Abstract
The intestinal microbiota plays a pivotal role in maintaining human health and well-being. Previously, we have shown that mice deficient in the brush-border enzyme intestinal alkaline phosphatase (IAP) suffer from dysbiosis and that oral IAP supplementation normalizes the gut flora. Here we aimed to decipher the molecular mechanism by which IAP promotes bacterial growth. We used an isolated mouse intestinal loop model to directly examine the effect of exogenous IAP on the growth of specific intestinal bacterial species. We studied the effects of various IAP targets on the growth of stool aerobic and anaerobic bacteria as well as on a few specific gut organisms. We determined the effects of ATP and other nucleotides on bacterial growth. Furthermore, we examined the effects of IAP on reversing the inhibitory effects of nucleotides on bacterial growth. We have confirmed that local IAP bioactivity creates a luminal environment that promotes the growth of a wide range of commensal organisms. IAP promotes the growth of stool aerobic and anaerobic bacteria and appears to exert its growth promoting effects by inactivating (dephosphorylating) luminal ATP and other luminal nucleotide triphosphates. We observed that compared with wild-type mice, IAP-knockout mice have more ATP in their luminal contents, and exogenous IAP can reverse the ATP-mediated inhibition of bacterial growth in the isolated intestinal loop. In conclusion, IAP appears to promote the growth of intestinal commensal bacteria by inhibiting the concentration of luminal nucleotide triphosphates.
Keywords: gut flora, microbiotal homeostasis, dysbiosis, intestinal loop model, CpG DNA, flagellin, lipopolysaccharides
humans and other metazoans have developed a symbiotic relationship with commensal microbiota that provide enormous benefits to the host including vitamin synthesis, food digestion, gut development, immunity, and resistance to pathogenic infections (6, 18, 19, 63). Under normal conditions, ∼1014 bacteria made up of 300–1,000 different species inhabit the human gut (37). Dysbiosis is defined as dysregulation of the normal homeostasis of the intestinal microbiota resulting in an imbalance of the number and composition of intestinal microbes (15, 37). Dysbiosis has been implicated in the pathogenesis of a myriad of disease conditions, including antibiotic-associated diarrhea (AAD), Clostridium difficile-associated disease, inflammatory bowel disease (IBD), metabolic syndrome, obesity, and cancer (3, 32, 33, 54, 59). Over the past years, probiosis, prebiosis, and fecal biotherapy have emerged as therapeutic approaches for the prevention and treatment of dysbiosis-associated diseases (3, 22, 47, 50, 51). Probiotics are live organisms that provide health benefits and mostly include Lactobacillus species, Bifidobacterium species, Escherichia coli, Saccharomyces boulardii, and Trichuris suis (10, 38, 50, 52, 53). Although clinical trials of probiotics in some instances of dysbiosis have shown promising results, the efficacy of these live organisms appears to be quite limited and there is concern about occasional harmful side effects (3, 5, 43, 46, 57).1
Prebiotics are defined as nondigestible food ingredients that promote the growth of bacteria, stimulate host immunity, prevent pathogenic infections, and facilitate host metabolism and mineral absorption (10, 11, 13, 30, 47, 51, 52). Some examples of prebiotics are xylooligosaccharides, galactooligosaccharides, oligofructose, lactulose, inulin, and pomegranate extracts (10, 11, 13, 27, 36, 51, 52, 55). Prebiotics may be beneficial to healthy persons, but they are not generally used in clinically ill patients (45, 60). Up until now, an endogenous factor that regulates the growth of a wide spectrum of gut bacteria has not been identified.
We have previously demonstrated that the brush-border enzyme intestinal alkaline phosphatase (IAP) preserves the normal homeostasis of intestinal microbiota (37). We found that compared with their wild-type (WT) littermates, IAP knockout (KO) mice harbor decreased numbers of both aerobic and anaerobic bacteria. Furthermore, WT mice receiving oral IAP supplementation and an antibiotic were found to rapidly restore the normal microbiota upon termination of antibiotic treatment, thus reducing the antibiotic-induced susceptibility to enteric pathogens such as Salmonella typhimurium and Clostridium difficile (1). On the basis of the above observations, we hypothesized that IAP functions as an endogenous bacterial growth-promoting factor. In the present study we sought to delineate this role of IAP and to define its underlying mechanisms of action. Here, we report that IAP is indeed an endogenous factor that preferentially favors the growth of specific bacterial species, and, furthermore, these effects occur because of IAP-mediated reduction of the intestinal luminal concentrations of ATP and probably other luminal nucleotide triphosphates. We believe that orally administered IAP could represent a novel treatment against dysbiosis in humans.
MATERIALS AND METHODS
Chemicals.
DNA isolation kit was purchased from Qiagen (Valencia, CA). Bovine IAP, ARL 67156 trisodium salt hydrate, LPS, ATP, ADP, AMP, GTP, CTP, TTP, UTP, p-nitrophenyl phosphate (pNPP), l-phenylalanine (Phe), ampicillin, and streptomycin were obtained from Sigma-Aldrich (St. Louis, MO). ATP analog 6-N,N-diethyl-d-β-γ-dibromomethylene ATP (ARL 67156), a selective inhibitor of ecto-ATPases, was also obtained from Sigma. CpG DNA and flagellin were obtained from Invivogen (San Diego, CA). ENLITEN ATP Assay System Bioluminescence Detection Kit was from Promega (Madison, WI). Isoflurane was obtained from Baxter (Deerfield, IL). dATP, dGTP, dCTP, dTTP, Luria-Bertani (LB) broth, LB agar, Brain Heart Infusion (BHI) broth, Brucella agar (5% horse blood) plates were purchased from Fisher Scientific (Pittsburgh, PA). The CO2-producing gas pack and anaerobic condition indicators were also obtained from Fisher Scientific.
Mice.
The construction of C57Bl/6 IAP-KO (Akp3−/−) mice (Mus musculus) has previously been described (41). Heterozygous mice were obtained from the Sanford Burnham Medical Research Institute (La Jolla, CA). These animals were subsequently bred at the Massachusetts General Hospital (MGH) animal facility to create homozygous IAP-KO, heterozygous, and wild-type C57Bl/6 (WT) littermates. Confirmation of genotype was performed by PCR analysis (41). The mice were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School (Boston, MA) and those prepared by the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council [Department of Health, Education and Human Services, publication no. 85e23 (National Institute of Health), revised 1985]. The animal experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at MGH.
Bacterial culture.
We observed that normal mouse stool does not contain any streptomycin or ampicillin-resistant aerobic bacteria. Therefore, for our experiments, where we wanted to use such bacteria in an intestinal loop model (see below; In vivo intestinal loop model) we selected representative gram-negative (Escherichia coli and Morganella morganii) and gram-positive (Enterococcus faecalis) bacteria from the normal gut flora and selected for streptomycin or ampicillin resistance. Spontaneous streptomycin-resistant E. coli and M. morganii mutants were isolated by culturing the bacterial sample in LB broth medium containing streptomycin (100 μg/ml) followed by subculturing the grown bacteria on MacConkey plates containing streptomycin (100 µg/ml). Spontaneous mutations in the rpsL gene render the bacteria streptomycin resistant and they are phenotypically stable (24). Similarly, ampicillin-resistant E. faecalis was acquired by growing the native E. faecalis in a culture containing ampicillin (100 µg/ml). To ensure the absence of possible contaminants, the phenotype of each bacterial species was confirmed by the Clinical Laboratory of MGH.
Streptomycin-resistant gram-negative bacteria (E. coli and M. morganii) were grown in LB broth and MacConkey agar plates containing streptomycin (100 μg/ml). Similarly, ampicillin-resistant bacteria (E. faecalis) were grown in LB broth and LB agar plates containing ampicillin (100 μg/ml). For stool culture, mouse stool samples were collected fresh directly in BHI media (500 μl) in a microfuge tube, kept on ice, and then vortexed to homogenize the sample, followed by serial dilution and plating on LB agar and Brucella (5% horse blood) agar plates. Plates were incubated in ambient air overnight at 37°C for aerobic bacterial growth. For anaerobic bacterial growth, plates were incubated in a sealed plastic bag containing CO2-producing gas pack and anaerobic condition indicators (Fisher Scientific) for 3 days at 37°C. Samples were plated on the bench and then placed in the plastic bag. The color change of the anaerobic indicator was strictly monitored. Colony forming units (CFU) were counted and expressed as mean CFU ± SE.
The concentrations of IAP targets LPS, CpG DNAs and flagellin used in bacterial cultures were 10 μg/ml, 10 μg/ml, and 100 ng/ml, respectively. Concentrations of ATP and other nucleotides were 10 mM unless otherwise specified. In an experiment showing IAP-mediated reversal of growth inhibitory effect of ATP (10 mM) on E. coli, S. typhimurium, S. aureus, and L. monocytogenes, these bacteria were grown in LB broth containing 10 μl/ml (100 U/ml) of bovine IAP (Sigma) or 10 μl/ml “vehicle for IAP” [50 mM KCl, 10 mM Tris·HCl (pH 8.2), 1 mM MgCl2, 0.1 mM ZnCl2, and 50% glycerol, also known as IAP storage buffer] (control group). All aerobic cultures (200 μl) were grown in 8 wells of a 96-well clear-bottom plate at 37°C in a shaking incubator, and absorbance (OD600) was determined hourly or as indicated. Bacterial growth in a culture at a definite time point was calculated as average absorbance (OD600) ± SD.
In vivo intestinal loop model.
WT and IAP-KO mice (n = 5 per group unless indicated otherwise) each weighing ∼25 g were used. The procedure was done under general anesthesia with inhalational isoflurane at 2% and 1 l/min maintenance flow of oxygen. After a midline incision and exteriorization of the gut, a 5-cm segment of proximal jejunum, distal ileum, or colon was carefully tied off at the proximal and distal ends, thus constructing the loop. One animal was used to construct only one loop. When indicated, mice (n = 5 per group) were fasted for 14 or 48 h to repress the expression of IAP as previously described (17) followed by construction of the loop. Using a 28-G insulin needle, we injected (total of 100 μl) into the loop ∼1,000 CFU of specific bacteria along with other reagents [ATP (100 μM), IAP (100 U/ml), ARL 67156 (10 mM)] as indicated. The abdomen of the mouse was closed and the bacteria were incubated in the isolated loop for 2 h, while the animal was still under anesthesia. The loop was then harvested and the animal was euthanized. The luminal contents were squeezed off, the loop was homogenized, and both were plated on selective agar plates and grown overnight at 37°C. Bacterial growth was calculated as fold increase, i.e., the total number of bacteria (CFU) in loop (luminal contents + luminal tissue) divided by the number of instilled bacteria.
IAP assay.
IAP activity was determined following the protocol as previously described (37). Briefly, contents of a loop were collected by gentle squeeze and centrifuged, and supernatant was collected. Twenty-five microliters of the supernatant (or aqueous IAP solution as a control) were mixed with 175 μl of phosphatase assay reagent containing 5 mM of pNPP. Optical density at 405 nm was determined ∼10 min later when the reaction samples usually turned yellowish because of release of p-nitrophenol. IAP activity is expressed as average units per milliliter sample ± SE.
Collection of intestinal luminal fluid.
Mice were euthanized and the entire section of intestine was dissected out. Luminal contents were collected by gentle squeezing and then centrifuged at 13,000 g to pellet the debris and bacteria. The supernatant was collected and E. coli bacteria were then added to it and incubated overnight at 37°C.
ATP assay.
ATP concentration was measured with the ENLITEN ATP Assay System Bioluminescence Detection Kit according to the manufacturer's guideline (Promega).
Oral supplementation of l-phenylalanine.
The amino acid Phe inhibits the enzymatic activity of IAP, and when indicated mice (n = 5 per group) were fed 10 mM Phe in autoclaved drinking water.
Statistical analysis.
Statistical significance between two groups was tested by the two-tailed Student's t-test. Statistical significance between more than two groups was tested by one-way ANOVA (analysis of variance). Pillai's trace multivariate test was performed for the repeated-measures ANOVA and P < 0.05 was considered significant. SPSS (version 13, Chicago, IL) software was used for the statistical analyses.
RESULTS
Bacterial growth is inhibited in the jejunal loop of IAP-KO mice.
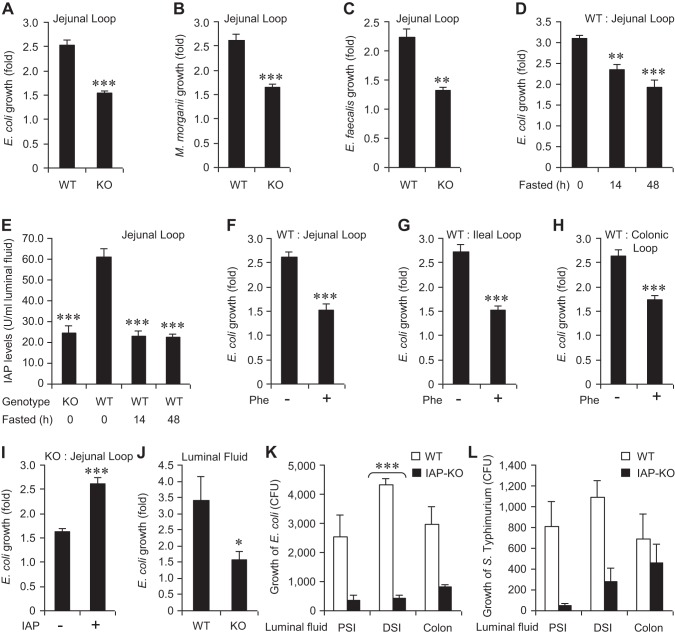
To assess the role of endogenous IAP in a pathophysiologically relevant manner, we employed an in vivo isolated intestinal loop model system. Under general anesthesia, mice underwent laparotomy and a 5-cm segment of the intestine (jejunum, ileum, or colon) was isolated and ligated at both ends to create the loop, with care taken to ensure that the vascular supply to the loop was not compromised. Approximately 1,000 CFU of specific bacteria were instilled into the loop by injection and allowed to incubate in vivo for 2 h. To ensure that we could distinguish from the commensal organisms, we instilled selective antibiotic-resistant strains into the loop (streptomycin-resistant E. coli and M. morganii and ampicillin-resistant E. faecalis). Following the incubation period, the loop was harvested, homogenized, and plated on the appropriate antibiotic-selective plates and grown overnight at 37°C. Figure 1, A and B, shows that growth of both E. coli and M. morganii was significantly higher in the WT compared with IAP-KO jejunal loop (∼1.5-fold differences in growth, P < 0.001 for both species). Similar to the results with these gram-negative species, the growth of E. faecalis, a native gram-positive inhabitant of the gut, was also significantly higher in the WT compared with IAP-KO mice (Fig. 1C). These results suggest that the endogenous IAP exerts a global-type growth promoting effect on these commensal gut bacteria.
Fig. 1.
Lower levels of endogenous intestinal alkaline phosphatase (IAP) reduce bacterial growth. Laparotomy was performed on mice under general anesthesia, a 5-cm loop was constructed, and ∼1,000 colony-forming units (CFU) of a specific bacterial species were instilled by injection (100 μl) into the loop. After 2 h, the loop was dissected out, homogenized, and plated on selective media for overnight growth at 37°C (see materials and methods for details). Bacterial growth in the jejunal loops of wild-type (WT) and IAP-knockout (KO) mice. A: growth of Escherichia coli (n = 10 in each group). B: growth of Morganella morganii (n = 5 in each group). C: growth of Enterococcus faecalis (n = 5 in each group). D: growth of E. coli in the jejunal loop of animals fasted for 14 and 48 h. E: IAP levels in the jejunal loops of animals fasted for 14 and 48 h. Growth of E. coli in the animals treated with phenylalanine (Phe). F: jejunal loop. G: ileal loop. H: colonic loop. I: exogenous IAP (10 U) enhances E. coli growth in the jejunal loop. J: E. coli growth is reduced in the intestinal luminal fluid of IAP-KO mice compared with WT mice. Bacterial growth in the luminal fluid from the proximal small intestine (PSI), distal small intestine (DSI), and colon of IAP-KO mice compared with WT mice. K: growth of E. coli was significantly reduced in DSI luminal fluid of IAP-KO mice compared with WT mice. L: growth of Salmonella typhimurium. Groups of mice were euthanized and the luminal fluid from different segments or the entire intestine of each mouse was collected. Approximately 1,000 CFU of bacteria were added to the fluid and incubated for 2 h at 37°C. Values are expressed as means ± SE. Statistical significance between 2 groups was tested by the 2-tailed Student's t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
Inhibition of endogenous IAP reduces bacterial growth.
To further delineate the impact of the endogenous IAP enzyme on luminal bacterial growth, we examined the effects of either silencing IAP expression or inactivating the IAP protein. We have previously shown that endogenous IAP expression is suppressed in rodents during starvation (14, 17). Accordingly, we fasted mice on a water-only diet for up to 48 h and compared the impact on E. coli growth in the intestinal loop to normally fed mice. Figure 1D shows the progressive inhibitory effects of fasting on the growth of E. coli in a jejunal loop. Compared with control-fed animals, E. coli growth was significantly lower under 14-h and 48-h fasted conditions [3.09 ± 0.09 (fed) vs. 2.35 ± 0.12 (14-h fasted) vs. 1.93 ± 0.16 (48-h fasted) fold increase; P = 0.001 (fed vs. 14-h fasted), P < 0.001 (fed vs. 48-h fasted)]. As expected, jejunal IAP levels were reduced by 70% during fasting (Fig. 1E).
We next investigated the effects of inactivating the endogenous IAP enzyme via treatment with oral Phe (14). Prior to isolating the intestinal loop, mice received 10 mM Phe in their drinking water for 48 h. Compared with the mice receiving no Phe, E. coli growth was significantly reduced in the jejunal loop of mice receiving Phe (2.61 ± 0.10 vs. 1.53 ± 0.12 fold increase, respectively; P < 0.001) (Fig. 1F). Similar reduction of E. coli growth was observed in the ileal loop (2.72 ± 0.14 vs. 1.53 ± 0.07 fold increase, respectively; P < 0.001) (Fig. 1G), as well as in the colonic loop (2.65 ± 0.12 vs. 1.74 ± 0.07 fold increase, respectively; P < 0.001) (Fig. 1H). To further confirm the role of the IAP enzyme we next instilled exogenous IAP into a jejunal loop of IAP-KO mice. We added 100 U of IAP or the vehicle control along with the bacteria into a jejunal loop of IAP-KO mice. As predicted, we observed a significant increase in E. coli growth when exogenous IAP was added to the intestinal loop (2.61 ± 0.12 vs. 1.63 ± 0.07 fold increase, respectively; P < 0.001) (Fig. 1I).
The intestinal luminal fluid from IAP-KO mice inhibits bacterial growth.
The above experiments established that IAP functions as a bacterial growth promoter within the intestine. We have previously shown that IAP does not directly affect the growth of bacteria (37), and therefore we hypothesized that IAP must exert its effects through some indirect mechanism, likely by modulating one or more of its known target molecules within the luminal fluid. To determine whether the luminal fluid itself is capable of promoting bacterial growth we examined the bacterial growth in vitro upon incubation with luminal fluids from WT and IAP-KO mice. The mice were euthanized and luminal fluid from the entire small intestine was gently squeezed out and collected. E. coli bacteria were then added to the luminal fluid and incubated for 2 h at 37°C. Figure 1J shows that E. coli growth was approximately twofold higher in the luminal fluid obtained from WT compared with IAP-KO mice. We then collected luminal fluids from proximal small intestine, distal small intestine, and colon and tested the growth of E. coli (Fig. 1K) and S. typhimurium (Fig. 1L), comparing the fluids from WT and KO mice. The results indicate that fluids from all these intestinal segments contain the growth regulating properties.
ATP inhibits the growth of stool aerobic bacteria in vitro.
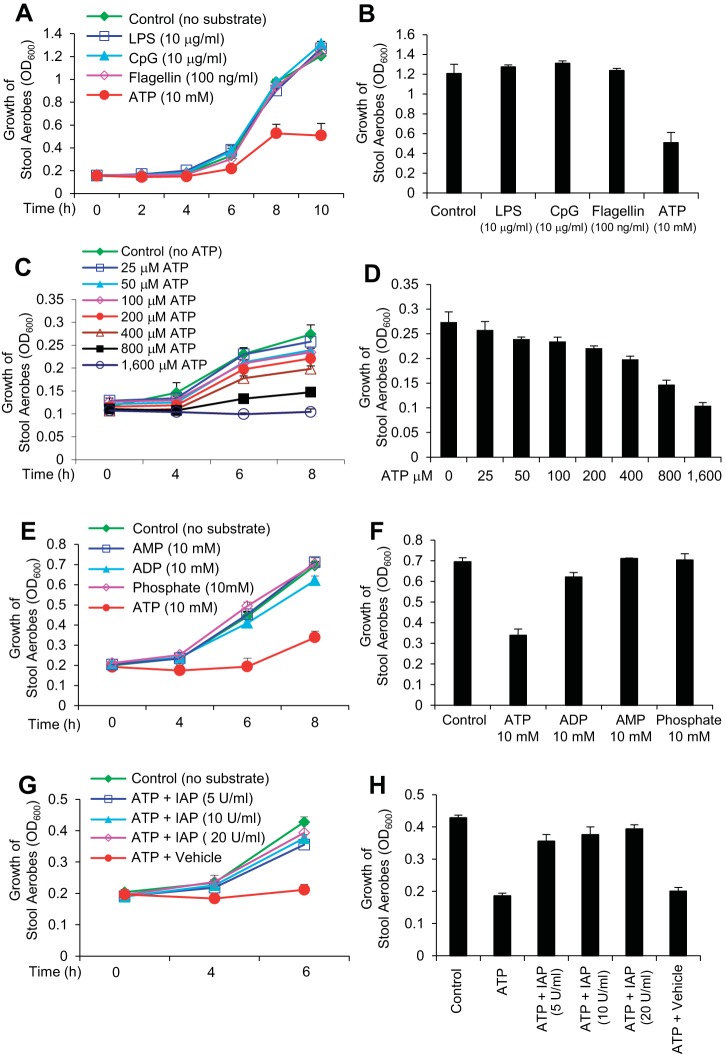
The prebiotic property of the WT luminal fluid along with the absence of any direct effects of IAP on bacterial growth indicated that IAP most probably inactivates the inhibitory effect of one or more of its target molecules on bacterial growth. Accordingly, we tested the growth of mouse stool aerobic bacteria in the presence of high concentrations of four known IAP targets: LPS, CpG DNA, flagellin, and ATP (9, 40). Figure 2A shows that LPS (10 μg/ml), CpG DNA (10 μg/ml), and flagellin (10 ng/ml) had no effect on the growth of aerobic stool bacteria. However, 10 mM ATP caused an ∼50% reduction in bacterial growth. ATP significantly attenuated bacterial growth [repeated-measures ANOVA: effect of time: F(5, 30) = 127.09, P value < 0.001; between-groups differences: F(4, 36) = 3.99, P value < 0.01; interaction of time and group: F(20, 132) = 1.58, P value = 0.07; Tukey's post hoc test: P value < 0.05]. Bacterial growth at the final time point is depicted in Fig. 2B.
Fig. 2.
ATP inhibits the growth of stool aerobic bacteria in vitro. Mouse stool samples were collected fresh directly in Brain Heart Infusion (BHI) media, homogenized, serially diluted and then cultured under aerobic conditions. For aerobic growth, each bacterial culture (200 μl) was grown in 8 wells of a 96-well clear-bottom plate at 37°C in a shaking incubator, and absorbance (OD600) was determined at indicated time points (see materials and methods). A: effects of different targets of IAP on stool aerobes. B: the effect of ATP on bacterial growth was most evident when optical densities (ODs) were compared after 10 h of incubation [1-way ANOVA F(4, 38) = 4.49, P value < 0.01; Tukey's post hoc test: P value < 0.05]. C: dose-response effects of ATP on stool aerobes. D: the highest effect of low concentration of ATP on bacterial growth was evident after 8 h of incubation [1-way ANOVA: F(9, 68) = 49.30, P value < 0.001; Tukey's post hoc test: P value = 0.015 for 200 μM of ATP, P values < 0.001 for 400, 800, 1,600 μM of ATP]. E: effects of ATP derivatives on stool aerobes. F: these effects are also illustrated at the final time point. G: IAP reverses the inhibitory effects of ATP on stool aerobic bacterial growth. H: IAP abolished the effects of ATP and dose dependently increased bacterial growth [1-way ANOVA: F(5, 83) = 13.97, P value < 0.001; Tukey's post hoc test: P values < 0.001]. Values for bacterial growth are expressed as average absorbance (OD600) ± SD. The experiment was repeated at least 3 times showing similar results.
We then determined the dose effects of ATP on a lower inoculum of stool aerobic bacteria and found that ATP inhibited growth even at lower concentrations and such inhibition was dose dependent [repeated-measures ANOVA: effect of time: F(3, 68) = 586.91, P value < 0.001; P value < 0.001; interaction of time and group: F(27, 210) = 4.53, P value < 0.001; Tukey's post hoc test: P value < 0.001 for 400, 800, 1,600 μM of ATP] (Fig. 2C). The highest effect of ATP on bacterial growth was evident after 8 h of incubation (Fig. 2D). As expected, we also observed the dose-dependent inhibitory effect of higher concentrations (mM) of ATP on the growth of higher inoculum of stool aerobic bacteria (data not shown).
We next determined the effects of ATP derivatives on the stool aerobic bacterial growth. The ATP derivatives ADP, AMP, and phosphate exerted little or no inhibition on the growth of the intestinal aerobic flora (Fig. 2, E and F). We also investigated the effects of the ATP derivative adenosine and observed that adenosine has no effect on stool bacterial growth (data not shown).
IAP reverses the inhibitory effect of ATP on aerobic bacterial growth.
The above data established that ATP can inhibit the growth of mouse stool aerobic bacteria in vitro. We next studied whether IAP would be able to reverse these inhibitory effects of ATP and found that, indeed, IAP is able to reverse the effects of ATP (10 mM) in a dose-dependent manner [repeated-measures ANOVA: effect of time: F(2, 77) = 287.97, P value < 0.001; between-groups differences: F(5, 78) = 5.10, P value < 0.001; interaction of time and group: F(10, 156) = 15.21, P value < 0.001; Tukey's post hoc test: P value < 0.05 for 50 U/ml of IAP and P values < 0.01 for 100 and 200 U/ml of IAP] (Fig. 2, G and H). These data suggest that IAP may exert its growth promoting effects by blocking the growth inhibitory effects of its target molecule ATP.
ATP inhibits the growth of stool anaerobic bacteria in vitro.
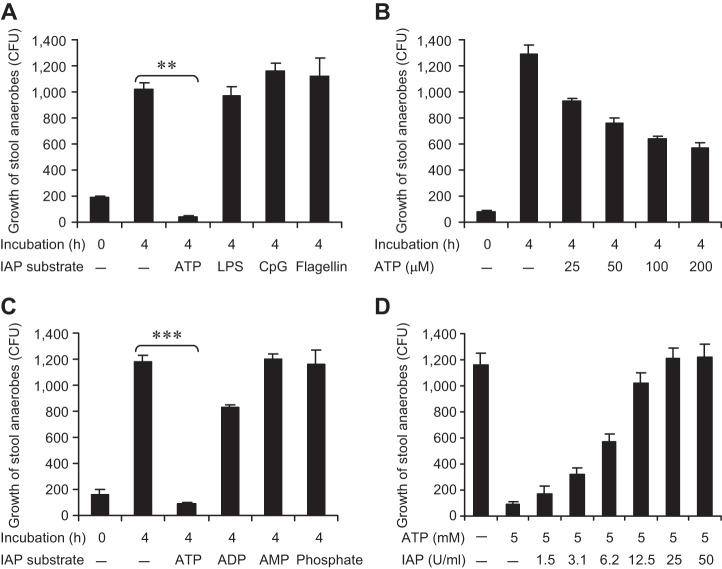
The aerobic bacterial population represents only a minor portion of the total gut bacterial population, most of which is anaerobic. Therefore, we sought to determine the effects of ATP and other IAP targets on the growth of mouse stool anaerobic bacteria. We grew the stool bacteria in the presence of ATP (10 mM), LPS (10 μg/ml), CpG DNA (10 μg/ml), or flagellin (100 ng/ml) under anaerobic conditions for 72 h at 37°C. In a manner similar to aerobes, we found that the growth of anaerobes was specifically inhibited by ATP (P < 0.01), whereas other IAP targets had no impact on the bacteria (Fig. 3A).
Fig. 3.
ATP inhibits the growth of stool anaerobic bacteria in vitro. Mouse stool samples were collected fresh directly in BHI media, homogenized, serially diluted, and then cultured under anaerobic conditions. For the growth of stool anaerobes, cultures were grown in a sealed plastic bag containing CO2-producing gas pack and anaerobic condition indicators for 4 h at 37°C. Then the cultures were plated on Brucella (5% horse blood) agar plates and incubated in a sealed plastic bag containing CO2-producing gas pack and anaerobic condition indicators for 3 days at 37°C (see materials and methods). A: effects of different targets of IAP on the growth of stool anaerobes. B: dose-response effects of ATP on the growth of stool anaerobes. C: effects of ATP derivatives on the growth of stool anaerobes. D: IAP reverses the inhibitory effects of ATP on the growth of stool anaerobes. Values are expressed as mean CFU ± SE. Statistics: 2-tailed Student's t-test; **P < 0.01, ***P < 0.001. The experiment was repeated at least 3 times showing similar results.
We then determined the dose-response effects of ATP (Fig. 3B) and found that the anaerobes were particularly sensitive to the inhibitory effects of ATP (P < 0.001). We found that 100 μM ATP caused 50% inhibition of anaerobic bacterial growth (Fig. 3B), whereas 1.2 mM ATP completely inhibited the bacterial growth and higher concentrations of ATP killed these bacteria (data not shown). We next investigated the effects of the ATP derivatives ADP, AMP, and phosphate on the growth of these anaerobes and found that, like aerobes, the ATP derivatives at 10 mM concentration did not have any significant impact on the growth of stool anaerobic bacteria (Fig. 3C).
IAP reverses the inhibitory effect of ATP on anaerobic bacterial growth.
The above data indicate that ATP has profound growth inhibitory effects on mouse stool anaerobes. We next studied whether IAP could reverse the effects of ATP. We treated the anaerobes with 5 mM ATP in the presence of different concentrations of IAP and found that IAP reverses the growth inhibitory effects of ATP in a dose-dependent manner (Fig. 3D).
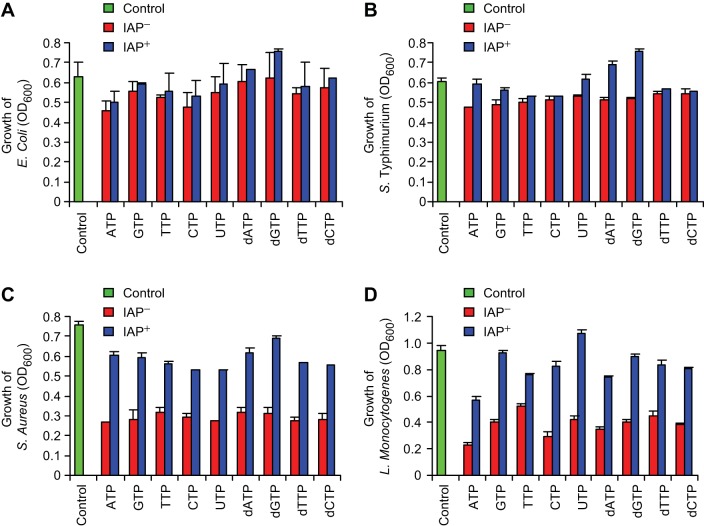
All nucleotide triphosphates inhibit bacterial growth.
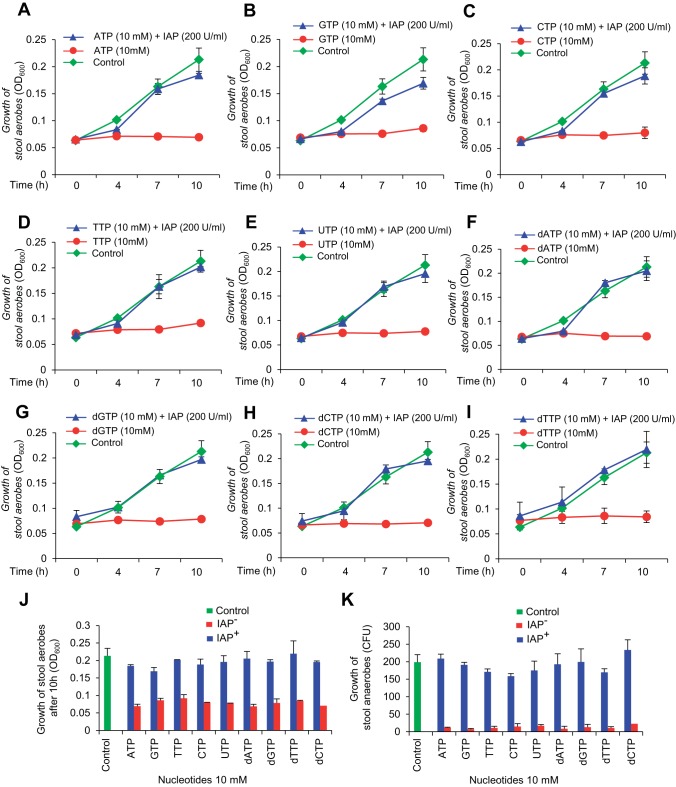
The inhibitory effects of the nucleotide ATP on bacterial growth raised the question whether other nucleotides (hydrolyzed products of luminal DNA and RNA) have any effect on bacterial growth. We observed that 10 mM nucleotides (ATP, GTP, CTP, TTP, and UTP) as well as deoxynucleotides (dATP, dGTP, dCTP, and dTTP) inhibit aerobic bacterial growth and these effects can be reversed by IAP (Figs. 4, A–J). Similar to aerobes, these nucleotides and deoxynucleotides at 10 mM concentration also inhibited or killed the stool anaerobes and the effects were reversed by IAP (Fig. 4K).
Fig. 4.
IAP reverses the bacterial growth inhibition by various nucleotide triphosphates. Mouse stool samples were collected and grown under aerobic and anaerobic conditions as described in Figs. 2 and 3 (also see materials and methods). Cultures were treated with a nucleotide (10 mM) ± IAP (200 U/ml). Growth of stool aerobes in different nucleotide triphosphates. A: ATP. B: GTP. C: CTP. D: TTP. E: UTP. F: dATP. G: dGTP. H: dCTP. I: dTTP. Values for aerobic bacterial growth are expressed as average absorbance (OD600) ± SD. The experiment was repeated at least 3 times showing similar results [2-way repeated-measures ANOVA: effect of time: F(3, 19) = 1,330.55, P value < 0.001; interaction of time and IAP: F(3, 19) = 449.87, P value < 0.001; interaction of time and nucleotide triphosphate: F(27, 63) = 2.32, P value < 0.01; interaction of time, IAP, and nucleotide triphosphate: F(24, 63) = 2.70, P value < 0.01; between-groups differences for IAP: F(1, 21) = 516.65, P value < 0.001; between-groups differences for nucleotide triphosphates: F(9, 21) = 11.61, P value < 0.001; Tukey's post hoc test: P value < 0.05 for dTTP; P values < 0.001 for all other nucleotide triphosphates]. J: dose-response curve at the end point of the experiment (10 h) [2-way ANOVA: effect of IAP: F(1, 39) = 586.13, P value < 0.001; Effect of nucleotide triphosphate: F(9, 39) = 13.65, P value < 0.001; interaction of IAP and nucleotide triphosphate: F(9, 39) = 8.44, P value < 0.001; Tukey's post hoc test: P value < 0.05 for dTTP; P values < 0.001 for all other nucleotide triphosphates; Tukey's post hoc test: P value < 0.01 for GTP; P values < 0.001 for all other nucleotide triphosphates]. K: growth of stool anaerobes in different nucleotides. Values are expressed as mean CFU ± SE. The experiment was repeated at least 3 times showing similar results.
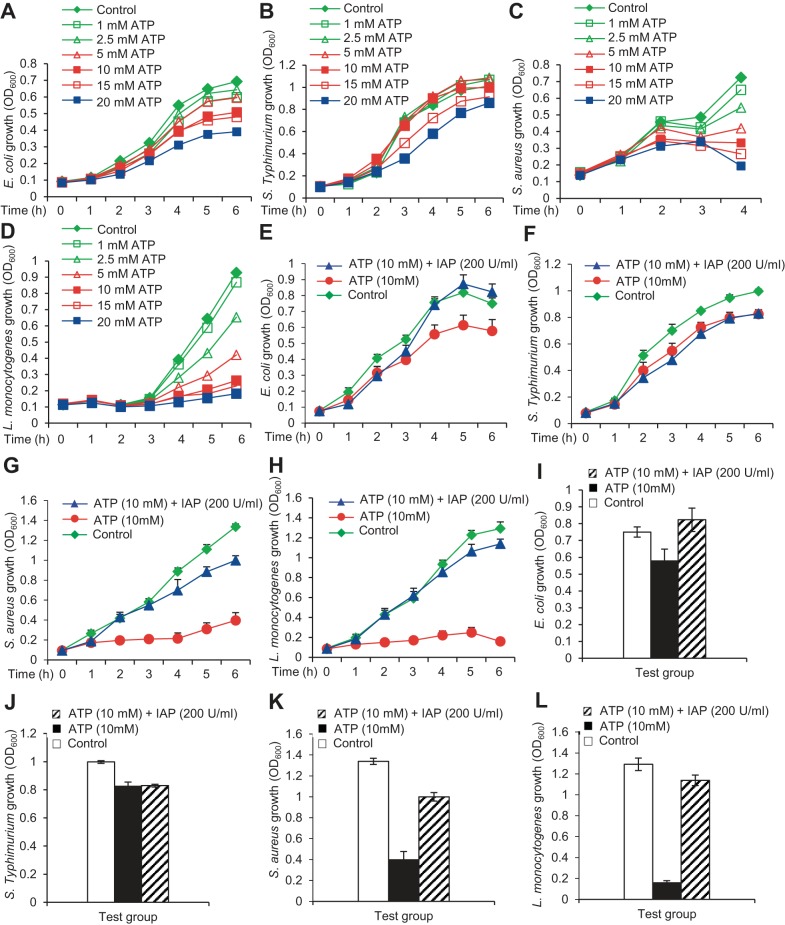
ATP differentially inhibits the growth of specific bacteria.
We were interested to know whether the growth-inhibitory effects of ATP were similar for all bacterial species. Accordingly, we exposed two gram-positive and two gram-negative bacterial species to different concentrations of ATP (1–20 mM). We observed that ATP slightly inhibited the growth of the gram-negative species E. coli (Fig. 5A) and S. typhimurium (Fig. 5B), but had more profound inhibition on the growth of the gram-positive species, S. aureus (Fig. 5C) and L. monocytogenes (Fig. 5D). Growth inhibition by ATP was greatest with L. monocytogenes > S. aureus > E. coli > S. typhimurium. These data indicate that ATP has variable effects on the growth of different bacterial species, and seems to affect gram-positive bacteria more than the gram-negative bacteria. As expected, IAP (200 U/ml) reversed the inhibitory effects of ATP (10 mM) on the growth of all these bacterial species (Fig. 5, E–L).
Fig. 5.
ATP differentially inhibits the growth of specific bacteria. Each bacterial culture (200 μl) was grown in 8 wells of a 96-well clear-bottom plate at 37°C in a shaking incubator, and absorbance (OD600) was determined at indicated time points. Dose-response effects of ATP (1–20 mM). A: E. coli. B: Salmonella typhimurium. C: Staphylococcus aureus. D: Listeria monocytogenes. Effects of ATP (10 mM) ± IAP (200 U/ml) on the growth of E. coli (E; P = 0.029), S. typhimurium (F, P = 0.18), S. aureus (G, P = 0.003), and L. monocytogenes (H, P = 0.007). I–L: these effects are also illustrated at the final time points. Values for bacterial growth are expressed as average absorbance (OD600) ± SD. The experiment was repeated at least 3 times showing similar results. Statistics: the statistical analysis was performed comparing the OD values in the IAP vs. Vehicle group using 2-tailed paired Student's t-test. Note: to avoid cluttering the error bars are not shown in A–D.
ATP derivatives do not exert significant effects on the growth of selective bacterial species.
We determined the effects of ATP derivatives on the growth of E. coli, S. typhimurium, S. aureus and L. monocytogenes. It is apparent that the growth of these bacterial species was not significantly affected by the ATP derivatives except 10 mM ADP inhibited the growth of S. aureus by 50% (P < 0.05) (data not shown).
All nucleotide triphosphates preferentially inhibit gram-positive bacteria.
We examined the effects of all nucleotide triphosphates and deoxynucleotides on the growth of gram-negative E. coli and S. typhimurium and gram-positive S. aureus and L. monocytogenes. It is evident that these nucleotides (10 mM) had minimal growth-inhibitory effects on the gram-negatives (Fig. 6, A and B); however, they substantially inhibited the growth of the gram-positive bacteria (Fig. 6, C and D). The growth-inhibitory effects of all nucleotide triphosphates were reversed by IAP (200 U/ml) treatment (Fig. 6, A–D).
Fig. 6.
Nucleotide triphosphates preferentially inhibit gram-positive bacteria. Each bacterial culture (200 μl) was grown in 8 wells of a 96-well clear-bottom plate at 37°C in a shaking incubator, and absorbance (OD600) was determined at indicated time points. Each culture was treated with an individual nucleotide (10 mM) ± IAP (200 U/ml). Growth was recorded every 2 h and the data presented here are from the 6-h time point. Values for bacterial growth are expressed as average absorbance (OD600) ± SD. The experiment was repeated at least 3 times showing similar results.
ATP inhibits bacterial growth in vivo.
The data presented above established that ATP and other nucleotide triphosphates inhibit bacterial growth in vitro. To define the physiological role for ATP and other nucleotide triphosphates on bacterial growth in vivo, we focused on determining the effects of ATP in the intestinal lumen. We determined the ATP concentrations in the small intestinal luminal fluids of WT and IAP-KO mice. We observed that compared with WT animals, there was nearly 10-fold more ATP in the intestinal luminal fluid of KO animals (Fig. 7A). Fasting silences IAP and hence we examined the concentration of ATP in fasted animals and found that ATP concentration increases approximately fourfold in the fasted (14 h) animals compared with the fed animals, consistent with the fact that IAP functions to dephosphorylate ATP (Fig. 7B). We then determined the concentrations of ATP in the loops of WT vs. IAP-KO mice. Figure 7C shows that compared with WT jejunal loop, ATP concentration was ∼4.3-fold higher in the IAP-KO jejunal loop. We next directly tested whether IAP destroys ATP in vivo. We instilled 100 U IAP into the jejunal loop in IAP-KO mice and observed that compared with the control animals receiving no IAP, the ATP concentration was significantly lower in the mice receiving IAP (∼80% reduction) (Fig. 7D).
Fig. 7.
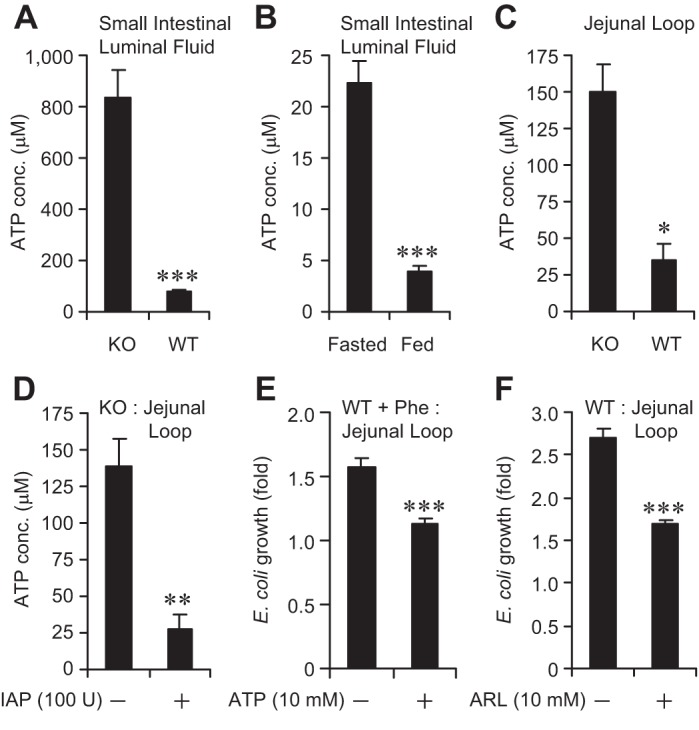
ATP inhibits bacterial growth in vivo. The small intestine was dissected out and luminal fluid was collected by gentle squeezing, then centrifuged, and the supernatant was obtained for ATP assay (see materials and methods). For determining ATP concentration and studying the effects of ATP in vivo, laparotomy was performed on mice (n = 5 per group) under general anesthesia and a 5-cm jejunal loop was constructed. Approximately 1,000 CFU of a specific bacterial species were instilled by injection into the loop. After 2 h, the loop was dissected out, homogenized, and plated on selective media for overnight growth at 37°C (see materials and methods for details). A: ATP concentrations (conc.) in the small intestinal luminal fluids of WT and IAP-KO mice. B: ATP concentrations in the small intestinal luminal fluids of WT mice fasted for 14 h. C: ATP concentrations in the jejunal loops of WT and IAP-KO mice. D: ATP concentrations in the jejunal loops of IAP-KO mice receiving IAP (100 U: injection directly into the loop). E: growth of E. coli in the jejunal loops of WT mice pretreated with 10 mM phenylalanine in the drinking water. F: growth of E. coli in the jejunal loops of WT mice receiving the ecto-ATPase inhibitor ARL 67156 (ARL; 10 mM) injection directly into the loop. Values are expressed as means ± SE. Statistics: 2-tailed Student's t-test; *P < 0.05, **P < 0.01, ***P < 0.001.
We next sought to determine whether exogenous ATP would inhibit bacterial growth within the context of the intact gut luminal environment. To prevent the destruction of exogenous ATP by the endogenous IAP, we fed WT mice 10 mM Phe in the drinking water for 48 h. We then instilled 100 μM ATP in the loop followed by E. coli. We observed that, compared with control mice receiving no ATP, E. coli growth was significantly lower in mice receiving ATP (1.57 ± 0.07 vs. 1.12 ± 0.05, respectively; P < 0.001) (Fig. 7E). To independently confirm this inhibitory effect of ATP on bacterial growth in vivo, we instilled into the loop an ecto-ATPase inhibitor ARL 67156 (10 mM), which is known to increase the ATP levels by inhibiting its hydrolysis by ecto-ATPases of CD39 family (31). Again, we observed that compared with mice receiving no ARL 67156, mice receiving ARL 67156 had a significant decrease in bacterial growth (2.71 ± 0.10 vs. 1.68 ± 0.06, respectively; P < 0.001) (Fig. 7F). Taken together, these data indicate that ATP inhibits bacterial growth in vivo and that IAP exerts its growth promoting effects by hydrolyzing ATP and probably other nucleotide triphosphates.
DISCUSSION
It has become clear in recent decades that intestinal dysbiosis plays a critical role in the pathogenesis of many diseases (3, 32, 33, 54, 59). A variety of different factors can be responsible for dysbiosis, including antibiotics; psychological and physical stress; diets rich in protein, simple sugar/refined carbohydrates, fat, or fructose; chemotherapy; radiation treatment; and intestinal infections (7, 15, 16, 42). Probiotics, prebiotics, synbiotics, and fecal biotherapy have emerged as therapies against dysbiosis, albeit with limited success (3, 22, 47, 50, 51). Prebiotics are known to modify and enhance the gut functionality (10, 11, 13, 30, 47, 48, 52), exerting their health benefits via alterations in the composition and/or activity of the intestinal microbiota. In the present study we provide evidence that the brush-border enzyme IAP promotes the growth of commensal bacteria. IAP exerts a number of beneficial effects on the gut, including the maintenance of local gut immunity (8), enhancing barrier function, reducing excessive inflammation, and inhibiting metabolic endotoxemia (4, 12, 14, 21, 26). The present study delineates another key beneficial impact of the IAP enzyme, promoting the growth of the commensal flora.
We have demonstrated the growth promoting effects of IAP in different in vivo settings using an isolated intestinal loop model. We compared commensal bacterial growth in high- vs. low-IAP environments by utilizing WT and IAP-KO, as well as fasted and fed mice. In addition, luminal IAP levels were altered either by directly adding exogenous IAP or by inhibiting the luminal IAP activity by adding its inhibitor Phe. Our data indicate that IAP is able to regulate a wide range of commensal bacteria, gram-positive, gram-negative, aerobes, and anaerobes.
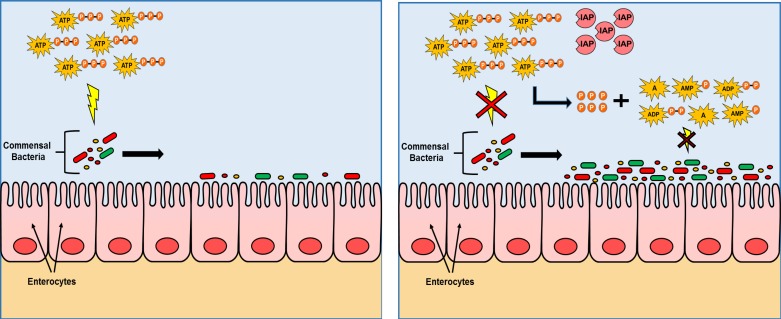
We have focused on determining the mechanisms by which IAP exerts its bacterial growth promoting effects. We have previously demonstrated in vitro that IAP does not exert any direct growth-promoting effect on specific aerobic bacterial species, such as E. coli, S. typhimurium, S. aureus, or L. monocytogenes (37). This observation led us to hypothesize that IAP must work through an indirect mechanism, likely by blocking one or more of its target molecules that otherwise inhibits bacterial growth (37). Accordingly, we tested the effects of known IAP targets and found only ATP to exhibit an inhibitory impact on bacterial growth. More importantly, these effects of ATP were completely blocked after incubation with IAP, suggesting that IAP works by inactivating ATP. It is interesting to note that the magnitude of the effects of extracellular ATP varies from one bacterial species to another. Where 10 mM ATP had minimal effects on the growth of the gram-negatives E. coli and S. typhimurium, the same concentration of ATP dramatically inhibited the growth of the gram-positive species, S. aureus and L. monocytogenes. Atarashi et al. (2) demonstrated that intestinal luminal ATP originates from both dead mammalian cells as well as the bacteria themselves, and Ivanova et al. (20) described that gram-negative bacteria in general secrete more ATP compared with gram-positive bacteria, which might be the reason why the gram-negative bacteria are relatively resistant to extracellular ATP. Although the precise mechanism by which ATP inhibits bacterial growth is beyond the scope of this study, it is worth noting that the extracellular ATP-mediated lysis of bacterial cells has been previously reported (62). Since the amount of ATP increases with higher bacterial count and turnover, we speculate that the growth inhibitory effects of ATP could be a mechanism by which bacteria self-regulate their population, avoiding overgrowth that would be detrimental to the host in which they reside, as well as to the growth of their bacterial symbiotic partners. Figure 8 is a schematic overview of the growth promoting role of IAP in the gastrointestinal luminal environment and the proposed mechanism.
Fig. 8.
Gut microbiota live in symbiosis with the host. The brush border enzyme IAP appears to play a central role in regulating the microbiota through a mechanism that involves the dephosphorylation of luminal ATP. ATP (and similarly other nucleotide triphosphates not shown in this figure) exerts an inhibitory effect on the growth and survival of a wide spectrum of bacteria. By dephosphorylating ATP, IAP blocks this inhibitory effect, resulting in greater numbers of gut bacteria. Both aerobes and anaerobes are affected by ATP. Note: The selected gram-positive bacteria (L. monocytogenes and S. aureus) were more significantly affected by ATP and consequently IAP compared with the tested gram-negatives (non-pathogenic E. coli and pathogenic Salmonella). A, adenosine, P, phosphate.
IAP is a brush border enzyme that is exclusively expressed in villus-associated enterocytes and it is therefore considered a marker for enterocyte differentiation (17). Much recent progress has been in made in delineating the physiological and pharmacological properties of the IAP enzyme. IAP has the ability to dephosphorylate a variety of bacterial and host-derived ligands (LPS, CpG DNA, flagellin, UDP), each of which works through a specific receptor to exert its inflammatory impact on target cells (8, 39). Here we show that IAP reverses the growth-inhibitory effects of various nucleotide triphosphates, indicating that these nucleotides are also IAP targets (see Figs. 2–7), and IAP probably destroys these targets by dephosphorylation (phosphohydrolysis). IAP has been shown to play an important role in fat absorption, and it has been established that the IAP-KO mice are obese compared with their WT littermates (41). Furthermore, IAP has been found to act as a gut mucosal defense factor and its expression is influenced by exposure to bacteria (4, 14, 25). IAP is also involved in the maintenance of normal gut barrier function (12, 21).
Pharmacologically, IAP has been found to be an effective therapy against various human as well as animal disease models including IBD, necrotizing enterocolitis, gram-negative sepsis, C. difficile, and S. typhimurium infections, and metabolic syndrome (1, 21, 34, 44, 56, 58, 61). Clinical trials using bovine IAP against colitis have shown that IAP has no or minimal side effects (34).
Recently, we have shown that oral supplementation with IAP prevents high-fat diet-associated metabolic syndrome in mice (21). We postulated that IAP detoxifies luminal LPS and therefore less LPS is available to be bound to fatty acids (chylomicron) and be translocated to systemic circulation, thus preventing metabolic endotoxemia and consequently the metabolic syndrome. However, it is also possible that more chronic alterations in the flora and/or levels of the extracellular nucleotide triphosphates caused by IAP contribute to the prevention of obesity and the metabolic syndrome.
Here we show that the luminal ATP and other nucleotide triphosphates play a critical role in preventing the bacterial growth. It is likely that the nucleotide triphosphates represent just one factor in the overall regulation of the commensal bacterial population within the gut, working in concert with other endogenous factors, e.g., antibacterial peptides (29, 49). The idea that extracellular nucleotide triphosphates inhibit bacterial growth in the intestine raises the question of whether these molecules play a similar role in other sites, e.g., the mouth, vagina, and urinary and respiratory tracts. Some associations have been noted between ATP and certain urinary and respiratory infections (28, 35), but a role in terms of regulating bacterial growth has not been previously reported.
In addition to the IAP, mammalians express other alkaline phosphatase (AP) forms, including placental, germ cell, neutrophil, and tissue nonspecific (liver/bone/kidney) (25). Furthermore, within the mouse intestine, three different IAP isozymes exist (Akp3, 5, and 6). All of the AP enzymes share significant structural homology as well as functional similarities, but it will be of interest in the future to determine whether these other forms of AP also have the capability to promote bacterial growth. ATP is also a known target of ecto-ATPases (23), which would be expected to be intact in the IAP-KO mice. Since ATP concentrations are increased in IAP-KO mice, it could be concluded that ecto-ATPases are less efficient than IAP in destroying/inactivating ATP. Further work will be needed to determine whether other ecto-nucleotidases play any role in regard to regulating the gut microflora.
In conclusion, although caution should always be taken extrapolating mouse studies to humans, the present data indicate that IAP functions as an endogenous promoter of commensal bacterial growth, likely through an inhibition of luminal ATP and other nucleotide triphosphates. As such, we believe that these findings lay the groundwork for developing IAP-based therapies in clinical settings of dysbiosis, either as a supplement or through a diet-related increase in endogenous levels. The concept of using an endogenous enzyme as a supplement to promote commensal bacteria not only is novel but also could represent a simple and safe way to impact the millions of people worldwide who are at risk for gut-derived infections and conditions associated with dysbiosis.
GRANTS
This work was supported by NIH grants R01DK050623 and R01DK047186 to R. A. Hodin, a Junior Faculty Award to M. S. Malo from the MGH Department of Surgery, a Grand Challenge Exploration Grant from the Bill and Melinda Gates Foundation to M. S. Malo, and a Feasibility Grant from Nutrition Obesity Research Center at Harvard (NORCH, NIH Grant P30 DK40561) to M. S. Malo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S.M. and R.A.H. conception and design of research; M.S.M., O.M., N.M., B.B., S.N.A., K.P.E., S.S.G., S.R.H., N.S.M., A.T., M.M.R.M., Q.T., S.N., J.L.M., E.L.H., H.S.W., S.C.R., and R.A.H. performed experiments; M.S.M., O.M., N.M., B.B., S.N.A., K.P.E., S.S.G., S.R.H., N.S.M., A.T., M.M.R.M., Q.T., S.N., J.L.M., E.L.H., H.S.W., S.C.R., and R.A.H. analyzed data; M.S.M., O.M., N.M., B.B., S.N.A., K.P.E., S.S.G., S.R.H., A.T., M.M.R.M., Q.T., S.N., J.L.M., E.L.H., H.S.W., S.C.R., and R.A.H. interpreted results of experiments; M.S.M. and R.A.H. prepared figures; M.S.M., O.M., S.S.G., and R.A.H. drafted manuscript; M.S.M., S.S.G., and R.A.H. edited and revised manuscript; M.S.M., O.M., N.M., B.B., S.N.A., K.P.E., S.S.G., S.R.H., N.S.M., A.T., M.M.R.M., Q.T., S.N., J.L.M., E.L.H., H.S.W., S.C.R., and R.A.H. approved final version of manuscript.
Footnotes
This article is the topic of an Editorial Focus by Jean-Paul Lalles (26a).
REFERENCES
- 1.Alam SN, Yammine H, Moaven O, Ahmed R, Moss AK, Biswas B, Muhammad N, Biswas R, Raychowdhury A, Kaliannan K, Ghosh S, Ray M, Hamarneh SR, Barua S, Malo NS, Bhan AK, Malo MS, Hodin RA. Intestinal alkaline phosphatase prevents antibiotic-induced susceptibility to enteric pathogens. Ann Surg 259: 715–722, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria TH17 cell differentiation. Nature 455: 808–812, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Balakrishnan M, Floch MH. Prebiotics, probiotics and digestive health. Curr Opin Clin Nutr Metab Care 15: 580–585, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2: 371–382, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengmark S. Nutrition of the critically ill — a 21st-century perspective. Nutrients 5: 162–207, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bik EM. Composition and function of the human-associated microbiota. Nutr Rev 67, Suppl 2: S164–S171, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris) 56: 305–309, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Chen KT, Malo MS, Beasley-Topliffe LK, Poelstra K, Millan JL, Mostafa G, Alam SN, Ramasamy S, Warren HS, Hohmann EL, Hodin RA. A role for intestinal alkaline phosphatase in the maintenance of local gut immunity. Dig Dis Sci 56: 1020–1027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen KT, Malo MS, Moss AK, Zeller S, Johnson P, Ebrahimi F, Mostafa G, Alam SN, Ramasamy S, Warren HS, Hohmann EL, Hodin RA. Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. Am J Physiol Gastrointest Liver Physiol 299: G467–G475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 69: 1052S–1057S, 1999 [DOI] [PubMed] [Google Scholar]
- 11.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 111: 1–66, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Geddes K, Philpott DJ. A new role for intestinal alkaline phosphatase in gut barrier maintenance. Gastroenterology 135: 8–12, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125: 1401–1412, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg RF, Austen WG, Jr, Zhang X, Munene G, Mostafa G, Biswas S, McCormack M, Eberlin KR, Nguyen JT, Tatlidede HS, Warren HS, Narisawa S, Millan JL, Hodin RA. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci USA 105: 3551–3556, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Altern Med Rev 9: 180–197, 2004 [PubMed] [Google Scholar]
- 16.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science 280: 1371–1374, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hodin RA, Graham JR, Meng S, Upton MP. Temporal pattern of rat small intestinal gene expression with refeeding. Am J Physiol Gastrointest Liver Physiol 266: G83–G89, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science 292: 1115–1118, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe 12: 496–508, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanova EP, Alexeeva YV, Pham DK, Wright JP, Nicolau DV. ATP level variations in heterotrophic bacteria during attachment on hydrophilic and hydrophobic surfaces. Int Microbiol 9: 37–46, 2006 [PubMed] [Google Scholar]
- 21.Kaliannan K, Hamarneh SR, Economopoulos KP, Nasrin Alam S, Moaven O, Patel P, Malo NS, Ray M, Abtahi SM, Muhammad N, Raychowdhury A, Teshager A, Mohamed MM, Moss AK, Ahmed R, Hakimian S, Narisawa S, Millan JL, Hohmann E, Warren HS, Bhan AK, Malo MS, Hodin RA. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci USA 110: 7003–7008, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 108: 500–508, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Kunzli BM, Berberat PO, Dwyer K, Deaglio S, Csizmadia E, Cowan P, d'Apice A, Moore G, Enjyoji K, Friess H, Robson SC. Variable impact of CD39 in experimental murine colitis. Dig Dis Sci 56: 1393–1403, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Lagatolla C, Dolzani L, Monti-Bragadin C. Characterization of the phenotype conferred by two different rpsL alleles coding for streptomycin dependence. New Microbiol 21: 105–111, 1998 [PubMed] [Google Scholar]
- 25.Lalles JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev 68: 323–332, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Lalles JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev 72: 82–94, 2014 [DOI] [PubMed] [Google Scholar]
- 26a.Lalles JP. Luminal ATP: the missing link between intestinal alkaline phosphatase, the gut microbiota and inflammation? Am J Physiol Gastrointest Cell Physiol (March 27, 2014). 10.1152/ajpgi.00435.2013 [DOI] [PubMed] [Google Scholar]
- 27.Lecerf JM, Depeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, Cayzeele A, Abdelnour G, Jaruga A, Younes H, Jacobs H, Lambrey G, Abdelnour AM, Pouillart PR. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 108: 1847–1858, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Lee BH, Hwang DM, Palaniyar N, Grinstein S, Philpott DJ, Hu J. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PloS One 7: e35812, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehrer RI, Lu W. α-Defensins in human innate immunity. Immunol Rev 245: 84–112, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Lenoir-Wijnkoop I, Sanders ME, Cabana MD, Caglar E, Corthier G, Rayes N, Sherman PM, Timmerman HM, Vaneechoutte M, Van Loo J, Wolvers DA. Probiotic and prebiotic influence beyond the intestinal tract. Nutr Rev 65: 469–489, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sevigny J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol 152: 141–150, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 10: 311–323, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukas M, Drastich P, Konecny M, Gionchetti P, Urban O, Cantoni F, Bortlik M, Duricova D, Bulitta M. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm Bowel Dis 16: 1180–1186, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Lundin A, Hallander H, Kallner A, Lundin UK, Osterberg E. Bacteriuria testing by the ATP method as an integral part in the diagnosis and therapy of urinary tract infection (UTI). J Biolumin Chemilumin 4: 381–389, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther 24: 701–714, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Malo MS, Alam SN, Mostafa G, Zeller SJ, Johnson PV, Mohammad N, Chen KT, Moss AK, Ramasamy S, Faruqui A, Hodin S, Malo PS, Ebrahimi F, Biswas B, Narisawa S, Millan JL, Warren HS, Kaplan JB, Kitts CL, Hohmann EL, Hodin RA. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut 59: 1476–1484, 2010 [DOI] [PubMed] [Google Scholar]
- 38.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 16: 2202–2222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss AK, Hamarneh SR, Mohamed MM, Ramasamy S, Yammine H, Patel P, Kaliannan K, Alam SN, Muhammad N, Moaven O, Teshager A, Malo NS, Narisawa S, Millan JL, Warren HS, Hohmann E, Malo MS, Hodin RA. Intestinal alkaline phosphatase inhibits the proinflammatory nucleotide uridine diphosphate. Am J Physiol Gastrointest Liver Physiol 304: G597–G604, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss DW, Walli AK. Intermediates in the hydrolysis of ATP by human alkaline phosphatase. Biochim Biophys Acta 191: 476–477, 1969 [DOI] [PubMed] [Google Scholar]
- 41.Narisawa S, Huang L, Iwasaki A, Hasegawa H, Alpers DH, Millan JL. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol 23: 7525–7530, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PloS One 8: e59470, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn's disease: a randomised controlled trial with Lactobacillus GG. Gut 51: 405–409, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramasamy S, Nguyen DD, Eston MA, Alam SN, Moss AK, Ebrahimi F, Biswas B, Mostafa G, Chen KT, Kaliannan K, Yammine H, Narisawa S, Millan JL, Warren HS, Hohmann EL, Mizoguchi E, Reinecker HC, Bhan AK, Snapper SB, Malo MS, Hodin RA. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm Bowel Dis 17: 532–542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao S, Srinivasjois R, Patole S. Prebiotic supplementation in full-term neonates: a systematic review of randomized controlled trials. Arch Pediatr Adolesc Med 163: 755–764, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Riquelme AJ, Calvo MA, Guzman AM, Depix MS, Garcia P, Perez C, Arrese M, Labarca JA. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. J Clin Gastroenterol 36: 41–43, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Leotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr 104, Suppl 2: S1–S63, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowland I. Functional food science and gastrointestinal physiology and function. Br J Nutr 80, Suppl 1: S147–S171, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Salzman NH. Paneth cell defensins and the regulation of the microbiome: detente at mucosal surfaces. Gut Microbes 1: 401–406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sartor RB. Therapeutic correction of bacterial dysbiosis discovered by molecular techniques. Proc Natl Acad Sci USA 105: 16413–16414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saulnier DM, Spinler JK, Gibson GR, Versalovic J. Mechanisms of probiosis and prebiosis: considerations for enhanced functional foods. Curr Opin Biotechnol 20: 135–141, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics—approaching a definition. Am J Clin Nutr 73: 361S–364S, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128: 825–832, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis as a prerequisite for IBD. Gut 53: 1057, 2004 [PMC free article] [PubMed] [Google Scholar]
- 55.Toward R, Montandon S, Walton G, Gibson GR. Effect of prebiotics on the human gut microbiota of elderly persons. Gut Microbes 3: 57–60, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Tuin A, Poelstra K, de Jager-Krikken A, Bok L, Raaben W, Velders MP, Dijkstra G. Role of alkaline phosphatase in colitis in man and rats. Gut 58: 379–387, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Van Kruiningen HJ, West AB. Potential danger in the medical use of Trichuris suis for the treatment of inflammatory bowel disease. Inflamm Bowel Dis 11: 515, 2005 [DOI] [PubMed] [Google Scholar]
- 58.van Veen SQ, van Vliet AK, Wulferink M, Brands R, Boermeester MA, van Gulik TM. Bovine intestinal alkaline phosphatase attenuates the inflammatory response in secondary peritonitis in mice. Infect Immun 73: 4309–4314, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vipperla K, O'Keefe SJ. The microbiota and its metabolites in colonic mucosal health and cancer risk. Nutr Clin Pract 27: 624–635, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Whelan K. Mechanisms and effectiveness of prebiotics in modifying the gastrointestinal microbiota for the management of digestive disorders. Proc Nutr Soc 72: 288–298, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Whitehouse JS, Riggle KM, Purpi DP, Mayer AN, Pritchard KA, Jr, Oldham KT, Gourlay DM. The protective role of intestinal alkaline phosphatase in necrotizing enterocolitis. J Surg Res 163: 79–85, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Xi C, Wu J. dATP/ATP, a multifunctional nucleotide, stimulates bacterial cell lysis, extracellular DNA release and biofilm development. PloS One 5: e13355, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut 57: 1605–1615, 2008 [DOI] [PubMed] [Google Scholar]