Abstract
Aging nephropathy is characterized by podocyte depletion accompanied by progressive glomerulosclerosis. Replacement of terminally differentiated podocytes by local stem/progenitor cells is likely a critical mechanism for their regeneration. Recent studies have shown that cells of renin lineage (CoRL), normally restricted to the kidney's extraglomerular compartment, might serve this role after an abrupt depletion in podocyte number. To determine the effects of aging on the CoRL reserve and if CoRL moved from an extra- to the intraglomerular compartment during aging, genetic cell fate mapping was performed in aging Ren1cCre × Rs-ZsGreen reporter mice. Podocyte number decreased and glomerular scarring increased with advanced age. CoRL number decreased in the juxtaglomerular compartment with age. There was a paradoxical increase in CoRL in the intraglomerular compartment at 52 and 64 wk of age, where a subset coexpressed the podocyte proteins nephrin, podocin, and synaptopodin. Transmission electron microscopy studies showed that a subset of labeled CoRL in the glomerulus displayed foot processes, which attached to the glomerular basement membrane. No CoRL in the glomerular compartment stained for renin. These results suggest that, despite a decrease in the reserve, a subpopulation of CoRL moves to the glomerulus after chronic podocyte depletion in aging nephropathy, where they acquire a podocyte-like phenotype. This suggests that they might serve as adult podocyte stem/progenitor cells under these conditions, albeit in insufficient numbers to fully replace podocytes depleted with age.
Keywords: regeneration, podocyte, focal segmental glomerulosclerosis, cells of renin lineage, glomerulus
as longevity increases, so does the prevalence and incidence of aging nephropathy (1, 41, 50, 55). Aging nephropathy is characterized by a progressive decline in podocyte number, which is accompanied by focal and global glomerulosclerosis (31, 52, 54, 57). In addition, there is a mismatch between glomerular tuft volume and total podocyte volume per tuft, leading to podocyte hypertrophy (8, 57). Although the precise causes of reduced podocyte number in aged kidneys is not entirely understood (56), numerous studies have shown that a progressive decline in podocyte number underlies the development of glomerulosclerosis (19, 28, 53, 58). Adult podocytes are terminally differentiated epithelial cells that do not readily engage or progress through the cell cycle (reviewed in Ref. 13). Adult podocytes are therefore typically unable to proliferate adequately to replace themselves (20).
Recent studies have shown that despite an absence of podocyte proliferation, under certain circumstances a decline in podocyte number can be reversed and even normalized in diabetic and nondiabetic glomerular diseases (4, 33, 60). These data suggest the possibility that local stem/progenitors might serve this regenerative function in adults (12). Seminal studies have implicated parietal epithelial cells (PECs) as a source of adult podocyte stem/progenitor cells (47). Romagnani and colleagues (3, 48, 25) showed that a subpopulation of adult human PECs located at the tubular pole coexpress markers considered stem/progenitor associated. Similar findings in humans and rats have been shown by Benigni and colleagues (4, 36). Additionally, a subset of cells lining Bowman's capsule coexpress both PEC and podocyte markers in rodents and humans (3, 4, 25, 33, 36, 48, 60, 61). This number can be increased in mice by certain experimental therapies such as corticosteroids (60), retinoids (30, 61), improvement in the diabetic milieu (33), and during aging in rats (59). Recent data from four reports, in different mouse model systems, do not support a role for PECs as podocyte progenitors. Sakamoto et al. (42), Hackl et al. (14), Miyazaki et al. (29), and Schulte et al. (43) each showed in diseased states that cells lining Bowman's capsule coexpressing podocyte and PEC proteins derive from a podocyte lineage and not from a PEC lineage. The discrepancies in results between human and mouse may reflect species and/or model system differences (47).
Recent studies have implicated cells of renin lineage (CoRL) as local stem/progenitor cells. Renin-producing cells in adult animals are normally restricted to the kidney's extraglomerular vascular smooth muscle compartment (22). However, recent data show that CoRL exhibit marked “stemness/plasticity” under certain circumstances (reviewed in Ref. 11). Examples include adult CoRL transdifferentiating into erythropoietin producing cells (21), smooth muscle cells (45), possibly mesangial cells (16, 45), and more recently into glomerular epithelial cells (34). After an abrupt depletion in podocyte number induced by experimental focal segmental glomerulosclerosis (FSGS) in four different reporter mice strains where CoRL were permanently genetically labeled, a subpopulation of CoRL moved from the extraglomerular compartment to the intraglomerular compartment (34). In the latter location, they began de novo to coexpress the podocyte proteins WT-1, nephrin, synaptopodin, and podocin (34).
The purpose of the current studies reported herein was to assess the impact of advancing age on the normal juxtaglomerular reservoir of CoRL to determine if CoRL migrate to the glomerulus when podocyte number is depleted during aging nephropathy and if they acquire features of podocytes in the intraglomerular compartment.
METHODS
Animals and Experimental Models
Aging nephropathy in Ren1cCre × Rs-ZsGreen mice.
The purpose of this experiment was to determine the effects of aging on the resident extraglomerular vascular smooth muscle pool of CoRL in reporter mice and to determine their pattern of localization during the evolution of aging nephropathy. Accordingly, a Cre-recombinase cassette was cloned into the Ren1c BAC using homologous recombination as we previously described (34). When the RenCre transgenic line was crossed with the commercially available B6.Cg-Gt (ROSA) 26Sortm6(CAG-ZsGreen1) Hze/J reporter mouse (abbreviated as Zsgreen), a floxed stop cassette was excised, allowing for constitutive ZsGreen expression driven by a CAG promoter. Ren1cCre × Rs-ZsGreen reporter mice were housed under physiological conditions and killed at age 4 (n = 3), 12 (n = 3), 52 (n = 3), and 64 wk(n = 8). After euthanasia at each time point kidneys were fixed in 10% buffered formalin for analysis. Experimental procedures were approved by and conducted in accordance with the Roswell Park Institute and the University of Washington Animal Care and Use Committees.
Immunostaining
The following immunostainings were performed at each time point.
p57 staining to measure podocyte number.
To quantify podocyte number, single immunostaining for p57 was performed as we reported (49). Rabbit antibody to p57 (Santa Cruz Biotechnology, Santa Cruz, CA) was followed with a biotin conjugated mouse anti-rabbit secondary antibody (Jackson Immunoresearch, West Grove, PA). The ABC kit (Vector Laboratories, Burlingame, CA) was used for signal amplification, and 3,3′-diaminobenzamidine (DAB) (Sigma, St. Louis, MO) was used as a chromogen. Slides were counterstained with hematoxylin (Sigma-Aldrich), dehydrated, and mounted in Histomount (National Diagnostics, Atlanta, GA).
Because of known changes in glomerular size with aging (9), Image J 1.48d software (National Institutes of Health, Bethesda, MD) was used to measure the glomerular tuft area according to The Image J User Guide. These measurements were then used as denominators for the number of positively stained podocytes. Briefly, Image J “set scale” dialog was used to define the spatial scale of the active image so measurement results could be presented in calibrated units (mm2). The freehand line selection tool was used to draw around the glomerular tuft. The “measure” dialog was then used to get the results for glomerular tuft area.
Glomerular scarring.
Glomerulosclerosis was determined by either periodic acid Schiff (PAS) or silver stained sections in an average of 70 ± 14 glomeruli per animal and was graded quantitatively based on the percentage of glomerular tuft area involvement as follows: grade 0 = 0% (normal glomerulus with no abnormalities); grade 1 <25% (glomerulus contains a few capillaries with dilation); grade 2 <25–50% (glomerulus contains multiple capillaries with dilation); grade 3 <50–75% (glomerulus contains multiple capillaries with dilation and some synechial attachments); grade 4 <75–100% (glomerulus contains multiple synechial attachments with focal segmental sclerosis) as previously reported (5, 17, 49, 60).
To determine if glomerular injury was altered by the presence of labeled CoRL, glomeruli with or without labeled CoRL were correlated with grade 0 (normal) or with glomeruli having a grade of 1 or higher (injured) by fluorescent and light microscopy in an average of 107 ± 6 glomeruli per animal.
Renin staining and quantification.
Renin staining was performed as previously described (34). The number of renin-expressing cells was quantified by capturing 49 ± 9 fluorescent field digital images of the kidney cortex from each animal using a 10 × 10 grid. The images were analyzed using Image J 1.48d (National Institutes of Health). Image thresholds were adjusted for positive renin staining, which was then divided by the total area. Data are expressed as percentage of kidney cortical area stained. One-way ANOVA was calculated and a P value <0.05 was considered significant.
Identifying the ZsGreen reporter.
No antibody is required to visualize the ZsGreen reporter. To visualize ZsGreen in Ren1cCre × Rs-ZsGreen-R mice, kidneys were perfusion fixed with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, pH 7.4, followed by emersion fixation for 60 min. Biopsies were then transferred to 30% sucrose, left at 4°C overnight, and frozen in Tissue-Tek Cryo-OCT Compound (VWR). Four-micrometer cryosections were used. The number of cells labeled by the ZsGreen reporter for cells of renin lineage were counted per area of the kidney cortex using a 10 × 10 grid. Reporter-positive cells were quantified two ways. First, the percentage of glomeruli containing one or more labeled CoRL were quantified in each animal. Second, reporter-positive cells were quantified based on their cortical location and classified as either extraglomerular vascular smooth muscle cells or intraglomerular cells located within the glomerular tuft. Tubular and interstitial cells were excluded from this quantitation. An average of 70 ± 8 fields from each animal was assessed. Results were expressed as mean percentage of glomeruli ± SE and the number of cells per cortical area ± SE.
Double-immunostaining of CoRL reporter with renin or podocyte markers.
Immunofluorescent double staining for ZsGreen was performed in combination with renin, nephrin, synaptopodin, or podocin as we reported (34). Antigen retrieval was performed in 1 mM EDTA, pH 6.0 or pH 8.0, for 10 min. Nonspecific protein binding was blocked with Background Buster (Accurate Chemical & Scientific, Westbury, NY), and endogenous biotin activity was quenched with the Avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). After blocking, tissue sections were incubated overnight at 4°C with the appropriate primary antibodies: biotinylated sheep antibody to renin (Innovative Research, Novi, MI), guinea pig antibody to nephrin (Fitzgerald Industries International, Concord, MA), mouse antibody to synaptopodin (Fitzgerald Industries International), or rabbit antibody to podocin (Abcam, Cambridge, MA). The appropriate biotinylated secondary antibody (Vector Laboratories) was applied followed by Streptavidin, AlexaFluor 594 conjugate (Life Technologies-Molecular Probes, Grand Island, NY). Negative controls consisted of omitting the primary antibody and using tissue from Ren1cCre × Rs-ZsGreen-R mice where Cre was absent.
Measuring S phase of cell cycle.
Ki-67 measures the S phase of the cell cycle where DNA synthesis occurs (51). Double staining was performed for Ki-67 and ZsGreen in aging nephropathy. Rabbit antibody to Ki67 (Lab Vision, Freemont, CA) was followed with Alexa Fluor 594 F(ab′)2 Fragment of Goat Anti-Rabbit IgG (H+L) (Invitrogen). ZsGreen was visualized directly. Ki67+/ZsGreen+ cells were quantified in the cortex using a 10 × 10 grid. The data are expressed as a percentage of all cells in the extraglomerular vascular smooth muscle compartment.
Immunoelectron microscopy.
To better visualize the morphology of CoRL when in a glomerular location, immunoperoxidase staining was performed with a rabbit antibody to ZsGreen (Clontech Laboratories, Mountain View, CA). The ZsGreen reporter was then visualized with electron dense Diaminobenzidine (DAB) that was reacted with 2% OsO4, dehydrated, and infiltrated with a 50/50 mixture of PolyBed (PolySciences, Warrington, PA) and propylene oxide. Ultrathin sections were prepared, mounted on grids, and examined by transmission electron microscopy as previously described (2). Positive controls for immunostaining consisted of RenCre-positive mice where ZsGreen expression had already been confirmed. Negative controls consisted of omission of the ZsGreen antibody and RenCre-negative tissue.
Statistical analysis.
One-way ANOVA was calculated, and a P value <0.05 was considered significant.
RESULTS
Ren1cCre × Rs-ZsGreen Mice Developed Aging Nephropathy
We first wanted to ensure that old Ren1cCre × Rs-ZsGreen-R mice displayed typical features of aging nephropathy. Figure 1A shows that a progressive increase in glomerular tuft area in Ren1cCre × Rs-ZsGreen-R mice with advancing age, similar to what was previously observed in aging rats (59). As expected, podocyte density, measured by the number of cells staining positive for p57 divided by the glomerular tuft area, decreased progressively with age (Fig. 1, B–F). Compared with younger mice aged 12 wk, podocyte density was reduced 1.6-fold at 52 wk of age (0.94 ± 0.04 vs. 1.52 ± 0.004, P < 0.001) and 1.7 fold at 64 wk (0.891 ± 0.03 vs. 1.52 ± 0.004, P < 0.001).
Fig. 1.
Changes in podocyte number in aging nephropathy. Ren1cCre × Rs-ZsGreen-R reporter mice were studied with advancing age at 4, 12, 52 and 64 wk. A: glomerular tuft area increased progressively and significantly at 52 and 64 wk of age. B: podocyte density, measured by the number of p57-stained cells/glomerular tuft area was significantly reduced at 52 and 64 wk compared with younger ages. C–F: examples of p57 staining (brown, nuclear) at ages 4 (C), 12 (D), 52 (E), and 64 (F) wk respectively.
Having shown reduced podocyte number, we next measured the impact of age on glomerular sclerosis on PAS-stained tissue (Fig. 2). Compared with younger mice, glomerulosclerosis was higher at 52 wk (0.24 ± 0.03 vs. 0.073 ± 0.01 at 12 wk, P = 0.003) and at 64 wk (0.26 ± 0.02 vs. 0.073 ± 0.01 at 12 wk, P < 0.001). Taken together, these data (reduced podocyte number, glomerulosclerosis) show that old Ren1cCre × Rs-ZsGreen-R reporter mice exhibit classical features of aging nephropathy (10, 52).
Fig. 2.
Glomerulosclerosis in aging nephropathy. A: glomerulosclerosis, quantitated on PAS-stained sections in aging Ren1cCre × Rs-ZsGreen-R reporter mice, increased significantly at 52 and 64 wk compared with younger ages. B–E: representative images of glomeruli at 4 (B), 12 (C), 52 (F), and 64 wk (G) show decreased podocyte number, glomerulosclerosis, and synchiae (indicated by arrows), features consistent with aging nephropathy.
Cells of Renin Lineage Increase in the Intraglomerular Compartment in Aged Ren1cCre × Rs-ZsGreen-R Mice
We previously reported that labeled CoRL were detected in glomeruli in young Ren1cCre × Rs-ZsGreen-R mice, albeit at low numbers (34). These data were validated in this cohort of young mice (Fig. 3, A and D). The percentage of glomeruli with at least one labeled CoRL in the intraglomerular compartment was 28 ± 0.67% at 12 wk and 29 ± 4.2% at 52 wk of age (Fig. 3, B and D). However, the percentage of glomeruli with at least one labeled CoRL increased at 64 wk of age to 39 ± 4% (P < 0.05) (Fig. 3, C and D). Of note was that not only did the percentage of glomeruli containing labeled CoRL increase, but the number of cells per glomerulus was also higher.
Fig. 3.
Cells of renin lineage increase in the intraglomerular compartment and glomerular injury is reduced in glomeruli containing cells of renin lineage in aged Ren1cCre × Rs-ZsGreen-R mice. A–C: representative pictures of kidneys from mice aged 12, 52, and 64 wk. Glomeruli with no labeled cells of renin lineage (CoRL) are indicated by solid circles, glomeruli containing labeled CoRL are indicated by dashed circles. D: quantification of the percentage of glomeruli containing labeled CoRL, percentage of glomeruli with injury, percentage of glomeruli with labeled CoRL with injury, and percentage of glomeruli with no labeled CoRL with injury.
Glomerular Injury Is Reduced in Glomeruli Containing Cells of Renin Lineage
Having shown that labeled CoRL were detected in glomeruli, we asked if glomerular injury was altered by the presence of labeled CoRL. Of the 9 ± 1.9% of glomeruli that displayed injury at 52 wk, only 1.8 ± 0.2% contained labeled CoRL. The remaining 7.2 ± 2.6% of injured glomeruli contained no labeled CoRL (P = 0.02 vs. glomeruli with labeled CoRL) (Fig. 3D). As shown above, glomerular injury was higher at 64 wk of age, where glomerular injury was present in 16.5 ± 2% of glomeruli. At this age, 4 ± 0.7% of injured glomeruli contained labeled CoRL, whereas 12.4 ± 2.9% contained no CoRL (P < 0.001 vs. glomeruli with labeled CoRL) (Fig. 3D). This data indicates that glomerular injury was lower in glomeruli containing CoRL.
Cells of Renin Lineage Decrease in the Extraglomerular Vascular Smooth Muscle Compartment in Aged Ren1cCre × Rs-ZsGreen-R Mice
Although advanced age affects nonrenal stem/progenitor cells (35), the impact of age on renin expressing cells, and on cells of renin lineage is not known. Accordingly, the number of labeled CoRL (green color) was quantitated both in the extra- and again in the intraglomerular location during aging, and the results are shown in Fig. 4. In young mice aged 4 and 12 wk (Fig. 4, A, B, F), the typical number of reporter-labeled CoRL that localized to the extraglomerular vascular smooth muscle compartment was 25.0 ± 0.21 and 25.6 ± 1.32 cells/mm2 kidney cortex, respectively. With age, there was a progressive decline in the density of labeled CoRL in the extraglomerular vascular smooth muscle compartment, with a 1.62-fold decrease at 52 wk (15.8 ± 2.2 vs. 25.6 ± 1.32, P = 0.02) and 1.73-fold at 64 wk (14.8 ± 0.43 vs. 25.6 ± 1.32, P < 0.001). The decrease in labeled CoRL at 52 wk was not simply due to a decrease in renin expression with age, because renin density was not changed (0.18 ± 0.01 vs. 0.19 ± 0.03, P = 0.621) (Fig. 4) at 52 wk. However, both renin density (0.13 ± 0.01 vs. 0.19 ± 0.03, P = 0.05) and labeled CoRL decreased at age 64 wk (Fig. 4).
Fig. 4.
Labeled CoRL decrease in the extraglomerular vascular smooth muscle compartment, and increase in the intraglomerular compartment in aging nephropathy. A–E: representative pictures of kidneys from mice aged 4, 12, 52, and 64 wk. A: low-magnification image (×100) from a 4-wk-old kidney shows that CoRL are permanently labeled with ZsGreen reporter in the extraglomerular vascular smooth muscle compartment. They were rarely detected in the intraglomerular compartment and are faintly detected in the tubular compartment. B: low magnification image (×100) from a 12 wk kidney shows a similar distribution of reporter labeled CoRL to 4 wk kidneys shown in A. C: at 52 wk, reporter labeled CoRL decrease in the extraglomerular vascular smooth muscle compartment. Labeled reporter cells were still rarely detected in glomeruli and in tubules. D: at 64 wk, reporter labeled CoRL remained decreased in the extraglomerular vascular smooth muscle compartment. However, there was a paradoxical increase in the number of reporter labeled CoRL in the intraglomerular compartment (labeled g). E: glomerulus from a 64-wk-old kidney viewed at higher magnification (×630) shows a number of reporter-labeled cells (green) in the intraglomerular compartment, which were in a characteristic podocyte distribution pattern. F: number of ZsGreen reporter-labeled CoRL was quantitated in Ren1cCre × Rs-ZsGreen-R reporter mice. Compared with 4 and 12 wk of age, the number of ZsGreen-labeled cells decreased significantly in the extraglomerular vascular smooth muscle compartment at 52 and 64 wk (shaded bars). In contrast, there was an increase in the number of reporter labeled CoRL in the glomerular tuft (black bars) at 64 wk. G–L: representative images of double staining for renin and ZsGreen reporter. G: lower magnification shows the distribution of CoRL reporter (green) was restricted to the extraglomerular vascular smooth muscle compartment (arrows indicate examples). H: distribution of renin staining (red) was also restricted to the extraglomerular vascular smooth muscle compartment (arrows indicate examples). I: merged image shows clear overlap of renin staining and the reporter (yellow, arrows indicate examples). Red blood cell autofluorescence appears orange in color. J–L: higher magnification images of the glomerulus indicated by the white boxes in G–I, respectively. These data show that renin staining is restricted to the extraglomerular compartment and that when labeled CoRL migrate to the glomerulus they do not stain for renin.
Labeled CoRL were also quantitated in the intraglomerular compartment. Similar to the results shown above, the number of labeled CoRL in glomeruli were similar at 12 wk (3.6 ± 0.74 labeled CoRL/mm2 kidney cortex) and 52 wk (3.0 ± 0.2) of age. However, the number of labeled CoRL was higher in the glomerular tuft at 64 wk of age (9.5 ± 1.33 vs. 3.6 ± 1.32, P = 0.007) (Fig. 4F). Low power images of kidney cortex from each group are shown to demonstrate the density of labeled CoRL (Fig. 4, A–D) as well as an image of a glomerulus from the 64 wk group with multiple labeled CoRL within the glomerular tuft with a podocyte distribution (Fig. 4E).
Taken together, these results show that the number of labeled CoRL decreases progressively in the extraglomerular vascular smooth muscle compartment with age. However, the number of labeled CoRL paradoxically increases in glomeruli in aging nephropathy, which accompanied chronic podocyte depletion.
Renin Staining Is Limited to the Extraglomerular Compartment in Aged Ren1cCre × Rs-ZsGreen-R Mice
Ren1cCre × Rs-ZsGreen-R mice constitutively report for CoRL. Therefore, to ensure that the observation of CoRL in the intraglomerular compartment was not due to de novo expression of renin, which normally localizes only to vascular smooth muscle cells in the extraglomerular compartment, renin staining was performed at each time point. Two results were noteworthy. First, renin staining was restricted to cells of the extraglomerular compartment in mice of all ages studied (Fig. 4, G–L, and Fig. 5, B–G). Renin staining was never detected in the glomerulus at any time point. As expected, cells coexpressing both the ZsGreen reporter and renin stained yellow in the extraglomerular compartment (Fig. 4, G–L). Second, the number of extraglomerular cells staining for renin was unchanged up to aged 52 wk. Thereafter, there was a significant decrease with age (Fig. 5A). These results show that the presence of labeled cells in aged glomeruli was not due to de novo expression of renin.
Fig. 5.
Quantification of Renin staining in aging nephropathy. A: quantitation of renin staining was expressed as a percentage of cortical area in aging Ren1cCre × Rs-ZsGreen-R reporter mice. No changes were detected between 4, 12, and 52 wk. The percentage area of renin staining decreased in mice with aging nephropathy at 64 wk. B–E: typical renin staining in 4-, 12-, 52-, and 64-wk-old mice. F and G: higher magnification images of the areas indicated in C and E, respectively, show renin staining was confined to the extraglomerular compartment and not present in the intraglomerular compartment (g).
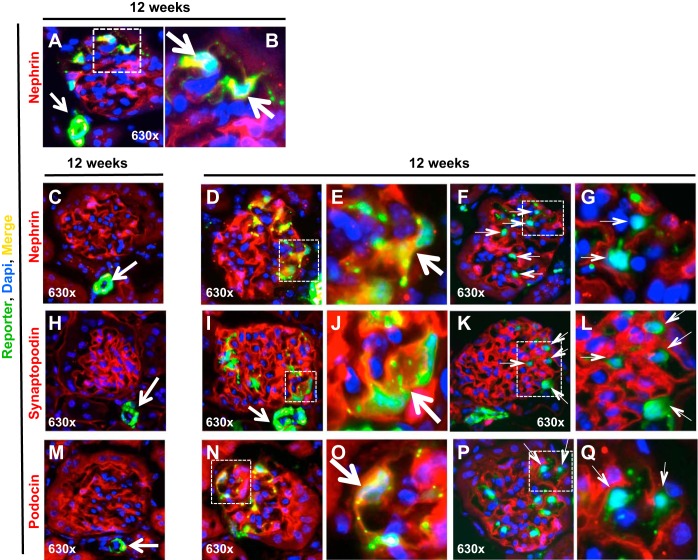
Cells of Renin Lineage in the Glomerulus Coexpress Slit Diaphragm Podocyte Proteins in Aging Nephropathy
We next determined if labeled CoRL that moved from the extraglomerulus to the glomerular tuft begin to express podocyte proteins, and the results are shown in Fig. 6. As expected, in younger mice aged 12 wk, CoRL (green) were mostly confined to the extraglomerular compartment and nephrin (red), synaptopodin (red), and podocin (red) to podocytes within the glomerulus (Fig. 6, C, H, and M). However, when labeled CoRL were present within glomeruli of young mice, a subset coexpressed nephrin (yellow color, Fig. 6, A and B). In mice aged 64 wk, ∼31 ± 4% of the 39% of glomeruli with labeled CoRL coexpressed nephrin (yellow color, Fig. 6, D and E), synaptopodin (yellow color, Fig. 6, I and J), or podocin (yellow color, Fig. 6, N and O). Some glomeruli contained labeled CoRL that did not coexpress either nephrin, synaptopodin, or podocin. Moreover, these cells had a different morphology on light microscopy and occupied different intraglomerular locations than a podocyte location. These cells may therefore represent an intermediate form of CoRL, prior to the de novo expression of podocyte proteins (Fig. 6, F and G, K and L, and P and Q). These data show that an increased subpopulation of CoRL de novo expresses three podocyte proteins when in the glomerular tuft in aging nephropathy.
Fig. 6.
Labeled CoRL costain for podocyte proteins and reporter in glomeruli in aging nephropathy. A: at 12 wk, reporter-labeled cells (green) were detected in the extraglomerular vascular smooth muscle compartment (white arrow), and a subset of reporter labeled cells present in the intraglomerular compartment costained for nephrin (yellow, represented by square). Cell nuclei were marked with DAPI (blue) B: portion of the glomerulus represented by the white square shows costaining of the reporter and nephrin at higher magnification, indicated by the arrows. C: typical glomerulus at 12 wk, where reporter labeled cells (green) were detected in the extraglomerular vascular smooth muscle compartment (white arrow), and nephrin (red) was limited to the glomerulus in a typical podocyte distribution. Cell nuclei were marked with DAPI (blue). D: at 64 wk, an increased subset of reporter labeled cells present in the intraglomerular compartment costained for nephrin (yellow, represented by square). E: portion of the glomerulus represented by the white square shows costaining of the reporter and nephrin at higher magnification, indicated by the arrow. F and G: lack of costaining for the reporter and nephrin. F: at 64 wk of age, a subset of reporter-labeled cells present in the intraglomerular compartment did not costain for nephrin (green, represented by square). G: portion of the glomerulus represented by the white square shows costaining of the reporter and nephrin at higher magnification, indicated by the thin arrows. H: typical glomerulus at 12 wk, reporter-labeled cells (green) were detected in the extraglomerular vascular smooth muscle compartment (white arrow), and synaptopodin (red) was limited to the glomerulus in a typical podocyte distribution. I: at 64 wk, increased reporter-labeled cells present in the intraglomerular compartment costained for synaptopodin (yellow, represented by square). J: portion of the glomerulus represented by the white square shows costaining at higher magnification, indicated by the arrow. K and L: lack of synaptopodin-reporter costaining. E: At 64 wk, a subset of reporter-labeled cells present in the intraglomerular compartment did not costain for synaptopodin (green, represented by square). F: portion of the glomerulus represented by the white square shows costaining of the reporter and synaptopodin at higher magnification, indicated by the thin arrows. M-O: podocin-reporter costaining. M: typical glomerulus at 12 wk, reporter-labeled cells (green) were detected in the extraglomerular vascular smooth muscle compartment (white arrow), and podocin (red) was limited to the glomerulus in a typical podocyte distribution. N: at 64 wk, increased reporter-labeled cells present in the intraglomerular compartment costained for podocin (yellow, represented by square). O: portion of the glomerulus represented by the white square shows costaining at higher magnification, indicated by the arrow. These data show that a subset of reporter-labeled CoRL in glomeruli of aged mice coexpress the podocyte proteins nephrin, synaptopodin, and podocin. P and Q: lack of costaining for the reporter and podocin. P: at 64 wk, a subset of reporter-labeled cells present in the intraglomerular compartment did not costain for podocin (green, represented by square). Q: portion of the glomerulus represented by the white square shows costaining of the reporter and podocin at higher magnification, indicated by the thin arrows.
Minimal Proliferation of CoRL in Aging Nephropathy
Double-staining for Ki-67 and the CoRL reporter ZsGreen was undertaken to determine if CoRL underwent proliferation. As expected in normal Ren1cCre × Rs-ZsGreen-R mice, Ki-67 was barely detected in ZsGreen-labeled cells in the extraglomerular vascular smooth muscle compartment (Fig. 7). This was not a false negative, because occasional tubular epithelial cells stain positive for Ki-67 (Fig. 7, A and B). During aging, Ki-67+/ZsGreen+ double-stained cells were detected in less than 1% of all cells in the extraglomerular vascular smooth muscle compartment. No Ki-67+/ZsGreen+ cells were detected in glomeruli in aging nephropathy (Fig. 7). These data show that CoRL barely proliferate in aging nephropathy and that the 1.62-fold (at 52 wk) and 1.73-fold (at 64 wk) decrease in CoRL exceeded the rate of proliferation observed.
Fig. 7.
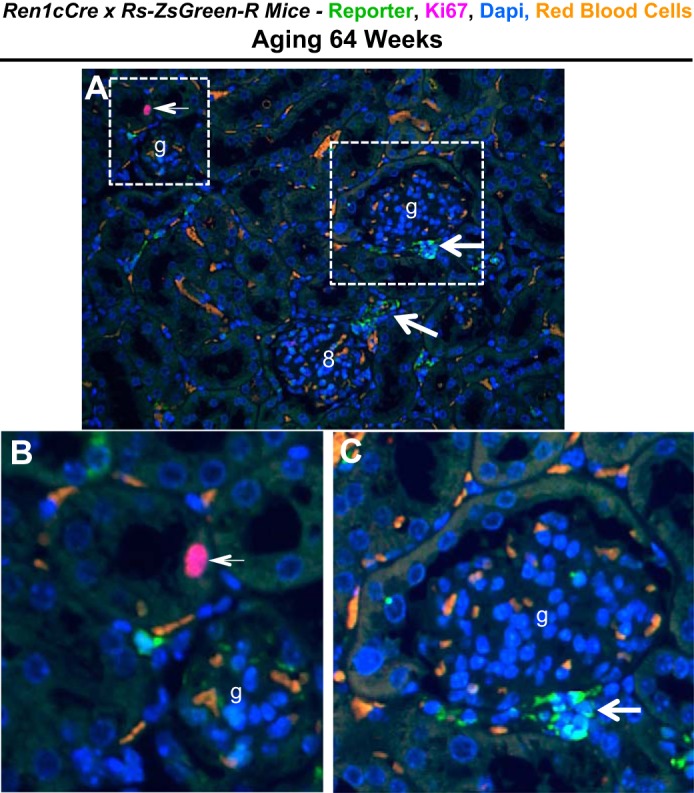
Reporter and Ki67 staining in aging nephropathy. A: lower power image of ZsGreen reporter (green, arrows) and Ki67 staining (purple, arrow) in aging Ren1cCre × Rs-ZsGreen-R reporter mice. Red blood cell autofluorescence appears orange in color. B and C: higher magnification images of the 2 areas indicated by the white boxes in A show a Ki67-positive cell within a tubule (B, arrow) and reporter staining in the extraglomerular vascular smooth muscle compartment (C, arrow); glomeruli are indicated with (g).
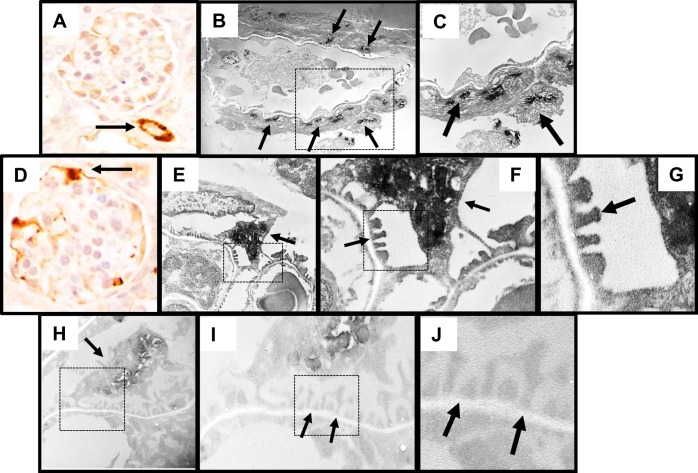
Labeled Cells of Renin Lineage in Glomeruli Acquire Ultrastructural Features Resembling Podocytes
To prove that one cell type is a stem/progenitor from another cell type requires data to include not only the de novo expression of proteins of the cell to which it is differentiating but also that the cell acquires its ultrastructural features (collectively called phenotype). To show that CoRL are likely stem/progenitors for adult podocytes in states of podocyte depletion, we determined if a subpopulation of labeled CoRL in glomeruli display ultrastructural features of podocytes, in addition to coexpressing three podocyte proteins. Immunoperoxidase staining was performed for ZsGreen with electron dense diaminobenzidine, and transmission electron microscopy was subsequently performed as previously described (2). As expected, immunoperoxidase staining viewed under light microscopy for Zsgreen was detected in the CoRL surrounding the arterioles outside the glomerulus, which was used as a positive control (Fig. 8A). Likewise, ultrathin sections viewed by transmission electron microscopy showed electron dense black staining in CoRL surrounding the arterioles outside the glomerulus (Fig. 8, B and C). The majority of podocytes lacked electron dense (black) staining in cell bodies and foot processes and represented native podocytes (Fig. 8, H–J). However, cells with reporter (ZsGreen) staining viewed under light microscopy were detected in the glomerulus (Fig. 8D) and clearly had electron dense black material within their cell bodies and within foot processes (Fig. 8, E–G). These results are consistent with a subset of CoRL within the glomerular tuft having ultrastructural features resembling podocytes.
Fig. 8.
Labeled CoRL acquire ultrastructural features of podocytes. To assess ultrastructural features labeled CoRL, immunoperoxidase staining for ZsGreen reporter was performed. A: image of immunoperoxidase staining for the ZsGreen reporter (brown) in the vascular smooth muscle compartment (indicated by arrow, positive control). B: immunoperoxidase staining for the ZsGreen reporter (black) on ultrathin section viewed by transmission electron microscopy (TEM) was detected in the vascular smooth muscle compartment, used as a positive control (arrows indicate examples). C: higher magnification image of reporter staining in the area indicated by the dashed square shown in B. D: immunoperoxidase staining for reporter (brown) in the intraglomerular compartment appears in a podocyte distribution (indicated by arrow). E: TEM image of podocyte with reporter staining (black) in both the cell body and within foot processes. F: higher magnification image of the area indicated by the dashed square shown in E. Arrows indicate staining in foot processes. G: even higher magnification image of the area indicated by the dashed square shown in F. Arrow indicates reporter staining in foot processes. H: lower magnification image of podocyte that lacked reporter staining in the cell body or within foot processes. I: higher magnification image of reporter staining (black) in the area indicated by the dashed square shown in H. Arrows indicate the lack of staining in foot processes. J: higher magnification image of the area indicated by the dashed square shown in I. Arrows indicate the lack of staining in foot processes.
DISCUSSION
Studies in glomerular diseases have recently focused on the possibility that a decrease in podocyte number in disease might improve because of regeneration by local stem/progenitor cells (12). Recent studies have shown that CoRL may serve as adult stem/progenitor cells for smooth muscle cells and glomerular epithelial cells (34, 45) and that they can differentiate into erythropoietin producing cells (21). However, the effect of advancing age on this cell population is poorly understood, their movement from the extra- to the intraglomerular compartment is not known in states of chronic podocyte depletion, and no studies to date have shown ultrastructural changes to support their plasticity to any cell type. The results of the current studies show that the number of cells of renin lineage in the extraglomerular compartment decreases progressively with age, yet paradoxically increases in glomeruli during the development of aging nephropathy. Evidence for their stem/progenitor nature is that in addition to coexpressing two podocyte slit-diaphragm proteins and an actin binding protein considered limited to podocytes, CoRL also display foot process and other ultrastructural features characteristic of podocytes when in an intraglomerular location.
Recent studies have shown that under certain circumstances in diabetic and nondiabetic glomerular diseases, a depletion of podocytes can be improved and even restored in the absence of podocyte proliferation (4, 26, 33). Grahammer and Huber (12) recently summarized several candidate mechanisms whereby adult podocytes might regenerate in disease (12). First, podocyte proliferation is unlikely because studies from numerous investigators have shown that terminally differentiated adult podocytes are unable to adequately proliferate to replace any decrease in their numbers (reviewed in Ref. 20). Wanner et al. (52) from Huber's group recently showed that despite a decrease in podocyte number in aging mice, they do not proliferate, and they did not find evidence for podocyte replacement in their model. Second, glomerular parietal epithelial cells (PECs) might serve such a biological role in human disease (reviewed in Ref. 39). Finally, recently published studies show that CoRL might transdifferentiate into glomerular epithelial cells after an abrupt decline in podocyte number (34).
In the current study we show evidence that advancing age in Ren1cCre × Rs-ZsGreen-R reporter mice was accompanied by a progressive decrease in podocyte number, a focal increase in glomerulosclerosis, and tubulointerstitial fibrosis. These features are consistent with aging nephropathy that has been described in experimental and human studies (10, 52, 56), thus validating this as a representative model of aging nephropathy in these reporter mice. The first finding in the current studies was that the reservoir of CoRL in the extra-glomerular compartment decreases with age. At 52 wk of age, the total number of labeled CoRL decreased significantly in the extraglomerular compartment. This was not due to a decrease in overall cell number in the extraglomerular compartment, because the number of cells exhibiting renin protein accumulation remained unchanged with age. At 64 wk of age, the number of labeled CoRL and the number of renin staining cells decreased in the extraglomerular compartment.
This is the first such report on this cell population in aging to our knowledge. Other stem/progenitors exhibit a similar fate with advanced age (37). The absence of the proliferation marker Ki-67 suggests a limited proliferative response at the time points studied, although Ki-67 has a short half-life (51). The reason for the decrease in labeled CoRL in the extraglomerular location was not studied. We speculate that this may be attributable to age-related abnormalities in autophagy or apoptosis. Another speculative reason is that in the absence of proliferation, the reservoir becomes depleted over time as cells move to the glomerulus. However, because the decrease in labeled CoRL in the juxtaglomerulus exceeded the number of labeled CoRL in glomeruli, this alone would not explain the decrease. It is not known why CoRL did not proliferate at the time points studied. However, Kurtz (23) described a general lack of CoRL proliferation.
A second major finding was that despite a decrease of CoRL in the extraglomerular compartment during the evolution of aging nephropathy, labeled CoRL increased in number within the intraglomerular compartment. We next asked if CoRL in glomeruli exhibited “stemness/plasticity,” defined by some as the de novo expression of several proteins considered unique to another cell type and acquiring the ultrastructure of that cell type. The data showed that a subset of CoRL that moved to the glomerulus in aging nephropathy began to coexpress two slit diaphragm proteins (nephrin, podocin), a podocyte-specific actin-binding protein (synaptopodin), and displayed ultrastructural features resembling podocytes (foot process on EM). Together these features are highly suggestive that a subpopulation of CoRL acquires an adult podocyte phenotype and support the paradigm that CoRL might serve as stem/progenitor cells for adult podocytes in aging nephropathy. This coincided with chronic podocyte depletion. However, the number of CoRL detected in glomeruli were less than the absolute decrease in podocyte number, suggesting that under these conditions, podocyte loss exceeds the capacity of CoRL to undergo adequate podocyte regeneration. A potential reason for this is the decrease in the extraglomerular reserve with aging (see earlier discussion).
In CoRL in inducible reporter mice, tamoxifen is sufficient to excise the STOP cassette in CoRL during the immediate period after inducible labeling, as we previously reported (34). Although the reporter mice used in the current studies was not an inducible strain, they offer several advantages for aging studies. First, the constitutive labeling is more robust than inducible mice, because the entire renal vasculature and juxtaglomerulus is labeled from early kidney development. Second, if any new CoRL came into existence after removal of tamoxifen (because of proliferation and/or recruitment), they would not be reporter labeled. These unlabeled cells (together with labeled cells) may move into the glomerulus with age but would not be detected because of the absence of reporter expression in the absence of tamoxifen excising the STOP cassette. Therefore, for the inducible model to be effective over long periods of time such as those used in this aging study, mice would need to be repeatedly administered tamoxifen to label the “new” members of the CoRL reservoir over time. Third, repeated tamoxifen injections over a 64-wk period is challenging for the animals and can even lead to deleterious effects such as the induction of covalent DNA adducts (15). Likewise, repeated injections of tamoxifen, an estrogen antagonist, throughout the life of the mouse could have confounding consequences. Finally, the major concern with noninducible strains is that detection of a reporter could simply reflect de novo or re-expression of the gene to which the reporter is used. There is some evidence of local renin expression in podocytes in disease. Rosenberg et al. (40) showed that in the rat remnant kidney model, renin mRNA was increased in RNA extracted from whole glomeruli and that renin immunostaining was increased “in cells in the glomerular tuft.” Because no cell identification methods clarified which cell type stained for renin, one cannot make any cell type assumptions. This is important, because studies have shown that all three resident cell types in the tuft are involved in remnant glomeruli (18, 27, 44, 46). We have shown that exposing cultured podocytes to high glucose concentration increases angiotensin II concentrations, AT1 receptor protein levels, and renin mRNA and protein levels, as well as activity (7). The renin results could not be replicated in vivo in an experimental diabetic model (7). Philips et al. (32) showed that the direct renin inhibitor aliskiren mitigates the profibrotic and apoptotic effects of high glucose on cultured mouse podocytes. To date, although there has been much interest in renin-angiotensin-aldosterone system in the aging kidney (6), there are no published data to show that renin mRNA, protein, and/or activity increases in glomeruli or podocytes in aged kidneys. Moreover, in the current study, renin staining was limited to cells of the juxtaglomerulus and was not detected in cells in the intraglomerular compartment. Thus, although possible, it is highly improbable that labeled CoRL in glomeruli of aged kidneys simply reflects renin (re)expression in aging. Of course, we cannot exclude an unknown activation of the renin promoter by unknown mechanisms independent of renin production. Taken together, the reporter strain used was well suited for the purposes of the questions posed in the current studies.
De novo expression of stem/progenitor cell “markers” in CoRL were not detected in the current study. This is not surprising, because expression of such markers is not required to prove “stemness” of a candidate cell. To date, all “markers” used in glomerular studies are not specific, nor functional, but are rather antigens used to denote stemness in nonkidney cells, such as CD133 and CD24 (38). The latter is not expressed in mice (24). We recognize that these studies were not functional and that the biological effects of CoRL in glomeruli were not fully tested. The mechanisms underlying the signals and pathways whereby CoRL are activated, migrate, and transdifferentiate in aging nephropathy are under active investigation. Temporally, the current data and a previously published report (34) suggest that a decrease in podocyte number is required for CoRL to move from the extra- to the intraglomerular compartments. The reasons for their decrease in the extraglomerular compartment are also unknown.
In summary, these studies show the reserve of CoRL in the extraglomerular compartment decreased with age. However, the chronic depletion in podocyte number in aging nephropathy was accompanied by the movement of a subpopulation of CoRL from their original extraglomerular location to glomeruli in a focal pattern, where a subset de novo coexpressed three podocyte-“specific” proteins and displayed ultrastructural features characteristic of podocytes. These data suggest that CoRL transdifferentiated into podocytes in a state of chronic podocyte depletion, as they do after acute podocyte depletion (34). However, the degree to which this occurs is likely not sufficient to match the depletion in podocyte number. The contribution of CoRL to glomerular regeneration needs to be further investigated and the impact of currently used therapies on these processes is being studied. Finally, the current data on podocytes adds to the increasing published literature that adult cells of renin lineage are pluripotent cells and, under different circumstances, can differentiate into smooth muscle cells (45, 52), mesangial cells (16, 45, 52), and erthyropoetin-producing cells (21).
GRANTS
Aspects of this work were supported by National Heart, Lung, and Blood Institute [Grant RO1HL048459 (to KG)], National Cancer Institute [Grants R21CA121212 and P30 CA016056 (to KG)] National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK87389, DK84077, and DK94768 (to JSD); Grants R01DK056799 and R21DK081835 (to SJS); and Grant DK83391 (to CEA)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.W.P., J.S.D., K.W.G., and S.J.S. conception and design of research; J.W.P., S.T.G., R.D.K., M.E.R., and K.L.H. performed experiments; J.W.P., S.T.G., M.E.R., C.E.A., K.L.H., and S.J.S. analyzed data; J.W.P., J.S.D., K.W.G., and S.J.S. interpreted results of experiments; J.W.P., K.L.H., and S.J.S. prepared figures; J.W.P., J.S.D., K.W.G., and S.J.S. drafted manuscript; J.W.P., S.T.G., C.E.A., J.S.D., K.W.G., and S.J.S. edited and revised manuscript; J.W.P., S.T.G., C.E.A., J.S.D., K.W.G., and S.J.S. approved final version of manuscript.
REFERENCES
- 1.Ahn SY, Ryu J, Baek SH, Kim S, Na KY, Kim KW, Chae DW, Chin HJ. Incident chronic kidney disease and newly developed complications related to renal dysfunction in an elderly population during 5 years: a community-based elderly population cohort study. PLos One 8: e84467, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpers CE, Hudkins KL, Floege J, Johnson RJ. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol 5: 201–209, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Angelotti ML, Lazzeri E, Lasagni L, Romagnani P. Only anti-CD133 antibodies recognizing the CD133/1 or the CD133/2 epitopes can identify human renal progenitors. Kidney Int 78: 620–621, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G. Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkkoetter PT, Wu JS, Ohse T, Krofft RD, Schermer B, Benzing T, Pippin JW, Shankland SJ. p35, the non-cyclin activator of Cdk5, protects podocytes against apoptosis in vitro and in vivo. Kidney Int 77: 690–699, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Conti S, Cassis P, Benigni A. Aging and the renin-angiotensin system. Hypertension 60: 878–883, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol 294: F830–F839, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glassock RJ. An update on glomerular disease in the elderly. Clin Geriatric Med 29: 579–591, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int 82: 270–277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez RA, Belyea B, Medrano S, Pentz ES, Sequeira-Lopez ML. Fate and plasticity of renin precursors in development and disease. Pediatr Nephrol 29: 721–726, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grahammer F, Wanner N, Huber TB. Podocyte regeneration: who can become a podocyte? Am J Pathol 183: 333–335, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Griffin SV, Petermann AT, Durvasula RV, Shankland SJ. Podocyte proliferation and differentiation in glomerular disease: role of cell-cycle regulatory proteins. Nephrol Dial Transplant 18, Suppl 6: vi8–13, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hackl MJ, Burford JL, Villanueva K, Lam L, Susztak K, Schermer B, Benzing T, Peti-Peterdi J. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med 19: 1661–1666, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han XL, Liehr JG. Induction of covalent DNA adducts in rodents by tamoxifen. Cancer Res 52: 1360–1363, 1992 [PubMed] [Google Scholar]
- 16.Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ. Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J Clin Invest 100: 786–794, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarad G, Pippin JW, Shankland SJ, Kreidberg JA, Miner JH. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am J Physiol Renal Physiol 300: F811–F820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitamura H, Shimizu A, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N. Apoptosis in glomerular endothelial cells during the development of glomerulosclerosis in the remnant-kidney model. Exp Nephrol 6: 328–336, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Kriz W. The pathogenesis of “classic” focal segmental glomerulosclerosis-lessons from rat models. Nephrol Dial Transplant 18, Suppl 6: vi39–44, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kriz W. Progressive renal failure—inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant 11: 1738–1742, 1996 [PubMed] [Google Scholar]
- 21.Kurt B, Paliege A, Willam C, Schwarzensteiner I, Schucht K, Neymeyer H, Sequeira-Lopez ML, Bachmann S, Gomez RA, Eckardt KU, Kurtz A. Deletion of von Hippel-Lindau protein converts renin-producing cells into erythropoietin-producing cells. J Am Soc Nephrol 24: 433–444, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz A. Control of renin synthesis and secretion. Am J Hypertens 25: 839–847, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Kurtz A. Renin release: sites, mechanisms, control. Annu Rev Physiol 73: 377–399, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA 111: 1527–1532, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasagni L, Ballerini L, Angelotti ML, Parente E, Sagrinati C, Mazzinghi B, Peired A, Ronconi E, Becherucci F, Bani D, Gacci M, Carini M, Lazzeri E, Romagnani P. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 28: 1674–1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasagni L, Romagnani P. Basic research: podocyte progenitors and ectopic podocytes. Nat Rev Nephrol 9: 715–716, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest 96: 953–964, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki Y, Shimizu A, Ichikawa I, Hosoya T, Pastan I, Matsusaka T. Mice are unable to endogenously regenerate podocytes during the repair of immunotoxin-induced glomerular injury. Nephrol Dial Transplant. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, Lombardi D, Giocaliere E, Della Bona M, Villanelli F, Parente E, Ballerini L, Sagrinati C, Wanner N, Huber TB, Liapis H, Lazzeri E, Lasagni L, Romagnani P. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol 24: 1756–1768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perico N, Remuzzi G, Benigni A. Aging and the kidney. Curr Opin Nephrol Hypertens 20: 312–317, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Phillips LM, Wang Y, Dai T, Feldman DL, LaPage J, Adler SG. The renin inhibitor aliskiren attenuates high-glucose induced extracellular matrix synthesis and prevents apoptosis in cultured podocytes. Nephron Exp Nephrol 118: e49–59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichaiwong W, Hudkins KL, Wietecha T, Nguyen TQ, Tachaudomdach C, Li W, Askari B, Kobayashi T, O'Brien KD, Pippin JW, Shankland SJ, Alpers CE. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol 24: 1088–1102, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ. Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol 183: 542–557, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raveh-Amit H, Berzsenyi S, Vas V, Ye D, Dinnyes A. Tissue resident stem cells: till death do us part. Biogerontology 14: 573–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzo P, Perico N, Gagliardini E, Novelli R, Alison MR, Remuzzi G, Benigni A. Nature and mediators of parietal epithelial cell activation in glomerulonephritides of human and rat. Am J Pathol 183: 1769–1778, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Rohani L, Johnson AA, Arnold A, Stolzing A. The aging signature: a hallmark of iPS cells? Aging Cell 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol 9: 137–146, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Romagnani P, Remuzzi G. Renal progenitors in non-diabetic and diabetic nephropathies. Trends Endocrinol Metab 24: 13–20, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg ME, Correa-Rotter R, Inagami T, Kren SM, Hostetter TH. Glomerular renin synthesis and storage in the remnant kidney in the rat. Kidney Int 40: 677–683, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Rosner M, Abdel-Rahman E, Williams ME, ASN Advisory Group on Geriatric Nephrology. Geriatric nephrology: responding to a growing challenge. Clin J Am Soc Nephrol 5: 936–942, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto K, Ueno T, Kobayashi N, Hara S, Takashima Y, Pastan I, Matsusaka T, Nagata M. The direction and role of phenotypic transition between podocytes and parietal epithelial cells in focal segmental glomerulosclerosis. Am J Physiol Renal Physiol 306: F98–F104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulte K, Berger K, Boor P, Jirak P, Gelman IH, Arkill KP, Neal CR, Kriz W, Floege J, Smeets B, Moeller MJ. Origin of parietal podocytes in atubular glomeruli mapped by lineage tracing. J Am Soc Nephrol 25: 129–141, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz MM, Evans J, Bidani AK. The mesangium in the long-term remnant kidney model. J Lab Clin Med 124: 644–651, 1994 [PubMed] [Google Scholar]
- 45.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Shankland SJ, Hamel P, Scholey JW. Cyclin and cyclin-dependent kinase expression in the remnant glomerulus. J Am Soc Nephrol 8: 368–375, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Shankland SJ, Smeets B, Pippin JW, Moeller MJ. The emergence of the glomerular parietal epithelial cell. Nat Rev Nephrol 10: 158–173, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Smeets B, Angelotti ML, Rizzo P, Dijkman H, Lazzeri E, Mooren F, Ballerini L, Parente E, Sagrinati C, Mazzinghi B, Ronconi E, Becherucci F, Benigni A, Steenbergen E, Lasagni L, Remuzzi G, Wetzels J, Romagnani P. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J Am Soc Nephrol 20: 2593–2603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taniguchi Y, Pippin JW, Hagmann H, Krofft RD, Chang AM, Zhang J, Terada Y, Brinkkoetter P, Shankland SJ. Both cyclin I and p35 are required for maximal survival benefit of cyclin-dependent kinase 5 in kidney podocytes. Am J Physiol Renal Physiol 302: F1161–F1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tonelli M, Riella MC. Chronic kidney disease and the aging population. Kidney Int 85: 487–491, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23: 7212–7220, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Wanner N, Hartleben B, Herbach N, Goedel M, Stickel N, Zeiser R, Walz G, Moeller MJ, Grahammer F, Huber TB. Unraveling the role of podocyte turnover in glomerular aging and injury. J Am Soc Nephrol 25: 707–716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Wiggins J. Podocytes and glomerular function with aging. Semin Nephrol 29: 587–593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiggins J, Bitzer M. Slowing the aging process. Clin Geriatric Med 29: 721–730, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Wiggins JE. Aging in the glomerulus. J Gerontol A Biol Sci Med Sci 67: 1358–1364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Hansen KM, Pippin JW, Chang AM, Taniguchi Y, Krofft RD, Pickering SG, Liu Z, Abrass CK, Shankland SJ. De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302: F571–F580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Pippin JW, Krofft RD, Naito S, Liu Z, Shankland SJ. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 304: F1375–F1389, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp Nephrol 121: e23–e37, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]