression at 7 days suggested these interacting catalytic and regulatory subunits may have a role in mediating the early stage collateral growth. To test this possibility, we first verified redox regulation of collateral growth by administering a general antioxidant and determining the effect on collateral artery diameter change. As shown in Fig. 2A, apocynin completely inhibited collateral growth compared with untreated controls. Because apocynin may act as a Nox2 inhibitor in vivo (29), in addition to other antioxidant properties (28), we next assessed effects of the selective Nox2 inhibitor Nox2ds-tat (gp91ds-tat), which inhibits interaction of Nox2 and p47phox (11, 53), on collateral growth. As depicted in Fig. 2B, administration of Nox2ds-tat, but not the scrambled peptide, prevented any significant collateral growth in young WKY rats.
Fig. 2.
Antioxidant treatment or Nox2 inhibition suppress rat mesenteric collateral growth. A: mesenteric artery control and collateral artery diameters (7-day model) were determined in rats receiving plain drinking water (untreated, n = 6) or water supplemented with 3 mM apocynin (n = 4). (*, +; P < 0.001 vs. untreated control and untreated collateral, respectively). B: control and collateral artery diameters were determined in rats receiving scramble ds-tat (n = 4) or Nox2ds-tat (1 mg·kg−1·day−1; n = 3) delivered via osmotic minipump (*, +; P < 0.001 vs. scramble ds-tat control and scramble ds-tat collateral, respectively). Collateral growth is expressed as percent change because the mesenteric ligation model allows for artery diameter measurements of the same vessel at time 0 and at 7 days postligation.
We next confirmed the role of the Nox2/p47phox interaction in collateral development using an alternative vascular bed. Apocynin or Nox2ds-tat was administered to separate groups of C57BL/6 mice that had undergone a femoral artery ligation to induce gracilis artery collateral growth. As shown in Fig. 3, both agents resulted in a significantly reduced capacity to develop gracilis artery collaterals, consistent with the results from the rat mesenteric model.
Fig. 3.
Antioxidant treatment or Nox2 inhibition suppresses mouse hindlimb collateral growth. A: gracilis artery diameters were determined in unligated (control) and ligated (collateral) limbs 14 days following femoral artery ligation in mice receiving plain drinking water (untreated, n = 8) or water supplemented with apocynin (n = 6). (*, +; P < 0.05 vs. control and untreated collateral, respectively). B: control and collateral artery diameters were determined in rats receiving scramble ds-tat (n = 6) or Nox2ds-tat (10 mg·kg−1·day−1; n = 5) delivered via osmotic minipump (*, +; P < 0.05 vs. control and scramble ds-tat collateral, respectively, determined by one-way ANOVA). Collateral growth is expressed as control and collateral artery diameters rather than percent diameter change because, unlike the rat mesentery, diameter measurements in the mouse hindlimb are only possible at death.
Regulation of collateral growth by p47phox expression.
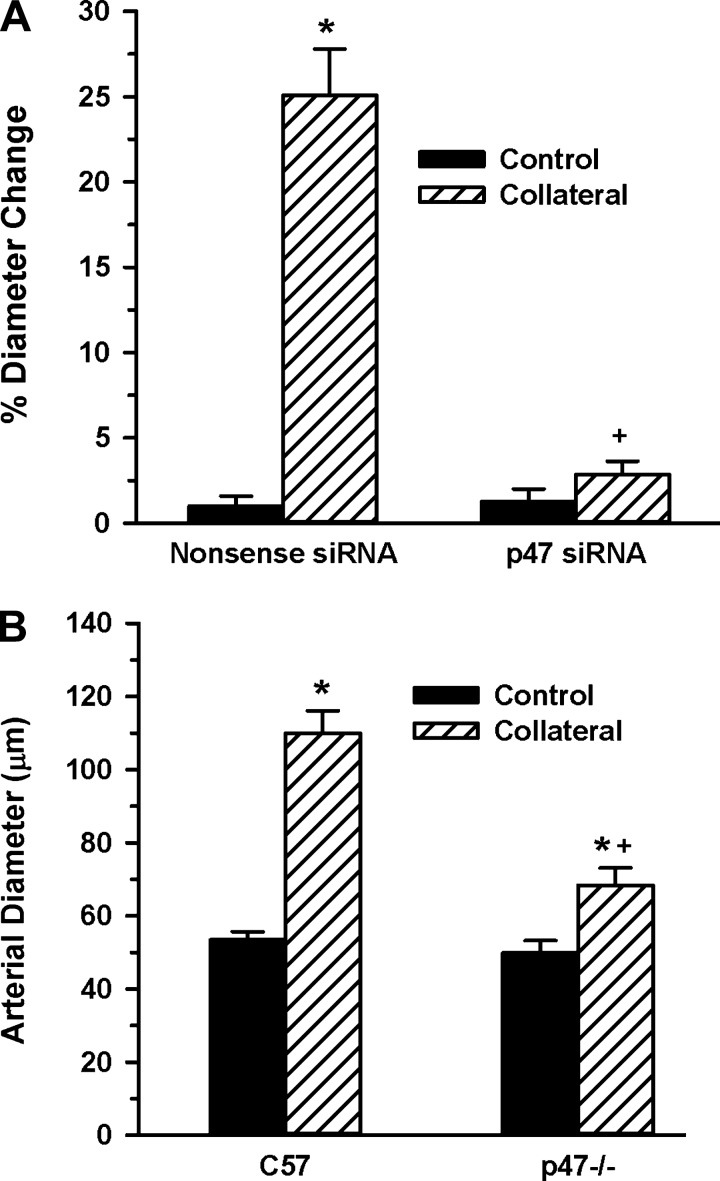
The p47phox subunit is a key regulator of Nox1 and -2 activity (39), and one study has shown that p47phox regulates flow-mediated conduit artery remodeling (7). Based on this study, along with our data showing temporal variations in Nox mRNA expression and the ability of Nox2ds-tat to inhibit collateral growth, we hypothesized that changes in p47phox expression levels could regulate early stage collateral growth. To address this hypothesis, we used both a molecular and genetic approach. First, we used a p47phox siRNA delivered systemically to WKY rats to suppress p47phox expression in conjunction with the mesenteric collateral model. The results in Fig. 4A show that administration of p47phox siRNA completely suppressed collateral growth in the young WKY rats compared with the nonsense (negative) siRNA control. To verify this finding both with genetic ablation and in an alternate collateral model, femoral artery ligation was performed in p47phox−/− mice and hindlimb gracilis artery collateral growth was assessed at 14 days. Similar to the results with the rat mesenteric model, hindlimb collateral growth was significantly attenuated in the p47phox−/− compared with the wild-type controls (Fig. 4B).
Fig. 4.
p47phox inhibition suppresses collateral growth. A: mesenteric artery control and collateral artery diameters (7-day model) were determined in rats receiving small interfering RNA (siRNA) to p47phox (n = 4) or to a nonsense (negative control) siRNA (n = 3). *, +; P < 0.001 vs. control and nonsense siRNA collateral, respectively. B: hindlimb collateral growth in p47phox−/− (p47−/−) mice. Gracilis artery collateral diameters were measured at 14 days following single ligation of the right femoral artery and compared with same animal control limbs in C57BL/6 (C57; same as untreated in Fig. 3; n = 8) and in p47−/− (n = 11). *, +; P < 0.05 vs. control, C57BL/6 collateral, respectively.
Role of peroxide in compensatory collateral growth.
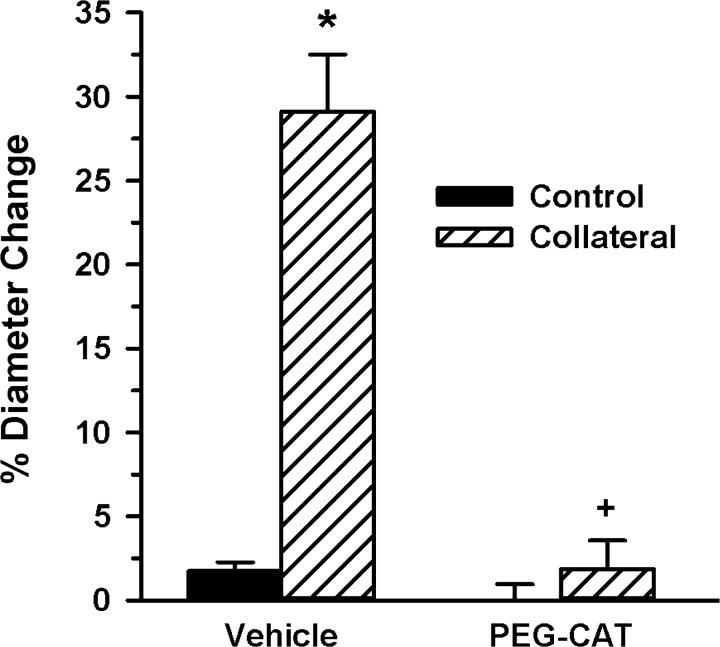
To assess a potential requisite role of hydrogen peroxide in mediating collateral growth, PEG-CAT was administered continuously via osmotic pump during the 7-day WKY rat mesenteric model. As shown in Fig. 5, PEG-CAT prevented any significant collateral growth from occurring compared with the vehicle control, indicating that peroxide is necessary for compensatory collateral growth. When PEG-CAT was administered to mice, significant collateral enlargement was observed (59.3 ± 1.1 vs. 82.5 ± 1.9 μm, control vs. collateral, respectively, P < 0.001, n = 4). However, this ∼20-μm increase in collateral diameter was significantly less (P < 0.02) than the ∼55-μm increase observed in the untreated mice (Fig. 3A), and the suppression in collateral enlargement was similar to results obtained with apocynin and Nox2ds-tat (Fig. 3, A and B).
Fig. 5.
Catalase inhibited rat mesenteric collateral growth. Polyethylene glycol-conjugated catalase (PEG-CAT) and vehicle (saline) administration via osmotic pump was started at the time of model creation and continued for 7 days. Collateral diameters in vehicle-treated rats (n = 3) significantly increased compared with in-animal control arteries, whereas no collateral growth occurred in the rats receiving PEG-CAT (n = 4). *, +; P < 0.001 vs. control and vehicle collateral, respectively.
DISCUSSION
Significant aspects of the current study findings may be summarized in four main points: 1) the temporal changes in expression of Nox subunits during early stages of collateral growth, 2) the ability of apocynin and Nox2ds-tat to inhibit collateral growth in both the rat and mouse models of collateral development, 3) decreased or absent p47phox expression and the inhibition of collateral growth, and 4) inhibition of collateral growth by PEG-CAT. The significance of these points is considered in detail below, following a brief discussion of the rodent models' relevance to the study.
Model considerations.
The rat mesenteric and mouse hindlimb models of collateral growth both use acute ligation of select arteries to allow investigation of responses of primary collaterals, which are the major determinants of resistance following arterial occlusion in humans (10, 43, 61) and animals (37, 68, 74). The rat mesenteric model is readily accessible and allows for repeated diameter measurements on the same artery. It also has been well characterized in terms of hemodynamic properties including shear stress (22, 63, 65, 67) and allows for molecular analyses of mRNA and/or protein expression (e.g., collateral Nox subunit mRNA expression, Fig. 1). The hemodynamics of collateral formation in the mouse hindlimb gracilis artery model are largely unknown, but this model is more relevant to the focal occlusion which occurs in the human leg and has the advantage of molecular knockouts to assess function of select proteins. These two different collateral models were used in this study to allow for a combination of pharmacological, molecular, and genetic approaches to investigate the role of specific Nox in redox-mediated physiological collateral growth and to ensure the applicability of the results to more than one type of vascular bed.
Temporal expression of Nox during collateral growth.
Changes in Nox expression were assessed by measuring relative mRNA expression changes for Nox1, -2, -4, and p47phox subunits at 1, 3, and 7 days, following the creation of the mesenteric collateral model. These time points were chosen because they represent stages 1) before remodeling has begun but transcriptional changes have occurred (day 1), 2) at which both protein synthesis and the earliest measureable increase in diameter have occurred (day 3), and 3) when flow-mediated remodeling is in a rapid period of development and significant artery diameter change can be easily detected (day 7) (63, 66, 67). Because of the relatively low expression level of Nox1, we assumed its contribution to redox regulation of collateral growth was negligible in this model. However, the possibility of posttranscriptional regulation of Nox1 or related subunit expression and/or activity cannot be ruled out. Nox2 and p47phox, but not Nox4, subunit expression was consistently increased at the earliest stages of collateral growth (days 1 and 3), and p47phox expression was normalized to control values at day 7 (Fig. 1). Taken together, these results suggested that Nox2 was potentially involved in early stages of collateral development by regulation via changes in p47phox expression/activity. These results are partially consistent with the findings of Duerrschmidt et al. (19) that showed acute temporal changes in predominantly Nox2-related ROS in response to elevated shear. Belin de Chantemele et al. (3) noted that Nox2, but not p47phox, expression was increased at 3 wk in a mesenteric collateral model, which is consistent with the drop in p47phox expression we observed at 7 days. Because the mRNA expression data implicated the Nox2/p47phox interaction in redox regulation of the early stages of collateral development, we investigated the relationship between Nox2 and collateral growth using functional approaches.
Nox2 and p47phox as regulators of collateral growth.
Because both apocynin and Nox2ds-tat prevent interaction of Nox2 and the p47phox subunit (11, 29, 53), the observed inhibition of collateral growth by these Nox inhibitors in both rat mesenteric (Fig. 2) and mouse hindlimb (Fig. 3) models of collateral formation strongly implicates the Nox2/p47phox interaction as a key component in redox-mediated collateral growth. Apocynin may have antioxidant actions independent from its action as an in vivo Nox inhibitor (28); nevertheless, its ability to suppress luminal expansion is consistent with the action of Nox2ds-tat, suggesting its primary action in these collateral models is via Nox inhibition. This result is consistent with that obtained by Henrion's group (3) who showed that chronic treatment with Tempol or apocynin blocked collateral growth in the rat mesenteric model. The Nox2 B-loop peptide, on which the Nox2ds-tat peptide is based, recently has been shown to be selective for the Nox2/p47phox interaction (11), and administration of Nox2ds-tat completely suppressed collateral growth in the young WKY rats (Fig. 2B). Nox2ds-tat also significantly reduced development of the gracilis collateral in the mouse hindlimb (Fig. 3B), suggesting Nox2 is an important component of redox signaling in small arteries with characteristics different from the mesenteric circulation. It is known that p47phox also can be a regulatory subunit for Nox1, but because the expression level of Nox1 was so low in the mesenteric arteries, we interpret this observation to mean that it is not a major source of vascular ROS involved in redox signaling, at least in this model. We also have observed that Nox1 mRNA expression in mouse femoral/iliac vessels was similar to rat mesenteric; insufficient to accurately quantify compared with the expression of other Nox subunits analyzed under the same conditions (S. J. Miller, unpublished observation).
The current study determined the role of Nox2 in resistance vessels forming primary collateral pathways using clinically relevant well-defined models of subcritical ischemia. The major stimulus for the outward remodeling of these collateral vessels is the wall shear stress associated with increases in volume flow through the collateral vessels (21, 63). We are aware of only two other studies that have examined the relationship between Nox2 and flow-dependent remodeling using Nox2−/− mice. Castier et al. (7) used a high-flow carotid model and found that Nox2−/− mice had normal outward remodeling compared with wild-type mice. However, the results of this study demonstrated a requirement for p47phox flow-mediated remodeling, consistent with our observations. DiStasi et al. (17) used a mouse hindlimb model with femoral artery excision and determined gracilis collateral artery growth to be unimpaired in Nox2−/− mice, which is inconsistent with our current results.
The explanation for the divergent role of Nox2 in these two studies compared with the current report is unknown but could relate to the magnitude of the stimulus and vascular heterogeneity in Nox expression. In the Castier et al. study (7), shear stress was increased >5× compared with control versus ∼2× in the rat mesenteric model (67). Likewise, the excision of the entire femoral artery as used by DiStasi et al. would be expected to elevate shear stress in collaterals to a greater degree than in the current paper with single ligation of the same artery. The level of shear stress could directly influence Nox activation in the endothelium and indirectly in the medial layer where circumferential wall stress could be influenced by flow-mediated dilation. In addition, the localization of the various Nox within the vascular wall is complex and may vary between vascular location and species (5). It has been reported that Nox2 typically present in resistance vessels may be replaced by Nox1 in conduit vessels (38), and this could also explain the observed difference between the carotid and mesenteric vessels exposed to elevated flow. In the carotid, p47phox could function as a regulatory subunit for Nox1, and the possibility of increased compensatory expression/activity of Nox1 in Nox2−/− mice exposed to higher levels of shear stress cannot be ruled out. Recent work also suggests that p47phox may have effects independent of Nox activity regulation (50). However, this possibility would seem unlikely in our collateral models because the Nox2 inhibitor data in both rat and mouse (Figs. 2 and 3) provide compelling evidence for p47phox regulation of the Nox2 complex as a mechanism for generating the ROS involved in normal collateral development. While a role for Nox4 in collateral growth cannot be entirely ruled out, especially in the mouse, the rat PCR data indicate minimal expression changes in early stages of mesenteric artery remodeling. Studies have indicated a role for Nox4 in compensatory vascular function, but primarily in the context of angiogenesis (13, 55, 75) or dilatation (51) and not remodeling of resistance vessels. It also has been suggested that the function of Nox4 is significant only during disease or stress conditions and not in normal physiological function (60). Clearly, further work is needed to delineate the role of the various Nox in compensatory and pathological adaptations in vascular tissues.
Potential role of hydrogen peroxide in collateral growth.
The ability of PEG-CAT to suppress collateral growth (Fig. 5) suggests hydrogen peroxide is an important form of ROS regulating compensatory collateral growth. Increased flow has been shown to stimulate acute peroxide production in rat coronary resistance arterioles (31), and it has been demonstrated that peroxide can participate in physiological processes such as angiogenesis and the regulation of vascular dilation. Schroder et al. (55) found that PEG-CAT inhibited angiogenesis in the hindlimbs of control mice, and Kang et al. (31) showed that flow-induced dilation of 4-mo-old F344 rat coronary arterioles was reduced by treatment with CAT. The current results extend these previous findings on peroxide function to the context of physiologically relevant chronically elevated flow and collateral growth.
A potential mechanism for flow-mediated peroxide-dependent collateral growth may be provided by the findings of Kumar et al. (34), who showed that a shear-mediated decrease in CAT (due to a decrease in PKC-δ activity) occurs in pulmonary aortic endothelial cells, which increases hydrogen peroxide signaling and results in elevated endothelial NO synthase activity and NO production. Several studies (6, 18, 58, 70, 76) have established that physiological concentrations of peroxide regulate endothelial NO synthase activity and the resultant production of NO, which is an important mediator of collateral development (12, 36). Peroxide has also been recently shown to stabilize the mRNA for soluble guanylate cyclase, which increases its expression and facilitates the action of NO (44). Another potential contributing mechanism for the action of peroxide on collateral growth is posttranscriptional regulation of placenta growth factor, a known regulator of arteriogenesis (56).
Although not a focus of this study, a related consideration for the role of peroxide in collateral growth is its primary source. While Nox4 may be a primary source for peroxide under certain conditions (16), our previous work showed that Nox2 is a source of peroxide production, at least in the context of risk factor-impaired vascular function (76, 77). In addition, cross talk between Nox2-derived ROS and mitochondria has been demonstrated to result in mitochondrial overproduction of ROS, including peroxide [see Dikalov (15) for a review]. Thus, while additional work will be needed to understand the specific pathways involved in Nox-mediated peroxide production and function, our results raise the possibility that Nox2-derived peroxide is a primary ROS involved in redox signaling associated with compensatory collateral growth.
Summary.
The results from the current study are consistent with the hypothesis that Nox2 is the primary Nox associated with the normal redox signaling necessary for flow-mediated compensatory remodeling in resistance vessels of young, healthy animals. Taken together with our previous work showing the ability of antioxidants to reverse the age- and hypertension-related impairment in collateral growth (45, 46), the current results also are consistent with the concept of an “optimal redox window,” where relative amounts of ROS and other redox-regulated molecules such as NO must be in balance for prevention of vascular dysfunction/disease and to allow vascular processes to proceed normally (24, 35, 52, 54). In addition, our results suggest that hydrogen peroxide is the key ROS mediating compensatory collateral growth. Due to the potential use of Nox pharmacological inhibitors and other antioxidants to treat pathological vascular conditions related to oxidant stress, it will be important to understand the role of specific Nox subunits and ROS in both the promotion and impairment of collateral growth. In particular, use of general or Nox-specific antioxidants as preventive therapy for young and/or healthy humans may be inappropriate due to potential suppression of normal vascular functions such as flow-mediated dilation or compensatory remodeling. More work will be needed to fully delineate the specific Nox-related redox signaling pathways involved in both physiological and risk-factor impaired compensatory collateral growth.
GRANTS
Research reported in this publication was supported by National Institutes of Health Grants R01-HL-092012 and R01-HL-92012-S1 (to S. J. Miller and J. L. Unthank) and T32-DK-007519 (to M. R. DiStasi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.R.D., J.L.U., and S.J.M. conception and design of research; M.R.D., J.L.U., and S.J.M. performed experiments; M.R.D., J.L.U., and S.J.M. analyzed data; M.R.D., J.L.U., and S.J.M. interpreted results of experiments; M.R.D., J.L.U., and S.J.M. prepared figures; M.R.D., J.L.U., and S.J.M. drafted manuscript; M.R.D., J.L.U., and S.J.M. edited and revised manuscript; M.R.D., J.L.U., and S.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Randal G. Bills for invaluable assistance in creating the rat mesenteric collateral models, Mary Jo Wenning for excellent work with the PCR and assisting in preparation of the siRNA reagents, Miguel Ortiz for assistance with the mouse surgeries and tissue harvests, and Tonia L. Miller for help in editing the manuscript. We also thank Dr. David A. Ingram, Jr., for supplying some of the C57BL/6 and p47phox−/− mice used in the study.
REFERENCES
- 1.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 99: 2239–2242, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Antoniucci D, Valenti R, Moschi G, Migliorini A, Trapani M, Santoro GM, Bolognese L, Cerisano G, Buonamici P, Dovellini EV. Relation between preintervention angiographic evidence of coronary collateral circulation and clinical and angiographic outcomes after primary angioplasty or stenting for acute myocardial infarction. Am J Cardiol 89: 121–125, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Belin de Chantemele EJ, Vessieres E, Dumont O, Guihot AL, Toutain B, Loufrani L, Henrion D. Reactive oxygen species are necessary for high flow (shear stress)-induced diameter enlargement of rat resistance arteries. Microcirculation 16: 391–402, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Belin de Chantemele EJ, Vessieres E, Guihot AL, Toutain B, Maquignau M, Loufrani L, Henrion D. Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood flow. Cardiovasc Res 81: 788–796, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16–27, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Li Z, Dikalov S, Holland SM, Hwang J, Jo H, Dudley SC, Jr, Harrison DG. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J Biol Chem 277: 48311–48317, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res 97: 533–540, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Castier Y, Ramkhelawon B, Riou S, Tedgui A, Lehoux S. Role of NF-kappaB in flow-induced vascular remodeling. Antioxid Redox Signal 11: 1641–1649, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol 289: H947–H959, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Conrad MC, Green HD. Hemodynamics of large and small vessels in peripheral vascular disease. Circulation 29: 847–853, 1964 [DOI] [PubMed] [Google Scholar]
- 11.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 106: 1870–1881, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol 27: 2319–2324, 2007 [DOI] [PubMed] [Google Scholar]
- 14.DeBakey ME, Lawrie GM, Glaeser DH. Patterns of atherosclerosis and their surgical significance. Ann Surg 201: 115–131, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Distasi MR, Case J, Ziegler MA, Dinauer MC, Yoder MC, Haneline LS, Dalsing MC, Miller SJ, Labarrere CA, Murphy MP, Ingram DA, Unthank JL. Suppressed hindlimb perfusion in Rac2−/− and Nox2−/− mice does not result from impaired collateral growth. Am J Physiol Heart Circ Physiol 296: H877–H886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 86: 347–354, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Duerrschmidt N, Stielow C, Muller G, Pagano PJ, Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J Physiol 576: 557–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol 169: 719–728, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res 99: 656–662, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Fath SW, Burkhart HM, Miller SC, Dalsing MC, Unthank JL. Wall remodeling after wall shear rate normalization in rat mesenteric arterial collaterals. J Vasc Res 35: 257–264, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Girerd X, London G, Boutouyrie P, Mourad JJ, Safar M, Laurent S. Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension 27: 799–803, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Gu W, Weihrauch D, Tanaka K, Tessmer JP, Pagel PS, Kersten JR, Chilian WM, Warltier DC. Reactive oxygen species are critical mediators of coronary collateral development in a canine model. Am J Physiol Heart Circ Physiol 285: H1582–H1589, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Haddad P, Dussault S, Groleau J, Turgeon J, Michaud SE, Menard C, Perez G, Maingrette F, Rivard A. Nox2-containing NADPH oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress. Arterioscler Thromb Vasc Biol 29: 1522–1528, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Hedera P, Bujdakova J, Traubner P, Pancak J. Stroke risk factors and development of collateral flow in carotid occlusive disease. Acta Neurol Scand 98: 182–186, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Herrmann LG, Cranley JJ, Preuninger RM. Importance of collateral circulation in obliterative arterial disease of the lower extremities. Geriatrics 9: 1–7, 1954 [PubMed] [Google Scholar]
- 28.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Johnson DK, Schillinger KJ, Kwait DM, Hughes CV, McNamara EJ, Ishmael F, O'Donnell RW, Chang MM, Hogg MG, Dordick JS, Santhanam L, Ziegler LM, Holland JA. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium 9: 191–203, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol Heart Circ Physiol 239: H14–H21, 1980 [DOI] [PubMed] [Google Scholar]
- 31.Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 109: 520–525, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Kilian JG, Keech A, Adams MR, Celermajer DS. Coronary collateralization: determinants of adequate distal vessel filling after arterial occlusion. Coron Artery Dis 13: 155–159, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Sud N, Fonseca FV, Hou Y, Black SM. Shear stress stimulates nitric oxide signaling in pulmonary arterial endothelial cells via a reduction in catalase activity: role of protein kinase C delta. Am J Physiol Lung Cell Mol Physiol 298: L105–L116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res 85: 753–766, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Lamblin N, Cuilleret FJ, Helbecque N, Dallongeville J, Lablanche JM, Amouyel P, Bauters C, Van Belle E. A common variant of endothelial nitric oxide synthase (Glu298Asp) is associated with collateral development in patients with chronic coronary occlusions. BMC Cardiovasc Disord 5: 27 (Sept 15, 2005). 10.1186/1471-2261-5-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lash JM, Nixon JC, Unthank JL. Exercise training effects on collateral and microvascular resistances in rat model of arterial insufficiency. Am J Physiol Heart Circ Physiol 268: H125–H137, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurindo FR, Pedro Mde A, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O, da Luz PL. Vascular free radical release Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res 74: 700–709, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol 23: 776–782, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Longland CJ. The collateral circulation of the limb; Arris and Gale lecture delivered at the Royal College of Surgeons of England on 4th February, 1953. Ann R Coll Surg Engl 13: 161–176, 1953 [PMC free article] [PubMed] [Google Scholar]
- 43.Ludbrook J. Collateral artery resistance in the human lower limb. J Surg Res 6: 423–434, 1966 [DOI] [PubMed] [Google Scholar]
- 44.Martin-Garrido A, Gonzalez-Ramos M, Griera M, Guijarro B, Cannata-Andia J, Rodriguez-Puyol D, Rodriguez-Puyol M, Saura M. H2O2 regulation of vascular function through sGC mRNA stabilization by HuR. Arterioscler Thromb Vasc Biol 31: 567–573, 2011. [DOI] [PubMed] [Google Scholar]
- 44a.Menard MT, Belkin M. Femoropopliteal and tibial occlusive disease. In: Greenfield's Surgery: Scientific Principles and Practice, edited by Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Upchurch GR. Philadelphia, PA: Lippincott Williams & Wilkins, p. 1649–1661, 2006 [Google Scholar]
- 45.Miller SJ, Coppinger BJ, Zhou X, Unthank JL. Antioxidants reverse age-related collateral growth impairment. J Vasc Res 47: 108–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller SJ, Norton LE, Murphy MP, Dalsing MC, Unthank JL. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat mesenteric collateral growth impairment. Am J Physiol Heart Circ Physiol 292: H2523–H2531, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Nakae I, Fujita M, Miwa K, Hasegawa K, Kihara Y, Nohara R, Miyamoto S, Ueda K, Tamaki S, Sasayama S. Age-dependent impairment of coronary collateral development in humans. Heart Vessels 15: 176–180, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Paliege A, Pasumarthy A, Mizel D, Yang T, Schnermann J, Bachmann S. Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290: R694–R700, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Patel VB, Wang Z, Fan D, Zhabyeyev P, Basu R, Das SK, Wang W, Desaulniers J, Holland SM, Kassiri Z, Oudit GY. Loss of p47phox subunit enhances susceptibility to biomechanical stress and heart failure because of dysregulation of cortactin and actin filaments. Circ Res 112: 1542–1556, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol 28: 61–67, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Shaw JH, Lloyd PG. Post-transcriptional regulation of placenta growth factor mRNA by hydrogen peroxide. Microvasc Res 84: 155–160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sousa T, Pinho D, Morato M, Marques-Lopes J, Fernandes E, Afonso J, Oliveira S, Carvalho F, Albino-Teixeira A. Role of superoxide and hydrogen peroxide in hypertension induced by an antagonist of adenosine receptors. Eur J Pharmacol 588: 267–276, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277: 6017–6024, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Touyz RM, Montezano AC. Vascular Nox4: a multifarious NADPH oxidase. Circ Res 110: 1159–1161, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Traupe T, Ortmann J, Stoller M, Baumgartner I, de Marchi SF, Seiler C. Direct quantitative assessment of the peripheral artery collateral circulation in patients undergoing angiography. Circulation 128: 737–744, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Tuttle JL, Hahn TL, Sanders BM, Witzmann FA, Miller SJ, Dalsing MC, Unthank JL. Impaired collateral development in mature rats. Am J Physiol Heart Circ Physiol 283: H146–H155, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol 281: H1380–H1389, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Tuttle JL, Sanders BM, Burkhart HM, Fath SW, Kerr KA, Watson WC, Herring BP, Dalsing MC, Unthank JL. Impaired collateral artery development in spontaneously hypertensive rats. Microcirculation 9: 343–351, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Unthank JL, Fath SW, Burkhart HM, Miller SC, Dalsing MC. Wall remodeling during luminal expansion of mesenteric arterial collaterals in the rat. Circ Res 79: 1015–1023, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Unthank JL, McClintick JN, Labarrere CA, Li L, Distasi MR, Miller SJ. Molecular basis for impaired collateral artery growth in the spontaneously hypertensive rat: insight from microarray analysis. Physiol Rep 1: (June 26, 2013). 10.1002/phy2.5.00005.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Unthank JL, Nixon JC, Burkhart HM, Fath SW, Dalsing MC. Early collateral and microvascular adaptations to intestinal artery occlusion in rat. Am J Physiol Heart Circ Physiol 271: H914–H923, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Unthank JL, Nixon JC, Lash JM. Early adaptations in collateral and microvascular resistances after ligation of the rat femoral artery. J Appl Physiol 79: 73–82, 1995 [DOI] [PubMed] [Google Scholar]
- 69.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M. Role of Nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urao N, Sudhahar V, Kim SJ, Chen GF, McKinney RD, Kojda G, Fukai T, Ushio-Fukai M. Critical role of endothelial hydrogen peroxide in post-ischemic neovascularization. PLoS One 8 (March 5, 2013) 10.1371/journal.pone.0057618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walden R, Adar R, Rubinstein ZJ, Bass A. Distribution and symmetry of arteriosclerotic lesions of the lower extremities: an arteriographic study of 200 limbs. Cardiovasc Intervent Radiol 8: 180–182, 1985 [DOI] [PubMed] [Google Scholar]
- 72.Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res 101: 627–635, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res 95: 1075–1081, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Yang HT, Ren J, Laughlin MH, Terjung RL. Prior exercise training produces NO-dependent increases in collateral blood flow after acute arterial occlusion. Am J Physiol Heart Circ Physiol 282: H301–H310, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou X, Bohlen HG, Miller SJ, Unthank JL. NAD(P)H oxidase-derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol 295: H1008–H1016, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou X, Bohlen HG, Unthank JL, Miller SJ. Abnormal nitric oxide production in aged rat mesenteric arteries is mediated by NAD(P)H oxidase-derived peroxide. Am J Physiol Heart Circ Physiol 297: H2227–H2233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, Murphy MP, George Akingba A, Sturek M, Dalsing MC, Unthank JL. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation 17: 3–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]