Abstract
Vascular endothelial growth factor (VEGF) is crucial for vascular development in several organs. However, the specific contribution of epithelial-VEGF signaling in the liver has not been tested. We used a mouse model to specifically delete Vegf from the liver epithelial lineages during midgestational development and assessed the cell identities and architectures of epithelial and endothelial tissues. We find that without epithelial-derived VEGF, the zonal endothelial and hepatocyte cell identities are altered. We also find decreased portal vein and hepatic artery branching coincident with an increase in hepatic hypoxia postnatally. Together, these data indicate that VEGF secreted from the hepatic epithelium is required for normal differentiation of cells and establishment of three-dimensional vascular branching and zonal architectures in both epithelial and endothelial hepatic tissues.
Keywords: vascular development, liver zonation, liver sinusoidal endothelial cells, platelet endothelical cell adhesion molecule, vascular endothelial growth factor
vascular endothelial growth factor (VEGF) signaling is an essential mediator of vascular growth and maturation in both development and disease. In the liver, the secretion of VEGF from hepatocytes and cholangiocytes is proposed to play an important role in liver protection and regeneration (15, 24, 30, 33) but can also promote the progression of liver tumors (26, 28, 35). The delicate balance of VEGF communication between hepatocytes, cholangiocytes, and endothelial cells (ECs) must be understood and regulated in a context-specific manner to harness the beneficial impacts of VEGF.
The architecture of the hepatic vascular systems, including the portal vein (PV), hepatic artery (HA), and central vein (CV), are highly precise and stereotypic. It is unknown what signals regulate the architecture of these structures and whether signaling interactions between epithelial and endothelial tissues is crucial to generate the proper vascular patterning. Previous studies have suggested that an epithelial-endothelial VEGF signal from the intrahepatic bile duct (IHBD) is crucial for the development of the HA (9, 27). Interestingly, several human diseases also display correlated IHBD and vascular paucities, posing the question of how the epithelial and endothelial tissues may interact during development.
VEGF has previously been shown to be important for normal liver development during embryonic and early postnatal periods (2, 11). However, the studies conducted so far have used VEGF inhibition methods that ubiquitously block all VEGF signaling either globally in postnatal mice or specifically in the liver of embryonic mice. These methods inhibited the baseline level of signaling required for EC survival and homeostasis (10, 22). Therefore, it is unsurprising that these mice showed decreases in ECs and reductions in vascular branching early in embryonic liver development. These studies also found disorganized sinusoids and hepatocyte cords, reduced numbers of liver sinusoidal endothelial cells (LSECs), and reduced lipid uptake into hepatocytes (2, 11). Because of the extreme impact on ECs in these mouse models, the studies conducted thus far have been unable to assess the role of VEGF in the growth and architectural establishment of the PV and HA.
In this study, we use a unique mouse model in which liver VEGF signaling is decreased but not completely deleted. We used a combination of the transgene Tg(Alb-cre)21Mgn and the Vegfatm2Gne allele (Albumin-Cre; Vegfflox/flox; hereafter referred to as VKO) to delete VEGF from hepatoblasts, the bipotential liver progenitor, beginning at midgestation. This results in both hepatocyte and cholangiocyte deletion of VEGF. The production of VEGF from nonepithelial cell types is not impaired by this genetic deletion. This mouse model allows us to specifically address the question of whether the hepatic epithelium drives the architectural establishment and growth of the PV and HA through VEGF signaling.
We find that mice are able to survive for several weeks postnatally after the midgestational VEGF deletion but display abnormalities in both the epithelial and endothelial tissues. VKO mice show an initial reduction in endothelium in the liver but recover postnatally without concomitant elevation of hepatic or serum VEGF. However, these mice display a progressive impairment in the postnatal elaboration of the PV and HA systems, in addition to disruptions in the sinusoidal network and in LSEC identity. These changes correlate with hypoxia in the liver and to alterations in hepatic zonation and gene expression.
We conclude that secretion of VEGF specifically from the hepatic epithelium is required for the postnatal architectural development of the liver vascular systems and for proper hepatic oxygenation and hepatocyte zonal fates. Additionally, epithelial VEGF is required to maintain LSEC identity and function and for the postnatal phase of PV and HA elaboration.
METHODS
Mouse lines.
VEGF knockout (VKO) mice were generated by crossing Tg(Alb-cre)21Mgn (Albumin-Cre) (29) and Vegfatm2Gne (VEGFflox) (11) mouse lines to generate Albumin-Cre;VEGFflox/flox mice. Mouse genotypes were confirmed by polymerase chain reaction using previously established primer pairs. All breeding and experimental procedures were performed with approval from the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital.
Mouse husbandry.
Mice were kept in a specific pathogen-free barrier facility and were kept on a 12:12-h light-dark cycle.
Immunohistochemistry.
Murine liver tissue was fixed overnight at 4°C in 4% paraformaldehyde, processed, and embedded in paraffin. Sodium citrate pH 6 antigen retrieval was performed in heat and high pressure for 15 min. Incubation in Tris pH 10 overnight at 60°C was used for antigen retrieval for the laminin antibody. For frozen sections, murine liver tissue was equilibrated in 30% sucrose and embedded in optimum cutting temperature compound (Tissue-Tek). Sections were incubated in 1° antibody overnight at 4°C and 2° antibody for 2 h at room temperature in 1% bovine serum albumin in phosphate-buffered saline. Antibodies and reagents are listed in Table 1. Mayer's hematoxylin or bisbenzimide was used as counterstains. Images were acquired using Axioplan2, an Olympus BX51 scope, and Olympus DP71 camera.
Table 1.
Antibodies and reagents used for immunostaining
| Retrieval | Dilution | Host | Company | Amplification | |
|---|---|---|---|---|---|
| Primary antibodies used for IHC | |||||
| Antigen | |||||
| Cytokeratin 19 (TROMA III) | Sodium citrate | 1:50 | Rat | DSHB | Vector Blue |
| CPS1 | Sodium citrate | 1:500 | Rabbit | Abcam | |
| DBA-biotin | Sodium citrate | 1:500 | Vector | ABC; DAB | |
| Endomucin (paraffin) | Sodium citrate | 1:200 | Goat | R&D | ABC; DAB |
| Endomucin (frozen) | 1:200 | Goat | R&D | ||
| Glutamine synthetase | Sodium citrate | 1:1,000 | Mouse | BD Transduction | Vector Blue |
| Hypoxyprobe | Sodium citrate | 1:100 | Mouse | NPI | Vector Blue |
| IsolectinB4-biotin | Sodium citrate | 1:100 | Sigma | ABC; DAB | |
| Laminin | Tris pH 10 | 1:200 | Rabbit | BioGenex | ABC; DAB |
| PECAM (frozen) | 1:100 | Rat | BD Pharmingen | ||
| Smooth muscle actin | Sodium citrate | 1:1,000 | Mouse | Sigma | ABC; DAB |
| Secondary antibodies used for IHC | |||||
| α−Rabbit-biotin | 1:500 | Donkey | Jackson ImmunoResearch | ||
| α-Rabbit-cy3 | 1:300 | Donkey | Jackson ImmunoResearch | ||
| α-Rat-Alexa488 | 1:300 | Donkey | Jackson ImmunoResearch | ||
| α-Rat-alkaline phosphatase | 1:500 | Donkey | Jackson ImmunoResearch | ||
| α-Goat-biotin | 1:500 | Donkey | Jackson ImmunoResearch | ||
| α-Mouse-biotin | 1:500 | Goat | Jackson ImmunoResearch | ||
| α-Mouse-alkaline phosphatase | 1:500 | Donkey | Jackson ImmunoResearch | ||
| Streptavidin-cy2 | 1:300 | Jackson ImmunoResearch | |||
| Reagents | |||||
| ABC | Vector | ||||
| Vector Blue | Vector | ||||
| DAB | Vector | ||||
IHC, immunohistochemistry; CPS1, carbamoyl phosphate synthetase 1; DBA, Dolichos biflorus agglutinin; ABC, avidin-biotin complex; DAB, 3,3′-diaminobenzidine; PECAM, platelet endothelial cell adhesion molecule.
Serum chemistry.
Blood was collected from postmortem mice and tested for serum total bilirubin (TB; TecoDiagnostics, Anaheim, CA), total bile acids (BA; Diazyme, Poway, CA), and alanine aminotransferase (ALT; TecoDiagnostics).
Visualization of hypoxia.
Hypoxyprobe (NPI, Burlington, MA) was used as directed. Approximately 0.6 mg/g Hypoxyprobe was injected into mice. Mice were killed 90 min after injection.
Quantification of VEGF protein.
Liver tissue [caudate lobes at postnatal day (P3) 0; caudate, right, and medial lobes at P15 and P3; whole liver at embryonic day (E) 16.5] was digested with Complete protease inhibitor cocktail (Roche, Mannheim, Germany). Serum was isolated from mouse blood at the time of harvest. VEGF protein was measured using ELISA as directed (R&D, Minneapolis, MN). Total protein for normalization was measured with a Pierce BCA Protein Assay Kit as directed (Thermo Scientific, Rockford, IL).
RNA preparation and semiquantitative reverse transcription PCR.
Total liver RNA was prepared using TRIZOL (Life Technologies, Carlsbad, CA) and a Turbo DNA-Free kit (Ambion, Austin, TX). Total RNA (1.5 μg) was used in complementary DNA synthesis, performed with SuperScript III First-Strand (Life Technologies). Semiquantitative reverse transcription PCR for isoforms of Vegf mRNA was performed using Vegf exon 3 forward 5′-CAG GCT GCT GTA ACG ATG AA-3′ and Vegf 3′-untranslated region reverse 5′-GAC ATG GTT AAT CGG TCT TTC C-3′ primers (14). Gapdh mRNA was used as a standard: forward primer 5′-GAC ACC CAC TCC TCC ACC TTT G and reverse primer 5′-GTC CAC CAC CCT GTT GCT GTA G. Three independent samples per genotype were analyzed after thermocycling times of 25, 30, 35, 40, 45.
Statistical analysis.
A one-tailed unpaired Student's t-test was used to analyze differences.
Circulating blood cell analysis.
Blood samples were collected in Microvette EDTA tubes at the time of death. Blood was analyzed with a Drew Hemavet 950FS.
RESULTS
Hepatoblast-specific deletion of VEGF reduces total VEGF levels in the liver at embryonic and adult time points.
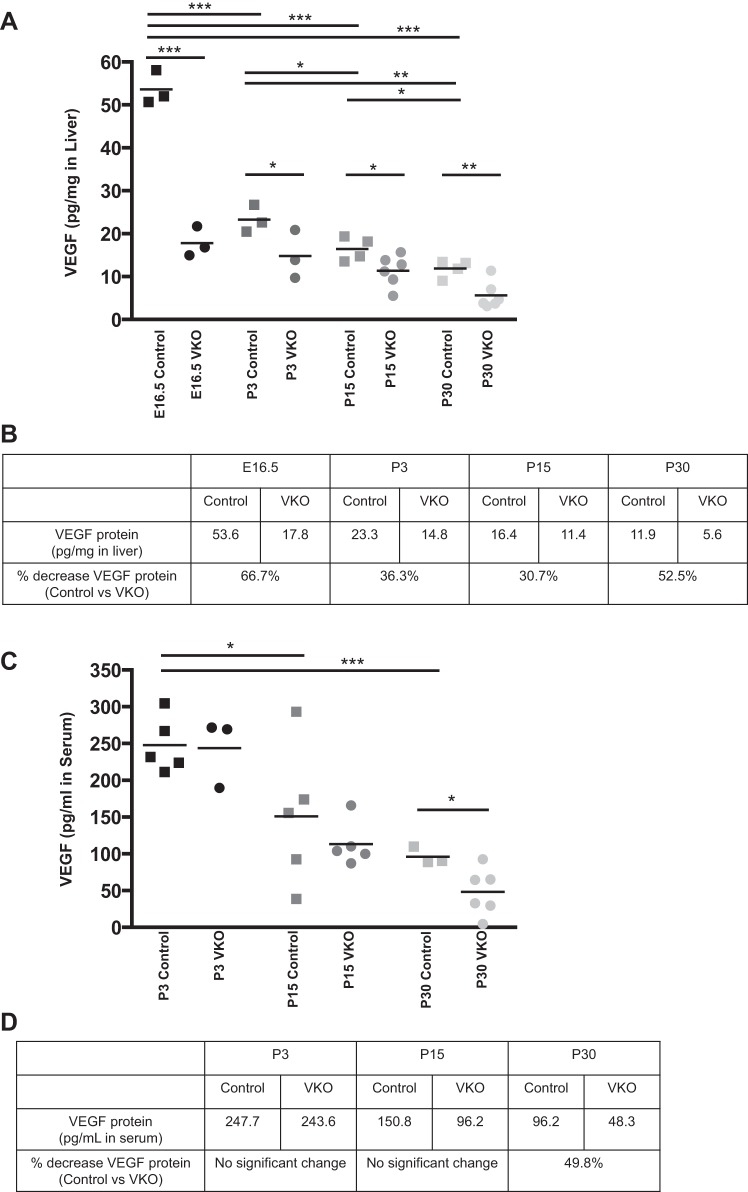
To determine the extent of VEGF protein reduction in VKO mice, VEGF protein levels in whole liver were analyzed through an ELISA assay. In control mice, the total liver VEGF was highest at E16.5 and was significantly decreased at each subsequent time point (Fig. 1A). The levels of VEGF in the liver of VKO mice were significantly reduced compared with control at all time points analyzed (Fig. 1, A and B). VEGF protein in the VKO liver was reduced, compared with control, 66.7% at E16.5, 36.3% at P3, 30.7% at P15, and 52.5% at P30.
Fig. 1.
Vascular endothelial growth factor knockout (VKO) mice have decreased liver vascular endothelial growth factor (VEGF) protein during embryonic and adult time points. VEGF protein levels in liver (A and B) and serum (C and D) were analyzed by ELISA in embryonic and postnatal control and VKO genotypes. Measurements were performed in duplicate and standardized relative to total liver protein (A) or total serum volume (C). A: each square or circle represents one biological sample. In controls, liver VEGF levels were highest at embryonic day (E) 16.5 and decreased progressively over time. At E16.5, postnatal day (P) 3, P15, and P30, VKO mice have significantly less VEGF protein in the liver than littermate controls. No compensation for the loss of liver VEGF was observed in the serum. There was no significant difference in serum VEGF levels between control and VKO mice at P3 or P15. However, at P30, VKO mice display a significant reduction in serum VEGF compared with controls. The average VEGF protein concentrations in liver tissue (B) and serum (D) are provided for control and VKO mice at all time points. The percentage reduction in relative VEGF protein levels between controls and VKO is shown for all time points. *P < 0.05, **P < 0.01, and ***P ≤ 0.001.
To determine whether the loss of VEGF in the liver could influence systemic VEGF levels, perhaps to compensate for the hepatic loss of VEGF or as a result of decreased VEGF secretion from the liver into the bloodstream, we also measured VEGF protein in the blood serum. Similar to the pattern in the liver, serum VEGF protein in control mice was highest at the first time point measured, P3, and was significantly decreased at P15 and P30 (Fig. 1, C and D). At P3 and P15, no change in serum VEGF protein levels was observed between control and VKO mice. At P30, VKO mice exhibited a significant decrease in serum VEGF protein levels compared with controls (Fig. 1, C and D).
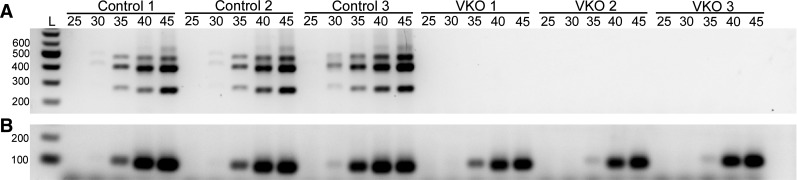
Additionally, we performed semiquantitative reverse transcription PCR of whole liver RNA from P45 control and VKO mice. A VEGF primer set that picks up all three isoforms (120, 164, and 188) was used along with gapdh primers to demonstrate cDNA concentration and quality (Fig. 2, A and B). All control samples tested amplified all VEGF isoforms; however, none of the VEGF isoforms amplified in the VKO samples (Fig. 2A). This result is consistent with our ELISA results that demonstrated significant loss of VEGF levels in the livers of VKO mice at various time points (Fig. 1). Furthermore, loss of VEGF expression at P45 demonstrates there is not compensation of VEGF but rather continuous VEGF expression loss in VKO mice (Fig. 2).
Fig. 2.
VEGF isoforms are absent in VKO mice at P45. Total liver RNA was isolated from P45 control and VKO mice and synthesized to cDNA. A semiquantitative reverse transcription PCR was performed to analyze loss of VEGF isoforms in VKO mice. A primer set that amplifies VEGF isoforms 120, 164, and 188 was used to assess VEGF (A), and GAPDH (B) was used to demonstrate cDNA concentrations and quality. In the control samples, all three VEGF isoforms amplified with isoform 164 being most predominant. All three VKO mice tested did not show any amplification of any of the VEGF isoforms, indicating a sufficient loss of VEGF as anticipated. Five amplification cycles were analyzed (25, 30, 35, 40, and 45). L corresponds to a 100-bp DNA ladder.
VKO mice display global phenotypes, including reduced body mass and indicators of hypertension.
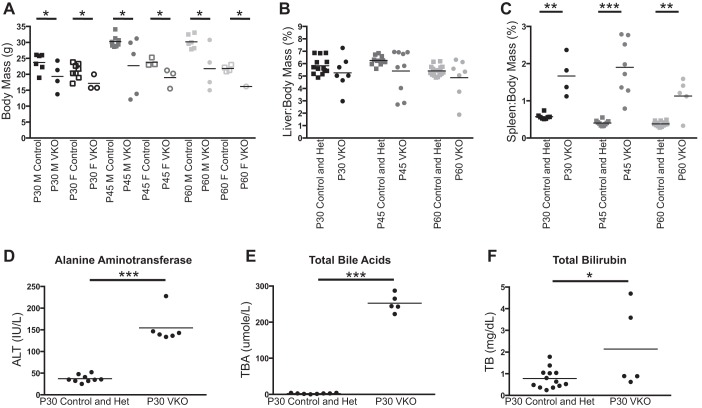
To determine the global effect of the hepatoblast-specific loss of VEGF on mice, we allowed mice to age until P60 and measured body, liver, and spleen mass at several postnatal time points. Several VKO mice display poor health and lethality between P30 and P60. Whereas some VKO mice have comparable body masses to their littermate controls, there was a significant decrease in body mass between sex-matched control and VKO mice at P30, P45, and P60 (Fig. 3A). Because of the high rate of lethality before P60, we only analyzed one female VKO mouse, which exhibited a lower body mass than all P60 control females.
Fig. 3.
VKO mice demonstrate decreased body mass, splenomegaly, and liver injury. To assess the phenotype of VKO mice, markers of global mouse health and physiology as well as liver health and function were assessed. The body mass and organ mass of P30, P45, and P60 control and VKO mice were measured, and the organ-to-body mass ratio was calculated for liver and spleen. A: at P30, P45, and P60, several VKO mice display total body masses that are much less than their sex-matched littermate controls. VKO mice, sorted by gender, have significantly lower body masses at all time points for both males (M) and females (F). Statistics were not performed for P60 females because of the high lethality before this time point and the consequent low number of P60 VKO females obtained. B: liver-to-body mass ratio (%) was not significantly different between control and VKO mice at P30, P45, and P60 despite several VKO mice displaying abnormally low liver-to-body mass ratios. C: VKO mice display splenomegaly, having significantly elevated spleen-to-body mass ratios at P30, P45, and P60. To assess liver health and function, serum from P30 control and VKO mice was tested for alanine aminotransferase (ALT), total bile acids (BA), and total bilirubin (TB). No difference was observed between control and heterozygous mice in any of these analyses. D: VKO mice have elevated levels of ALT in serum at P30. E: VKO mice have elevated levels of BA in serum at P30. F: some VKO mice have elevated levels of TB in serum at P30. Although some VKO mice display normal levels of TB, the difference between control and VKO mice was statistically significant. *P < 0.05, **P < 0.01, and ***P ≤ 0.001.
VKO mice at P30 and older time points displayed an obvious and consistent phenotype of peritoneal vertices (data not shown). Blood vessels around the abdominal organs, including the stomach, intestine, and pancreas, were enlarged. Occasionally, this phenotype was accompanied by death of the gut and/or a pink-toned pancreas, indicative of blood retention in the organ. Taken together, these phenotypes indicate the potential of hypertension.
To assess hypertension in VKO mice, we used the surrogate measurement of splenomegaly. At P30, P45, and P60, VKO mice displayed splenomegaly, having a significantly increased spleen-to-body mass ratio (Fig. 3C). This suggests that there is hypertension in VKO mice P30 and older. No differences in body mass or spleen-to-body mass ratio were observed in VKO mice P15 or younger (data not shown).
Because of the enlarged abdominal blood vessels and a difficulty in extracting serum from VKO mouse blood, we examined whether there were any changes in the circulating blood cell populations (data not shown). Inhibiting VEGF systemically or specifically in the liver has been shown to cause an upregulation of hepatocyte-produced erythropoietin, leading to increased hematocrit in a matter of weeks in adult mice (32). We first looked at P60, when mice are visibly sick, but because of the high lethality before P60, we did not collect enough mice to collect blood and perform statistical analysis. The one P60 VKO mouse analyzed displayed a large increase in hematocrit, explaining the phenotype of thickened blood with reduced serum. We also analyzed mice at P30 and found a smaller (average of 59.93% circulating red blood cells in 6 mice) but significant increase in hematocrit in VKO over controls (average of 44.33% in 8 mice) at that time. P30 VKO mice also displayed an increase in the number of neutrophils (data not shown).
VKO mice have altered liver morphology, health, and function by P30.
To assess the health and function of the liver, we performed blood serum measurements for ALT, BA, and TB in P30 control and VKO mice (Fig. 3, D–F). In P30 VKO mice, ALT and BA were consistently and significantly increased over control littermates. Levels of TB were inconsistent in P30 VKO mice, with some mice having normal or near-normal levels of TB and some mice displaying a mild but abnormal increase in TB. However, the increase in TB in P30 VKO mice over controls was statistically significant.
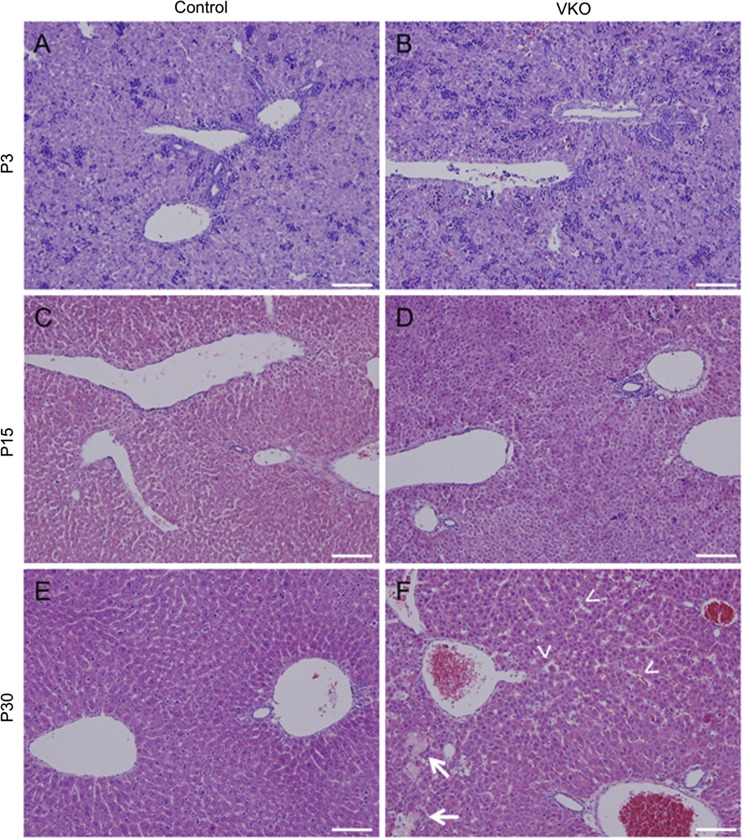
With the serum tests providing evidence that liver health and function was impaired (Fig. 3, D–F), we assessed whether VKO mice displayed any changes in liver morphology. We assessed liver histopathology by hematoxylin and eosin (H&E) staining (Fig. 4). The H&E stain did not reveal any differences in liver morphology between control and VKO mice at P3 (Fig. 4, A and B). However, some differences in hepatocyte cord morphology were observed at P15 (Fig. 4, C and D), and, by P30, VKO mice displayed small areas of focal necrosis and dilated sinusoids (Fig. 4, E and F).
Fig. 4.
VKO mice display disrupted hepatocyte cords and dilated sinusoids. Hematoxylin and eosin (H&E) stains were done on P3, P15, and P30 control and VKO livers. At P3, no obvious visual differences were observed between control (A) and VKO (B) mice. At P15, VKO mice exhibited abnormal hepatocyte cord morphology (D) compared with controls (C). By P30, VKO mice demonstrate dilated sinusoids (F, arrowheads) compared with control (E). There is also a loss of hepatocyte zonal morphology and focal necrosis (arrows). In the P30 control, an H&E shows subtle differences in the periportal and pericentral hepatocytes; the periportal hepatocytes are more densely associated than the pericentral hepatocytes (E). However, no zonation is apparent in the P30 VKO liver (F). Scale bar is 100 μm.
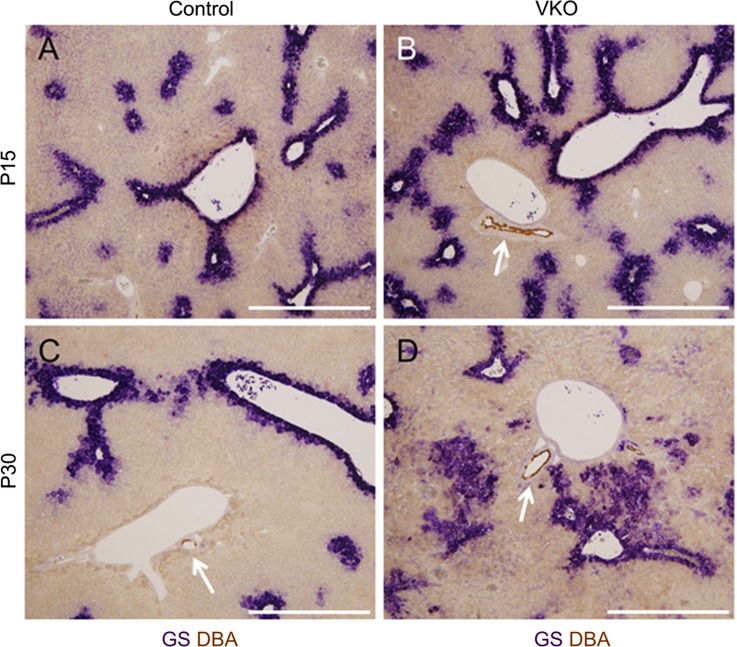
To examine the zonation of hepatocytes, we assessed the expression of the zone-specific hepatocyte enzymes glutamine synthetase (GS) (Figs. 5 and 6) and carbamoyl phosphate synthetase 1 (CPS1) (Fig. 6). GS and CPS1 are enzymes involved in glutamine formation and urea formation, respectively, in hepatocytes (16). GS expression in both VKO and control mice was observed in its normal location, in the hepatocytes surrounding CVs (Fig. 5). However, the expression region of GS was abnormally expanded in VKO mice; at P15, there was a slight increase in the area of expression in the pericentral zone, and, at P30, GS expression was observed in large clusters of hepatocytes not surrounding a CV (Fig. 5).
Fig. 5.
VKO mice display expanded pericentral hepatocyte gene expression. To assess hepatocyte zonation in VKO mice, expression of the pericentral hepatocyte marker glutamine synthetase (GS, purple) was assessed with immunohistochemistry. The lectin Dolichos biflorus agglutinin (DBA, brown, white arrows) was used to mark hilar intrahepatic bile ducts (IHBDs) next to hilar portal vein branches. In control mice at P15 (A) and P30 (C), the expression of GS is restricted to hepatocytes within a few cell diameters from a central vein. No GS expression is observed around portal veins. In VKO mice, however, a slight visual increase in the area of GS expression is observed at P15 (B). In P30 VKO mice, there is a large increase in the area of GS expression, with several large patches of hepatocytes displaying abnormal GS expression (D). Scale bar is 500 μm.
Fig. 6.
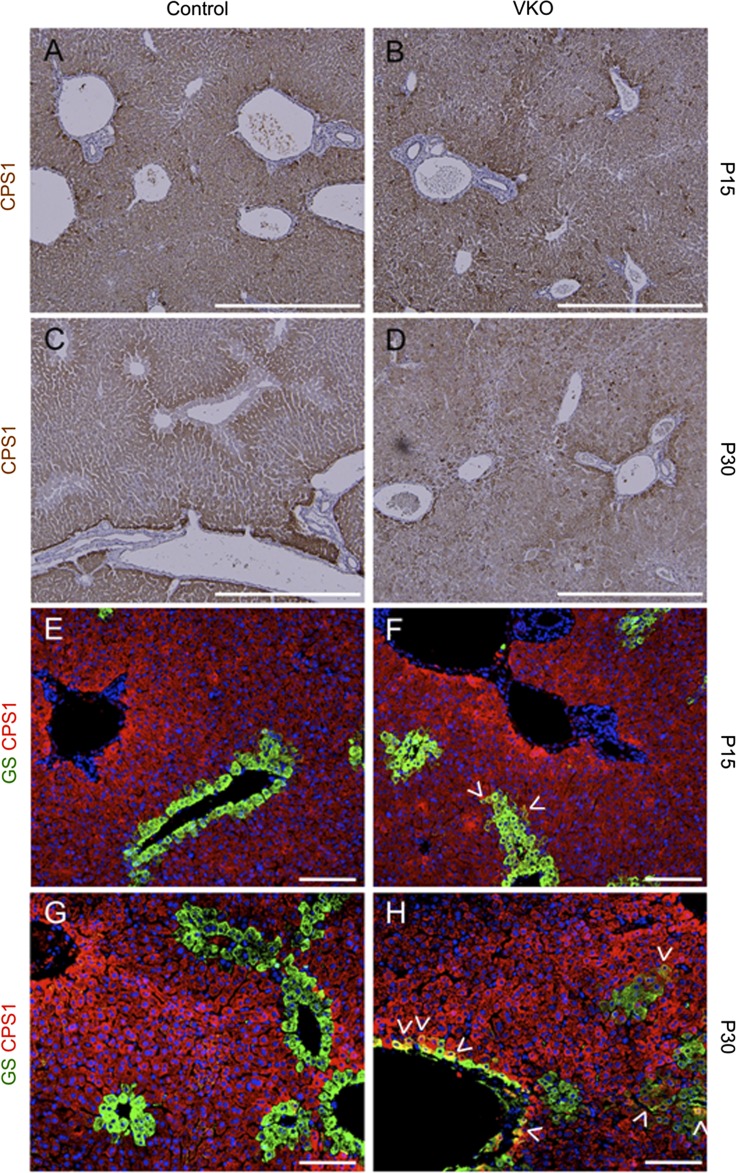
VKO mice display a loss of hepatocyte zonation and abnormal overlap of hepatocyte zonal markers. To assess hepatocyte zonation in VKO mice, we assessed the expression of carbamoyl phosphate synthetase 1 (CPS1), a marker of periportal and intermediate zone hepatocytes, and the coexpression of CPS1 and GS. In control mice, CPS1 begins to exhibit zonal restriction at P15 (A), and, by P30, expression of CPS1 is excluded from pericentral hepatocytes (C). At both P15 and P30, control mice do not display overlap in the expression patterns of CPS1 and GS (E and G). In VKO mice, similar to controls, rough zonal restriction of CPS1 expression is observed at P15 (B). However, by P30, there is a loss of zonal pattern of CPS1 expression in VKO mice (D). VKO mice also display the abnormal coexpression of GS and CPS1 in both pericentral hepatocytes and in intermediate zone hepatocytes that abnormally express GS (arrowheads). This coexpression occurs in rare hepatocytes at P15 (F) but is increased at P30 (H). Scale bar is 500 μm (A–D) and 100 μm (E–H).
In control adult mouse livers, GS and CPS1 expression is mutually exclusive, with GS expressed only in pericentral hepatocytes and CPS1 expressed in periportal and intermediate hepatocytes (Fig. 6). In P15 VKO mice, in line with the expansion of GS expression, there are a small number of hepatocytes present that coexpress GS and CPS1 (Fig. 6). At P30, the number of GS and CPS1 coexpressing cells has visually increased, and these cells are found both juxtaposing CVs and in the abnormal GS-expressing hepatocyte clusters that do not juxtapose CVs (Fig. 6). This altered gene expression indicates a loss of zonation and a disruption in zone-specific hepatocyte identity.
VKO mice display hypoxia in the liver by P15.
In the consideration that a liver morphogenesis phenotype is already apparent by P30, and increasing hematocrit after P30 may cause secondary defects confounding the immediate role of VEGF in liver development, we hereafter focus on P30 and earlier time points. Hepatocyte zonation has been hypothesized to result, at least in part, from the steep gradient in blood oxygen pressure across the hepatic lobule (4). To determine if hypoxia may be playing a causative role in the altered zonal identity of hepatocytes, we used Hypoxyprobe to visualize regions of hypoxia in the livers of P15 and P30 VKO and control mice (Fig. 7).
Fig. 7.
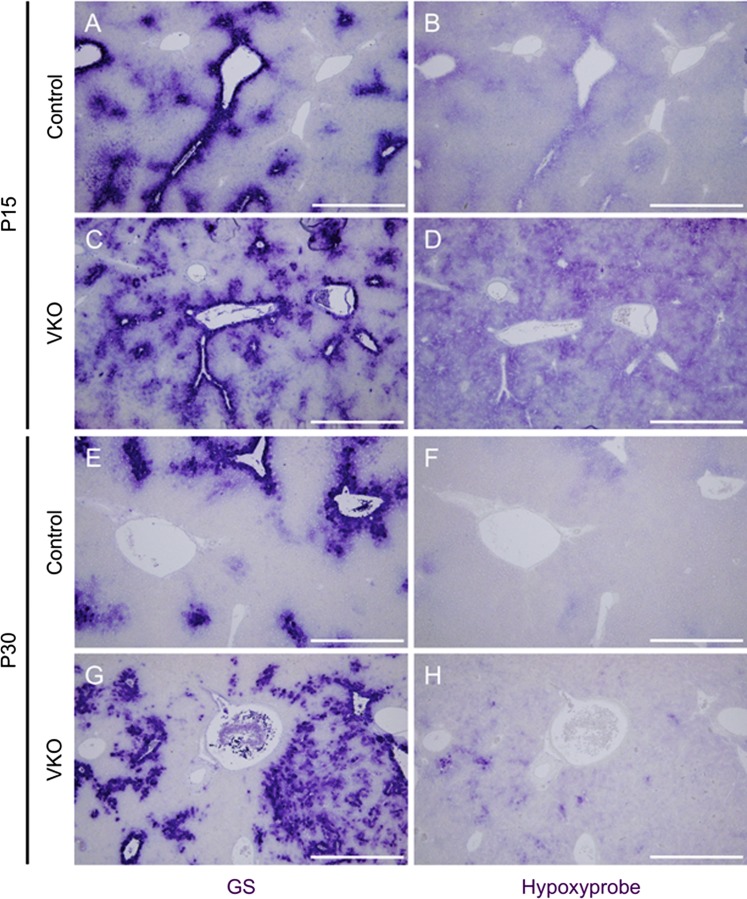
VKO mice demonstrate increased hypoxia in the liver that occurs in close proximity to, but does not necessarily overlap, regions of GS expression. Serial sections were used to assess the association of oxygen levels and GS expression in the livers of VKO mice. Hypoxyprobe was injected into mice 90 min before death and assessed by immunohistochemistry (purple, B, D, F, and H). At P15 and P30, control mice display low levels of regional hypoxia, specifically around central veins (B and F). In P15 VKO mice, however, the regions of hypoxia are markedly expanded compared with that observed in control (D). This hypoxia is still present in P30 VKO mice but is not as widespread (H). The regions of hypoxia correspond with regions of GS expression in P15 and P30 controls (A and E). In P15 VKO livers, GS expression correlates with the strongest Hypoxyprobe staining (C and D), but not all hypoxic areas express GS (E and F). In P30 VKO livers, Hypoxyprobe occurs in close spatial association with GS expression, but GS and Hypoxyprobe expression frequently occurs in different cells (G and H). Scale bar is 500 μm.
In control mice, hypoxia is faintly apparent in a zonal pattern, specifically around CVs marked by GS expression, at P15 and P30 (Fig. 7, B and F). In VKO mice, however, there is a visual increase in the area of hypoxia over controls at both P15 and P30; and an apparent gradient is less apparent (Fig. 7, D and H). This indicates that the VKO mice do have abnormally hypoxic livers, which may be contributing to the observed altered hepatocyte zonal identities.
To assess whether regions of hypoxia correlate with the areas of expanded GS expression, serial liver sections were stained for GS and Hypoxyprobe (Fig. 7). In P15 and P30 controls, the areas of GS expression very closely lined up with the areas of faint Hypoxyprobe staining (Fig. 7, A, B, E, and F). In P15 VKO livers, hypoxia is dispersed throughout the tissue, but the regions of GS staining do still correlate with the darkest Hypoxyprobe staining (Fig. 7, C and D). In P30 livers, hypoxia is observed in areas of expanded GS staining; however, there is not a complete overlap between GS and Hypoxyprobe staining (Fig. 7, G and H). Large areas of GS staining exist that are not positive or only weakly positive for Hypoxyprobe. Interestingly, the strongest Hypoxyprobe staining is frequently seen in cells on the border of a GS+ patch, or in the middle of a GS+ patch in cells that juxtapose GS staining but do not express GS themselves (Fig. 7, G and H).
VKO mice display an embryonic decrease in endothelial-lineage cells.
After determining that VKO mice did have a liver phenotype in which hypoxia is increased and hepatocyte zonal identities are altered, we examined the different vascular compartments to see if any were abnormal in VKO mice and could be responsible for the aforementioned liver phenotypes. We focused our examination on the PV, the HA, and the hepatic sinusoidal ECs.
Previous reports have demonstrated that inhibiting VEGF signaling ubiquitously in the liver results in a reduction in the number of ECs (2, 11). To determine if there is a similar reduction in EC number when only epithelial VEGF expression is reduced from a midgestational time point, we stained with IsolectinB4 (IsoB4) in embryonic and postnatal livers (data not shown). At E16.5 in VKO mice, there is a visible reduction in the number of cells that stain positive for IsoB4 compared with control. However, this reduction is no longer observed at P3 or at P15. This indicates that, although there was an initial defect in the number of IsoB4-expressing endothelial-lineage cells, the loss is compensated for postnatally.
VKO mice have reduced PV branching at P30.
To determine whether epithelial VEGF plays a role in PV branching, we analyzed the number of PV branches in P15 and P30 VKO and control mice. We analyzed both the number of PV branches per liver area as a measure of vessel density and the number of PV branches per transverse section as an assessment of the vessel architectural pattern independent of any consequent reductions in liver size.
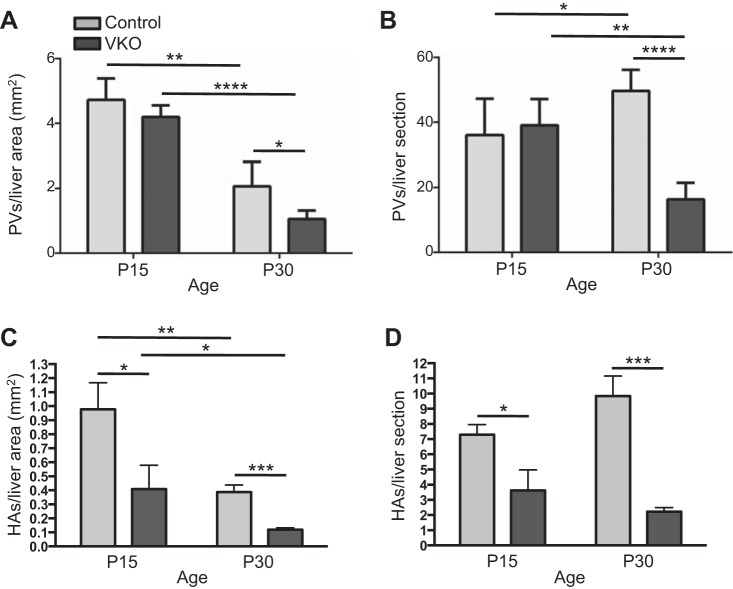
Between P15 and P30, control mice exhibit an increase in the number of PVs/liver section but a decrease in the number of PVs/liver area. This indicates that new PV branches are being formed during this time, but the rate of PV branch addition is relatively less than the rate of liver growth. At P15, there is no observable decrease in PVs/liver section or PVs/liver area in VKO mice compared with control. However, there is a reduction in both PVs/liver section and in PVs/liver area in P30 VKO mice compared with controls (Fig. 8, A and B).
Fig. 8.
VKO mice display a reduction in portal vein (PV) branches and hepatic artery (HA) branches. To count PV branches, liver sections were stained with GS to demarcate central veins (CVs). PVs, defined as any vein not lined by GS+ hepatocytes, were counted. The number of branches per liver area (A) and the number of branches per liver cross section (B) were analyzed. At P15, no difference in PVs/liver area or PV/liver section between control and VKO mice was observed. At P30, VKO mice had significantly fewer PVs per liver area and per section. The number of PVs per area decreased between P15 and P30 in both control and VKO mice (A). The number of PVs per liver section increased in control mice but decreased in VKO mice (B). HA branches were visualized by immunohistochemistry for smooth muscle actin (SMA). Expression of SMA in the arterial mesenchyme marks the major HA branches. The number of branches per liver area (C) and the number of branches per liver cross section (D) were analyzed. At both P15 and P30, VKO mice have fewer HA branches per liver area and per liver section compared with controls (C and D). At least six liver sections per animal were counted. N = 3 (P15) or 4 (P30) for PV counts and N = 4 (P15) or 6 (P30) for HA counts for both genotypes. *P < 0.05, **P < 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.
VKO mice have reduced HA branching at P15 and P30.
To determine whether epithelial VEGF is required for HA branching, we assessed HA branches as normalized to liver tissue area and per liver section, analyzed by the same method as PV branches.
Similar to the PV, we found that, in control mice between P15 and P30, the number of HA branches/liver area decreases. This indicates that, similarly to the PV, the rate of HA branch addition is less than that of liver parenchymal expansion (Fig. 8, C and D). In VKO mice compared with controls, there were fewer HAs/liver area and HAs/liver section at P15 and P30.
VKO mice demonstrate a loss of LSEC identity.
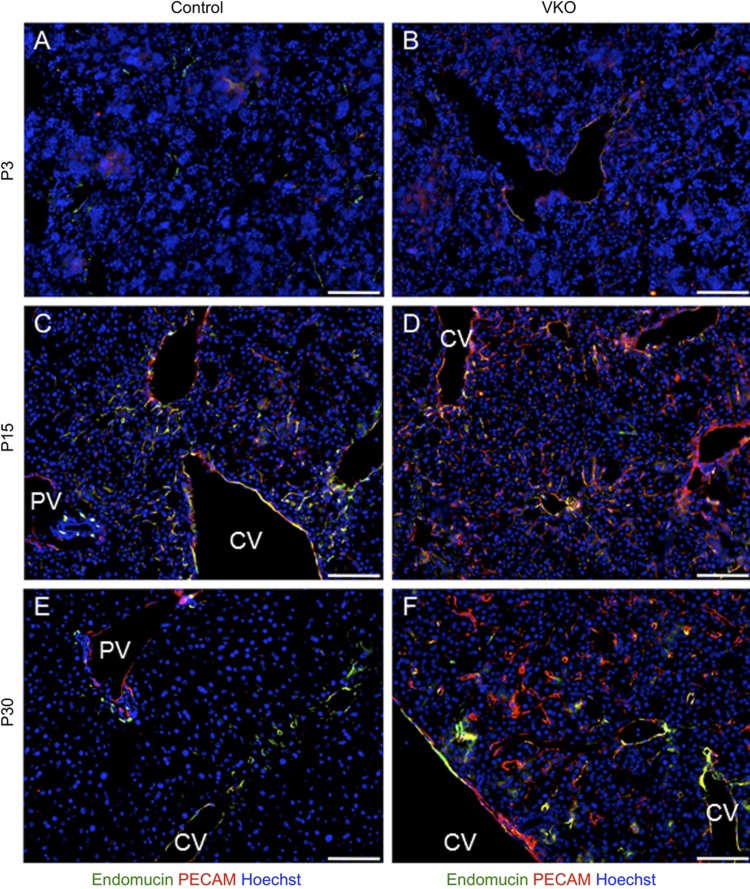
To determine whether epithelial VEGF plays a role in the development of the sinusoid network, we assessed the expression of endothelial markers in the liver. First, we analyzed the expression of endomucin in P3, P15, and P30 VKO and control mouse livers. In control livers, the expression of endomucin is not uniform over all hepatic ECs: endomucin is expressed in the endothelium of the HA, the CV, and the pericentral sinusoids (Fig. 9). No endomucin expression is observed in the endothelium of the PV or periportal sinusoids. In P15 VKO mice, a slight expansion of endomucin expression is observed compared with controls. By P30, however, the pattern of endomucin in P30 VKO is highly abnormal (Fig. 9). The restriction of expression within the hepatic zones is lost, and endomucin is now observed in the periportal sinusoidal endothelium. Additionally, the presence of endomucin+ cells is decreased in the regions of pericentral hepatocytes, with additional hepatocyte cords between the endomucin+ sinusoidal endothelium.
Fig. 9.
VKO sinusoid endothelial cells display an abnormal expression of platelet endothelial cell adhesion molecule (PECAM) and endomucin. To assess the differentiation status of sinusoid endothelial cells, the expression of PECAM (red) and endomucin (green) was assessed by immunofluorescence. In P3 control mice, low amounts of PECAM and endomucin are expressed in venous endothelium (A). No difference is observed between control and VKO (B) mice at this time. In P15 control mice, CV endothelium expresses PECAM and endomucin, whereas portal vein endothelium only expresses PECAM (C). A subset of sinusoid endothelial cells expresses both PECAM and endomucin. In P15 VKO mice, PECAM is similarly expressed in both portal vein and CV endothelium, whereas endomucin is expressed in the CV but not portal vein endothelium (D). Differently from controls, however, P15 VKO mice display an expansion in the expression of PECAM in sinusoid endothelial cells. By P30, control mice express PECAM only in portal and CV endothelium and express endomucin only in CV, pericentral sinusoid, and peribiliary plexus endothelium (E). PECAM is not expressed in sinusoid endothelial cells. P30 VKO mice, however, have a large expansion of PECAM expression, with PECAM abnormally expressed in a large number of sinusoid endothelial cells (F). The expression of endomucin is similarly expanded, compared with control, to sinusoid endothelial cells that are not pericentral. Scale bar is 100 μm.
To assess the differentiated identity of the LSECs, we assessed expression of platelet endothelial cell adhesion molecule (PECAM). Normally, PECAM is not expressed in LSECs in adult mice. At P3, both PECAM and endomucin (Fig. 9) were expressed only within venous endothelium in control and VKO mice. In P15 controls, PECAM is expressed primarily in venous endothelium and in some sinusoidal endothelium that coexpresses endomucin (Fig. 9C). In P15 VKO mice, PECAM is not only expressed in venous endothelium, but the expression of PECAM is also expanded to a greater number of sinusoidal ECs (Fig. 9D). As previously noted, the expression of endomucin is slightly expanded in sinusoids in the P15 VKO liver compared with control, and the pattern of PECAM shows a similar effect (Fig. 9D). By P30, the expression of PECAM is highly restricted in control mice: PECAM is expressed within the PV and CV endothelium, but not within the sinusoidal endothelium (Fig. 9E). In contrast, PECAM is expressed not only in venous endothelium but is also very highly associated with the sinusoidal endothelium in P30 VKO mice (Fig. 9F). There does not seem to be any zonal or regional restrictions to PECAM expression within the sinusoidal endothelium. The expression of PECAM within LSECs indicates that these cells have lost features of their sinusoidal identity and may be indicative of “capillarization.”
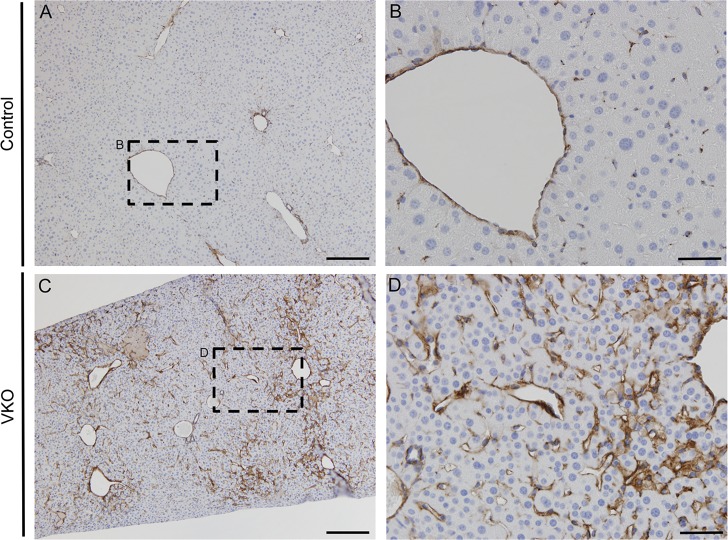
One characteristic of capillarization is the formation of an organized basement membrane. Therefore, we used laminin, a basement membrane marker, to further characterize possible capillarization of the LSECs in VKO mice. In control mice at P30 there is very little laminin staining, especially in the LSECs (Fig. 10, A and B). Conversely, there is a dramatic increase in laminin in the LSECs around the CV in VKO mice littermates (Fig. 10, C and D). This finding along with the increase and expansion of PECAM expression (Fig. 9) further demonstrates capillarization of LSECs in VKO mice.
Fig. 10.
VKO mice exhibit an increase of basement membrane in the sinusoids. To further understand changes in sinusoid endothelial cells, we analyzed the basement membrane marker laminin. VKO mice demonstrated a dramatic abundance of laminin (C and D) compared with littermate controls (A and B) at P30. Laminin was increased primarily around the CV in the surrounding sinusoid endothelial cells. Slides were counterstained with Mayer's hematoxylin. Scale bar is 200 μm (A and C) and 50 μm (B and D).
VKO mice exhibit a reduction in the peribiliary vascular plexus and sinusoidal endothelial capillary network.
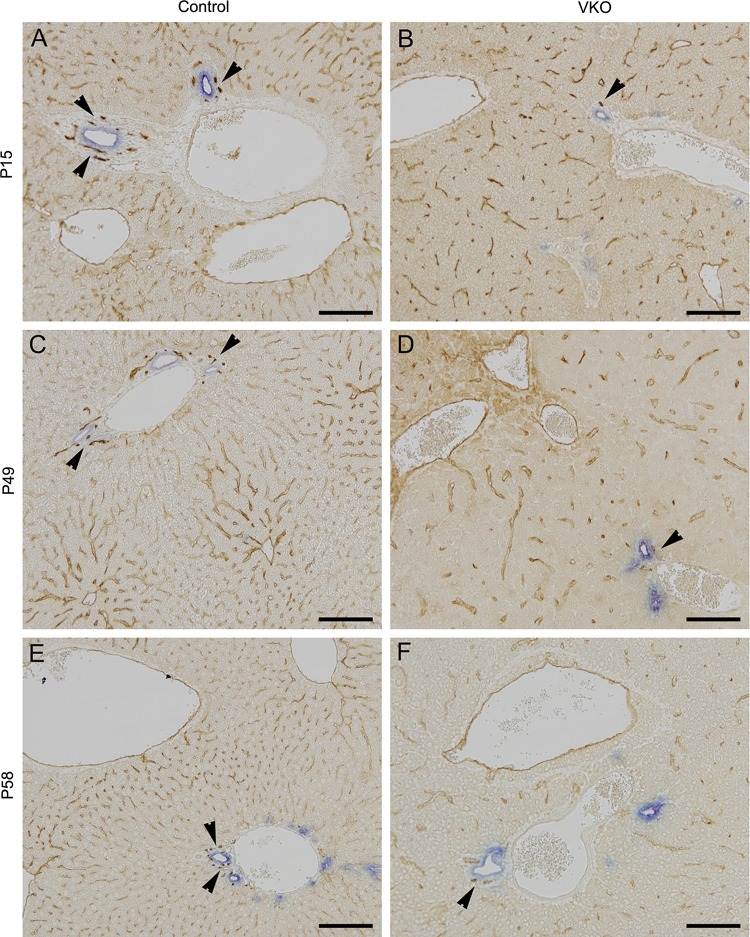
To assess the evolution of the biliary structures and peribiliary plexus in VKO mice, we performed a chromogenic immunohistochemistry for cytokeratin 19, marker of bile ducts, and endomucin, an endothlial marker, at various time points. At all ages analyzed (P15, P49, and P58) in the control mice the peribiliary plexus forms an intricate pattern around the bile ducts, and a dense sinusoidal endothelial capillary network is apparent (Fig. 11, A, C, and E). In VKO littermates, the peribiliary plexus forms, however, it is greatly simplified, and the sinusoidal endothelial capillary network is less dense (Fig. 11, B, D, and F). The IHBD structures were visibly unaffected by loss of VEGF in all time points analyzed in the VKO mice. These results suggest VEGF is necessary for proper formation of the peribiliary plexus and sinusoidal endothelial capillary network.
Fig. 11.
VKO mice exhibit a slight reduction in the peribiliary plexus and sinusoidal endothelial capillary network. To analyze the formation of the biliary structures and peribiliary plexus, we used cytokeratin 19 (CK19), a marker of bile ducts, and endomucin, an endothelial marker. At all stages analyzed (P15, P49, and P58), the control mice exhibit an intricate peribiliary vascular plexus (A, C, and E, arrowheads) and dense sinusoidal endothelial capillary network. In VKO mice, the peribiliary vascular plexus and sinusoidal endothelium do form. However, the peribiliary vascular plexus is simplified (B, D, and F, arrowheads), and the sinusoidal endothelial capillary network appears less dense in the VKO mice compared with littermate controls. Scale bar is 100 μm.
DISCUSSION
Hepatic VEGF levels differentially affect the PV, HA, peribiliary plexus, and sinusoids.
The VKO displays alterations in PV, HA, peribiliary plexus, and sinusoids compared with control, but the abnormal phenotypes appear in the different vascular tissues at different times. VKO mice display alterations in IsoB4+ endothelial-lineage cells as early as E16.5, but recovery is visible by P3 (data not shown). HAs, peribiliary plexus, and LSECs show abnormal phenotypes by P15 that persist at P30, and PVs show abnormal phenotypes at P30 (Figs. 8 and 9).
These differences can be explained by a variety of explanations. First, it may be that there are different required levels of VEGF for each vascular tissue. As the levels of VEGF decrease as development proceeds in VKO mice, it may be that the absolute hepatic VEGF levels drop below the required level for HA/peribiliary plexus development and LSEC identity sooner than they drop below the level required for PV morphogenesis. This explanation fits with the known role for VEGF in arterial-venous differentiation (31); high levels of VEGF promote arterial fates, whereas ECs not receiving high VEGF signaling adopt a venous fate.
A second explanation is that the different vessels rely primarily on VEGF derived from different tissues. Perhaps the PV receives the majority of its VEGF signal from the mesenchyme, whereas the sinusoids depend on VEGF from hepatocytes and the HA is directed by VEGF secreted from the IHBD. These alternate potential sources of VEGF could also contribute to the levels of VEGF that are normally received by each vascular tissue and that are required for normal development and function.
Third, it may be that VEGF serves a different function for the different vascular tissues based on the identity and specific mode of development of each tissue. VEGF has been described as having roles in endothelial proliferation, differentiation, branching morphogenesis, cell survival, and vascular permeability (2, 5, 12, 13, 18, 22, 23). The main role of VEGF may be different in the different endothelium, or may change at different stages of development. At this point in time it is not possible to distinguish between these possibilities.
Loss of epithelial VEGF results in an inability to generate and maintain PV branches during postnatal growth.
Between P15 and P30, control mice decrease the number of PVs per liver area but increase the number of PV branches per section (Fig. 8B). This indicates that the PV system is expanding and adding new branches, but it is doing so at a rate that is slower than the overall expansion of the liver parenchymal mass and area.
VKO mice do not exhibit a similar addition of new PV branches during this period and instead actually exhibit a loss of PV branches in section (Fig. 8B). This may indicate that the PV branches formed before P15 are not maintained in the VKO model. Alternatively, it may be that the decrease in ECs initially observed at E16.5 (data not shown) results in a failure to produce enough venous endothelial progenitors, limiting the elaboration of the PV system past P15. Although the average number of HA branches per liver section also decreases in VKO mice over time, this decrease is not significant (Fig. 8D). Hence, the failure to maintain branches may illuminate a unique role for epithelial VEGF in the homeostasis of the PV.
LSECs undergo capillarization in vivo as a result of deficient epithelial VEGF signaling.
Capillarization is the loss of LSEC-specific features and involves a reduction in fenestrae, a reduction in scavenger behavior, formation of an organized basement membrane, and an upregulation of PECAM (7). This process is seen in vivo in the liver during cirrhosis and as a result of aging. In vitro, if LSECs are not either treated with VEGF or cocultured with a cell type that produces VEGF, LSECs undergo capillarization (7, 20, 36).
In agreement with in vitro studies, the decrease in VEGF protein in the VKO liver results in the capillarization of LSECs. In P15 and P30 control mice, PECAM expression is restricted to the major vessels and excluded from the LSECs. However, in VKO mice, the LSECs express high levels of PECAM at P15 and P30 (Fig. 9). Additionally, VKO mice exhibit an increase in laminin expression in LSECs, indicative of formation of an organized basement membrane, a characteristic of capillarization (Fig. 10). Importantly, the loss of LSEC identity in VKO mice may impede the transfer of particles between the blood stream and the hepatocytes, potentially explaining the observed decreased liver function as seen in serum tests of VKO mice (20, 25).
Hypoxia may occur as a result of alterations to HA paucity or LSEC capillarization.
By P15, VKO mice display a vast increase in hypoxia in the liver compared with controls (Fig. 7). At this time, there are alterations observed in both the HA and the sinusoids that could contribute to the hypoxic phenotype (Figs. 8 and 9).
Previous studies have indicated that LSEC capillarization correlates with changes in high-energy phosphates and other metabolites in hepatocytes and a decreased ability to perform oxygen-dependent drug metabolism, consistent with a decrease in oxygen availability (20). It may be that the LSECs in the VKO liver have a decreased capacity for the transfer of oxygen to hepatocytes, contributing to hypoxia in the parenchyma.
Alternatively, the decrease in HA branches provides a simple explanation for the decrease of oxygen in the liver parenchyma. The blood supplied to the liver by the HA has a much higher oxygen tension than that supplied by the PV, indicating that a reduction in HA input into the liver or HA density could have a large effect on the oxygenation of the blood in the liver (34). The reduction in both HAs per liver area and per liver section provides a simple explanation for the increased liver hypoxia observed in the VKO mice, especially since recent studies have been unable to find a definitive link between LSEC capillarization and hepatocyte hypoxia (3).
Hepatocyte zonation is tied to hypoxia, but hypoxia does not account for defects in VKO hepatocyte zonation.
Whereas the regions of expanded GS expression are closely associated with hypoxia, Hypoxyprobe and GS expression do not always necessarily overlap (Fig. 7). Instead, the two tend to frequently be juxtaposed in P30 VKO mice (Fig. 7, G and H). This indicates that hypoxia likely does not directly control GS expression in a cell-autonomous way. However, because of the close spatial association between GS and Hypoxyprobe, it remains likely that the two are connected and that hypoxia does play a role in the GS expression expansion and zonation abnormalities.
There are several potential explanations for the increased GS expression observed in P30 VKO mice, including: 1) VEGF may play a direct role on restricting GS expression in periportal hepatocytes; 2) the altered vasculature may be signaling abnormally to hepatocytes, resulting in hepatocyte zonal fate changes; 3) the hypoxia may be regulating GS expression in a non-cell-autonomous way by designating a boundary between GS+ and GS− hepatocytes; and 4) the immature hepatocyte structure, resulting from lack of epithelial-endothelial signaling or reduced oxygen levels, may make hepatocytes less competent to signal to each other and establish a zonal boundary. There are no highly relevant published studies supporting any of these possibilities, so the explanation remains unclear. Interestingly, however, VEGF and GS expressions are both frequently upregulated in cirrhosis and hepatocellular carcinoma (6, 19, 21). This provides some support against the idea that VEGF is a direct negative regulator of GS in hepatocytes.
Hepatocyte nuclear factor factor 4α and Wnt/β-catenin signaling has been found to regulate the expression of GS (1). It is possible that these signaling pathways are affected by the hypoxia in the liver, resulting in abnormal interhepatocyte signaling and zonal boundaries. One surprising finding is that hypoxia very closely overlaps with GS expression in postnatal control livers but does not in the expanded GS+ regions of the P30 VKO liver. This suggests that there is a different mechanism of GS regulation that emerges in the P30 VKO mice and differs from the normal mechanism of GS regulation in postnatal liver.
Influence of epithelial VEGF provides insightful information for the use of antiangiogenic agents in the treatment of liver disease.
An upregulation of VEGF is observed in liver diseases, including hepatocellular carcinoma (8, 26, 28, 35). There are several VEGF inhibitor drugs approved by the Food and Drug Administration for the treatment of specific types of cancers; however, the use of VEGF inhibitors has been shown to have negative side effects in both preclinical and clinical studies (17). Global side effects include EC apoptosis and capillary regression, reduction in EC fenestrations, hypertension, hemorrhage, and thrombosis (17). The current study supports the finding that reducing hepatic VEGF levels can result in vascular regression, and, specifically in the liver, we find impaired growth of the HA and PV as well as failure to maintain PV branches. We also add to this knowledge by demonstrating that reducing VEGF levels in the liver can have effects on hepatocyte zonal identity and LSEC identity. Importantly, this study does not use the complete blockage of VEGF, so we avoid disrupting the homeostasis of ECs. Use of this experimental model also allows us to distinguish that disruptions in the liver epithelial and endothelial tissues does not require a complete blockage of VEGF signaling but can instead occur when VEGF is simply at lower levels than normal. This suggests that dosage will be very important to minimize side effects on the liver in any VEGF inhibitor treatment.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01-DK-078640 (to S. S. Huppert), from the Cincinnati Children's Research Foundation to S. S. Huppert, and the Cincinnati Children's Hospital Medical Center Digestive Health Center (P30-DK-078392) for providing financial support for Integrative Morphology Core services.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.J.W. and S.S.H. conception and design of research; T.J.W. and A.E.C. performed experiments; T.J.W., A.E.C., and K.A.H. analyzed data; T.J.W., A.E.C., K.A.H., and S.S.H. interpreted results of experiments; T.J.W. and A.E.C. prepared figures; T.J.W. drafted manuscript; T.J.W., A.E.C., K.A.H., and S.S.H. edited and revised manuscript; T.J.W., A.E.C., K.A.H., and S.S.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Peter Campochiaro, Napoleone Ferrara, and Mark Magnuson for mice; Dr. Senad Divanovic for use of Hemavet; and Holly Poling for technical assistance.
REFERENCES
- 1.Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, Kitajewski J, Kahn A, Perret C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene 21: 8293–8301, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Carpenter B, Lin Y, Stoll S, Raffai RL, McCuskey R, Wang R. VEGF is crucial for the hepatic vascular development required for lipoprotein uptake. Development 132: 3293–3303, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cheluvappa R, Hilmer SN, Kwun SY, Jamieson HA, O'Reilly JN, Muller M, Cogger VC, Le Couteur DG. The effect of old age on liver oxygenation and the hepatic expression of VEGF and VEGFR2. Exp Gerontol 42: 1012–1019, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Colnot S, Perret C. Liver zonation. In: Molecular Pathology of Liver Diseases, edited by Monga SPS. Molecular Pathology Library, 2011, p. 7–16 [Google Scholar]
- 5.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 84: 1470–1478, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, Colombo M, Bedossa P. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology 56: 532–543, 2012 [DOI] [PubMed] [Google Scholar]
- 7.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol 287: G757–G763, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, Joplin RE, Okolicsanyi L, Strazzabosco M. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 43: 1001–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Fabris L, Cadamuro M, Libbrecht L, Raynaud P, Spirli C, Fiorotto R, Okolicsanyi L, Lemaigre F, Strazzabosco M, Roskams T. Epithelial expression of angiogenic growth factors modulate arterial vasculogenesis in human liver development. Hepatology 47: 719–728, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 118: 2906–2917, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417: 954–958, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris S, Craze M, Newton J, Fisher M, Shima DT, Tozer GM, Kanthou C. Do anti-angiogenic VEGF (VEGFxxxb) isoforms exist? A cautionary tale. PloS one 7: e35231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa K, Mochida S, Mashiba S, Inao M, Matsui A, Ikeda H, Ohno A, Shibuya M, Fujiwara K. Expressions of vascular endothelial growth factor in nonparenchymal as well as parenchymal cells in rat liver after necrosis. Biochem Biophys Res Commun 254: 587–593, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Jungermann K, Keitzmann T. Zonation of parenchymal and nonparenchymal metabolism in liver. Ann Rev Nutr 16: 179–203, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96: 1788–1795, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krueger J, Liu D, Scholz K, Zimmer A, Shi Y, Klein C, Siekmann A, Schulte-Merker S, Cudmore M, Ahmed A, le Noble F. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development 138: 2111–2120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon SH, Jeong SW, Jang JY, Lee JE, Lee SH, Kim SG, Kim YS, Cho YD, Kim HS, Kim BS, Jin SY. Cyclooxygenase-2 and vascular endothelial growth factor in chronic hepatitis, cirrhosis and hepatocellular carcinoma. Clin Mol Hepatol 18: 287–294, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Couteur DG, Cogger VC, Markus AM, Harvey PJ, Yin ZL, Ansselin AD, McLean AJ. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology 33: 537–543, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Yang J, Newell P, Singh S, Parwani A, Friedman SL, Nejak-Bowen KN, Monga SP. beta-Catenin signaling in hepatocellular cancer: Implications in inflammation, fibrosis, and proliferation. Cancer Lett 343: 90–97, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell 130: 691–703, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung A, Ciau-Uitz A, Pinheiro P, Monteiro R, Zuo J, Vyas P, Patient R, Porcher C. Uncoupling VEGFA functions in arteriogenesis and hematopoietic stem cell specification. Dev Cell 24: 144–158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancinelli R, Onori P, Gaudio E, Franchitto A, Carpino G, Ueno Y, Alvaro D, Annarale LP, Demorrow S, Francis H. Taurocholate feeding to bile duct ligated rats prevents caffeic acid-induced bile duct damage by changes in cholangiocyte VEGF expression. Exp Biol Med 234: 462–474, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell SJ, Huizer-Pajkos A, Cogger VC, McLachlan AJ, Le Couteur DG, Jones B, de Cabo R, Hilmer SN. Age-related pseudocapillarization of the liver sinusoidal endothelium impairs the hepatic clearance of acetaminophen in rats. J Gerontol 66: 400–408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Modern Pathol 16: 552–557, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Morell CM, Fabris L, Strazzabosco M. Vascular biology of the biliary epithelium. J Gastroenterol Hepatol 28, Suppl 1: 26–32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med 124: 1061–1065, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26: 149–150, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Shimizu H, Miyazaki M, Wakabayashi Y, Mitsuhashi N, Kato A, Ito H, Nakagawa K, Yoshidome H, Kataoka M, Nakajima N. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol 34: 683–689, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res 104: 576–588, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, Yuan J, Hefner C, Chartier C, Lee JS, Jiang S, Nayak NR, Kuypers FA, Ma L, Sundram U, Wu G, Garcia JA, Schrier SL, Maher JJ, Johnson RS, Yancopoulos GD, Mulligan RC, Kuo CJ. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med 12: 793–800, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem 49: 121–130, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Tygstrup N, Winkler K, Mellemgaard K, Andreassen M. Determination of the hepatic arterial blood flow and oxygen supply in man by clamping the hepatic artery during surgery. J Clin Invest 41: 447–454, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Marschall Z, Cramer T, Hocker M, Finkenzeller G, Wiedenmann B, Rosewicz S. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut 48: 87–96, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokomori H, Oda M, Yoshimura K, Nagai T, Ogi M, Nomura M, Ishii H. Vascular endothelial growth factor increases fenestral permeability in hepatic sinusoidal endothelial cells. Liver Int 23: 467–475, 2003 [DOI] [PubMed] [Google Scholar]