Abstract
We previously demonstrated increased villus height following genetic deletion, or knockout, of retinoblastoma protein (Rb) in the intestinal epithelium (Rb-IKO). Here we determined the functional consequences of augmented mucosal growth on intestinal fat absorption and following a 50% small bowel resection (SBR). Mice with constitutively disrupted Rb expression in the intestinal epithelium (Rb-IKO) along with their floxed (wild-type, WT) littermates were placed on a high-fat diet (HFD, 42% kcal fat) for 54 wk. Mice were weighed weekly, and fat absorption, indirect calorimetry, and MRI body composition were measured. Rb-IKO mice were also subjected to a 50% SBR, followed by HFD feeding for 33 wk. In separate experiments, we examined intestinal fat absorption in mice with conditional (tamoxifen-inducible) intestinal Rb (inducible Rb-IKO) deletion. Microarray revealed that the transcriptional expression of lipid absorption/transport genes was significantly reduced in constitutive Rb-IKO mice. These mice demonstrated greater mucosal surface area yet manifested paradoxically impaired intestinal long-chain triglyceride absorption and decreased cholesterol absorption. Despite attenuated lipid absorption, there were no differences in metabolic rate, body composition, and weight gain in Rb-IKO and WT mice at baseline and following SBR. We also confirmed fat malabsorption in inducible Rb-IKO mice. We concluded that, despite an expanded mucosal surface area, Rb-IKO mice demonstrate impaired lipid absorption without compensatory alterations in energy homeostasis or body composition. These findings underscore the importance of delineating structural/functional relationships in the gut and suggest a previously unknown role for Rb in the regulation of intestinal lipid absorption.
Keywords: retinoblastoma protein, CD36, fat metabolism
retinoblastoma protein (Rb) is a prototype tumor suppressor and cell cycle regulator (6, 22, 33). Rb is expressed in virtually all tissues and regulates the G1/S check point and cell cycle progression via interactions with the E2F family of transcription factors (1, 5). Germline Rb knockouts are lethal; therefore the effects of Rb deficiency have been studied through conditional and/or tissue-specific deletion models (27, 30).
An important role for Rb in intestinal homeostasis has been established using mutant mice in which Rb expression has been selectively disrupted within the intestinal epithelium in the fetal period. Using villin-Cre transgenics crossed with Rb(flox/flox) mice, we established that constitutive enterocyte deletion of Rb (intestinal Rb knockout, Rb-IKO) exhibits a hyperplastic intestinal phenotype associated with enhanced rates of enterocyte proliferation, taller villi, and deeper crypts (9, 13). However, whereas the structural effects of Rb deletion on intestinal morphology have been established, the role of Rb in intestinal function remains unexplored. Here we examined whether enterocyte-specific Rb deficiency, which leads to expanded intestinal mucosal surface area, might also alter nutrient absorption. Additionally, we sought to examine Rb-deficient intestinal function when intestinal length is significantly reduced by performing small bowel resection (SBR) on Rb-IKO mice. Finally, as Rb-IKO mice have a constitutive Rb deletion since birth, we produced a conditional Rb-deleted line to determine whether these inducible mice may have similar results when Rb is acutely deleted in adult mice.
MATERIALS AND METHODS
Animals.
The protocol for this study was approved by the Washington University Institutional Animal Care and Use Committee (Protocol no. 20100103) and was in accordance with the National Institute of Health laboratory animal care and use guidelines. Mice in which Cre recombinase expression is driven by the villin promoter within the intestinal epithelium were intercrossed with mice in which the exon 19 of Rb gene is flanked by loxP sites [Rb(flox/flox)]. The offspring of this breeding strategy resulted in mice with constitutive intestine-specific disruption of Rb expression, referred to as Rb-IKO. Littermates lacking Cre but containing a floxed Rb allele were used as wild-type (WT) controls. Weaned 3-wk-old Rb-IKO mice and WT littermates were placed on a high-fat diet (HFD, 42% kcal fat; Harlan Teklad TD.88137; Harland Laboratories, Madison, WI) and were fed this diet ad libitum daily up until the time of harvest 54 wk later. Inducible, intestine-specific Rb-null mice were generated by crossing mice with a tamoxifen-inducible Cre-fusion protein under control of the villin promoter (vil-Cre-ERT2) (obtained via generous donation from Sylvie Robine, Curie Institute, Paris, France) with mice in which the floxed Rb had been tagged for recombination (Jackson Laboratories, Bar Harbor, ME). Hereafter, mice with Cre [vil-Cre-ERT2(+); Rb(flox/flox)] are referred to as iRb-IKO, whereas littermates lacking Cre [vil-Cre-ERT2(-); Rb(flox/flox)] were used as WT controls. Cre recombinase expression was induced by intraperitoneal injection of 1 mg/100 μl tamoxifen (Sigma LifeSciences T5648–5G; Sigma, St. Louis, MO) 1 mg/day for 3 days, as previously described (7). All mice were housed in a WUSM animal facility and kept on a 12-h:12-h light/dark schedule.
Real-time quantitative PCR.
RNA was prepared from harvested ileal crypts and villi as previously described (8) and were homogenized in lysis buffer (RNAqueous kit; Ambion, Austin, TX). The RNA was extracted according to kit instructions and stored at −80°C. Total RNA concentration was determined using a NanoDrop Spectrophotometer (ND-1000; NanoDrop Technologies, Wilmington, DE). CD36, microsomal triglyceride transfer protein (MTTP), diacylglycerol O-acyltransferase 2 (DGAT2), Apolipoprotein B, ATP-Binding Cassette subfamily G member 5 (ABCG5), and ABCA1 primers were obtained from Life Technologies (Carlsbad, CA). β-Actin was used as the endogenous control (Applied Biosystems, Foster City, CA), and whole bowel RNA was used as a calibrator. Real-time PCR reagents were obtained from Applied Biosystems (Foster City, CA), and an Applied Biosystems 7500 Fast Real-Time PCR system was used to obtain relative RNA expression. All RT-PCR results are normalized to the β-actin endogenous control for each sample and shown relative to the whole bowel calibrator (= 1, which is not presented on the graph) for each gene studied. Three separate WT or Rb-IKO samples were used for gene expression analysis by Agilent microarray (Santa Clara, CA) in our Washington University DDRDC core facility. Array results were analyzed using Partek Genomics Suite software (Partek, St. Louis, MO).
Cholesterol and fat absorption studies.
Cholesterol was measured by fecal dual-isotope ratio method (29). Mice were gavaged with 150 μl of corn oil mixed with 1 μCi of [14C] cholesterol and 2 μCi of [3H] β-sitostanol and then individually housed in metabolic cages. Feces were collected over 2 days, and the ratio of 14C to 3H in each fecal sample was determined (29). Fecal fat was measured at a separate time from cholesterol absorption. Mice were again placed in individual metabolic cages. After 3 days of acclimation, food intake was measured, and feces were collected for a 2–3-day period for all animals. One gram of dried feces was solubilized in water and extracted in 40 ml of a 2:1 chloroform/methanol solution. After extraction, lipid content of the feces was determined gravimetrically. Lipid mass was normalized to food consumption to determine percent fat absorption (21).
Body composition analysis and metabolic rate measurements.
Body composition measurements were recorded on awake mice using magnetic resonance imaging (MRI; EchoMRI 3–1, Echo Medical Systems, Houston, TX). Oxygen consumption and carbon dioxide production (used to calculate respiratory quotient, RQ) as well as energy expenditure were determined using an Oxymax Indirect Calorimeter (Columbus Instruments, Columbus, OH). Mice were monitored individually in the Oxymax chamber for 24-h periods, during which they had free access to food and water.
Small bowel harvest and enterocyte isolation.
A 2-cm segment of tissue was removed 12 cm proximal to the terminal ileum and fixed for histology in 10% neutral-buffered formalin. The next 10-cm segment of ileum was cut open and transferred into tubes containing 5 ml of ice-cold PBS with protease inhibitors for Western blot analysis. Crypts and villi were separated from the resected intestine using our previously described technique of enterocyte isolation involving calcium chelation and mechanical dissociation (8).
Histology.
All histological measurements were performed by one investigator, who was blinded with regard to mouse strain. Five-micron-thick longitudinal sections of paraffin-embedded tissue sections were mounted on glass slides. Sections were stained with hematoxylin and eosin and used to measure villus height and crypt death with a video-assisted computer program (Metamorph; UIC, Dowington, PA). At least 20 well-oriented crypts and villi were counted per slide. Crypts were counted only if the crypt-villus junctions on both sides of the crypt were intact and if Paneth cells were present at the base of the crypt. Villi were counted only if the central lymphatic channel extended from the villus base to the tip and if the mucosal surface was in continuity with an intact crypt.
Western blotting.
Isolated villus samples were lysed with sodium dodecyl sulfate sample buffer. The lysate was then heated for 5 min at 100°C, and the protein concentration was determined by using the RC-DC kit (Bio-Rad, Hercules, CA). Proteins were loaded in equal amounts for Western blotting. Rb (5541436; BD Pharmingen, San Diego, CA) and actin (Cell Signaling Technology, Danvers, MA) antibodies were used.
Enterocyte proliferation and immunohistochemical analysis.
Ninety minutes before death, mice were given an intraperitoneal injection of 5-bromodeoxyuridine (BrdU; 0.1 ml/10 g body wt; Zymed Laboratories, San Francisco, CA). Formalin-fixed tissue sections were embedded in paraffin, sectioned, deparaffinized, and blocked with 3% hydrogen peroxide in methanol. Antigen retrieval was performed using Diva Decloaking solution (Biocare Medical, Concord, CA) (120°C for 5 min followed by 100°C for 10 min). Slides were blocked with avidin-pink and biotin-blue (Biocare Medical), treated with anti-BrdU antibody (1:500; Accurate Chemical, Westbury, NY) in DaVinci Green (Biocare Medical), stored overnight at 4°C, and visualized with biotinylated goat anti-rat IgG (Accurate Chemical) followed by streptavidin-horseradish peroxidase (Invitrogen, Camarillo, CA), diaminobenzidine (Sigma-Aldrich), and hematoxylin counterstaining. The numbers of positively staining cells and the total number of cells per crypt were counted from at least 20 well-oriented crypts by blinded scoring. A proliferative index was calculated from the ratio of these measurements.
SBR.
Mice underwent 50% proximal SBR as previously described (11). Briefly, mice that underwent SBR had transection of the bowel at a point 12 cm proximal to the ileal-cecal junction and also at a point 1 to 2 cm distal to the ligament of Treitz. The mesentery was ligated, and the intervening bowel was removed. Intestinal continuity was restored with an end-to-end anastomosis using 9–0 monofilament suture. In mice undergoing sham operation, the bowel was transected at a point 12 cm proximal to the ileal-cecal junction, and intestinal continuity was restored with an end-to-end reanastomosis. For 90% resection, mice underwent transection of the bowel ∼3 cm proximal to the ileal-cecal junction and the intestine resected to a point 1–2 cm distal to the ligament of Treitz (28). After the operation, mice were provided free access to water for the first 24 h after surgery. Mice were then fed with standard liquid diet (PMI Micro-Stabilized Rodent Liquid Diet LD 101; TestDiet, Richmond, IN) until postoperative day 7 and then switched to HFD for 33 wk.
Statistics.
For most experiments, means were calculated and compared using a Student's t-test. For comparison of weight gain over time, ANCOVA was performed (1.10; statistiXL, Nedlands, Western Australia). Values in the text are means ± SE. Differences were considered significant at P ≤ 0.05.
RESULTS
Rb-IKO mice have reduced expression of genes involved in lipid absorption and digestion and exhibit reduced fat absorption.
We initially performed an Agilent 44k microarray of mRNA expression in villus epithelial cells harvested from Rb-IKO and WT mice. Whereas there were no significant alterations (i.e., less than 2-fold, P < 0.05, data not shown) in the expression of mRNAs encoding genes involved in protein or carbohydrate transport or digestion, the expression of mRNAs encoding genes involved in lipid absorption/transport was significantly reduced in Rb-IKO mice. These findings were confirmed using RT-PCR (Fig. 1). CD36 and MTTP mRNA expression decreased ∼70% and 30%, respectively, whereas ABCA1 and ABCG5 mRNA expression decreased 70% and 50%, respectively, when compared with WT mice.
Fig. 1.
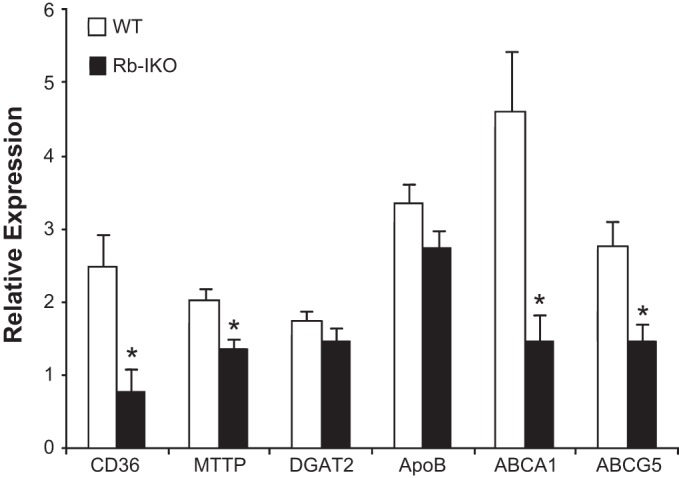
mRNA expression of CD36, microsomal triglyceride transporter protein (MTTP), diacylglycerol O-acyltransferase 2 (DGAT2), Apolipoprotein B (ApoB), ATP-Binding Cassette subfamily A member 1 (ABCA1), and ABCG5 in villus enterocytes as analyzed by RT-PCR. β-Actin was used as the endogenous control, and samples were calibrated to whole bowel signal. Mice were unoperated and on high-fat diet for 1 yr. WT, wild-type mice (n = 12); Rb-IKO, intestinal retinoblastoma protein (Rb) knockout mice (n = 9). *P < 0.05.
Consistent with our findings of diminished expression of several genes involved in lipid absorption, we found that Rb-IKO mice exhibited subtle but significantly increased fecal fat excretion compared with their WT littermates on regular chow (4.3% ± 0.7 vs. 3.6% ± 0.2, P < 0.05).
Impaired lipid absorption in Rb-IKO mice does not prevent HFD-induced obesity.
Because the Rb-IKO mice demonstrated both reduced fat absorption and attenuated mRNA expression of genes involved with intestinal lipid transport, we hypothesized that this may have a protective effect against diet-induced obesity, defined as excessive weight gain relative to the age of the mice. In other words, it was expected that Rb-IKO mice would not gain as much weight as WT littermates when placed on an HFD. Although food intake was not different between the groups (data not shown), Rb-IKO mice gained weight at the same rate as WT mice over the course of nearly a year (Fig. 2A). In the face of this finding, Rb-IKO mice again demonstrated decreased fat absorption and increased fecal fat (Table 1) as well as decreased cholesterol absorption (not shown).
Fig. 2.
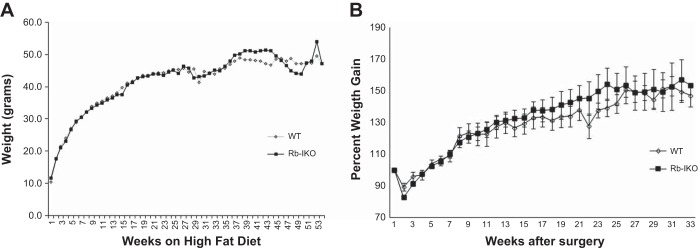
Body weight measurements while on a high-fat diet (42% kcal fat). A: weight on unoperated mice. WT (n = 11), Rb-IKO (n = 8). No statistical differences existed at any time point. B: percent weight gain in mice after small bowel resection. WT (n = 8), Rb-IKO (n = 10). No statistical differences existed at any time point.
Table 1.
Fat absorption, indirect calorimetry, and body composition measurements in unoperated and operated mice on HFD
| Unoperated |
50% Small Bowel Resection |
|||
|---|---|---|---|---|
| WT (n = 11) | Rb-IKO (n = 8) | WT (n = 8) | Rb-IKO (n = 10) | |
| Fat absorption, % | 95.6 ± 0.2 | 92.5 ± 0.8* | 88.6 ± 0.5* | 85.4 ± 0.8†‡ |
| Fecal fat, % | 4.6 ± 0.2 | 7.9 ± 0.8* | 12.7 ± 1.1* | 13.8 ± 0.9‡ |
| Respiratory quotient | 0.7 ± 0.02 | 0.7 ± 0.03 | 0.8 ± 0.3 | 0.8 ± 0.3 |
| Energy expenditure, kcal·h−1·g−1 | 0.01 ± 0.0006 | 0.01 ± 0.001 | 0.02 ± 0.006 | 0.02 ± 0.005 |
| Body fat, % | 41.7 ± 2.8 | 44.8 ± 3.3 | 23.9 ± 1.2* | 27.0 ± 1.9‡ |
| Lean mass, % | 56.6 ± 2.2 | 55.0 ± 3.6 | 75.6 ± 1.3* | 72.5 ± 1.9‡ |
Values are means ± SE. Studies were conducted on unoperated mice after 7 mo of high-fat diet (HFD, 42% kcal fat). Fat absorption studies on operated mice were conducted on postoperative week 3, and indirect calorimetry and body composition studies were measured on operated mice after postoperative week 10. Small bowel resection (SBR) represents a 50% proximal resection with primary anastomosis. WT, wild-type mice; Rb-IKO, intestinal Retinoblastoma protein knockout mice.
P < 0.05 compared with unoperated WT;
P < 0.05 compared with 50% SBR WT;
P < 0.05 compared with unoperated Rb-IKO.
Rb-IKO and WT mice exhibit similar metabolic rate and body composition.
Because Rb-IKO mice continued to gain the same amount of weight as WT mice despite impaired fat absorption, we employed indirect calorimetry to determine whether Rb-IKO and WT mice differed in baseline energy expenditure. No differences were found with regard to 24-h energy expenditure or respiratory quotient (Table 1). Furthermore, we analyzed body composition with the expectation that Rb-IKO mice would have decreased body fat compared with WT attributable to their fat malabsorption. Instead, we found that percent body fat and lean mass were equivalent in both groups (Table 1). At the end of the experimental period, there were no differences in body weight between Rb-IKO and WT mice. These findings suggest that, despite the small but statistically significant decrease in fat absorption efficiency in Rb-IKO mice, there are no obvious effects on overall energy capture or expenditure.
These Rb-IKO and WT mice were harvested after a year of HFD. Disrupted expression of Rb was confirmed by Western blot (Fig. 3A). Furthermore, histological examination confirmed that Rb-IKO mice manifest the expected hyperplastic phenotype with increased villus height and increased rates of enterocyte proliferation (Fig. 3, B and C).
Fig. 3.
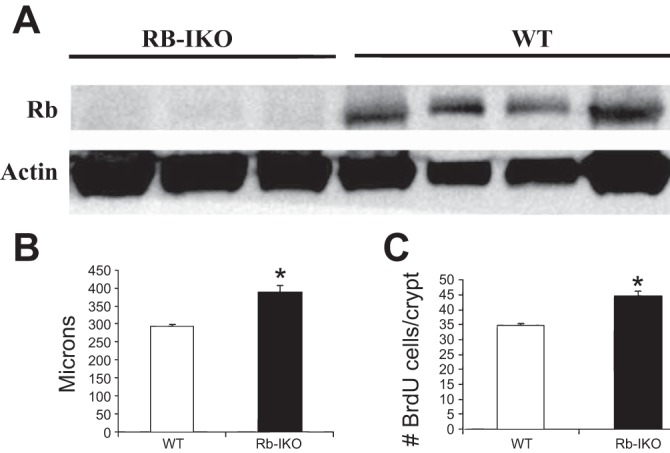
A: disrupted Rb protein expression is confirmed in Rb-IKO mice and present in WT mice. Western blot of protein from ileal villus enterocytes probed for Rb. Actin was used as protein-binding control. Villus height (B) and rates of enterocyte proliferation (C) as measured by percent of 5-bromodeoxyuridine (BrdU)-positive cells per total number of crypt cells. Samples from unoperated mice after 1 yr of high-fat diet. WT (n = 11), Rb-IKO (n = 8). *P < 0.05.
Similar adaptive responses to small bowel resection in Rb-IKO and WT mice.
To further explore the adaptive mechanisms in play with regard to intestinal function in Rb IKO mice, we undertook 50% SBR to determine whether the acute response to diminished intestinal length would magnify differences in intestinal function by genotype. Our previous work had shown that, whereas WT mice adapt in response to SBR with a usual 20–25% increase in villus height, Rb-IKO mice exhibit (at baseline) taller villi yet do not undergo further structural adaptation in response to intestinal resection (13).
There were no differences in either average daily food consumption (data not shown) or weight gain over time between Rb-IKO mice and controls (Fig. 2B). At postoperative week (POW) 3, fat absorption was again impaired in Rb-IKO mice (Table 1). Fat absorption was also significantly decreased in these resected mice compared with unoperated mice. By POW 10, a trend of decreased fat absorption in Rb-IKO mice was still evident; however, the difference was no longer statistically significant (not shown). Respiratory quotient and energy expenditure were also similar in both groups of resected mice, as was body composition (Table 1). Whereas respiratory quotient and energy expenditure were no different between resected and unoperated mice, percent body fat was significantly decreased with a corresponding increase in percent lean mass in resected mice (Table 1). Although Rb-IKO mice had not previously shown adaptive growth after SBR on normal chow (13), we found that feeding the mice an HFD postoperatively resulted in significant increases in villus height in both Rb-IKO and WT mice (Fig. 4).
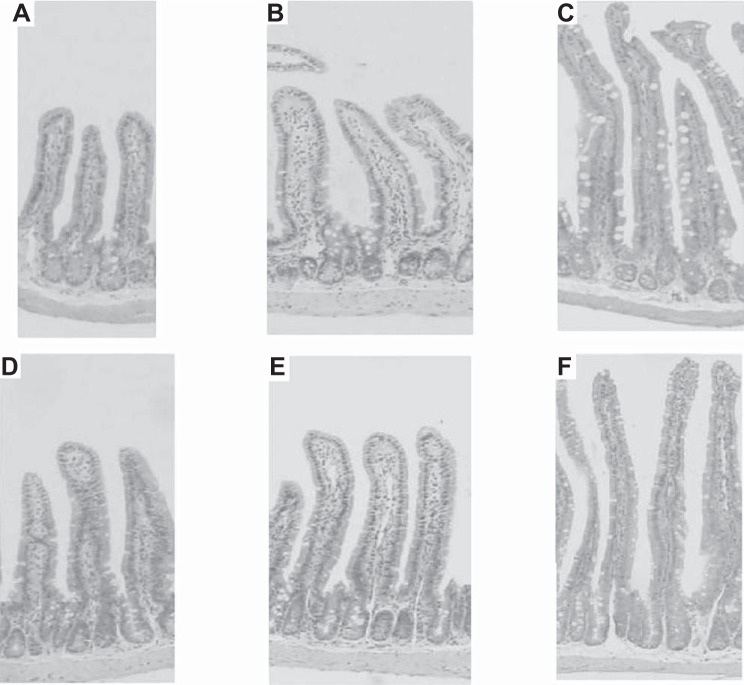
Fig. 4.
Ileal histology at ×4 magnification. Unoperated (A), 50% small bowel resection (SBR) on regular diet (B), 50% SBR on high-fat diet (C) of WT mice as well as unoperated (D), 50% SBR on regular diet (E), and 50% SBR on high-fat diet (F) of Rb-IKO mice. SBR represents a 50% proximal resection with primary anastomosis.
Because we did not demonstrate a significant difference in phenotype between the two groups of mice after 50% SBR, we performed 90% SBR on Rb-IKO mice and WT littermates to see whether a dramatic reduction in bowel length would reveal any significant differences. Survival was equally poor in both groups, which is consistent with our previous experience with such significant loss of bowel length (28). However, postoperative weight, fat absorption, or body composition were again similar in both genotypes (data not shown).
Attenuated fat absorption following inducible intestinal Rb deletion.
To further confirm the role of short-term (vs. from birth) intestinal Rb deletion in fat absorption, we generated an inducible intestinal Rb knockout strain (iRb-IKO). One month after Tamoxifen injection, we conducted fat absorption studies. As in the constitutive Rb-IKO, we observed decreased fat absorption (91.2% ± 0.7 vs. 93.5% ± 0.2, P < 0.05) in iRb-IKO mice compared with WT. We confirmed that these iRb-IKO mice exhibited augmented villus height and attenuated CD36 mRNA expression in villus enterocytes (data not shown). Absent Rb protein in villus enterocytes was also confirmed by Western blot (not shown).
DISCUSSION
In the present study, we have found that intestine-specific deletion of Rb results in subtle fat malabsorption with an associated decrease in CD36, MTTP, ABCA1, and ABCG5 mRNA expression. However, this degree of fat malabsorption was not protective against HFD induced-obesity. We could not attribute this disparity to alterations in metabolic rate or body composition. These results were reproduced when Rb was inducibly deleted in the intestinal epithelium of adult mice (iRb-IKO). Furthermore, stressing these Rb-IKO mice with SBR did not elicit a metabolic disadvantage. Rather Rb-IKO mice exhibited enhanced adaptation in response to HFD despite relative fat malabsorption at baseline. Together, our data suggest that Rb perturbs intestinal fat absorption and implies that compensatory mechanisms must be in place to maintain weight gain.
The finding that Rb-IKO mice exhibit fat malabsorption despite a hyperplastic phenotype was surprising. This demonstrates an ability to compensate for this subtle defect in fat absorption in other ways, perhaps by shifting to more distal regions of intestine in Rb-IKO mice. We did not formally examine the regional efficiency of lipid transport in the small intestine by genotype, and so this suggestion will await rigorous confirmation. Nevertheless, although we demonstrated significantly that reduced mRNA expression of several genes involved fat metabolism, the expressions of multiple other intestinal carbohydrate and protein transporter mRNAs were unaffected by Rb deficiency. Although it is possible that the Rb-IKO mice consumed more food to compensate for impaired fat absorption, we found no difference in food intake.
It is worth noting that Rb function has also been implicated in other aspects of metabolism. Rb inhibition is believed to be involved in glutamine metabolism, as cell cultures of mouse embryonic fibroblasts isolated from mice with global Rb deletion were found to have enhanced glutamine uptake (19). Additionally, deletion of Rb is directly and indirectly involved with numerous mediators of the glucose pathway, including enzymes of glycolysis, glutaminolysis, and the electron transport chain (4). Thus it is not unreasonable to believe that there may be alternate enhanced metabolic pathways to compensate for a fat malabsorption.
There have been several studies where targeted mutations within the fat metabolism network have resulted in decreased weight gain when mice are placed on an HFD. MTTP intestinal knockout mice exhibit frank steatorrhea compared with WT controls and also gain significantly less weight even on a low-fat chow diet (30). In regards to the present study, it is possible that the 20–30% reduction in MTTP mRNA expression noted in Rb-IKO mice yielded only subtle effects on fat absorption.
It must be considered that the measurement of fat absorption alone is insufficient to elucidate the total effect on whole body metabolism. There are several examples of mutant mice with normal intestinal fat absorption resulting in protection against diet-induced obesity. l-Fatty acid-binding protein knockout mice have normal fecal fat levels but instead have slower intestinal lipid secretion kinetics, which may contribute to their decreased weight gain in response to HFD compared with WT (17). Tis7 knockout mice also do not exhibit overt alterations in fecal fat or basal metabolic rate but nevertheless manifest decreased weight gain on HFD (32). It is possible that that these changes may be due to intestinal lipid trafficking and hepatic lipid metabolism, as these mice have lower serum and hepatic cholesterol, triglyceride, and free fatty acid levels. Dgat1-null mice are also resistant to diet-induced obesity(23) in a manner that is not attributed to fat malabsorption but rather impaired intestinal TAG absorption as suggested by reduced postabsorptive chylomicronemia (2).
A further novel finding of this study is the reduced CD36 expression in Rb-IKO mice. CD36 is a transmembrane glycoprotein most prominently found on the brush-border membrane and implicated in fatty acid uptake (14, 15, 18). CD36-null mice do not exhibit fat malabsorption although fat absorption was shifted to the distal intestine. CD36-null mice were also protected against diet-induced obesity, possibly due to impaired intestinal lipid secretion (10, 16) The finding that disrupted expression of CD36 itself does not impair net fat absorption but is still protective against diet-induced obesity would suggest that other factors besides CD36 are able to compensate.
We also asked whether Rb-IKO mice exhibited a reduced metabolic rate in the face of decreased fat absorption, as an explanation for the observation of comparable weight gain by genotype. There is a precedent for alterations of metabolic rate in animals with alteration in fat metabolism. Pharmacological inhibition of DGAT1 of rats in the setting of dietary high fat resulted in decreased RQ although energy expenditure was not affected, suggesting an increase in whole body fatty acid oxidation (20). However, we found that intestinal Rb deficiency did not affect rates of either energy expenditure or respiratory quotient. Additionally, although we expected that Rb-IKO mice would have decreased body fat content, our findings revealed that percent body fat and lean mass were similar in both genotypes. It is of course possible that the parameters we chose to examine in relation to whole body energy homeostasis may not discriminate subtle alterations.
We performed SBR to determine whether features of the aforementioned adaptive mechanisms in Rb-IKO mice would be revealed when a significant amount of intestine was removed. However, both genotypes gained the same amount of weight without any alterations in metabolic rate and body composition, and we observed that both WT and Rb-IKO mice demonstrated augmented adaptation after SBR when fed an HFD. There are multiple studies that have demonstrated that enteral fat after SBR results in enhanced villus height after resection beyond what would be seen in normal adaptation (3, 12, 24, 26). This adaptive response in Rb-IKO mice is novel for two reasons: 1) our previous work with Rb-IKO mice after SBR generally failed to show further intestinal villus growth after resection (13), and 2) this HFD stimulated-increased villus height took place despite a baseline defect in fat absorption. These findings together underscore the influence of HFD in the structural adaptation response of the remnant bowel to massive SBR.
We have also found that fat absorption is decreased after SBR in both WT and Rb-IKO mice compared with their unoperated counterparts despite the presence of HFD. This is in contrast to the findings of Sukhotnik et al. (25), who found rats given a 50% kcal fat diet after a mid-75% SBR had increased fat absorption compared with sham-operated animals. It is possible that differences in procedure may have contributed to these disparate results including a different operation (mid vs. proximal SBR), diverse HFDs, and variation in the fat absorption studies. Sukhotnik also used sham-operated rats on a normal chow diet, whereas we have made comparisons between resected and unresected mice on HFD. Additionally, we found that resected mice had greater lean mass and less body fat than unresected mice. This is consistent with our finding of decreased fat absorption after SBR.
In summary, intestinal Rb deficiency is associated with an expanded mucosal surface area, attenuated expression of several genes involved with fat absorption, and a subtle defect in intestinal fat absorption. Despite these features, weight gain and body composition are indistinguishable following HFD feeding as well as after surgical removal of a significant length of small bowel. These findings together underscore the complexity of the adaptive pathways in intestinal lipid metabolism. Although we have not established causality in that it is possible that the fat malabsorption may have caused adaptive villus growth, our results also suggest that taller villi and expanded mucosal surface area are not necessarily associated with enhanced capacity for nutrient absorption.
GRANTS
This work was supported by National Institutes of Health Grants T32 GM008795 (P. Choi), HL-38180, DK-56260, and P30DK52574 (N. Davidson), Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine, and the Children's Surgical Sciences Institute of the St. Louis Children's Hospital Foundation. Dr. Choi is also supported by The Marion and Van Black Endowed Pediatric Surgical Research Fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.M.C., J.G., C.R.E., W.S.W., J.A.L., and Y.X. performed experiments; P.M.C. analyzed data; P.M.C., N.O.D., and B.W.W. interpreted results of experiments; P.M.C. prepared figures; P.M.C. drafted manuscript; J.G., C.R.E., N.O.D., and B.W.W. edited and revised manuscript; N.O.D. and B.W.W. conception and design of research; B.W.W. approved final version of manuscript.
REFERENCES
- 1.Attwooll C, Lazzerini Denchi E, Helin K. The E2F family: specific functions and overlapping interests. EMBO J 23: 4709–4716, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV., Jr DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem 277: 25474–25479, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Choi PM, Sun RC, Guo J, Erwin CR, Warner BW. High-fat diet enhances villus growth during the adaptation response to massive proximal small bowel resection. J Gastrointest Surg 18: 286–294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clem BF, Chesney J. Molecular pathways: regulation of metabolism by RB. Clin Cancer Res 18: 6096–6100, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Cobrinik D. Pocket proteins and cell cycle control. Oncogene 24: 2796–2809, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene 24: 2810–2826, 2005 [DOI] [PubMed] [Google Scholar]
- 7.el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Guo J, Longshore S, Nair R, Warner BW. Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J Biol Chem 284: 134–140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haigis K, Sage J, Glickman J, Shafer S, Jacks T. The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J Biol Chem 281: 638–647, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56: 1872–1880, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg 183: 441–449, 1996 [PubMed] [Google Scholar]
- 12.Kollman KA, Lien EL, Vanderhoof JA. Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastroenterol Nutr 28: 41–45, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Leinicke JA, Longshore S, Wakeman D, Guo J, Warner BW. Regulation of retinoblastoma protein (Rb) by p21 is critical for adaptation to massive small bowel resection. J Gastrointest Surg 16: 148–155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobo MV, Huerta L, Ruiz-Velasco N, Teixeiro E, de la Cueva P, Celdran A, Martín-Hidalgo A, Vega MA, Bragado R. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J Histochem Cytochem 49: 1253–1260, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 282: 19493–19501, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 131: 1197–1207, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology 44: 1191–1205, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Poirier H, Degrace P, Niot I, Bernard A, Besnard P. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). FEBS J 238: 368–373, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Reynolds MR, Lane AN, Robertson B, Kemp S, Liu Y, Hill BG, Dean DC, Clem BF. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 33: 556–566, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schober G, Arnold M, Birtles S, Buckett LK, Pacheco-Lopez G, Turnbull AV, Langhans W, Mansouri A. Diacylglycerol acyltransferase-1 inhibition enhances intestinal fatty acid oxidation and reduces energy intake in rats. J Lipid Res 54: 1369–1384, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz M, Lund EG, Setchell KD, Kayden HJ, Zerwekh JE, Bjorkhem I, Herz J, Russell DW. Disruption of cholesterol 7alpha-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J Biol Chem 271: 18024–18031, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell 2: 103–112, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25: 87–90, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Sukhotnik I, Mor-Vaknin N, Drongowski RA, Miselevich I, Coran AG, Harmon CM. Effect of dietary fat on early morphological intestinal adaptation in a rat with short bowel syndrome. Pediatr Surg Int 20: 419–424, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Sukhotnik I, Mor-Vaknin N, Drongowski RA, Coran AG, Harmon CM. Effect of dietary fat on fat absorption and concomitant plasma and tissue fat composition in a rat model of short bowel syndrome. Pediatr Surg Int 19: 385–390, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Vanderhoof JA, Park JH, Herrington MK, Adrian TE. Effects of dietary menhaden oil on mucosal adaptation after small bowel resection in rats. Gastroenterology 106: 94–99, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Vidal A, Carneiro C, Zalvide JB. Of mice without pockets: mouse models to study the function of Rb family proteins. Front Biosci 12: 4483–4496, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Wakeman D, Longshore SW, McMellen ME, Santos JA, Guo J, Erwin CR, Warner BW. Extent of small bowel resection does not influence the magnitude of intestinal adaptation in the mouse. J Pediatr Surg 45: 1274–1279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang DQ, Paigen B, Carey MC. Genetic factors at the enterocyte level account for variations in intestinal cholesterol absorption efficiency among inbred strains of mice. J Lipid Res 42: 1820–1830, 2001 [PubMed] [Google Scholar]
- 30.Wikenheiser-Brokamp KA. Retinoblastoma family proteins: insights gained through genetic manipulation of mice. Cell Mol Life Sci 63: 767–780, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem 281: 4075–4086, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Yu C, Jiang S, Lu J, Coughlin CC, Wang Y, Swietlicki EA, Wang L, Vietor I, Huber LA, Cikes D, Coleman T, Xie Y, Semenkovich CF, Davidson NO, Levin MS, Rubin DC. Deletion of Tis7 protects mice from high-fat diet-induced weight gain and blunts the intestinal adaptive response postresection. J Nutr 140: 1907–1914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng L, Lee WH. The retinoblastoma gene: a prototypic and multifunctional tumor suppressor. Exp Cell Res 264: 2–18, 2001 [DOI] [PubMed] [Google Scholar]