Abstract
Metabolic and functional abnormalities in the kidney precede or coincide with the initiation of overt hypertension in the Dahl salt-sensitive (SS) rat. However, renal histological injury in SS rats is mild before the development of overt hypertension. We performed electron microscopy analysis in 7-wk-old SS rats and salt-insensitive consomic SS.13BN rats and Sprague-Dawley (SD) rats fed a 4% NaCl diet for 7 days. Long mitochondria (>2 μm) accounted for a significantly smaller fraction of mitochondria in medullary thick ascending limbs in SS rats (4% ± 1%) than in SS.13BN rats (8% ± 1%, P < 0.05 vs. SS rats) and SD rats (9% ± 1%, P < 0.01 vs. SS rats), consistent with previous findings of mitochondrial functional insufficiency in the medulla of SS rats. Long mitochondria in proximal tubules, however, were more abundant in SS rats than in SS.13BN and SD rats. The width of the endoplasmic reticulum, an index of endoplasmic reticulum stress, was significantly greater in medullary thick ascending limbs of SS rats (107 ± 1 nm) than in SS.13BN rats (95 ± 2 nm, P < 0.001 vs. SS rats) and SD rats (74 ± 3 nm, P < 0.01 vs. SS or SS.13BN rats). The tubules examined were indistinguishable between rat strains under light microscopy. These data indicate that ultrastructural abnormalities occur in the medullary thick ascending limbs of SS rats before the development of histological injury in renal tubules, providing a potential structural basis contributing to the subsequent development of overt hypertension.
Keywords: electron microscope, hypertension, kidney, medullary thick ascending limb, mitochondria
the dahl salt-sensitive (SS) rat is the most widely used polygenic model of human salt-sensitive forms of hypertension and renal injury, which are particularly prevalent in African-American and elderly patients. The kidney plays a key role in the development of hypertension in the SS rat (3, 5, 20). Numerous studies have identified structural and histological abnormalities in the kidneys of SS rats that have developed substantial hypertension on high-salt diets. These abnormalities, which could be the consequence of overt hypertension, could further exacerbate the progression of hypertension (6, 16).
Renal abnormalities, however, would have to precede or coincide with the initiation of substantial, overt hypertension if the kidney plays a role in increasing blood pressure salt sensitivity and predisposing animals to the development of overt hypertension. Several studies (15, 22, 23, 25) have indeed indicated that extensive molecular, metabolic, and functional alterations are present in the kidneys of young SS rats before or at the early stage of high-salt exposure. Moreover, genetic studies (4, 19) have identified several candidate genes or genomic segments that may underlie the salt sensitivity in the SS rat.
What is not clear is whether a structural basis exists for the metabolic or functional abnormalities in the kidneys of SS rats before or at the initial stage of the development of overt hypertension. Proteinuria is mild, and interstitial fibrosis and tubular casts are confined to small regions of the kidney in young SS rats before or at the early stage of high-salt exposure (8, 11, 15, 25), which are unlikely to fully account for the functional or metabolic abnormalities.
The ultrastructure of the kidney, especially the renal tubules, however, has been overlooked. We reasoned that genetic or molecular abnormalities in the SS rat could lead to alterations in the ultrastructure of the renal tubules before any abnormalities could be observed at the light microscopy level. Identification of such ultrastructural alterations, if they exist, could provide novel insights into the early mechanisms that underlie blood pressure salt sensitivity or drive the development of overt hypertension.
To test the hypothesis, we carried out an extensive electron microscopy analysis of renal tubules in 7-wk old-SS rats, consomic SS.13BN rats, which are genetically highly similar to SS rats but show an attenuation in salt sensitivity (15, 22), and widely used Sprague-Dawley (SD) rats, which are generally salt insensitive. We found significant abnormalities in the structure of mitochondria and the endoplasmic reticulum (ER), particularly in the medullary thick ascending limb (mTAL) of SS rats, even though the tubules appeared normal under light microscopy.
METHODS
Animals
Male Dahl SS rats, consomic SS.13BN rats, and SD rats were used. Inbred SS and SS.13BN rats were produced at the Medical College of Wisconsin (15, 22, 23). SD rats were purchased from Harlan. Rats of each strain were divided into two groups. One group was maintained on an AIN-76A diet containing 0.4% NaCl from weaning to the time of tissue collection (n = 6 SS rats, 5 SS.13BN rats, and 4 SD rats, respectively). The other group was switched to a 4% NaCl diet at 6 wk of age for 7 days (n = 5 SS rats, 6 SS.13BN rats, and 4 SD rats, respectively). All groups were euthanized for tissue collection at 7 wk of age.
Electron Microscopy
The cortex and outer stripe of the outer medulla (OM) from the left kidney were separated and cut into pieces of ∼1 mm3. The tissue pieces, obtained at a consistent depth from the kidney surface across all rats, were fixed in 2.5% gluteraldehyte and 4% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) for 60 min at 4°C, washed with 0.1 M cacodylate buffer, postfixed in 1% OsO4 for 2 h on ice, dehydrated in ascending concentrations of acetone, and embedded in resin for routine electron microscopy. Fine slices of 60 nm thickness were stained with uranyl acetate and lead citrate and then examined under a Hitachi H-600 electron microscope at an accelerating voltage of 75 kV.
For each rat, five tubule cross-sections from at least three tissue blocks were selected randomly for morphological quantitative analysis. Metamorph Offline (version 7.7.0.0) software was used to analyze all images. The following parameters were examined.
Mitochondrial morphology.
Under ×8,000 magnification, ∼100 mitochondria were measured in each tubule segment to determine the percent distribution of mitochondria with the following various lengths: <1 μm (short), 1–2 μm (medium), and >2 μm (long). Mitochondrial shape was assessed by calculating the ratio of the short axis over the long axis.
Volume density of mitochondria as a percentage of cytoplasmic volume.
The volume density of mitochondria was determined by a point-counting procedure using a grid (with each point spaced by 2.048 mm) with a final magnification of × 8,000. The equation applied was as follows: Vmit = (Pmit/Pcyt) × 100, where Vmit is the volume density of mitochondria as a percentage of cytoplasmic volume, Pmit is the number of grid points that fell within mitochondria, and Pcyt is the number of points that fell within the cell but outside of the nucleus.
Surface density of the inner mitochondrial membrane.
The surface density of the inner mitochondrial membrane (IMM) was calculated using the following equation: SIMM = 4/π × I/(P × d), where SIMM is the surface density of the IMM, I is the number of intersections of the IMM with the test line, P is the number of points that fell within the mitochondrial reference area, and d is the scale dimension between points. The final magnification used was ×60,000.
Width of the ER.
The area and length of >50 rough ER cisternae were measured to calculate the average width. The width did not include the ER membrane or attached ribosomes.
Light Microscopy
The right kidney was removed without flushing, fixed in 10% phosphate-buffered formalin for 24 h at room temperature, and then embedded in paraffin. Tissue sections of 4 μm thickness were prepared, deparaffined, and stained with Masson's trichrome staining. Sections were observed under a Nikon Eclipse E400. In each section, 5 fields in the superficial cortex and 5 fields in the juxtamedullary cortex were randomly selected to examine proximal convoluted tubules (PCTs), 10 fields in each region were randomly selected to examine distal tubules, and the entire section was used to examine glomeruli. Five fields in the outer stripe and five fields in the inner stripe of the OM were randomly selected to examine mTALs. Tubular diameter and cell height were measured under ×200 and ×400 magnifications, respectively. Images were taken using a SPOT Insight camera program and SPOT Advanced (version 3.5.9) for Windows software. Metamorph Offline (version 7.7.0.0) software was used to analyze all images. The parameters evaluated were as follows.
Glomerular density.
Glomerular density was calculated as the total glomerular number divided by the total renal cortex area.
Tubular diameter.
Tubular diameter was defined as the length of the shortest possible straight line that passed through the center of a symmetrically sectioned tubule. Asymmetrically sectioned tubules were excluded from measurements. The diameter did not include the basement membrane.
Tubular cell height.
Tubular cell height was measured along the diameter axis from the base to the apical surface of a tubular cell crossing the nucleus, not including the brush border.
Tubular cell number.
Tubular cell number was calculated as the number of cell nuclei in a tubular cross-section.
Renal interlobular artery thickness.
Symmetrically sectioned round renal interlobular arteries (10–15 arteries/rat) were selected. Artery, artery wall, and artery lumen areas were measured separately.
Cast area.
Cast area was expressed as the total cast area in the cortex divided by the total renal cortex area and the total cast area in the OM divided by the total renal OM area.
OM interstitial collagen.
The OM interstitial collagen area was graded according to the following semiquantitative scoring from 0 to 5: 0 = normal tubular basement membrane and connective tissue, 1 = scattered tubular basement membrane thickening affecting <5% tubules and/or increased deposition of fibrous connective tissue affecting <5% of the OM area, and 2–5 = multifocal tubular basement membrane thickening affecting 5–10%, 10–25%, 25–50%, and >50% tubules, respectively, and/or increased deposition of fibrous connective tissue affecting 5–10%, 10–25%, 25–50%, and >50% of the OM area, respectively.
OM inflammation.
OM interstitial inflammatory cell infiltration was graded according to the following semiquantitative scoring from 0 to 5: 0 = no inflammatory cells infiltration, 1 = scattered infiltration of inflammatory cells affecting <5% of the OM area, 2–4 = multifocal or patchy distribution of inflammatory cells affecting 5–10%, 10–25%, and 25–50% of the OM area, respectively, and 5 = flaky or diffused distribution of inflammatory cells affecting >50% of the OM area.
Statistical Analysis
Data are expressed as means ± SE. Comparisons among multiple groups were examined by one-way or two-way ANOVA followed by a Student-Newman-Keuls test. P < 0.05 was considered significant, and P < 0.01 was considered highly significant.
RESULTS
Electron Microscopy
The renal cortex and outer stripe of the OM from 7-wk-old SS, SS.13BN, and SD rats fed a 4% NaCl diet for 7 days were examined by electron microscopy. A previous study (22) has shown that mean arterial blood pressure at this time point is elevated by <15 mmHg compared with baseline and is just beginning to diverge significantly between SS and SS.13BN rats. No signs of fibrosis or inflammation were observed in the areas randomly selected for electron microscopy examination. Cell edema, however, was observed in some PCT and mTAL cells in SS rats under electron microscope. In some PCT cells with edema, the basal plasma membrane infoldings were widened.
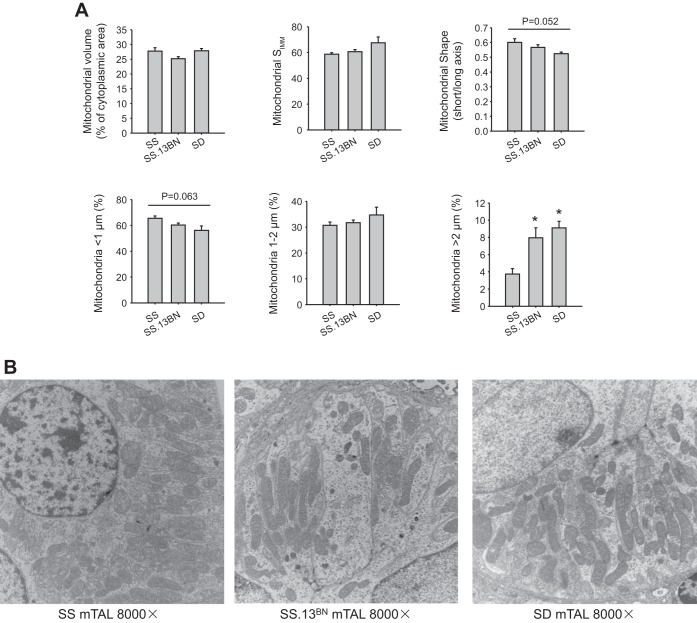
mTALs of SS rats contained a smaller fraction of long mitochondria than in the other two rat strains (Fig. 1, A and B). Long mitochondria (>2 μm), an indication of healthy mitochondria (14), accounted for 4 ± 1% of all mitochondria in mTALs in SS rats, 8 ± 1% in SS.13BN rats (P < 0.05 vs. SS rats), and 9 ± 1% in SD rats (P < 0.01 vs. SS rats). The fraction of short mitochondria (<1 μm) tended to be greater in SS rats than in the other two strains, although the difference did not reach statistical significance. Similarly, the ratio of the short axis to the long axis of mitochondria tended to be greater in SS rats and lower in SD rats. Mitochondrial volume density (as a percentage of cytoplasmic area) was ∼25% in mTALs and was not significantly different among the three rat strains. The surface density of the IMM was also not significantly different.
Fig. 1.
The medullary thick ascending limb (mTAL) of Dahl salt-sensitive (SS) rats contained a smaller proportion of long mitochondria than SS.13BN or Sprague-Dawley (SD) rats. A: mitochondrial volume, surface density of the inner mitochondrial membrane (SIMM), mitochondrial shape, and length distribution of mitochondria. B: representative electron microscopic images. n = 4–6. *P < 0.05 vs. SS rats.
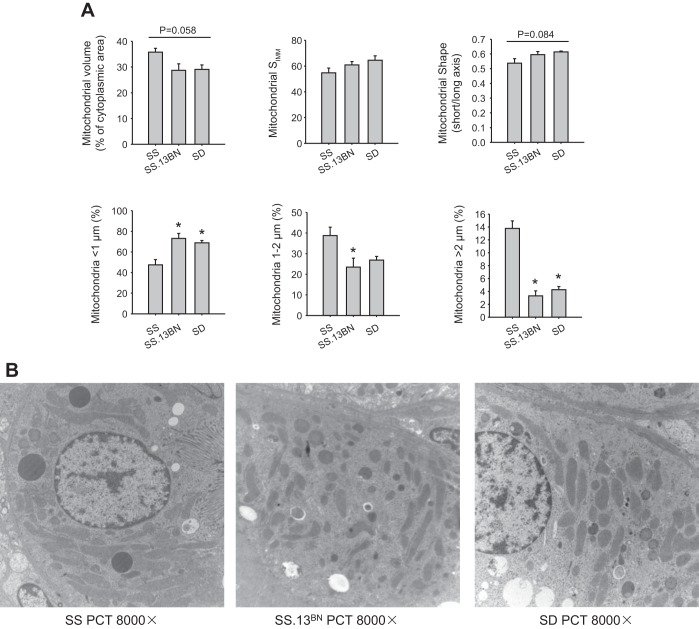
In contrary, PCTs of SS rats contained a significantly larger fraction of long mitochondria and a smaller fraction of short mitochondria than SS.13BN or SD rats (Fig. 2, A and B). The ratio of the short axis to the long axis of mitochondria tended to be lower in SS rats. Mitochondrial volume density was ∼35% in PCTs of SS rats, which tended to be higher than in SS.13BN and SD rats.
Fig. 2.
The proximal convoluted tubule (PCT) of SS rats contained a larger proportion of long mitochondria than in SS.13BN or SD rats. A: mitochondrial volume, SIMM, mitochondrial shape, and length distribution of mitochondria. B: representative electron microscopic images. n = 4–6. *P < 0.05 vs. SS rats.
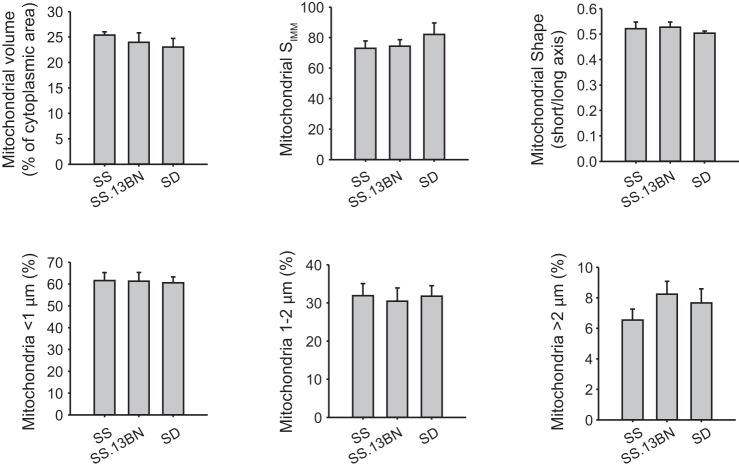
Mitochondria in the distal tubule appeared similar between SS, SS.13BN, and SD rats (Fig. 3).
Fig. 3.
Mitochondria in the distal tubule (DT) appeared similar in SS, SS.13BN, and SD rats. Mitochondrial volume, SIMM, mitochondrial shape, and length distribution of mitochondria are shown. n = 4–6.
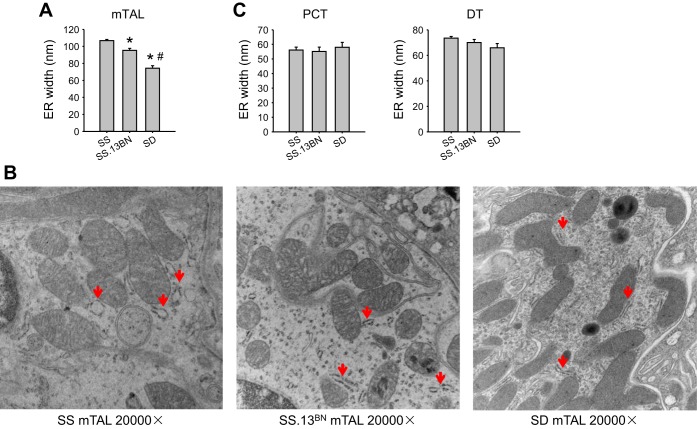
As shown in Fig. 4, A and B, the width of the rough ER, an index of ER stress, was significantly larger in mTALs in SS rats (107 ± 1 nm) than in SS.13BN rats (95 ± 2 nm) by 13% and SD rats (74 ± 3 nm) by a substantial 45%. The width in SS.13BN rats was significantly larger than in SD rats by 28%. The width of the rough ER in the distal tubule tended to show the same trend as the mTAL, but the difference between rat strains did not reach statistical significance (Fig. 4C). The width of the ER in PCTs was not significantly different between rat strains.
Fig. 4.
The width of the endoplasmic reticulum (ER) in the mTAL was largest in SS rats, medium in SS.13BN rats, and smallest in SD rats. A: width of the ER in mTALs. B: representative electron microscopic images of mTALs. Red arrows indicate the rough ER. C: width of the ER in PCTs and DTs. n = 4–6. *P < 0.05 vs. SS rats; #P < 0.05 vs. SS.13BN rats.
Light Microscopy
No significant differences were observed for the diameter or tubular cell height of PCTs or mTALs, glomeruli density, or lumen or wall areas of interlobular arteries among the three rat strains on the 0.4% NaCl diet or the 4% NaCl diet for 7 days (Table 1). The diameter of the distal tubule was significantly larger in SS rats on the 4% NaCl diet than in the other groups by ∼10%. The height or number of cells in the distal tubule was not significantly different among groups.
Table 1.
Morphometric analysis of the kidney under light microscopy
| SS Rats |
SS.13BN Rats |
SD Rats |
||||
|---|---|---|---|---|---|---|
| 0.4% NaCl diet | 4% NaCl diet | 0.4% NaCl diet | 4% NaCl diet | 0.4% NaCl diet | 4% NaCl diet | |
| Glomeruli, number of glomeruli/mm2 cortex | 3.7 ± 0.2 | 3.5 ± 0.1 | 3.6 ± 0.3 | 3.8 ± 0.2 | 3.4 ± 0.1 | 4.0 ± 0.2 |
| PCT diameter, μm | ||||||
| Superficial | 38.0 ± 0.6 | 38.2 ± 0.1 | 38.0 ± 0.6 | 36.5 ± 0.8 | 40.7 ± 0.8 | 40.4 ± 1.1 |
| Juxtamedullary | 38.7 ± 0.4 | 39.1 ± 0.6 | 39.0 ± 0.5 | 38.0 ± 0.8 | 38.6 ± 0.5 | 36.6 ± 1.1 |
| PCT cell height, μm | ||||||
| Superficial | 11.7 ± 0.3 | 11.3 ± 0.2 | 11.3 ± 0.2 | 10.9 ± 0.3 | 11.7 ± 0.4 | 11.4 ± 0.2 |
| Juxtamedullary | 11.8 ± 0.3 | 11.2 ± 0.2 | 11.5 ± 0.1 | 11.5 ± 0.2 | 12.1 ± 0.1 | 10.6 ± 0.4 |
| DT diameter, μm | 26.2 ± 0.3 | 29.2 ± 0.1* | 26.4 ± 0.2 | 25.9 ± 0.4 | 26.8 ± 0.1 | 26.8 ± 0.1 |
| DT cell height, μm | 8.9 ± 0.2 | 8.6 ± 0.1 | 8.8 ± 0.1 | 8.8 ± 0.1 | 8.7 ± 0.3 | 8.6 ± 0.2 |
| DT cell number, number of cells/DCT | 9.6 ± 0.2 | 10.0 ± 0.2 | 9.5 ± 0.2 | 9.9 ± 0.2 | 9.6 ± 0.4 | 9.6 ± 0.2 |
| mTAL diameter, μm | ||||||
| Outer stripe | 26.1 ± 0.2 | 27.2 ± 0.5 | 26.7 ± 0.4 | 27.2 ± 0.5 | 25.9 ± 0.6 | 28.9 ± 0.9 |
| Inner stripe | 26.2 ± 0.4 | 26.7 ± 0.8 | 25.5 ± 0.4 | 24.7 ± 0.5 | 25.1 ± 0.2 | 25.8 ± 0.4 |
| mTAL cell height, μm | ||||||
| Outer stripe | 8.8 ± 0.2 | 8.9 ± 0.1 | 9.3 ± 0.2 | 8.9 ± 0.2 | 8.5 ± 0.2 | 9.2 ± 0.2 |
| Inner stripe | 7.9 ± 0.3 | 8.1 ± 0.2 | 8.1 ± 0.3 | 8.0 ± 0.1 | 7.6 ± 0.1 | 8.6 ± 0.2 |
| Interlobular artery | ||||||
| Lumen/vessel | 0.20 ± 0.02 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.19 ± 0.01 | 0.22 ± 0.01 | 0.19 ± 0.01 |
| Wall/vessel | 0.80 ± 0.02 | 0.81 ± 0.02 | 0.81 ± 0.02 | 0.81 ± 0.01 | 0.79 ± 0.01 | 0.81 ± 0.01 |
Values are means ± SE; n = 4–6 rats/group.
PCT, proximal convoluted tubule; DT, distal tubule; mTAL, medullary thick ascending limb.
P < 0.05 vs. Dahl salt-sensitive (SS) rats on a 0.4% NaCl diet and SS.13BN and Sprague-Dawley (SD) rats on a 4% NaCl diet.
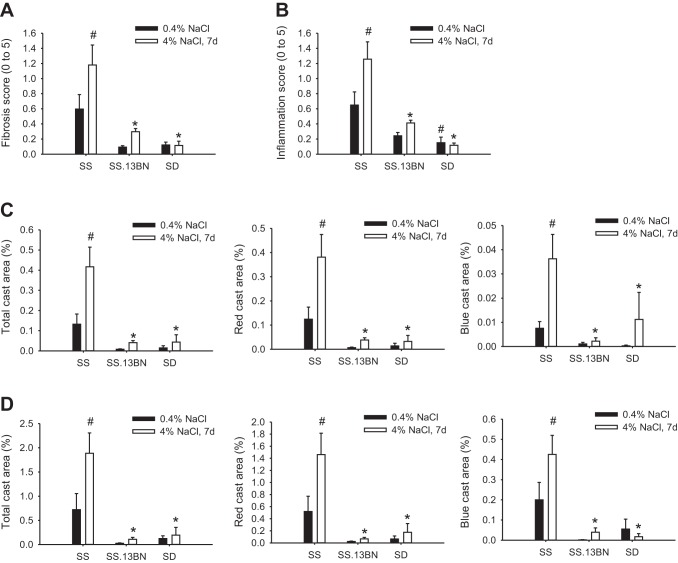
Fibrosis and inflammation scores in the OM and cast areas in the cortex and OM were significantly higher in SS rats fed the 4% NaCl diet for 7 days than in SS.13BN or SD rats on the 4% NaCl diet or SS rats maintained on the 0.4% NaCl diet (Fig. 5). The histological injury appeared to be higher in SS rats on the 0.4% NaCl diet than in the other two strains, but the difference did not reach statistical significance except for inflammation scores in SS and SD rats. Red casts accounted for ∼90% of the casts in the cortex and 75% in the OM (Fig. 5, C and D). The remaining casts were blue casts. Both types of casts showed similar differences among rat groups.
Fig. 5.
Fibrosis, inflammation, and tubular casts affected <5% of the kidney area in SS, SS.13BN, and SD rats on 0.4% NaCl diets or 7 days (7d) of 4% NaCl diets. A: fibrosis scores. A score of 1 indicates that <5% of the area was affected. B: inflammation scores. A score of 1 indicates that <5% of the area was affected. C: cast area (expressed as a percentage of the total area) in the cortex. D: cast area (expressed as a percentage of the total area) in the outer medulla. n = 4–6. *P < 0.05 vs. SS rats on the 4% NaCl diet; #P < 0.05 vs. SS rats on the 0.4% NaCl diet.
Despite the significant differences among rat groups, renal histological injury was mild in all conditions. Even in SS rats fed the 4% NaCl diet for 7 days, fibrosis and inflammation affected not much more than 5% of the renal OM (average score: around 1). Fibrosis and inflammation only occurred sporadically in the cortex and were not sufficient for reliable quantification. Cast areas were <2% of the OM area and ∼0.4% of the cortex area in SS rats on the 4% NaCl diet and lower in other groups.
DISCUSSION
The present study is the first systematic analysis of renal tubular ultrastructure in a model of hypertension. The results indicate that shortening of mitochondria and dilation of the rough ER occurred in the mTALs of 7-wk-old SS rats exposed to a high-salt diet for just 7 days. It provides a potential structural basis for mitochondrial functional deficiency and ER stress that might participate in driving the development of substantial, overt hypertension.
Mitochondrial and metabolic deficiencies are emerging as a new mechanism contributing to the development of hypertension (13). Intravenous infusion of succinate, an intermediate in the tricarboxylic acid cycle, or inducible overexpression of uncoupling protein 1 in vascular smooth muscle cells, which uncouples respiration and oxidative phosphorylation, caused hypertension in mice (2, 10). Succinate activation of the GPR91 receptor is a potential link between high glucose and renin release (17). Fumarase, an enzyme in the tricarboxylic acid cycle, was genetically altered and functionally insufficient in the renal medulla of SS rats compared with SS.13BN rats before blood pressure substantially diverged between the two rat strains (22, 23). Experimental elevation of fumaric acid, the substrate of fumarase that mimics the condition in SS rats, exacerbates salt-induced hypertension in SS.13BN rats (23). Mitochondria isolated from mTALs of SS rats contained several proteins with altered abundance compared with SS.13BN rats and exhibit a lower capability of O2 utilization (27). The shortening of mitochondria found in the present study would be consistent with compromised mitochondrial function in the mTALs of SS rats, although it may not completely explain the functional differences between rat strains. In some cases, cellular failure to remove defective mitochondria may result in extensively extended mitochondria with poor function. However, short and fragmented mitochondria are associated with poor mitochondrial function in most cases (14).
Mitochondria in PCTs, however, are longer in SS rats than in salt-insensitive rat strains. Mitochondrial fission was prominent in the cerebral cortex of SS rats after 9 wk of an 8% NaCl diet (18). Cardiac mitochondria from SS rats consume more O2 and produce more H2O2 than BN rats (1). Substitution of mitochondrial genomes from spontaneously hypertensive rats into SS rats improved aerobic treadmill running capacity and survival, whereas mitochondrial genomes from SS rats did not appear to have an effect on spontaneously hypertensive rats (12). Furosemide-sensitive O2 consumption in isolated mTALs did not appear to differ between SS rats and Dahl salt-resistant rats maintained on a Purina diet containing 0.22% Na+ (9). Together, these findings suggest mitochondrial dysfunction in SS rats might be cell type dependent and influenced by genomic context.
ER stress is a cellular response to the accumulation of unfolded or misfolded proteins, which could result from several conditions including aberrant redox states or Ca2+ levels in the cell (24). ER stress leads to cell cycle arrest, alterations of protein production, and, eventually, cell death. The role of ER stress in the development of hypertension has been recently recognized. Young et al. (26) demonstrated that ER stress in the brain subfornical organ contributed to ANG II-induced hypertension in mice. The results of the present study suggest that ER stress in the mTAL could contribute to the development of hypertension in the SS rat. It remains to be determined what initiates ER stress in the mTAL of the SS rat and what might mediate its effect on blood pressure. Oxidative stress, which could initiate ER stress, occurs in the renal medulla, including the mTAL, of SS rats and is known to contribute to the development of hypertension in SS rats (7, 21). The oxidative stress in the medulla of SS rats could, in part, be due to mitochondrial insufficiencies (23). It would be interesting to examine whether mitochondrial insufficiencies contribute to the dilation of the ER in the mTAL of the SS rat. mTALs of SS rats on a high-salt diet contain an increased number of proliferative cells that might be arrested in the G1/S phase of the cell cycle (25), which could result, in part, from ER stress.
It would not be practical to ascertain the cause-effect relationship between an ultrastructural alteration and hypertension with any single experiment. That is because it is difficult to specifically change any ultrastructural feature with an experimental intervention, especially in vivo. Evidence for a pathophysiological role of ultrastructural alterations would have to come from multiple lines of studies that correlate ultrastructural alterations with biochemical and functional changes whose contribution to hypertension can be experimentally tested. Such evidence in mTALs of SS rats is currently preliminary for the ER but strong for mitochondria, as discussed above.
It is a crucial challenge in salt-sensitive hypertension research to understand the mechanisms underlying the development of hypertension, not just the consequences of later-phase, overt hypertension. However, it is difficult, probably impossible, to find a time point where the kidney structure has stabilized and blood pressure is still completely normal in SS rats on the AIN-76A diet. Nephrogenesis in rats is not complete until 2–3 wk postnatal, and kidneys continue to mature for another 2–3 wk after that. However, small increases in blood pressure and mild renal injury likely already occur in 6-wk-old SS rats even if they have been maintained on a low-salt diet (8, 11, 15, 25). What we could reasonably look for was structural changes that precede or coincide with the initiation of substantial, overt hypertension. Exposure of 6- to 7-wk-old SS rats to the 4% salt diet for 7 days represents a critical time point where substantial, overt hypertension is just beginning to develop (22). We cannot rule out the possibility that some of the ultrastructural changes we observed were the consequence of small increases in blood pressure in SS rats. However, the time point we chose to study was a reasonable compromise and was informative for identifying structural changes that might contribute to the subsequent development of overt hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-116264 (to M. Liang), HL-082798 (to M. Liang), and HL-029587 (to A. W. Cowley, Jr.), Tongji Hospital, and National Science Foundation of China Grants 81228002 and 31071029 (to Z. Tian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.H. and M.L. conception and design of research; X.H., Y.L., and K.U. performed experiments; X.H. and M.L. analyzed data; X.H., Z.T., A.W.C.J., and M.L. interpreted results of experiments; X.H. and M.L. prepared figures; X.H. and M.L. drafted manuscript; X.H. and M.L. edited and revised manuscript; X.H., Y.L., K.U., Z.T., A.W.C.J., and M.L. approved final version of manuscript.
REFERENCES
- 1.An J, Du J, Wei N, Guan T, Camara AK, Shi Y. Differential sensitivity to LPS-induced myocardial dysfunction in the isolated brown Norway and Dahl S rat hearts: roles of mitochondrial function, NF-κB activation, and TNF-α production. Shock 37: 325–332, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature 435: 502–506, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA 275: 1581–1589, 1996 [PubMed] [Google Scholar]
- 4.Cowley AW., Jr. The genetic dissection of essential hypertension. Nat Rev Genet 7: 829–840, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Dahl LK, Heine M, Thompson K. Genetic influence of the kidneys on blood pressure. Evidence from chronic renal homografts in rats with opposite predispositions to hypertension. Circ Res 40: 94–101, 1974 [DOI] [PubMed] [Google Scholar]
- 6.Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGF-β antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283: R757–R767, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, Cowley AW., Jr. Increased expression of NAD(P)H oxidase subunit p67phox in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrett MR, Dene H, Rapp JP. Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J Am Soc Nephrol 14: 1175–1187, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Haque MZ, Ares GR, Caceres PS, Ortiz PA. High salt differentially regulates surface NKCC2 expression in thick ascending limbs of Dahl salt-sensitive and salt-resistant rats. Am J Physiol Renal Physiol 300: F1096–F1104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Johnson RJ, Gordon KL, Giachelli C, Kurth T, Skelton MM, Cowley AW., Jr. Tubulointerstitial injury and loss of nitric oxide synthases parallel the development of hypertension in the Dahl-SS rat. J Hypertens 18: 1497–505, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Kumarasamy S, Gopalakrishnan K, Abdul-Majeed S, Partow-Navid R, Farms P, Joe B. Construction of two novel reciprocal conplastic rat strains and characterization of cardiac mitochondria. Am J Physiol Heart Circ Physiol 304: H22–H32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang M. Hypertension as a mitochondrial and metabolic disease. Kidney Int 80: 15–16, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89: 799–845, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Li P, Yang C, Kurth T, Misale M, Skelton M, Moreno C, Roman RJ, Greene AS, Jacob HJ, Lazar J, Liang M, Cowley AW., Jr. Dynamic convergence and divergence of renal genomic and biological pathways in protection from Dahl salt-sensitive hypertension. Physiol Genomics 41: 63–70, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW., Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol 19: 1472–1482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peti-Peterdi J, Gevorgyan H, Lam L, Riquier-Brison A. Metabolic control of renin secretion. Pflügers Arch 465: 53–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase Cδ under oxidative stress conditions in vivo. Mol Biol Cell 22: 256–265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapp JP. Genetic analysis of inherited hypertension in the rat. Physiol Rev 80: 135–172, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Roman RJ. Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol Renal Fluid Electrolyte Physiol 251: F57–F65, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Taylor NE, Glocka P, Liang M, Cowley AW., Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Tian Z, Greene AS, Usa K, Matus IR, Bauwens J, Pietrusz JL, Cowley AW, Jr, Liang M. Renal regional proteomes in young Dahl salt-sensitive rats. Hypertension 51: 899–904, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Tian Z, Liu Y, Usa K, Mladinov D, Fang Y, Ding X, Greene AS, Cowley AW, Jr, Liang M. Novel role of fumarate metabolism in dahl-salt sensitive hypertension. Hypertension 54: 255–260, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115: 2656–2664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, Stingo FC, Ahn KW, Liu P, Vannucci M, Laud PW, Skelton M, O'Connor P, Kurth T, Ryan RP, Moreno C, Tsaih SW, Patone G, Hummel O, Jacob HJ, Liang M, Cowley AW., Jr. Increased proliferative cells in the medullary thick ascending limb of the loop of Henle in the Dahl salt-sensitive rat. Hypertension 61: 208–215, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheleznova NN, Yang C, Ryan RP, Halligan BD, Liang M, Greene AS, Cowley AW., Jr. Mitochondrial proteomic analysis reveals deficiencies in oxygen utilization in medullary thick ascending limb of Henle in the Dahl salt-sensitive rat. Physiol Genomics 44: 829–842, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]