Abstract
Overactivation of hypoxia-inducible factor (HIF)-1α is implicated as a pathogenic factor in chronic kidney diseases (CKD). However, controversy exists regarding the roles of HIF-1α in CKD. Additionally, although hypoxia and HIF-1α activation are observed in various CKD and HIF-1α has been shown to stimulate fibrogenic factors, there is no direct evidence whether HIF-1α is an injurious or protective factor in chronic renal hypoxic injury. The present study determined whether knocking down the HIF-1α gene can attenuate or exaggerate kidney damage using a chronic renal ischemic model. Chronic renal ischemia was induced by unilaterally clamping the left renal artery for 3 wk in Sprague-Dawley rats. HIF-1α short hairpin (sh) RNA or control vectors were transfected into the left kidneys. Experimental groups were sham+control vector, clip+control vector, and clip+HIF-1α shRNA. Enalapril was used to normalize blood pressure 1 wk after clamping the renal artery. HIF-1α protein levels were remarkably increased in clipped kidneys, and this increase was blocked by shRNA. Morphological examination showed that HIF-1α shRNA significantly attenuated injury in clipped kidneys: glomerular injury indices were 0.71 ± 0.04, 2.50 ± 0.12, and 1.34 ± 0.11, and the percentage of globally damaged glomeruli was 0.02, 34.3 ± 5.0, and 6.3 ± 1.6 in sham, clip, and clip+shRNA groups, respectively. The protein levels of collagen and α-smooth muscle actin also dramatically increased in clipped kidneys, but this effect was blocked by HIF-1α shRNA. In conclusion, long-term overactivation of HIF-1α is a pathogenic factor in chronic renal injury associated with ischemia/hypoxia.
Keywords: collagen, α-smooth muscle actin, renal fibrosis, chronic kidney diseases
reduced renal tissue oxygen levels have been demonstrated in a large variety of chronic kidney diseases (CKD) in both human patients and in experimental animal models. Hypoxia in CKD results from a combination of structural and functional changes (12, 36). As a result, hypoxia-inducible factor (HIF)-1α has been reported to be consistently upregulated in almost all types of CKD (7, 16, 17, 36–38). However, it is unclear whether upregulation of HIF-1α is beneficial or deleterious in progressive CKD. HIF-1α is a transcription factor and has been shown to stimulate collagen accumulation (7, 15, 43, 44) and promote the epithelial-to-mesenchymal transition (EMT) (11, 35), an important mechanism involved in the progression of CKD (3, 33, 57, 67). Therefore, although upregulation of HIF-1α is protective in acute kidney injury (9, 18, 37, 53), ample evidence indicates that long-term overactivation of HIF-1α may be a pathogenic factor in CKD (10, 16, 21, 24, 36, 45).
Previous studies have shown that genetic ablation of renal epithelial HIF-1α inhibits the development of renal tubulointerstitial fibrosis in unilateral ureteral obstruction rats (15) and that overexpression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis in ⅚ nephrectomy mice (23). We have also reported that silencing HIF-1α gene expression attenuates angiotensin II-induced profibrotic effects and transforming growth factor (TGF) β1-induced EMT in renal cells in vitro and in vivo (13, 62, 71). A more recent study has shown that increasing HIF-1α level exacerbates the kidney damage in a rat model of hypertension induced by a high-salt diet and nitric oxide withdrawal (5). Taken together, these studies suggest that overactivation of HIF-1α is an injurious factor in CKD.
However, there have been controversial reports regarding the role of HIF-1α in CKD. Induction of HIF-1α by CoCl2 ameliorates the renal injury in rats with nephritis (60) and hypertensive type 2 diabetes (46). In contrast to the deleterious effects of overexpressed HIF-1α in ⅚ nephrectomy mice (23), other reports have shown that upregulation of HIF-1α by pharmacological agents protects the kidneys using the same ⅚ nephrectomy model of CKD (6, 56, 59). Thus more detailed investigations are required regarding the role of the HIF-1α pathway in CKD under different situations.
Because of these disparate observations, it is imperative to clarify the role of HIF-1α in CKD. This clarification is critical for the application of HIF-1α activation or inhibition as a potential therapeutic strategy. The present study was to further elucidate whether ischemia-induced activation of HIF-1α is a beneficial or injurious factor in chronic kidney damage. We used a two-kidney, one-clip (2K1C) rat model treated with an angiotensin-converting enzyme (ACE) inhibitor so as to eliminate the possible effect of activation of the renin-angiotensin system in this model. By using this model, we attempted to minimize other impacts and evaluate the effect of increased HIF-1α on chronic renal injury in clipped kidneys. The present study determined whether silencing HIF-1α gene expression by short hairpin (sh) RNA attenuates or exaggerates renal injury in the clipped kidneys in 2K1C rats. To our knowledge, the present study provides the first direct evidence that chronic ischemic/hypoxic activation of HIF-1α produces chronic kidney damage.
MATERIALS AND METHODS
Animals.
Experiments were performed using male Sprague-Dawley rats (250–350 g, Harlan, Madison, WI) with free access to food and water throughout the study. All animal procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Plasmids expressing rat HIF-1α shRNA.
Predesigned rat HIF-1α siRNA was purchased from Sigma-Aldrich. Sequences of HIF-1α siRNA were sense, GGA AAG AGA CUC AUA GAA A; and antisense, UUU CUA UGA CUC UCU UUC C. After confirmation of effective knockdown of HIF-1α genes by these siRNAs in cultured rat renal cells, the sequences were constructed into a pRNA-CMV3.2 vector (Genscript, Piscataway, NJ) to produce shRNA. The effective silencing of the renal HIF-1α gene by shRNA in vivo was verified in our previous studies (62, 71). Plasmids expressing luciferase were used in control animals.
Transfection of DNA into the kidney.
DNA was transfected into the rat kidneys as we described previously (66, 71). In brief, rats were anesthetized with 2% isoflurane, and 50 μg of plasmids mixed with 8 μl of in vivo jetPEI (Polyplus Transfection, New York, NY) in 10% glucose (600 μl) were injected into the kidneys via the left renal artery when the renal artery and vein were temporarily blocked (<5 min). After injection, an ultrasound transducer (Sonitron 2000, Rich-Mar) was applied directly onto the kidney with an output of 1 MHz at 10% power for a total of 60 s with 30-s intervals, and then the renal artery and vein were unblocked to recover renal blood flow. This technique has been shown to effectively deliver DNA into the renal cells without toxicity to the kidney (26, 27, 41, 66, 71). The transfection reagent in vivo jetPEI, a polyethylenimine derivative, has been used to successfully deliver DNA into renal cells in vivo in previous studies, including ours (8, 29, 34, 63, 69, 70). In addition, a combination of ultrasound and different transfection reagents (19, 30, 40), including polyethylenimine nanoparticles (4), has been shown to significantly enhance the DNA transfection. We showed before that the expression of a transgene in the kidney peaked on around days 5–7 and gradually decreased thereafter, while the mRNA levels in transfected animals remained 4.5 times higher than that in control animals 4 wk after transfection (66). The in vivo expression time period of the transgene in our studies is consistent with reports by others using nonviral vectors and different DNA delivery methods, which have shown that in vivo overexpression of transgenes lasts for at least 2 or 4 wk (41, 52, 65). In addition, it has been shown that by using DNA-based small interfering (si) RNA expression vectors, target gene knockdown can endure for 20 wk in vivo (64). We therefore utilized this technique for in vivo gene silencing in the kidneys.
Induction of chronic renal ischemia in the left kidney.
To produce chronic renal ischemia, the 2K1C model was utilized. In the same surgery as above, after DNA transfection, the left renal artery was clipped by placing of a U-shaped silver clip with an internal diameter of 0.30 mm as described before (14, 28). The same surgical procedures were performed in sham rats, in which the clips were removed in the surgical procedures. Three groups of animals were included: sham+control plasmids, clip+control plasmids, and clip+HIF-1α shRNA plasmids.
Chronic monitoring of arterial blood pressure in conscious rats.
A telemetry transmitter (Data Sciences International) was implanted for the measurement of mean arterial blood pressure (MAP) as we described previously (31). One week after clamping the renal artery, all clipped rats showed increases in MAP, indicating a successful generation of the model, and then an ACE inhibitor enalapril (5 mg·kg−1·day−1) was given in drinking water for the rest of the experiment. Two weeks later, animals were humanely euthanized and kidneys were removed. The clipped kidneys were cut longitudinally. Half of the kidney was fixed in 10% neutral buffered formalin and the other half frozen in liquid N2 and stored in −80°C.
Preparation of tissue homogenate and nuclear extracts and Western blot analyses for protein levels of HIF-1α, collagen I/III, and α-smooth muscle actin.
Renal tissue homogenates and nuclear protein were prepared, and Western blot analyses were performed as we described previously (32). Primary antibodies used in the present study included anti-rat HIF-1α (monoclonal, 1:300 dilution, Novus Biologicals), collagen I/III (rabbit polyclonal, 1:300, Calbiochem), and α-smooth muscle actin (α-SMA; rabbit polyclonal, 1:1,000, Abcam). The intensities of the blots were determined using an imaging analysis program (ImageJ, free download from http://rsbweb.nih.gov/ij/).
Morphological and immunohistochemical analysis.
The fixed kidneys were paraffin-embedded and cut into 4-μm sections. For morphological analysis, the tissue sections were stained with periodic acid-Schiff (PAS). Glomerular damage was morphologically evaluated by two independent examiners who were blinded as to animal groups. The damage was semiquantitatively scored based on the degree of glomerular damage as described previously (39, 51). In brief, a minimum of 50 glomeruli in each specimen were examined, and the severity of the lesion was graded from 0 to 4 according to the percentage of glomerular involvement. Thus 0 = normal; 1 = 1–25% of glomerular area involved; 2 = 26–50%; 3 = 51–75%; and 4 = >75% of tuft area involved. The averaged scores from counted glomeruli were used as the glomerular damage index for each animal.
Immunostaining was performed as we described before (32) using antibodies against rat α-SMA (rabbit polyclonal, 1:200, Abcam). Collagen I/III was stained using picro sirius red, and the percentage of positive-stained area was calculated using a computer program (Image-Pro Plus) as described previously (61).
Statistics.
Statistics were performed using SigmaStat. Data are presented as means ± SE. The significance of differences in mean values within and among three experimental groups was evaluated using ANOVA, and any significant differences revealed by this procedure were further investigated using appropriate post hoc tests as indicated in results. P < 0.05 was considered statistically significant.
RESULTS
Changes in arterial pressure.
MAP was significantly increased in 2K1C rats, suggesting the successful generation of a renal ischemic model (Fig. 1). Treatment with an ACE inhibitor, enalapril, normalized MAP in clipped rats (Fig. 1), which eliminated the potential impact of the activated angiotensin system on renal injury in clipped kidneys.
Fig. 1.
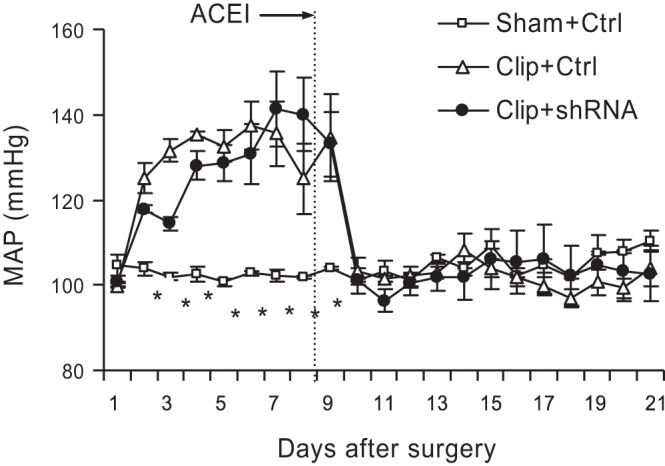
Changes in mean arterial blood pressure (MAP). Angiotensin-converting enzyme-1 (ACEI) indicates the start of enalapril treatment. *P < 0.001 vs. other 2 groups by 2-way repeated measures ANOVA (n = 7).
Effects of HIF-1α shRNA on hypoxia-induced HIF-1α accumulation in clipped kidneys.
To confirm the successful inhibition of HIF-1α accumulation in the clipped kidneys by shRNA, HIF-1α protein levels were determined. Renal HIF-1α protein levels were significantly upregulated in clipped kidneys. In rats transfected with shRNA plasmids, HIF-1α protein levels in the clipped kidneys were much lower than that in rats treated with control vectors in both renal cortical and medullary areas (Fig. 2), indicating a successful inhibition of HIF-1α accumulation in clipped kidneys.
Fig. 2.
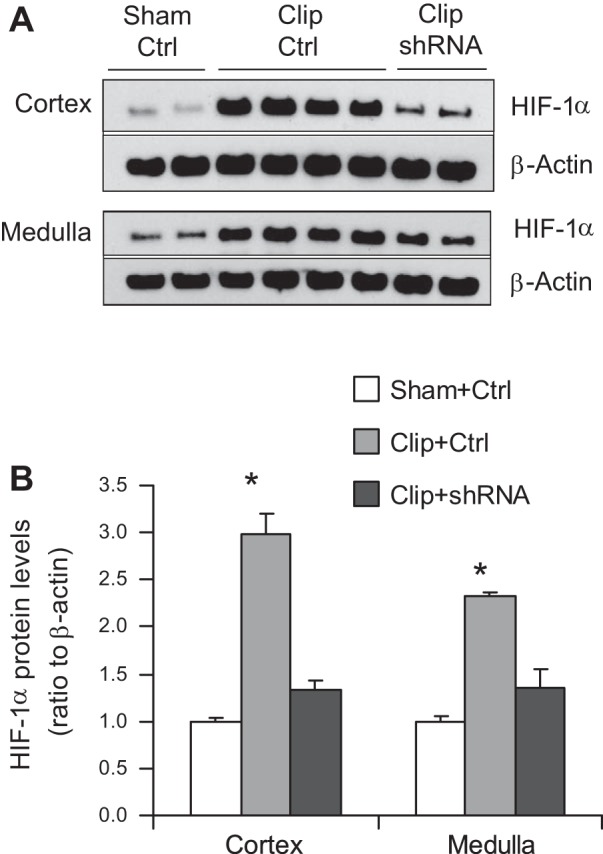
Effects of hypoxia-inducible factor (HIF)-1α short hairpin (sh) RNA on HIF-1α accumulation in clipped kidneys. A: representative enhanced chemiluminescence (ECL) gel documents of Western blot analyses depicting protein levels of HIF-1α. B: summarized intensities of HIF-1α blots (normalized to Sham+Ctrl). *P < 0.001 vs. other 2 groups by 1-way ANOVA with Tukey's post hoc test (n = 7).
Effects of HIF-1α shRNA on histological changes in the glomeruli in clipped kidneys.
Morphological analysis showed significant glomerular damage in clipped kidneys as indicated by glomerular mesangial expansion with hypercellularity, capillary collapse, and fibrous deposition in glomeruli (Fig. 3A). The glomerular damage index was substantially greater in clipped kidneys (Fig. 3B). However, HIF-1α shRNA transfection significantly attenuated glomerular damage in clipped kidneys (Fig. 3, A and B). Strikingly, the significantly larger number of globally damaged glomeruli in clipped kidneys was dramatically reduced by HIF-1α shRNA (Fig. 4). These results suggested that activation of HIF-1α mediates ischemia-induced glomerular injury.
Fig. 3.
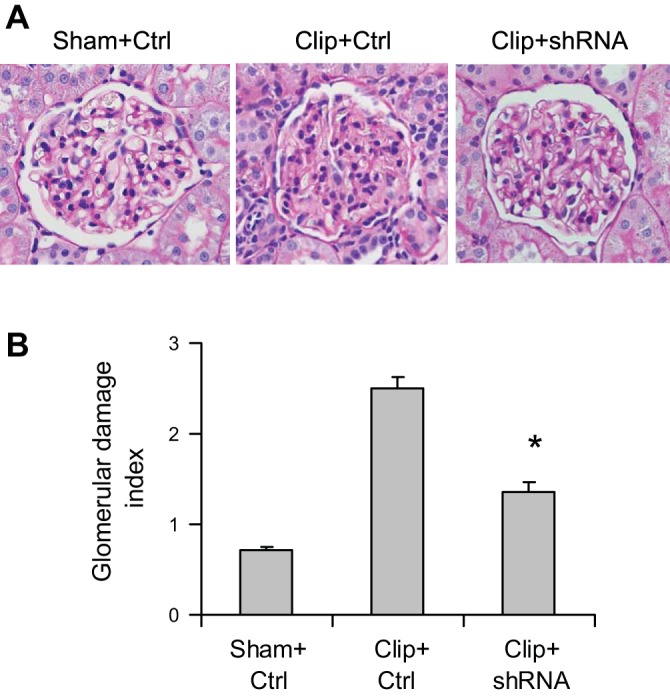
Effect of HIF-1α shRNA on morphological changes in the glomeruli in clipped kidneys. A: representative photomicrographs showing glomerular structures [periodic acid-Schiff (PAS) staining, ×400]. B: summarized glomerular damage index by semiquantitation of scores in different groups. *P < 0.001 vs. Clip+Ctrl and P = 0.003 vs. Sham+Ctrl by 1-way ANOVA with Tukey's post hoc test (n = 7).
Fig. 4.
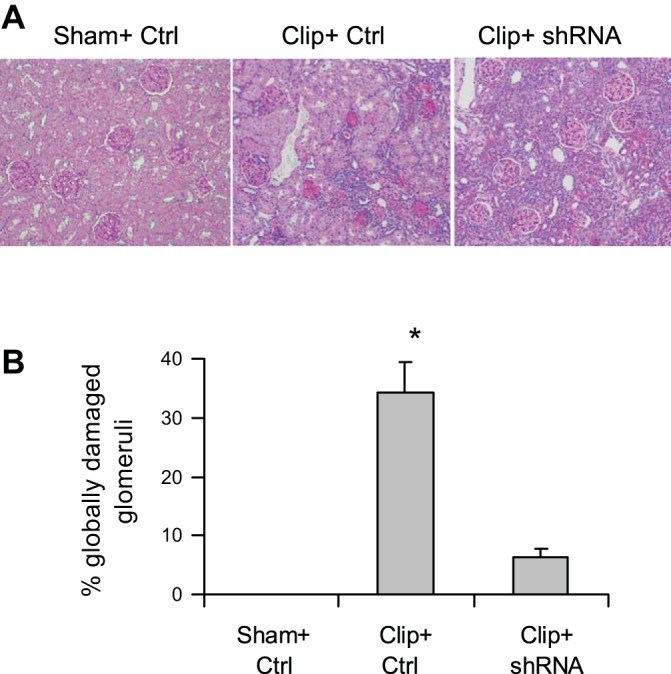
Effect of HIF-1α shRNA on the percentage of globally damaged glomeruli in clipped kidneys. A: representative photomicrographs showing glomeruli (PAS staining, ×100). B: calculated percentage of globally damaged glomeruli. *P < 0.001 vs. other 2 groups by 1-way ANOVA with Tukey's post hoc test (n = 7).
Effects of HIF-1α shRNA on interstitial injuries in clipped kidneys.
The positive staining of collagen and α-SMA in the outer medulla was used as the index of interstitial injury. The positive-stained areas of collagens were significantly larger in clipped kidneys than those in sham rats (Fig. 5). In rats treated with HIF-1α shRNA, the positive-stained areas of collagens were much smaller than those in control vector-treated rats (Fig. 5). The analysis of α-SMA staining showed the same pattern as that of collagens: the positive-stained areas of α-SMA in clipped kidneys were much larger compared with those in sham rats, whereas the positive-stained areas of α-SMA were significantly less in HIF-1α shRNA-treated rats compared with control vector-treated rats (Fig. 6). Further quantitation of collagen I/III and α-SMA expression in the kidneys by Western blot analyses also showed that the protein levels of collagens and α-SMA were significantly higher in clipped kidneys and much lower in the clipped kidneys treated with HIF-1α shRNA (Fig. 7), which is consistent with the results of collagen and α-SMA staining (Figs. 5 and 6). These data demonstrate that HIF-1α activation mediates the fibrotic damage in the renal tubulointerstitial area in chronic ischemic/hypoxic renal injury.
Fig. 5.
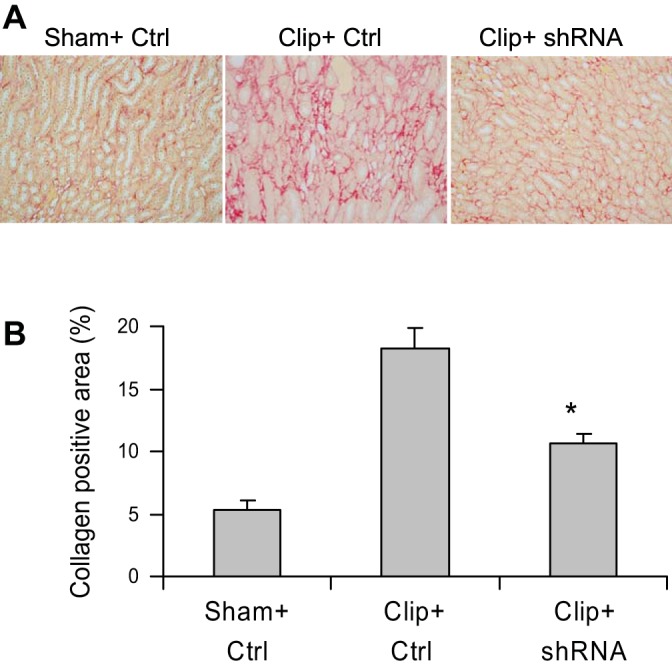
Effect of HIF-1α shRNA on collagen I/III staining in clipped kidneys. A: representative photomicrographs showing staining of collagens in outer medulla (red color). B: calculated percentage of positively stained area. *P = 0.009 vs. Clip+Ctrl and P = 0.004 vs. Sham+Ctrl by 1-way ANOVA with Tukey's post hoc test (n = 6).
Fig. 6.
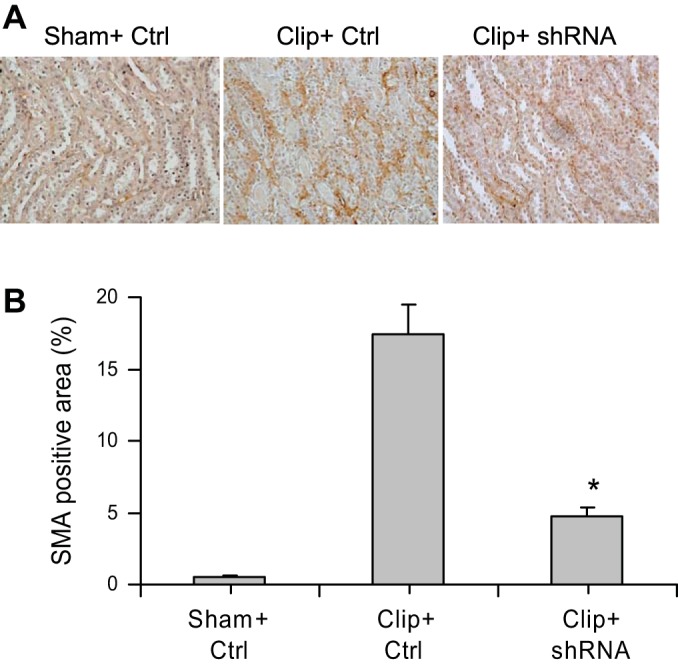
Effect of HIF-1α shRNA on α-smooth muscle actin (SMA) staining in clipped kidneys. A: representative photomicrographs showing staining of α-SMA in outer medulla (brown color). B: calculated percentage of positively stained area. *P < 0.001 vs. Clip+Ctrl and P = 0.029 vs. Sham+Ctrl by 1-way ANOVA with Tukey's post hoc test (n = 7).
Fig. 7.
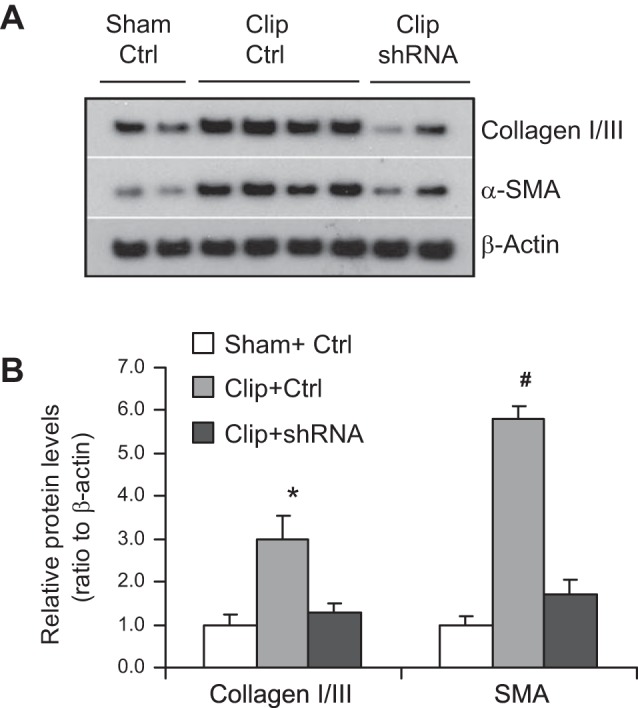
Effect of HIF-1α shRNA on protein levels of collagen I/III and α-SMA in clipped kidneys. A: representative ECL gel documents of Western blot analyses depicting the protein levels. B: summarized blot intensities (normalized to Sham+Ctrl). *P = 0.001 vs. Sham+Ctrl and P = 0.01 vs. Clip+shRNA. #P < 0.001 vs. other 2 groups by 1-way ANOVA with Tukey's post hoc test (n = 7).
DISCUSSION
The present study showed that chronic renal ischemia/hypoxia increased HIF-1α levels and that gene silencing of HIF-1α significantly attenuated the renal morphological changes and blocked the upregulation of α-SMA and collagen accumulation in clipped kidneys. These effects were independent of angiotensin II and hypertension. It is suggested that overactivation of HIF-1α in the kidney is a crucial mediator in chronic renal injury associated with hypoxia.
Although hypoxia and upregulation of HIF-1α are observed in a variety of CKD models, there is no direct evidence whether HIF-1α is an injurious or protective factor in kidney damage under chronic hypoxic conditions. Different CKD models may exhibit complex mechanisms and signaling pathways as well as interactions among the signaling pathways. It may therefore be difficult to eliminate other factors and dissect the effects of HIF-1α in these CKD models. The model used in the present study has been shown to produce hypoxia in the kidneys (49, 50). By using this model, we attempted to minimize other possible influences on kidney damage in addition to ischemia-induced HIF-1α accumulation. It is well known that the 2K1C model activates the renin-angiotensin system and increases angiotensin II levels (1). Angiotensin II significantly affects renal function and has been shown to cause kidney damage (2, 38, 54). Normalization of MAP by enalapril indicated that the potential effect of angiotensin II on chronic renal injury was minimized in the present study. It allowed us to evaluate the effect of HIF-1α with minimal influence of other factors on the kidney damage in clipped kidneys, although we could not totally rule out the possible effects of other factors, if any. It should be noted that ACE inhibition has been shown not to improve the reduced oxygenation in the clipped kidney in the 2K1C model (49, 50), and therefore ACE inhibition would not affect HIF-1α levels beyond its action on the angiotensin II system in the present study.
The present study showed that chronic ischemia/hypoxia caused an increase in HIF-1α levels, which was accompanied by both glomerular and tubulointerstitial damage in clipped kidneys. Analyses of the glomerular damage index and the percentage of globally damaged glomeruli showed that chronic ischemia produced glomerular injury and that inhibition of HIF-1α expression substantially attenuated glomerular injury in clipped kidneys, demonstrating that activation of HIF-1α importantly participated in the glomerular injury under ischemic conditions. In addition, it has been shown that renal tubulointerstitial damage contributes to the progression of chronic renal injury. The present study also demonstrated that inhibition of HIF-1α expression blocked tubulointerstitial damage as indicated by the abolition of the increase in collagen I/III and α-SMA expressions in clipped kidneys in HIF-1α shRNA-treated rats. These results suggest that overactivation of HIF-1α is a pathogenic factor producing both glomerular and tubulointerstitial damage in the kidneys under chronic ischemic conditions.
There is a limitation in the present study in that the improvement in histological damage in the clipped kidney was not verified by renal functional parameters. The present study wanted to limit other influences and focus on the role of HIF-1α in chronic kidney damage. The assessment of single kidney function of the clipped kidney requires anesthesia, surgery, and instrumentation of the kidneys, which may significantly complicate the measurements. Despite lack of support by functional parameters, the dramatic recovery in the histological parameters in HIF-1α shRNA-treated rats provides compelling evidence of an injurious effect of HIF-1α overactivation in the kidneys under chronic ischemic conditions.
An interesting finding in the present study was that HIF-1α shRNA blocked the increase in α-SMA levels in the clipped kidneys. α-SMA is a well-known marker of cell transdifferentiation, including the EMT and fibroblast activation into myofibroblasts. Cell transdifferentiation importantly contributes to the progression of CKD (3, 21, 33, 57, 67). Although in vitro studies have shown that hypoxia induces cell transdifferentiation via HIF-1α in renal cells (58, 68), the present study, for the first time, provided in vivo evidence that ischemia-induced HIF-1α mediates cell transdifferentiation in kidneys. These data suggest that the HIF-1α pathway may be involved in the relatively early stage of the profibrotic process in chronic hypoxic renal injury.
The present study demonstrated that chronic ischemia-induced overactivation of HIF-1α in the kidney mediates chronic renal damage. It is important to notice that HIF-1α overactivation can be stimulated by many other mechanisms independently of oxygen levels, such as oxidative stress and proinflammatory factors (13, 20, 22, 47, 48, 55, 62). These oxygen-independent mechanisms are well known to participate in chronic kidney damage in a variety of CKD. Therefore, the observation in the present study may be applied to other types of CKD and indicate that HIF-1α is very possibly also a crucial mediator of kidney damage in pathological conditions associated with HIF-1α activation independently of hypoxia.
The results of the present study did not provide an explanation of the reason some previous reports have provided evidence that HIF-1α accumulation is a beneficial factor in CKD. It is interesting to note that all the studies using genetic approaches to locally manipulate HIF-1α levels within the kidneys demonstrate that HIF-1α is an injurious mediator and that almost all reports using pharmacological approaches to systemically increase HIF-1α levels show that HIF-1α is a protective factor. A more recent study demonstrated that globally genetic activation of HIF-1α suppressed renal inflammation and fibrogenesis in mice subjected to unilateral ureteral obstruction and that HIF-1α activation exhibited an anti-inflammatory effect, which was associated with inhibition of inflammatory cell recruitment by myeloid cell-derived HIF-1α (25). This study suggested that cell type-specific action of HIF-1α may impact inflammation and fibrosis differentially. The beneficial effect of HIF-1α activation by pharmacological approaches may be produced by actions outside the kidneys. Therefore, local and global activation of HIF-1α may play different roles in the progression of CKD, which requires further investigation. Nevertheless, the present study provided strong evidence that long-term overactivation of HIF-1α within the kidney mediates renal damage.
In summary, the present study demonstrated that inhibition of HIF-1α overactivation in the kidneys attenuated renal injury under chronic ischemic/hypoxic conditions. It is suggested that overactivation of HIF-1α-mediated gene regulation in the kidney may constitute a pathogenic pathway mediating renal injury under various pathological conditions associated with ischemia and that normalization of overactivated HIF-1α in the kidneys may be a useful strategy in the treatment of chronic kidney damage associated with elevated levels of HIF-1α.
GRANTS
This work was supported by National Institutes of Health Grants HL-89563, HL-106042, and DK-54927.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.W., Q.Z., P.-L.L., F.Z., and N.L. provided conception and design of research; Z.W., Q.Z., R.D., and N.L. performed experiments; Z.W., Q.Z., R.D., and N.L. analyzed data; Z.W., Q.Z., P.-L.L., R.D., F.Z., T.W.G., and N.L. interpreted results of experiments; Z.W., Q.Z., and R.D. prepared figures; Z.W. drafted manuscript; Z.W., Q.Z., P.-L.L., R.D., F.Z., T.W.G., and N.L. approved final version of manuscript; Q.Z., P.-L.L., F.Z., T.W.G., and N.L. edited and revised manuscript.
REFERENCES
- 1.Atkinson AB, Brown JJ, Fraser R, Lever AF, Morton JJ, Riegger AJ, Robertson JI. Angiotensin II and renal hypertension in dog, rat and man: effect of converting enzyme inhibition. Clin Exp Hypertens 2: 499–524, 1980 [DOI] [PubMed] [Google Scholar]
- 2.Burns KD, Li N. The role of angiotensin II-stimulated renal tubular transport in hypertension. Curr Hypertens Rep 5: 165–171, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Burns WC, Kantharidis P, Thomas MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs 185: 222–231, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Chumakova OV, Liopo AV, Andreev VG, Cicenaite I, Evers BM, Chakrabarty S, Pappas TC, Esenaliev RO. Composition of PLGA and PEI/DNA nanoparticles improves ultrasound-mediated gene delivery in solid tumors in vivo. Cancer Lett 261: 215–225, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Dallatu MK, Choi M, Oyekan AO. Inhibition of prolyl hydroxylase domain-containing protein on hypertension/renal injury induced by high salt diet and nitric oxide withdrawal. J Hypertens, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Deng A, Arndt MA, Satriano J, Singh P, Rieg T, Thomson S, Tang T, Blantz RC. Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade. Am J Physiol Renal Physiol 299: F1365–F1373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Gao S, Dagnaes-Hansen F, Nielsen EJ, Wengel J, Besenbacher F, Howard KA, Kjems J. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol Ther 17: 1225–1233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunaratnam L, Bonventre JV. HIF in kidney disease and development. J Am Soc Nephrol 20: 1877–1887, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Haase VH. Hypoxia-inducible factor signaling in the development of kidney fibrosis. Fibrogenesis Tissue Repair 5, Suppl 1: S16, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haase VH. Oxygen regulates epithelial-to-mesenchymal transition: insights into molecular mechanisms and relevance to disease. Kidney Int 76: 492–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase VH. Pathophysiological consequences of HIF activation. Ann NY Acad Sci 1177: 57–65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han WQ, Zhu Q, Hu J, Li PL, Zhang F, Li N. Hypoxia-inducible factor prolyl-hydroxylase-2 mediates transforming growth factor beta 1-induced epithelial-mesenchymal transition in renal tubular cells. Biochim Biophys Acta 1833: 1454–1462, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helle F, Vagnes OB, Iversen BM. Angiotensin II-induced calcium signaling in the afferent arteriole from rats with two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 291: F140–F147, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle 7: 1128–1132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens 17: 266–270, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hill P, Shukla D, Tran MGB, Aragones J, Cook HT, Carmeliet P, Maxwell PH. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 19: 39–46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseinkhani H, Aoyama T, Ogawa O, Tabata Y. Ultrasound enhances the transfection of plasmid DNA by non-viral vectors. Curr Pharm Biotechnol 4: 109–122, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol 345: 105–120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens 13: 279–284, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kietzmann T, Görlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol 16: 474–486, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: F1023–F1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klahr S, Morrissey J. Progression of chronic renal disease. Am J Kidney Dis 41: S3–S7, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Gilbert V, Liu Q, Kapitsinou PP, Unger TL, Rha J, Rivella S, Schlondorff D, Haase VH. Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol 188: 5106–5115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koike H, Tomita N, Azuma H, Taniyama Y, Yamasaki K, Kunugiza Y, Tachibana K, Ogihara T, Morishita R. An efficient gene transfer method mediated by ultrasound and microbubbles into the kidney. J Gene Med 7: 108–116, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535–1548, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Leenen FH, de Jong W. A solid silver clip for induction of predictable levels of renal hypertension in the rat. J Appl Physiol 31: 142–144, 1971 [DOI] [PubMed] [Google Scholar]
- 29.Li L, Wu Y, Wang C, Zhang W. Inhibition of PAX2 gene expression by siRNA (polyethylenimine) in experimental model of obstructive nephropathy. Ren Fail 34: 1288–1296, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Li N, Chen L, Yi F, Xia M, Li PL. Salt-sensitive hypertension induced by decoy of transcription factor hypoxia-inducible factor-1α in the renal medulla. Circ Res 102: 1101–1108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Yi F, dos Santos EA, Donley DK, Li PL. Role of renal medullary heme oxygenase in the regulation of pressure natriuresis and arterial blood pressure. Hypertension 49: 148–154, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li PL. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol 292: F207–F216, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Singh RJ, Usa K, Netzel BC, Liang M. Renal medullary 11β-hydroxysteroid dehydrogenase type 1 in Dahl salt-sensitive hypertension. Physiol Genomics 36: 52–58, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manotham K, Tanaka T, Matsumoto M, Ohse T, Inagi R, Miyata T, Kurokawa K, Fujita T, Ingelfinger JR, Nangaku M. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int 65: 871–880, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med 85: 1325–1330, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res 31: 175–184, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Nangaku M, Yamada K, Gariepy CE, Miyata T, Inagi R, Kurokawa K, Yanagisawa M, Fujita T, Johnson RJ. ETB receptor protects the tubulointerstitium in experimental thrombotic microangiopathy. Kidney Int 62: 922–928, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Newman CMH, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther 14: 465–475, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Ng YY, Hou CC, Wang W, Huang XR, Lan HY. Blockade of NF[kappa]B activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int 67: S83–S91, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int 58: 2351–2366, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Norman JT, Orphanides C, Garcia P, Fine LG. Hypoxia-induced changes in extracellular matrix metabolism in renal cells. Exp Nephrol 7: 463–469, 1999 [DOI] [PubMed] [Google Scholar]
- 45.O'Donnell MP. Renal tubulointerstitial fibrosis. New thoughts on its development and progression. Postgrad Med 108: 159–162, 165, 171–152, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Ohtomo S, Nangaku M, Izuhara Y, Takizawa S, Strihou CvYd, Miyata T. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol Dial Transplant 23: 1166–1172, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Olson N, van der Vliet A. Interactions between nitric oxide and hypoxia-inducible factor signaling pathways in inflammatory disease. Nitric Oxide 25: 125–137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Page EL, Chan DA, Giaccia AJ, Levine M, Richard DE. Hypoxia-inducible factor-1α stabilization in nonhypoxic conditions: role of oxidation and intracellular ascorbate depletion. Mol Biol Cell 19: 86–94, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension 51: 345–351, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Palm F, Onozato M, Welch WJ, Wilcox CS. Blood pressure, blood flow, and oxygenation in the clipped kidney of chronic 2-kidney, 1-clip rats: effects of tempol and Angiotensin blockade. Hypertension 55: 298–304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984 [DOI] [PubMed] [Google Scholar]
- 52.Richard-Fiardo P, Payen E, Chevre R, Zuber J, Letrou-Bonneval E, Beuzard Y, Pitard B. Therapy of anemia in kidney failure, using plasmid encoding erythropoietin. Hum Gene Ther 19: 331–342, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt KU, Flippin LA, Arend M, Klaus SJ, Heyman SN. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant 23: 3472–3478, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant 21: 16–20, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Shatrov VA, Sumbayev VV, Zhou J, Brüne B. Oxidized low-density lipoprotein (oxLDL) triggers hypoxia-inducible factor-1α (HIF-1α) accumulation via redox-dependent mechanisms. Blood 101: 4847–4849, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Song YR, You SJ, Lee YM, Chin HJ, Chae DW, Oh YK, Joo KW, Han JS, Na KY. Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol Dial Transplant 25: 77–85, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Strutz FM. EMT and proteinuria as progression factors. Kidney Int 75: 475–481, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Sumual S, Saad S, Tang O, Yong R, McGinn S, Chen XM, Pollock CA. Differential regulation of Snail by hypoxia and hyperglycemia in human proximal tubule cells. Int J Biochem Cell Biol 42: 1689–1697, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest 85: 1292–1307, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Tanaka T, Matsumoto M, Inagi R, Miyata T, Kojima I, Ohse T, Fujita T, Nangaku M. Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int 68: 2714–2725, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Turnberg D, Lewis M, Moss J, Xu Y, Botto M, Cook HT. Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J Immunol 177: 4094–4102, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li PL, Li N. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int 79: 300–310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension 55: 1129–1136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wooddell CI, Van Hout CV, Reppen T, Lewis DL, Herweijer H. Long-term RNA interference from optimized siRNA expression constructs in adult mice. Biochem Biophys Res Commun 334: 117–127, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Dai C, Liu Y. Systemic administration of naked plasmid encoding hepatocyte growth factor ameliorates chronic renal fibrosis in mice. Gene Ther 8: 1470–1479, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Yi F, Xia M, Li N, Zhang C, Tang L, Li PL. Contribution of guanine nucleotide exchange factor Vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension 53: 90–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 82: 175–181, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Zeng R, Yao Y, Han M, Zhao X, Liu XC, Wei J, Luo Y, Zhang J, Zhou J, Wang S, Ma D, Xu G. Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc Nephrol 19: 380–387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Q, Liu M, Han WQ, Li PL, Wang Z, Li N. Overexpression of HIF prolyl-hydoxylase-2 transgene in the renal medulla induced a salt sensitive hypertension. J Cell Mol Med 16: 2701–2707, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Q, Wang Z, Xia M, Li PL, Zhang F, Li N. Overexpression of HIF-1α transgene in the renal medulla attenuated salt sensitive hypertension in Dahl S rats. Biochim Biophys Acta 1822: 936–941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu Q, Wang Z, Xia M, Li PL, Van Tassell BW, Abbate A, Dhaduk R, Li N. Silencing of hypoxia-inducible factor-1α gene attenuated angiotensin ii-induced renal injury in Sprague-Dawley rats. Hypertension 58: 657–664, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]


