Abstract
ANG (1-7) contributes to the blood pressure (BP)-lowering effect of angiotensin receptor blockers (ARBs) in male experimental animals. Females have greater ANG (1-7) concentrations than males; however, the contribution of ANG (1-7) to ARB-mediated decreases in BP in females is unknown. The current study tested the hypothesis that female spontaneously hypertensive rats (SHR) have a larger ANG (1-7) contribution to the BP-lowering effects of the ARB candesartan than male SHR. Twelve-week-old male and female SHR were randomized to receive candesartan (0.5 mg·kg−1·day−1; 7 days), candesartan plus ANG II (200 ng·kg−1·min−1; 7 days), the ANG (1-7) antagonist A-779 (48 μg·kg−1·h−1) plus candesartan and ANG II. Candesartan decreased basal BP in males and females (baseline vs. candesartan: 142 ± 2 vs. 122 ± 3 and 129 ± 1 vs. 115 ± 1 mmHg, respectively; P < 0.05); however, the decrease was greater in males. ANG II increased BP in males in the presence of candesartan (149 ± 2 mmHg; P < 0.05); candesartan blocked ANG II-induced increases in BP in females (116 ± 1 mmHg). Pretreatment with A-779 abolished candesartan-mediated decreases in BP in females, but not males. A-779 also exacerbated ANG II-induced proteinuria (26 ± 6 vs. 77 ± 11 μg·kg−1·day−1, respectively; P < 0.05) and nephrinuria (20 ± 5 vs. 202 ± 58 μg·kg−1·day−1, respectively; P < 0.05) in candesartan-treated female SHR, with no effect in males. In conclusion, females are more sensitive to the BP-lowering effect of ARBs during ANG II infusion, whereas males are more sensitive under basal conditions. In addition, ANG (1-7) has a greater contribution to ARB-mediated decreases in BP, protein, and nephrin excretion in females relative to males.
Keywords: gender, SHR, blood pressure, renin-angiotensin system, ANG (1-7)
the renin-angiotensin system (RAS) is critical in the regulation of blood pressure (BP), and RAS overactivation has been implicated in the development and maintenance of hypertension. Spontaneously hypertensive rats (SHR) are an established model of human hypertension, and BP in SHR is RAS-dependent (29). Angiotensin (ANG) II activation of AT1 receptors mediates most well-known biological functions of the RAS (4, 39), and male SHR have greater AT1 receptor expression in the renal cortex (36, 38), mesenteric arteries, and aorta (35) compared with females. However, there is controversy in the literature regarding the functional implications of sex differences in AT1 receptor expression in experimental models of hypertension, and whether it affects BP control. Male SHR have been reported to be either more, less, or equally sensitive to the BP-lowering effects of AT1 receptor blockade (ARB) than females (34, 42), while male Sprague-Dawley rats are either more or equally sensitive to ARB-mediated decreases in BP than females (30, 42).
ANG (1-7) is a peptide in the RAS pathway that opposes AT1-mediated effects leading to vasodilation (3), improved renal blood flow (32), and enhanced pressure natriuresis (19). Inhibition of ANG (1-7) in male SHR attenuates the effectiveness of RAS inhibitors to lower BP (24, 25), supporting a role for ANG (1-7) in contributing to the BP-lowering effects of RAS inhibitors in male SHR. We recently published that female SHR have higher concentrations of renal cortical ANG (1-7) under basal conditions, as well as following chronic ANG II infusion compared with males (36). Moreover, inhibition of ANG (1-7) increased BP sensitivity to ANG II-induced hypertension only in females (36). There are numerous established sex differences in both the expression of RAS components and in the physiological responses to perturbations of the RAS (43); therefore, the question arises whether there is also a sex difference in the relative contribution of ANG (1-7) in ARB-mediated decreases in BP.
Therefore, the goals of this study were to determine 1) whether there is a sex difference in the sensitivity to ARBs under basal conditions and during ANG II-mediated hypertension, and 2) whether there is a sex difference in the contribution of ANG (1-7) to ARB-mediated decreases in BP and proteinuria. Based on our previous publications and the work of others establishing a greater role for the vasodilatory components of the RAS in females compared with males (20), we hypothesized that the contribution of ANG (1-7) to the BP-lowering effects of the ARB candesartan will be greater in female SHR compared with male SHR. In addition, we previously published that chronic ANG II infusion results in renal injury in both male and female SHR (36); however, pretreatment with the ANG (1-7)-mas receptor blocker A-779 exacerbated proteinuria only in the female SHR. Therefore, we further hypothesize that ANG (1-7) will offer greater protection against ANG II-induced increases in protein, nephrin, and KIM-1 excretion in females relative to males.
METHODS
Animals.
Male and female SHR were used in this study (Harlan Laboratories, Indianapolis, IN, and colony rats maintained at Georgia Reagents University). All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Georgia Reagents University Institutional Animal Care and Use Committee. A subset of male and female SHR were implanted with telemetry transmitters (Data Sciences, St. Paul, MN) at 10 wk of age while anesthetized with isoflurane (1.5%). Rats were allowed 1 wk to recover from surgery before being placed on receivers. Baseline BP was measured for 1 wk, and then candesartan (0.5 mg·kg−1·day−1) was administered for 1 additional wk via drinking water (n = 7–8). Rats were individually housed throughout the study. Water intake and body weights were measured every other day, and the dose of candesartan in the drinking water was adjusted as needed to maintain consistent dosing between sexes; metabolic parameters are listed in Table 1. Drinking water containing candesartan was prepared separately for each sex to account for sex differences in weight and daily water volume intake. To assess the efficacy of candesartan, animals received subcutaneous osmotic minipumps (ALZET) to deliver ANG II (200 ng·kg−1·min−1; Phoenix, Burlingame, CA) while anesthetized with isoflurane (1.5%), for an additional 7 days in the presence of candesartan. To assess the contribution of ANG (1-7)-mas receptor activation on candesartan-mediated decreases in BP, separate groups of male and female SHR (n = 4–5, respectively) were implanted with osmotic minipumps to deliver the ANG (1-7)-mas receptor antagonist d-alanine-[Ang-(1-7)] (A-779) (48 μg·kg−1·h−1; Bachem, Torrance, CA) for 4 days before candesartan and ANG II treatment was initiated as described above. All rats were placed in metabolic cages for 24-h urine collection before any change in drug treatments, and at the end of the study. Animals were euthanized via exsanguination under ketamine/xylazine (50 mg/kg per 10 mg/kg ip) anesthesia before tissue was harvested and placed in liquid nitrogen.
Table 1.
Metabolic characteristics in vehicle and treated male and female SHR
| Urine Output, ml/day |
Water Intake, ml/day |
Body Wt, g |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Baseline | 15 ± 1 | 11 ± 1† | 31 ± 2 | 22 ± 1† | 303 ± 3 | 182 ± 3† |
| Cand | 15 ± 2 | 15 ± 1* | 31 ± 1 | 25 ± 1* | 327 ± 3* | 191 ± 3†,* |
| Cand+ANG II | 17 ± 1 | 15 ± 1* | 33 ± 2 | 29 ± 1* | 337 ± 4* | 195 ± 3†,* |
| Baseline | 12 ± 1 | 7 ± 1† | 27 ± 5 | 25 ± 1 | 276 ± 5 | 172 ± 4† |
| A-779 alone | 9 ± 1 | 10 ± 1 | 26 ± 2 | 23 ± 1 | 285 ± 2 | 185 ± 7† |
| A-779+Cand | 12 ± 1 | 13 ± 2* | 29 ± 2 | 25 ± 2 | 291 ± 5* | 180 ± 5† |
| A-779+Cand+ANG II | 13 ± 3 | 10 ± 1 | 37 ± 2 | 23 ± 2† | 302 ± 5* | 185 ± 5† |
Values are means ± SE.
Significant difference from baseline in the same sex,
significant difference from males, P < 0.05 for all comparisons. N = 4–6. Cand, candesartan.
A separate set of animals was used for biochemical analyses allowing for tissue measurements in rats that had been treated with candesartan alone. Male and female (n = 7–10) SHR were randomly assigned to the following groups: 1) candesartan alone (0.5 mg·kg−1·day−1), 2) candesartan + ANG II, or 3) vehicle control. Group 1 was treated with candesartan for 1 wk; group 2 was treated for 1 wk with candesartan and then an additional week of treatment with coadministration of candesartan and ANG II.
Urinary biochemical measurements.
Urinary protein concentration was determined by standard Bradford assay (Bio-Rad, Hercules, CA). Enzyme immunoassays measured kidney injury molecule-1 (KIM-1; R&D Systems, St. Paul, MN), and nephrin (Exocell, Philadelphia, PA) via the manufacturer's protocols.
Peptide analysis.
ANG (1-7) concentrations were measured by enzyme immunoassay after methanol extraction of the renal cortex, as described previously (38) via the manufacturer's protocol III (n = 7–10; Bachem). According to the kit manufacture, cross reactivity for this EIA is 100% for angiotensin I/II (1-7), and 0% for angiotensin I, II, III, and A.
Renal cortical homogenization and Western blot analysis.
Renal cortical samples (n = 4–6/group) were homogenized, as previously described (36). Protein concentrations were determined via standard Bradford assay (Bio-Rad) using BSA as the standard. Two-color immunoblots were performed using a polyclonal primary antibody to the mas receptor (Alomone Labs, Jerusalem, Israel). Specific bands were detected using the Odyssey Infrared Imager in conjunction with the appropriate IRDye secondary antibody (LI-COR Biosciences, Lincoln, NE). Actin (monoclonal, Sigma, St. Louis, MO) was used to verify equal protein loading, and all of the densitometric results were normalized to actin and reported as fold change from control.
Statistical analysis.
All data are presented as means ± SE. BP, protein, KIM-1, and nephrin excretion data within each sex were analyzed using repeated-measures ANOVA; between-sex comparisons in Figs. 1 and 5 were made using a Student's t-test. ANG (1-7) peptide data and mas receptor expression in treated males and females were compared using a two-way ANOVA; factor 1 was sex of the animal, and factor 2 was treatment. Mas receptor expressions within each sex were compared using a one-way ANOVA. Differences were considered statistically significant with P < 0.05. Analyses were performed using GraphPad Prism version 4.0 software (GraphPad Software, La Jolla, CA).
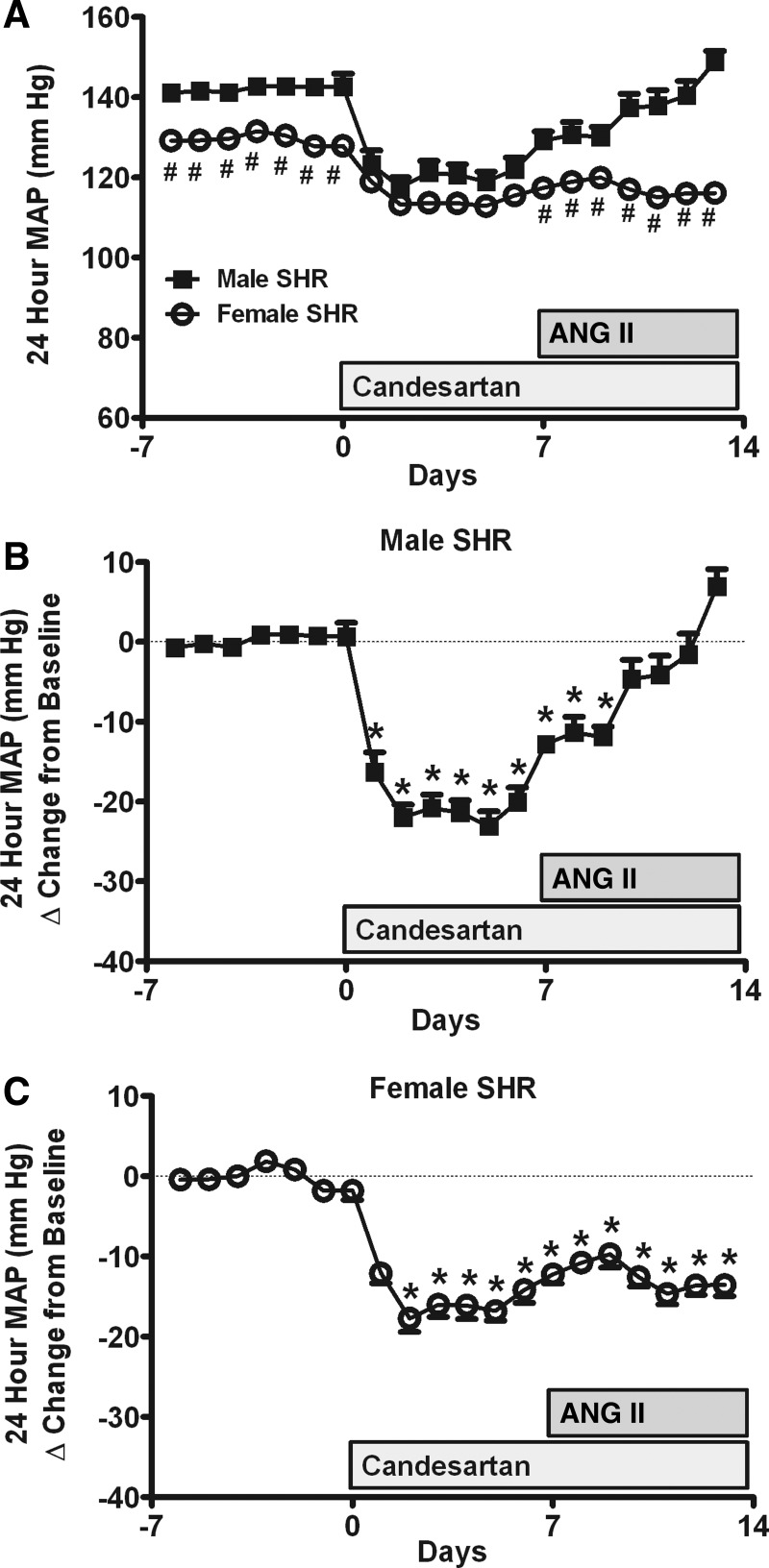
Fig. 1.
Effect of candesartan and ANG II infusion on 24-h mean arterial pressure (MAP) as measured by telemetry in male and female spontaneously hypertensive rats (SHR; A). Delta change in 24-h MAP from baseline in response to candesartan and ANG II in males (B) and females (C). Candesartan was initiated on day 0, followed by ANG II infusion on day 7. #Significant difference in blood pressure (BP) from males; *significant difference from baseline in the same sex, P < 0.05 for all comparisons. N = 7–8.
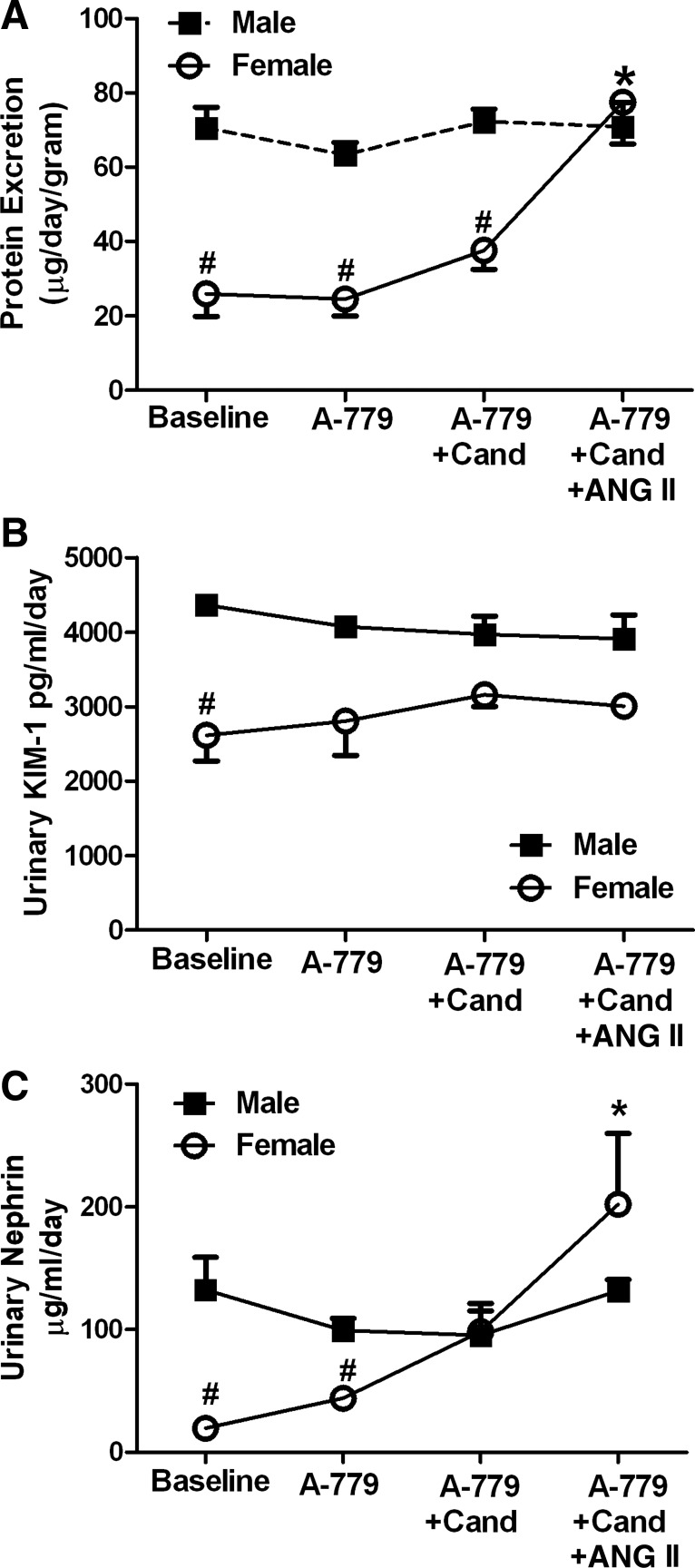
Fig. 5.
Effect of A-779 during candesartan and ANG II treatment on protein (A), kidney injury molecule-1 (KIM-1; B), and nephrin (C) excretion in male and female SHR. #Significant difference from males and *significant difference from baseline in the same sex, P < 0.05 for all comparisons. N = 4.
RESULTS
Effect of ARB on BP in male and female SHR.
Baseline BP was significantly greater in males compared with females (Fig. 1A). Candesartan rapidly decreased BP in both sexes; however, the decrease in BP was greater in males, as evidenced by the loss of the sex difference in BP. ANG II infusion in the presence of candesartan resulted in a steady increase in BP in male SHR (Fig. 1B); candesartan blocked ANG II-induced increases in BP in female SHR (P < 0.05 vs. males; Fig. 1C). As a result, BP in male SHR was significantly greater than in females at the end of the study. Increasing candesartan to 2 mg·kg−1·day−1 further decreased BP and blocked ANG II-induced increases in BP in male SHR (140 ± 6 to 117 ± 3 mmHg; P < 0.05); female SHR exhibited a further decrease in BP to the higher dose of candesartan despite the presence of ANG II (122 ± 3 to 106 ± 3 mmHg; P < 0.05).
Urinary protein excretion was also measured. Baseline protein excretion was greater in males compared with females (61 ± 1 vs. 11 ± 2 μg·day−1·g−1 body wt, respectively; P = 0.002). Protein excretion was unchanged in males following treatment with either candesartan [50 ± 6 μg·day−1·g−1 body wt; not significant (ns)] or ANG II (52 ± 5 μg·day−1·g−1 body wt; ns). Similarly, treatment with candesartan did not alter protein excretion in female SHR (13 ± 1 μg·day−1·g−1 body wt; ns); however, ANG II increased protein excretion from baseline levels (19 ± 2 μg·day−1·g−1 body wt; P = 0.028). Regardless, protein excretion remained significantly greater in males compared with females under all treatment conditions (P < 0.05).
Effect of ARB on ANG (1-7) peptide concentrations and Mas receptor expression.
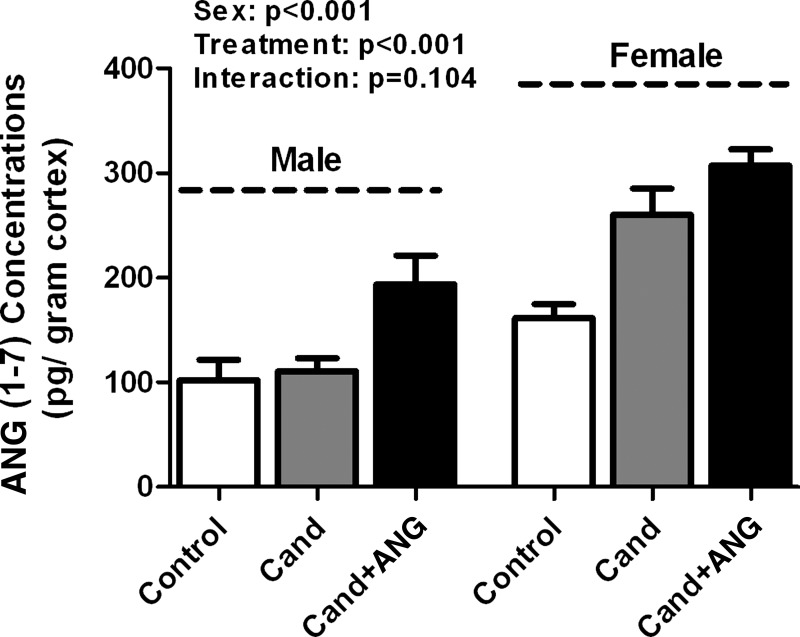
To gain mechanistic insight into sex differences in BP responses and proteinuria following treatment with candesartan and ANG II, renal cortical ANG (1-7) peptide levels were measured in male and female SHR under basal conditions, following treatment with candesartan alone, and following candesartan and ANG II (Fig. 2). Male SHR had lower basal renal cortical ANG (1-7) concentrations compared with females (102 ± 7 vs. 161 ± 13 pg/g cortex), although this did not reach statistical significance when compared using a two-way ANOVA. ANG (1-7) concentrations were not altered in male SHR by treatment with candesartan alone; however, ANG (1-7) concentrations were increased following ANG II infusion (P < 0.01). In females, renal cortical ANG (1-7) concentrations significantly increased following treatment with candesartan alone (P < 0.01); ANG II infusion did not result in a further significant increase. Female SHR maintained higher concentrations of ANG (1-7) following candesartan alone (P < 0.001), and with ANG II infusion (P < 0.001) compared with males. Mas receptor expression was also measured in the renal cortex under control, candesartan alone, and candesartan plus ANG II treatments. There were no significant changes in mas protein expression due to the effect of treatment in either males or females (Fig. 3, A and B; respectively). Similarly, mas receptor expression was comparable between the sexes regardless of treatment (Fig. 3C).
Fig. 2.
Renal cortical ANG (1-7) concentrations in vehicle control, candesartan (cand), and candesartan + ANG II-infused male and female SHR. N = 7–10.
Fig. 3.
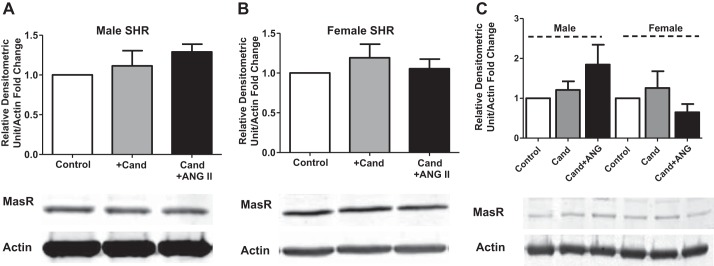
Renal cortical mas protein expression in vehicle control, candesartan, and candesartan + ANG II-infused male (A) and female (B) SHR. C: allows for the direct comparison of sex on mas receptor protein expression. N = 4–6.
Effect of ANG (1-7) blockade on ARB-mediated decreases in BP.
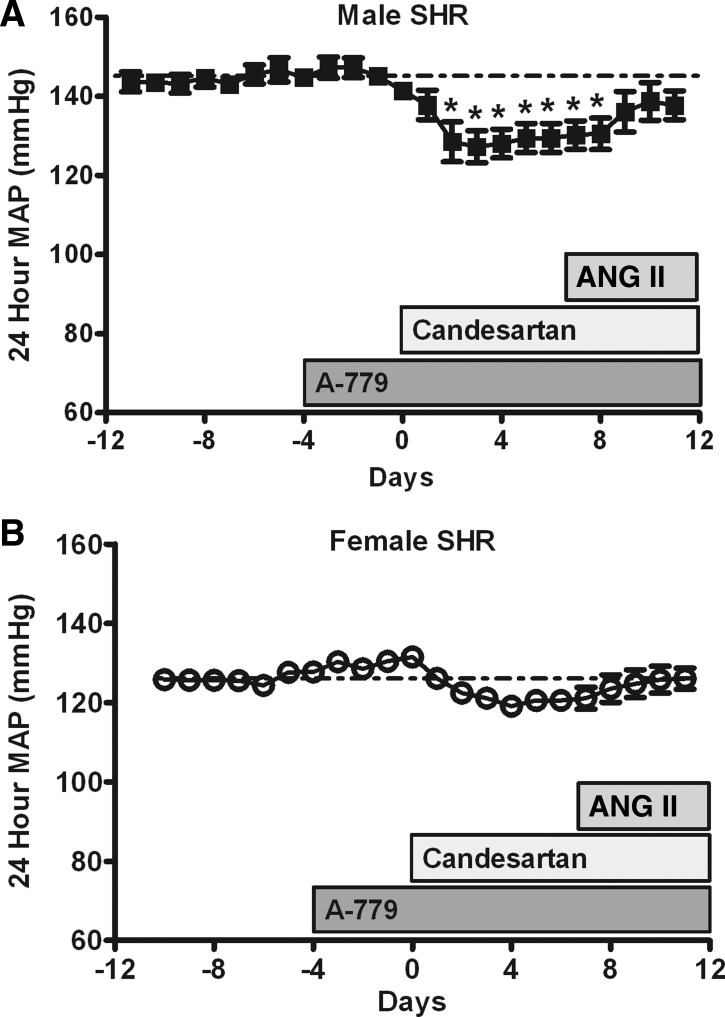
Additional studies assessed the contribution of ANG (1-7) to candesartan-mediated decreases in BP. Consistent with previous publications, A-779 alone did not alter baseline BP in either male or female SHR (1, 7, 36, 40). Candesartan significantly decreased BP from baseline in male SHR in the presence of A-779 (Fig. 4A; P < 0.05). In contrast, treatment with A-779 blocked candesartan-mediated decreases in BP from baseline in female SHR (Fig. 4B). Infusion of ANG II increased BP in male SHR treated with A-779 in the presence of candesartan, although ANG II infusion had no effect on BP in female SHR.
Fig. 4.
Effect of A-779 during candesartan and ANG II treatment on 24-h MAP as measured by telemetry in male (A) and female (B) SHR. A-779 infusion began 4 days before candesartan treatment, candesartan was initiated on day 0, and ANG II infusion was started on day 7. *Significant difference from baseline in the same sex, P < 0.05. N = 4–5.
Effect of ANG (1-7) on proteinuria, nephrinuria, and KIM-1 excretion.
Urinary excretion of protein, KIM-1, and nephrin were assessed in animals pretreated with A-779 during treatments with candesartan alone and following ANG II infusion (Fig. 4). Males had greater proteinuria compared with females at baseline. Protein excretion in males was not altered by any of the treatments (Fig. 5A). Neither A-779 alone, or in combination with candesartan, altered proteinuria in female SHR. However, inclusion of ANG II increased proteinuria in females treated with A-779 and candesartan, abolishing the sex difference (P = 0.004). Baseline excretion of KIM-1 was greater in males compared with females (P = 0.029; Fig. 5B). A-779 did not alter excretion of KIM-1 during treatment with candesartan alone or in the presence of ANG II in either sex. Nephrin excretion was also greater in males compared with females at baseline (P = 0.029; Fig. 5C), and pretreatment with A-779 had no effect on nephrinuria in male SHR under any conditions. However, the addition of ANG II significantly increased nephrinuria in females.
DISCUSSION
The primary novel finding of the current study is that while males are more sensitive to candesartan-mediated decreases in basal BP, females are more sensitive to candesartan-mediated blockade of ANG II-induced increases in BP. Greater sensitivity to ARB-mediated blockade of ANG II hypertension in females was associated with greater increases in renal cortical ANG (1-7) levels. Moreover, blocking ANG (1-7) exacerbated proteinuria and nephrinuria only in candesartan-treated females following ANG II. Collectively, these data suggest that ANG (1-7) plays a greater role in modulating BP in females compared with males, particularly when the RAS is perturbed by either an ARB or ANG II infusion.
Despite consistent reports that AT1 receptor expression is greater in males (35, 36, 38), the impact of sex on BP responses to ARBs remains unclear. BP responses in experimental animal models to losartan have been reported to be greater in young Sprague-Dawley rats (30) and aged (42) male SHR, comparable between the sexes in both Sprague-Dawley rats and SHR (10, 31), or reduced in male SHR (34). In the current study, BP was continuously measured using telemetry, and we used a lower dose of an ARB compared with other studies that allowed us to detect subtle changes in BP. Our results confirm that AT1 receptors are critical in maintaining elevated basal BP in both sexes of SHR. However, AT1 receptors contribute more to the maintenance of hypertension in male SHR, as evidenced by the greater decrease from baseline BP in male SHR during ARB treatment. To assess the efficacy of AT1 receptor blockade with candesartan in male and female SHR, studies challenged the blockade with ANG II infusion. A higher dose of the ARB losartan (15 mg·kg−1·day−1) was previously shown to abolish ANG II-induced increases in vasoconstriction (10) and BP in male and female SHR (42). Sex differences in BP sensitivity to candesartan likely reflect sex differences in AT1 receptor expression (35, 36); females have fewer AT1 receptors than males. Alternatively, sex differences in the pharmacokinetics of candesartan could explain sex differences in BP responses to candesartan. However, the published half-life and metabolism of candesartan are comparable between men and women (8, 16) and personal communications with lead scientists at AstraZeneca verified no differences in the metabolism of candesartan between male and female experimental animals. Therefore, sex differences in the BP response to candesartan are independent of sex differences in pharmacokinetics.
ANG (1-7) contributes to the BP-lowering effects of RAS inhibitors in male SHR (24, 25), and female SHR have higher concentrations of ANG (1-7) than males (36). We previously published that chronic ANG II infusion in the absence of candesartan increased ANG (1-7) concentrations in the renal cortex of male SHR, and the values were comparable to those found in the current study in male SHR treated with ANG II plus candesartan, further supporting the inability of low-dose candesartan to increase ANG (1-7) in male SHR. It should be noted that renal cortical ANG (1-7) localization was not examined; therefore, it is plausible that differences in localization may contribute to our observed functional differences. To our knowledge, there are no reports of sex differences in ANG (1-7) localization, although ANG (1-7) immunostaining has been reported in renal tubules of the inner cortex of virgin and pregnant rats (6). Candesartan alone did not increase ANG (1-7) in male SHR, although the inclusion of ANG II resulted in a significant increase, suggesting that ANG II acts as a substrate for ACE2 to increase ANG (1-7) in male SHR. Future studies will be designed to assess the mechanisms by which ANG (1-7) levels are increased in both sexes. Higher doses of RAS inhibitors than those used in the current study increase ANG (1-7) concentrations in male rats (12, 14, 24, 41). Therefore, a higher dose of candesartan may increase ANG (1-7) concentrations in male SHR (13).
Consistent with our previously published results in SHR (36), and reports in male diabetic SHR, WKY, and 2K1C Goldblatt hypertensive rats (1, 7, 40), A-779 had no effect on basal BP in either sex, although blocking ANG (1-7) abolished candesartan-mediated decreases in BP in female SHR. It should be noted that consistent with our previous publication; a role for ANG (1-7) in BP control in the current study was not uncovered until the RAS had been perturbed by AT1 receptor blockade and ANG II infusion. Our experimental design did not allow for the direct comparison of candesartan-mediated decreases in BP within each sex. Therefore, we cannot conclude that ANG (1-7) does not contribute to the BP-lowering effect of candesartan in males; it was simply less pronounced than in females. Our results are in agreement with studies indicating that female Dahl salt-sensitive rats have a prolonged decrease in BP to ANG (1-7) compared with males (11). We hypothesize that ANG (1-7) has less impact on BP in males relative to females. As a result, even a greater increase in ANG (1-7) in the males following ANG II infusion was not able to overcome ANG II-AT1-mediated increases in BP.
Inhibition of ANG (1-7) during treatment also had greater effects on proteinuria and nephrinuria in female SHR compared with males. None of the treatments altered protein, KIM-1, or nephrin excretion in males, which is likely related to the fact that although there was an increase in BP in male SHR with ANG II following candesartan treatment, BP did not significantly increase above baseline BP values at the beginning of the study. However, A-779 treatment significantly increased protein and nephrin excretion in female SHR abolishing the sex differences in both parameters. Increases in protein and nephrin excretion in female SHR occurred in the absence of an increase in BP, supporting our conclusion that ANG (1-7) plays a role in maintaining the integrity of renal barrier function in female SHR.
The mechanisms responsible for sex differences in BP responses to candesartan and ANG (1-7) remain unknown. To explore this, we measured mas receptor expression and found that total protein expression was unaltered by treatments and comparable between the sexes. However, sex differences in the functional contribution of ANG (1-7) to ARB-mediated decreases in BP may also be the result of sex differences in cellular or subcellular localization of mas, posttranslational modification of the receptor, or differences in signaling pathways downstream of receptor activation that would not be reflected by measurement of total protein alone. For example, females are known to have greater levels of nitric oxide (NO) compared with males (28, 37), and NO is a key signaling molecule downstream of ANG (1-7)-mediated receptor activation (18). Alternatively, losartan upregulates AT2 mRNA in mesenteric arteries from female SHR, but not males (10). Additionally, AT2 receptor activation has been suggested to enhance the anti-hypertensive effects of ARBs (20) and AT2 receptor activation contributes more to BP control in female Sprague-Dawley rats (22). Therefore, enhanced AT2 receptor activation may contribute to the lack of a BP response to ANG II in the presence of candesartan in female SHR. However, in the presence of A-779 and candesartan, there was no decrease in BP in female SHR during ANG II infusion to indicate a shunting of ANG II to the AT2 receptor. Female sex hormones are also a likely suspect due to tissue-specific regulation by estradiol of ACE/ACE2 and AT1/AT2 receptor genes (5); however, loss of female sex hormones does not alter renal cortical AT1 protein expression in females (2, 38). Future studies will be designed to further examine the molecular mechanisms responsible for sex differences in BP responses to ARBs.
In conclusion, there are sex differences in the response of hypertensive experimental animals to AT1 receptor blockade. Females are more sensitive to the BP-lowering effect of ARBs during ANG II infusion, while males are more sensitive under basal conditions. In addition, ANG (1-7) has a greater contribution to ARB-mediated decreases in BP, protein, and nephrin excretion in females relative to males.
Perspectives
Recent focus has shifted from the established idea that ANG II is the sole principal effector in mediating physiological responses to RAS activation to incorporate the biologically active vasodilatory peptide ANG (1-7). Inhibition of the AT1 receptor is a common and widely prescribed treatment for hypertension among both sexes; however, survival is higher among women with heart failure treated with ARBs (23) and women tend to have greater decreases in BP to ARBs compared with men (9, 27, 33). Our data suggest that there are sex differences in the effectiveness of RAS inhibitors to modulate BP and maintain the integrity of renal barrier function, as well as the mechanisms by which ARBs lower BP. These findings suggest that males need higher doses of candesartan than females to fully antagonize the RAS when activated.
GRANTS
This work was supported by the National Institutes of Health Grant (1R01 HL-093271-01A1) to J. C. Sullivan and the American Heart Association predoctoral fellowship (12PRE11470003) to M. A. Zimmerman. R. A. Harris is supported in part by the American Heart Association (10SDG3050006).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.Z., R.A.H., and J.C.S. conception and design of research; M.A.Z. performed experiments; M.A.Z. and J.C.S. analyzed data; M.A.Z., R.A.H., and J.C.S. interpreted results of experiments; M.A.Z. and J.C.S. prepared figures; M.A.Z. and J.C.S. drafted manuscript; M.A.Z., R.A.H., and J.C.S. edited and revised manuscript; M.A.Z., R.A.H., and J.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank AstraZeneca for the donation of candesartan and acknowledge the excellent technical assistance of Vanessa Kemp and G. Ryan Crislip.
REFERENCES
- 1.Al-Maghrebi M, Benter IF, Diz DI. Endogenous angiotensin-(1-7) reduces cardiac ischemia-induced dysfunction in diabetic hypertensive rats. Pharmacol Res 59: 263–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept 124: 7–17, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Benter IF, Ferrario CM, Morris M, Diz DI. Antihypertensive actions of angiotensin-(1-7) in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 269: H313–H319, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med 116: 263–272, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol 93: 658–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, Penninger J, Ferrario CM. Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension 42: 749–753, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Burgelova M, Vanourkova Z, Thumova M, Dvorak P, Opocensky M, Kramer HJ, Zelizko M, Maly J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1-7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens 27: 1988–2000, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Cabaleiro T, Roman M, Ochoa D, Talegon M, Prieto-Perez R, Wojnicz A, Lopez-Rodriguez R, Novalbos J, Abad-Santos F. Evaluation of the relationship between sex, polymorphisms in CYP2C8 and CYP2C9, and pharmacokinetics of angiotensin receptor blockers. Drug Metab Dispos 41: 224–229, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Canzanello VJ, Baranco-Pryor E, Rahbari-Oskoui F, Schwartz GL, Boerwinkle E, Turner ST, Chapman AB. Predictors of blood pressure response to the angiotensin receptor blocker candesartan in essential hypertension. Am J Hypertens 21: 61–66, 2008 [DOI] [PubMed] [Google Scholar]
- 10.de PRSF, dos Santos RA, Silva-Antonialli MM, Scavone C, Nigro D, Carvalho MH, de Cassia Tostes R, Fortes ZB. Differential effect of losartan in female and male spontaneously hypertensive rats. Life Sci 78: 2280–2285, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Eatman D, Wang M, Socci RR, Thierry-Palmer M, Emmett N, Bayorh MA. Gender differences in the attenuation of salt-induced hypertension by angiotensin (1-7). Peptides 22: 927–933, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Iskandar SS, Dean RH, Diz DI. Vasopeptidase inhibition and Ang-(1-7) in the spontaneously hypertensive rat. Kidney Int 62: 1349–1357, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ferrario CM, Jessup J, Gallagher PE, Averill DB, Brosnihan KB, Ann Tallant E, Smith RD, Chappell MC. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int 68: 2189–2196, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol 295: C1169–C1174, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gleiter CH, Jagle C, Gresser U, Morike K. Candesartan. Cardiovasc Drug Rev 22: 263–284, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Villalobos R, Klassen RB, Allen PL, Johanson K, Baker CB, Kobori H, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin-(1-7). Am J Physiol Renal Physiol 290: F1270–F1275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1-7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol 299: F983–F990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1-7): in vivo and in vitro studies. Am J Physiol Renal Fluid Electrolyte Physiol 270: F141–F147, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Hilliard LM, Mirabito KM, Denton KM. Unmasking the potential of the angiotensin AT2 receptor as a therapeutic target in hypertension in men and women: what we know and what we still need to find out. Clin Exp Pharmacol Physiol 40: 542–550, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Hilliard LM, Sampson AK, Brown RD, Denton KM. The “his and hers” of the renin-angiotensin system. Curr Hypertens Rep 15: 71–79, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Hudson M, Rahme E, Behlouli H, Sheppard R, Pilote L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure–a population study. Eur J Heart Fail 9: 602–609, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1-7) unmasked during combined treatment with lisinopril and losartan. Hypertension 31: 699–705, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1-7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension 31: 356–361, 1998 [DOI] [PubMed] [Google Scholar]
- 26.McCollum LT, Gallagher PE, Ann Tallant E. Angiotensin-(1-7) attenuates angiotensin II-induced cardiac remodeling associated with upregulation of dual-specificity phosphatase 1. Am J Physiol Heart Circ Physiol 302: H801–H810, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McInnes GT. Angiotensin II antagonism in clinical practice: experience with valsartan. J Cardiovasc Pharmacol 33, Suppl 1: S29–32; discussion S41–23, 1999 [DOI] [PubMed] [Google Scholar]
- 28.McIntyre M, Hamilton CA, Rees DD, Reid JL, Dominiczak AF. Sex differences in the abundance of endothelial nitric oxide in a model of genetic hypertension. Hypertension 30: 1517–1524, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35: 480–483, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Reverte V, Tapia A, Baile G, Gambini J, Gimenez I, Llinas MT, Salazar FJ. Role of angiotensin II in arterial pressure and renal hemodynamics in rats with altered renal development: age- and sex-dependent differences. Am J Physiol Renal Physiol 304: F33–F40, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Saez F, Castells MT, Zuasti A, Salazar F, Reverte V, Loria A, Salazar FJ. Sex differences in the renal changes elicited by angiotensin II blockade during the nephrogenic period. Hypertension 49: 1429–1435, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol 284: H1985–H1994, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Saunders E, Cable G, Neutel J. Predictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: a secondary analysis of the irbesartan/hydrochlorothiazide blood pressure reductions in diverse patient populations (INCLUSIVE) study. J Clin Hypertens (Greenwich) 10: 27–33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva-Antonialli MM, Fortes ZB, Carvalho MH, Scivoletto R, Nigro D. Sexual dimorphism in the response of thoracic aorta from SHRs to losartan. Gen Pharmacol 34: 329–335, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1-7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 298: R61–R69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000 [PubMed] [Google Scholar]
- 40.Widdop RE, Sampey DB, Jarrott B. Cardiovascular effects of angiotensin-(1-7) in conscious spontaneously hypertensive rats. Hypertension 34: 964–968, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Yamada K, Iyer SN, Chappell MC, Brosnihan KB, Fukuhara M, Ferrario CM. Differential response of angiotensin peptides in the urine of hypertensive animals. Regul Pept 80: 57–66, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 291: R383–R390, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman MA, Sullivan JC. Hypertension: what's sex got to do with it? Physiology (Bethesda) 28: 234–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]