Abstract
Renal ammonia metabolism is a fundamental element of acid-base homeostasis, comprising a major component of both basal and physiologically altered renal net acid excretion. Over the past several years, a fundamental change in our understanding of the mechanisms of renal epithelial cell ammonia transport has occurred, replacing the previous model which was based upon diffusion equilibrium for NH3 and trapping of NH4+ with a new model in which specific and regulated transport of both NH3 and NH4+ across renal epithelial cell membranes via specific membrane proteins is required for normal ammonia metabolism. A major advance has been the recognition that members of a recently recognized transporter family, the Rhesus glycoprotein family, mediate critical roles in renal and extrarenal ammonia transport. The erythroid-specific Rhesus glycoprotein, Rh A Glycoprotein (Rhag), was the first Rhesus glycoprotein recognized as an ammonia-specific transporter. Subsequently, the nonerythroid Rh glycoproteins, Rh B Glycoprotein (Rhbg) and Rh C Glycoprotein (Rhcg), were cloned and identified as ammonia transporters. They are expressed in specific cell populations and membrane domains in distal renal epithelial cells, where they facilitate ammonia secretion. In this review, we discuss the distribution of Rhbg and Rhcg in the kidney, the regulation of their expression and activity in physiological disturbances, the effects of genetic deletion on renal ammonia metabolism, and the molecular mechanisms of Rh glycoprotein-mediated ammonia transport.
Keywords: acid-base, ammonia, collecting duct, intercalated cell, principal cell
a major advance in our understanding of renal ammonia1 metabolism has been the identification of Rhesus (Rh) glycoproteins as important ammonia transporters. Rh glycoproteins are ammonia-specific transporters and are expressed in those distal epithelial sites responsible for secretion of 60–80% of total urinary ammonia. They are regulated in parallel with changes in renal ammonia excretion, and their expression is necessary for normal ammonia metabolism under both basal conditions and in many pathophysiological conditions. Heterologous expression studies show that Rh glycoproteins transport ammonia and its related compound methylammonia, but no other solutes transported in the kidneys, with the possible exception of CO2. Controversy exists as to the specific molecular form of ammonia transported, NH3 or NH4+ or both. X-ray crystallography and molecular dynamic simulation studies have provided important suggestions as to the molecular mechanisms through which Rh glycoproteins transport ammonia. The goal of this review is to summarize important findings regarding Rh glycoproteins in each of these aspects.
Ammonia Metabolism Summary
Acid-base homeostasis is fundamental to normal health and requires a continuous response by the kidneys to ongoing, yet variable, acid and/or alkali loads. Failure of acid-base regulation leads to a wide variety of complications, including failure to thrive, growth retardation, osteoporosis and osteopenia, and nephrolithiasis, and possibly contributes to the progression of chronic kidney disease (1, 2, 53, 107). Kidneys have a central role in this through the processes of filtered bicarbonate reabsorption and new bicarbonate generation. Reabsorbing filtered bicarbonate is necessary for acid-base homeostasis, but is not sufficient. New bicarbonate must be generated to replace that used to buffer fixed acid loads. New bicarbonate generation results from renal net acid excretion, which involves ammonia and titratable acid excretion.
In many ways, changes in renal ammonia metabolism are the predominant mechanism underlying acid-base homeostasis. Under basal conditions, the majority of new bicarbonate generation occurs through ammonia metabolism. In response to acid-base disturbances, such as metabolic acidosis and hypokalemia, changes in ammonia excretion account for as much as 80% in humans (19), and >95% in rodents, of the change in new bicarbonate generation (8, 9, 59).
Renal ammonia metabolism is an integrative process involving almost all renal epithelial cell segments. Glutamine metabolism, predominantly in the proximal tubule, results in generation of two NH4+ and two HCO3− molecules from each glutamine molecule, and accounts for almost all of the ammonia excreted in the urine (109). Ammonia undergoes a complex set of transport events in the different epithelial cell segments of the kidney, and these transport events determine the proportion of generated ammonia that is excreted in the urine (104, 106, 109). Importantly, only the ammonia excreted in the urine contributes to new bicarbonate generation.
Distal renal segments have a critical role in ammonia excretion because 60–80% of ammonia excreted in the urine is secreted in these segments (29, 104). All segments of the collecting duct, cortical collecting duct (CCD), outer medullary collecting duct (OMCD), and inner medullary collecting duct (IMCD), secrete ammonia (22, 51, 52, 90, 91). It is also likely that the distal convoluted tubule (DCT) and connecting segment (CNT) transport ammonia, because they express similar transport proteins as does the collecting duct, but direct experimental data are lacking. Comparing transport rates in the different collecting duct regions is difficult because of the lack of direct knowledge of interstitial/peritubular ammonia concentrations. However, mathematical modeling suggests that the rate of ammonia secretion is similar in the CCD and the OMCD, and is less in the IMCD (110).
A number of studies have shown that collecting duct ammonia secretion involves parallel H+ and NH3 secretion, without significant transepithelial transport of NH4+ (reviewed in Refs. 17, 29, and 50). H+ secretion involves at least two families of proteins, the vacuolar-type H+-ATPase and the P-type H+-K+-ATPase. Although NH3 transport has often been thought to involve diffusive NH3 movement across lipid bilayers, substantial evidence now indicates that specific proteins transport NH3 and that this transport is essential for normal renal ammonia metabolism. In the kidney, Rh glycoproteins mediate central roles in ammonia transport.
Initial Identification of Rh Glycoproteins as Ammonia Transporters
A wide variety of studies have led to the identification of Rh glycoproteins as critically important mammalian ammonia transporters. The first report of an ammonia-specific transport activity in yeast in 1970 (27) was followed quickly by multiple studies showing that both plant and yeast cells exhibit ammonia-specific transport activities (16, 23, 49, 93, 100, 101). In many cases, multiple ammonia transport activities, with different rates and affinities for ammonia, were identified in the same cells. In 1994, simultaneous publications reported the cloning of the first ammonia-specific transporters from plants and yeast, Amt1 and Mep12, respectively (70, 79). Over the next several years, multiple plant and yeast orthologs of Amt1 and Mep1 were identified and shown to be members of a rapidly expanding family of ammonia transporters (24, 68, 72, 96).
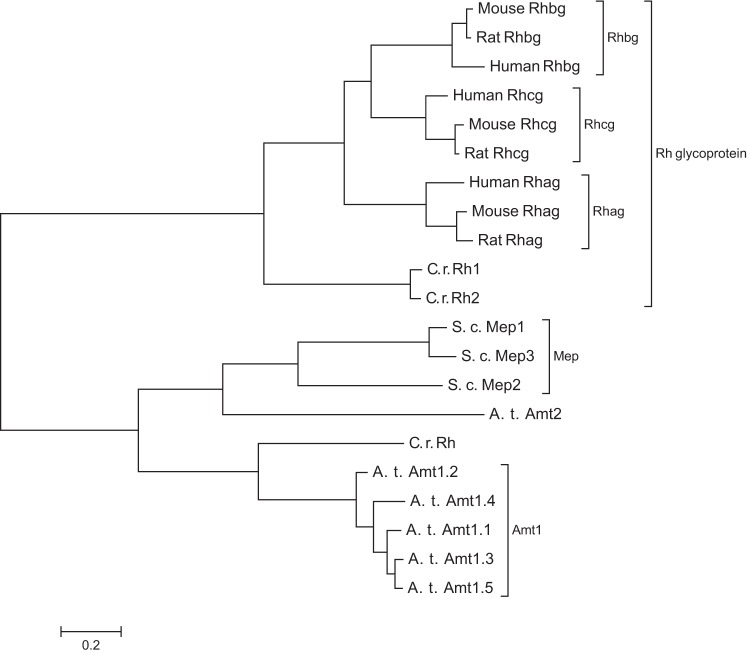
Identification of mammalian orthologs of Mep and Amt proteins initially proceeded slowly. In 1997, Marini and colleagues (69) reported that mammalian Rhesus proteins exhibited weak homology, with ∼25% amino acid sequence identity, to Amt and Mep family members, and suggested that Rhesus proteins might be mammalian ammonia-specific transport proteins. Three years later, Marini and colleagues showed that the Rhesus-associated glycoprotein (Rhag) and a glycosylated kidney homolog, initially named “Rhesus glycoprotein kidney” and now known as Rhesus C glycoprotein (Rhcg), could transport ammonia and methylammonia when expressed in yeast cells deficient in endogenous Mep proteins (67) (Fig. 1). Within the next year, Huang and colleagues (61, 62) reported the cloning of Rh B Glycoprotein (Rhbg) and Rh C Glycoprotein (Rhcg) and showed that they exhibited significant primary and secondary structural homology to Mep and Amt family members, and to Rhag. These three Rhesus glycoproteins, Rhag, Rhbg, and Rhcg, are the known mammalian members of the extended Mep-AMT-Rh glycoprotein ammonia transporter family. Figure 2 shows a family tree of representative members from the Mep, AMT, and Rh glycoprotein family of proteins.
Fig. 1.
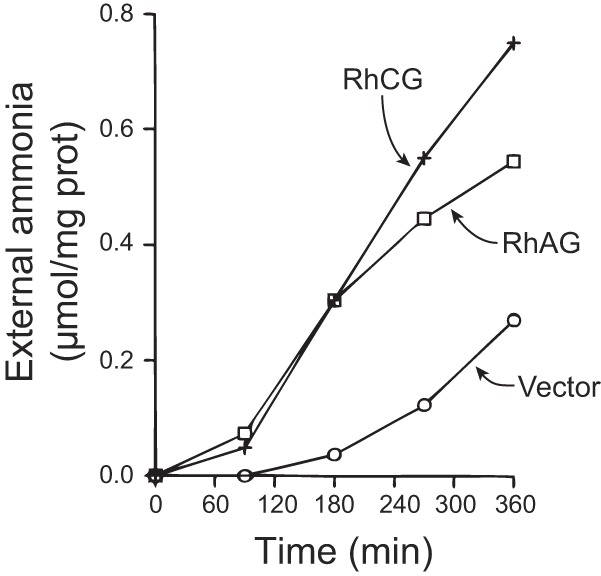
Ammonia transport by Rh A Glycoprotein (Rhag) and Rh C Glycoprotein (Rhcg). Saccharomyces cerevisiae deficient in endogenous ammonia transporters, Mep1, Mep2, and Mep3, were transfected either with vector, Rhag, or Rhcg. Cells were loaded with ammonia and then diluted into ammonia-free media. Ammonia's appearance in the media was quantified as a measure of ammonia transport. Expression of either Rhag or Rhcg dramatically increased the exit of ammonia, indicating increased ammonia transport. [Figure from Ref. 67, used with permission.]
Fig. 2.
Phylogenetic tree of Mep-AMT-Rh glycoproteins showing representative members. Sequences of representative Mep-Amt-Rh glycoproteins from human, mouse, rat, S. cerevisiae (S. c.), Arabidopsis thaliana (A. t.), and Chlamydomonas rhenhardtii (C. r.) were aligned using Clustal Omega (www.ebi.ac.uk/Tools/msa/clustalo, accessed 12/27/2013). Molecular phylogenetic analysis was inferred by using the Maximum Likelihood method based on the JTT matrix-based model (44) using MEGA6 software (92).
Cellular Expression of Rhesus Glycoproteins
Identifying the specific cellular localization of Rhbg and Rhcg is critical for understanding their physiological and pathophysiological roles. The mammalian Rhesus glycoproteins, Rhag, Rhbg, and Rhcg, exhibit distinct cellular expression patterns and important differences in their plasma membrane localization in polarized epithelial cells.
Rhag.
Rhag is an essential component of the erythrocyte “Rhesus complex” (4, 73, 83). The Rhesus complex consists of Rhag in association with RhD and RhCE subunits. Initial studies, utilizing density ultracentrifugation, suggested the Rhesus complex consisted of two Rhag proteins in a complex with RhD and RhCE subunits in a 2:1:1 stoichiometric ratio (5). More recent findings, however, suggest that it is more likely that the Rhesus complex consists of Rhag, RhD, and RhCE in a 1:1:1 stoichiometric ratio (26). RhD and RhCE do not appear to transport ammonia (66). In humans, deficiency of RhAG expression leads to Rhnull disease, which is characterized by hemolytic anemia, spherocytosis, and lack of erythrocyte expression of RhAG, RhD, and RhCE (40, 111).
Rhag protein is present in erythrocytes and in erythrocyte precursor cells present in the bone marrow and, in rodents, the spleen. To date, Rhag protein has not been reported in any nonerythroid tissues. In particular, Rhag protein has not been identified in the kidney, except in residual erythrocytes (103), or in the liver (105). Studies reporting Rhag mRNA expression can be difficult to interpret because sufficient Rhag mRNA can be present in the reticulocytes retained in tissues to yield false-positive findings by RT-PCR (Hong SP and Weiner ID, unpublished observations).
Rhbg.
Rhbg is found in a wide variety of ammonia-transporting and -metabolizing tissues. There is prominent expression in the kidney, and the initial report of the cloning of Rhbg also identified mRNA expression in the liver and sweat glands in the skin (62). Subsequent studies have identified Rhbg expression in a variety of additional tissues, including the lungs, gastrointestinal tract, testis, and epididymis (32, 37, 55).
Our understanding of Rhbg distribution in the kidney has not been straightforward. Initial studies, based upon mRNA hybridization results, suggested that proximal convoluted tubule cells express Rhbg (62). However, subsequent immunohistochemistry studies showed Rhbg protein was expressed solely in distal epithelial cell populations, with no detectable expression in the proximal tubule (82, 94). In distal epithelial cells, Rhbg expression was limited to the basolateral plasma membrane. Low-level expression was present in the DCT, with higher-level expression in the CNT and in the CCD, OMCD, and IMCD. The CNT and the collecting duct have heterogeneous epithelial cell populations, and there are significant cell-specific differences in Rhbg expression in the different cell types in these segments (82, 94). In particular, type A and non-A, non-B intercalated cells, principal cells, and CNT cells express Rhbg, but expression is greater in intercalated cells. Type B intercalated cells do not express detectable Rhbg immunolabel. Table 1 summarizes these findings.
Table 1.
Expression of Rhbg and Rhcg by immunohistochemistry in renal epithelial cells in the mouse kidney (8, 9, 48, 54, 56–58, 82, 94)
| Segment | Cell Type | Rhbg | Rhcg |
|---|---|---|---|
| DCT | DCT cell | +, Basolateral | +, Apical and basolateral |
| CNT | CNT cell | ++, Basolateral | ++, Apical and basolateral |
| Type A intercalated cell | ++, Basolateral | ++, Apical and basolateral | |
| Non-A, non-B cell | — | ++, Apical | |
| CCD | Type A intercalated cell | +++, Basolateral | +++, Apical and basolateral |
| Type B intercalated cell | — | — | |
| Principal cell | +, Basolateral | +, Apical and basolateral | |
| OMCD | Type A intercalated cell | +++, Basolateral | +++, Apical and basolateral |
| Principal cell | +, Basolateral | +, Apical and basolateral | |
| IMCD | Type A intercalated cell | ++, Basolateral | ++, Apical and basolateral |
| IMCD cell | — | — |
RHbg and Rhcg, Rhesus B and C Glycoprotein, respectively; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; OMCD and IMCD, outer and inner medullary collecting duct, respectively.
+, ++, and +++ Indicate low, medium, and high relative expression, as identified by immunohistochemistry.
The identification of Rhbg expression in the human kidney also has not been straightforward. Initial studies showed high levels of Rhbg mRNA expression in human and mouse kidney, but did not examine Rhbg protein expression (62). A subsequent report did not detect Rhbg protein in the human kidney, either by immunoblot analysis or by immunohistochemistry (12). Most recently, our studies identified an error in the initial cloning and sequencing of human Rhbg mRNA (33). This error resulted in a frame-shift alteration in the translated protein carboxy terminus. In the report that did not find human RhBG protein in the kidney, some of the antibodies used were directed against peptide sequences from the incorrect carboxy-terminus sequence. Generation of antibodies against the correct carboxy terminus, and development of new immunohistochemical techniques, which enabled use of antibodies directed against extracellular glycosylation sites, showed expression of Rhbg protein in the human kidney and that its expression in the human kidney was almost identical to its expression pattern in the rat and mouse kidneys (33).
This identification of an error in the initial cloning of human Rhbg has important implications beyond the presence or absence of Rhbg in the human kidney. Several studies examining regulation of human Rhbg used expression of the incorrect mRNA sequence. The putative Tyr-429 phosphorylation site (86) and the postulated PDZ-binding motif at 455-DTQA-458 (86) do not exist in native human RhBG. Whether the tertiary structure of the correct carboxy-tail alters the tertiary structure of the putative ankyrin binding site at 419-FLD-421 (63) is unknown.
Rhcg.
The initial cloning of Rhcg identified mRNA expression in the kidney, testis, and central nervous system (61). Subsequent detailed studies defined its cellular expression in the kidney and testis, and other studies identified Rhcg expression in a number of other organs, including the liver, skeletal muscle, lung, gastrointestinal tract, and epididymis (10, 32, 37, 105).
Renal Rhcg expression.
In the kidney, Rhcg is expressed in distal epithelial cells and has no detectable expression in glomeruli, proximal tubule, loop of Henle, vasculature, or interstitial cells (18, 31, 84, 85, 94). Rhcg expression is prominent in cells in the CNT, initial collecting tubule, CCD, OMCD, and IMCD and is expressed weakly in late DCT cells. Although initial studies identified only apical Rhcg expression, subsequent studies have clearly shown both apical and basolateral expression in the mouse, rat, and human kidney (31, 46, 84, 85). In the mouse, there is substantial strain-specific variation in the extent of basolateral plasma membrane expression. Basolateral Rhcg is present at very low levels in the Balb/C mouse, which was used in our initial report (94) and likely led to the initial failure to recognize basolateral Rhcg expression in the mouse. In contrast, basolateral Rhcg expression is prominent in the C57BL/6 mouse strain (46), and this increased expression correlates with increased ammonia secretion in response to an acid load compared with the Balb/c mouse (108).
Rhcg expression differs among renal epithelial cell types. In general, throughout the collecting duct type A intercalated cells express higher levels of Rhcg than do principal cells. Rhcg is not detectable by immunohistochemistry in type B intercalated cells, consistent with this cell's role in bicarbonate secretion and chloride reabsorption, and not in acid secretion. In the CNT, apical Rhcg expression in non-A, non-B intercalated cells is similar to that in CNT cells, although non-A, non-B intercalated cells have very little or no basolateral Rhcg. IMCD cells do not express detectable Rhcg. Table I summarizes these findings.
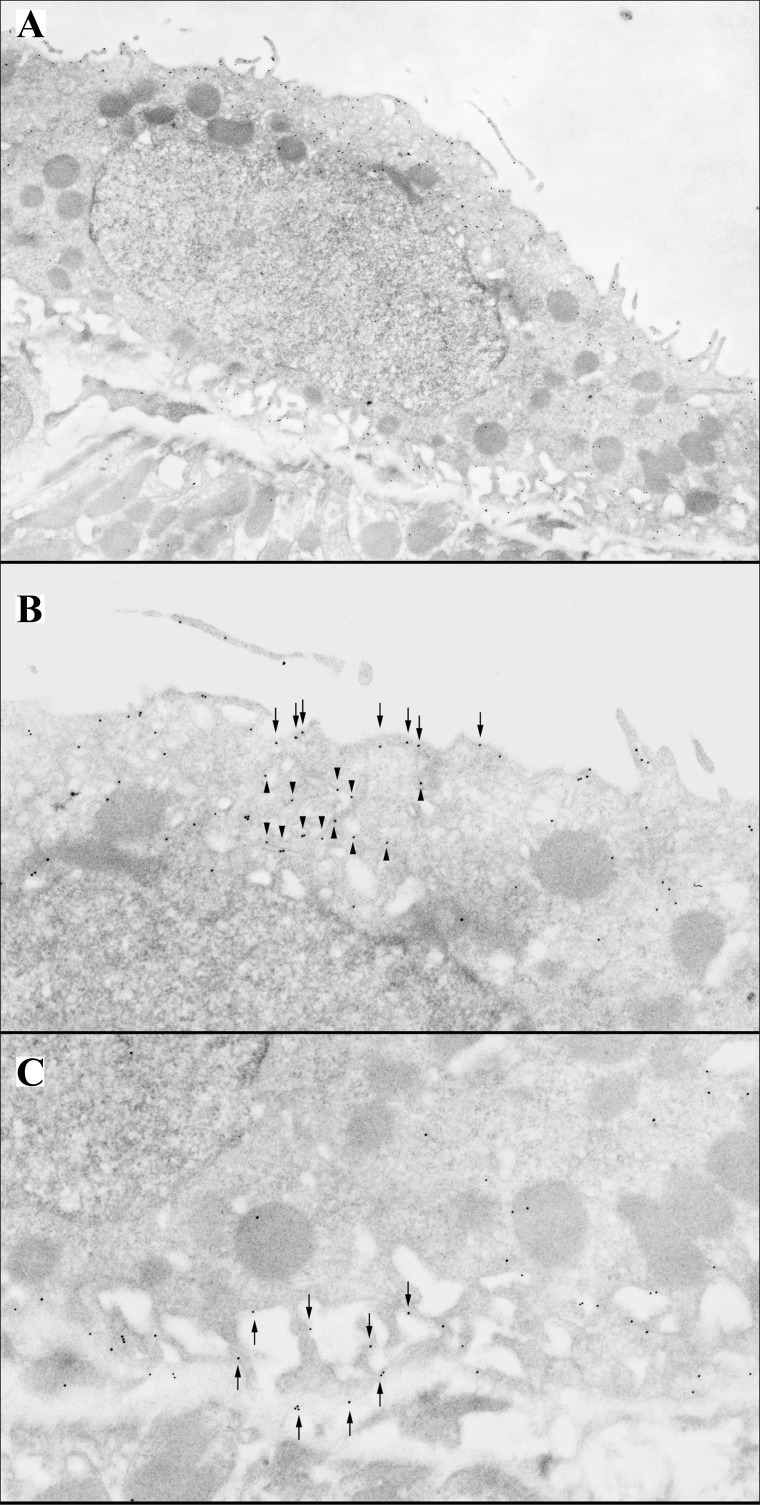
Rhcg is expressed in multiple distinct subcellular sites in renal epithelial cells (Fig. 3). In most cells, Rhcg is present in the apical plasma membrane, in the subapical region, and in the basolateral plasma membrane. In type A intercalated cells under basal conditions, abundant Rhcg is present in subapical vesicles (46, 85). In principal cells, Rhcg is expressed in the subapical cytoplasm, in addition to the apical and basolateral plasma membranes (85). Figure 4 shows representative ultrastructural localization of Rhcg.
Fig. 3.
Subcellular localization of Rhcg in intercalated cells. A: low-power electron micrograph of an intercalated cell in the inner stripe of the outer medulla. B: high-power micrograph of the apical region of this cell. Immunogold label for Rhcg is present both in the apical plasma membrane and in subapical vesicles. C: high-power micrographs of basolateral region of this cell. Immunogold label for Rhcg is present only in the basolateral plasma membrane. Figure is from Ref. 85.
Fig. 4.
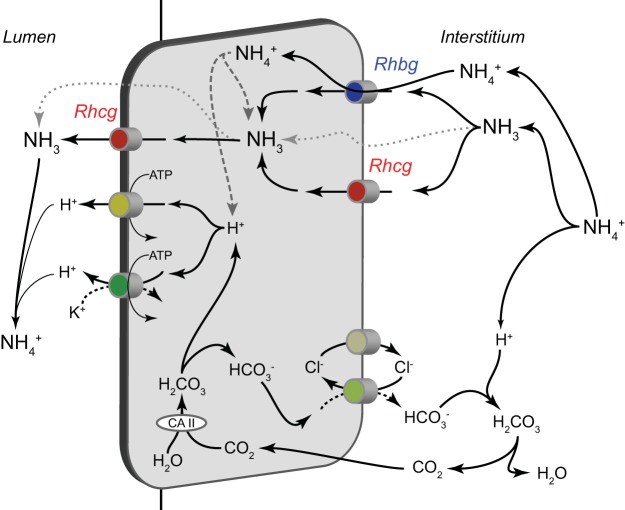
Role of Rh glycoproteins in collecting duct ammonia secretion. Rhbg and Rhcg likely both contribute to basolateral ammonia uptake. As described in the text, whether Rhbg mediates transport of NH4+ or NH3 remains controversial, but it is likely to mediate uptake of either ammonia molecular species across the basolateral membrane. Rhcg is generally believed to primarily mediate electroneutral NH3 uptake across the basolateral plasma membrane. NH3 is then secreted across the apical plasma membrane down its electrochemical gradient through processes involving both apical Rhcg transport and through a separate, undefined, and presumably diffusive transport mechanism. H+ ions are secreted across the apical plasma membrane by both H+-ATPase and H+-K+-ATPase. Cytoplasmic H+ ions are supplied by either dissociation of NH4+ or via a carbonic anhydrase II (CA II)-dependent bicarbonate shuttle mechanism involving basolateral chloride/bicarbonate exchange and basolateral chloride channel-mediated bicarbonate shuttling. Not shown is NH4+ uptake by basolateral Na+-K+-ATPase, which contributes to ammonia secretion in the inner medullary collecting duct (IMCD) (97), where the majority of cells do not express Rh glycoproteins. Pharmacological inhibitor studies indicate that Na+-K+-ATPase is unlikely to contribute to ammonia secretion by the cortical collecting duct (CCD) (51). Also not shown is Na+-K+-Cl− cotransporter 1 (NKCC1)-mediated NH4+ uptake, as inhibitor studies suggest that this transport mechanism does not contribute substantially to collecting duct ammonia secretion (98).
Role of Rh Glycoproteins in Renal Ammonia Excretion
Important insights into the roles of Rhbg and Rhcg in renal ammonia excretion have been obtained through a variety of approaches. Ion transport studies have examined the functional characteristics of collecting duct ammonia secretion and shown critical roles for nondiffusive NH3 transport. Several studies have examined Rhbg and Rhcg expression in conditions that alter renal ammonia metabolism. Finally, the effects of global and cell-specific Rhbg or Rhcg gene deletion on renal ammonia metabolism and collecting duct ammonia transport have been determined. Below, we will try to integrate these studies and use them to summarize the likely roles of Rhbg and Rhcg in renal acid-base homeostasis under basal conditions and in response to a variety of experimental models.
Characterization of collecting duct ammonia transport.
The collecting duct secretes 60–80% of the ammonia excreted by the kidneys (29). Theoretically, ammonia secretion could involve either transepithelial NH3 or NH4+ transport. A number of studies utilizing in vitro microperfused collecting duct segments have clearly shown the presence of parallel H+ and NH3 secretion and have shown that there is no significant transepithelial NH4+ permeability (reviewed in Refs. 17, 50, and 106). Collecting duct NH3 plasma membrane permeability is finite (30, 112), suggesting that NH3 movement across plasma membranes can be a limiting factor in ammonia secretion and that facilitated NH3 transport can be an important regulatory mechanism.
Two sets of studies have shown that diffusive NH3 permeability cannot explain fully collecting duct ammonia secretion. Studies examining the turtle urinary bladder, a model system for the mammalian collecting duct, showed that transepithelial ammonia secretion was saturable and involved competitive transport with methylammonia, functional characteristics indicating the presence of transport mechanisms other than diffusive NH3 movement (3). Studies examining cultured collecting duct epithelial cells showed that ammonia transport across the apical and basolateral plasma membranes involved saturable, i.e., transporter-mediated, NH3 transport (35, 36). Functional characterization showed that both apical and basolateral transport were Na+ and K+ independent and involved electroneutral NH3 transport activity, without detectable NH4+ transport activity (35, 36). A diffusive NH3 permeability was also present, but, at ammonia concentrations similar to those observed in the renal cortex and outer medulla, was only a minor component mechanism of ammonia transport.
Transport mechanisms involving basolateral NH4+ uptake may also contribute to collecting duct ammonia secretion. The ubiquitous basolateral Na+-K+-ATPase can transport NH4+ at the K+ binding site (99), and, at least in the IMCD, it can contribute to transepithelial ammonia secretion (97). However, in the CCD inhibiting Na+-K+-ATPase does not alter ammonia secretion (51). A second basolateral NH4+ transport mechanism may involve NKCC1, which can transport NH4+ at the K+ binding site, and is found in intercalated cells. However, pharmacological studies show that inhibiting NKCC1 does not alter OMCD ammonia secretion (98).
Basal conditions.
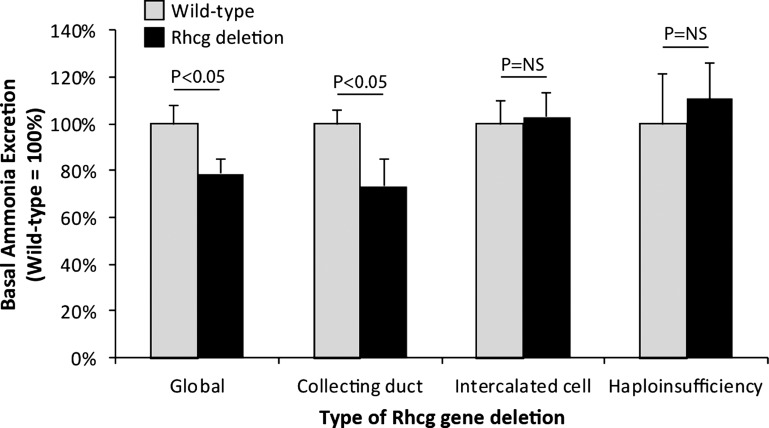
The presence of Rhcg in the plasma membranes of type A intercalated cells, which are acid-secreting cells, suggested that Rhcg is involved in ammonia secretion. The importance of Rhcg's role in ammonia excretion under basal conditions was confirmed by studies examining the effect of gene deletion; pharmacological inhibitor studies have not been performed as specific inhibitors are not available currently. Two studies, one utilizing global Rhcg deletion and the other utilizing collecting duct-specific deletion, both showed an ∼30% decrease in basal ammonia excretion (Fig. 5) (10, 54). The diminished basal ammonia excretion was apparently not due to decreased ammoniagenesis, as expression of the renal ammoniagenic enzymes phosphate-dependent glutaminase and phosphoenolpyruvate carboxykinase, was not altered (54). Haploinsufficiency of Rhcg, in contrast, did not alter basal ammonia excretion, nor did intercalated cell-specific Rhcg deletion (11, 57). The finding that Rhcg expression is necessary for basal ammonia excretion indicates its critical role in basal ammonia secretion.
Fig. 5.
Effect of Rhcg deletion on basal urinary ammonia excretion. Data from studies examining effects of global, collecting duct-, and intercalated cell-specific Rhcg deletion and Rhcg haploinsufficiency on basal urinary ammonia excretion are shown. Because of differing basal ammonia excretion rates in different studies, possibly reflecting either strain-specific or diet-specific differences, excretion rates are normalized such that wild-type mouse ammonia excretion equals 100%. NS, not significant. Data are calculated from Refs. 10, 11, 54, and 57.
Two studies have examined Rhbg's role in basal ammonia excretion. An initial study, utilizing global Rhbg deletion, identified no change in basal ammonia excretion or urine pH, and in vitro microperfused collecting duct studies showed no alteration in basolateral permeability to either NH3 or NH4+ (15). However, whether basolateral Rhcg could compensate for the absence of basolateral Rhbg was not examined. A second study utilized intercalated cell-specific deletion of Rhbg and also found no alterations in basal rates of urinary ammonia excretion (9). However, the expression of the ammonia-metabolizing enzyme glutamine synthetase was decreased, which likely enabled sufficient Rhbg-independent ammonia secretion to maintain ammonia excretion rates (9).
Metabolic acidosis.
RHCG.
The major renal acid-base response to metabolic acidosis is increased renal ammonia excretion (19, 104, 109). This response involves integrated changes in multiple components of ammonia metabolism, including increased glutamine transport, increased expression of the key enzymes involved in ammoniagenesis, increased proximal tubule Na/H exchanger 3 (NHE3) expression, and, most relevant for this review, increased expression of both Rhbg and Rhcg. In response to metabolic acidosis, there is increased Rhcg protein expression in both the outer medulla and the base of the inner medulla (84). Rhcg steady-state mRNA levels do not change, suggesting that regulation of Rhcg protein abundance involves posttranscriptional mechanisms (84).
A second mechanism regulating Rhcg function in transepithelial ammonia transport involves changes in its subcellular distribution. As noted previously, Rhcg is present in the apical plasma membrane, in subapical sites, and in the basolateral plasma membrane. After induction of metabolic acidosis, there is a relative decrease in the proportion of total cellular Rhcg present in the subapical Rhcg compartment and a simultaneous increase in apical plasma membrane Rhcg expression (85). Thus changes in the subcellular localization of Rhcg, involving redistribution from the subapical compartment to the apical plasma membrane, contributes to the regulation of renal ammonia transport.
The relative roles of the two major mechanisms regulating Rhcg apical plasma membrane expression, changes in steady-state protein expression and changes in the subcellular distribution, differ in intercalated cells and principal cells. In the OMCD, changes in Rhcg's subcellular distribution accounts for the majority of the increase in intercalated cell apical plasma membrane expression. This contrasts with principal cells, where the increase in total cellular expression is the quantitatively greater mechanism (85). These differences are summarized in Fig. 6.
Fig. 6.
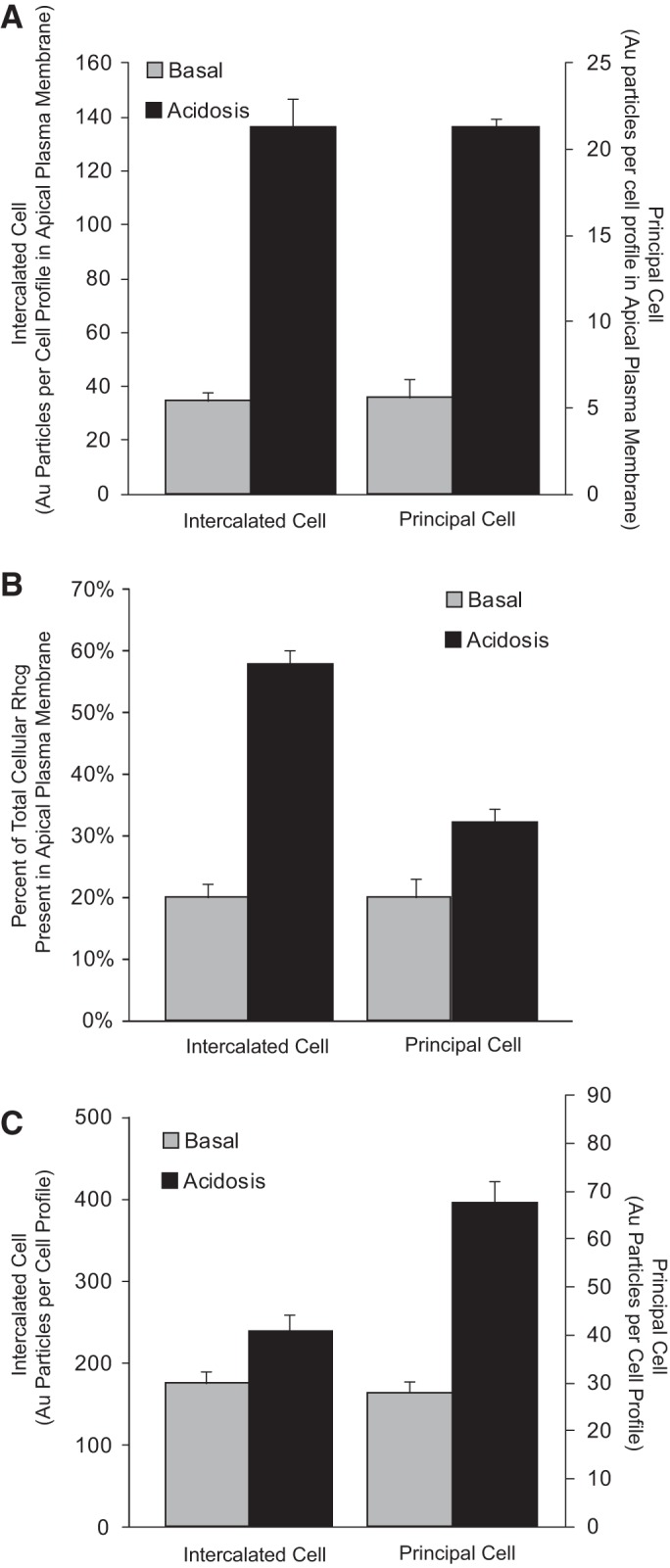
Effect of metabolic acidosis on Rhcg expression in intercalated cells and principal cells. Top: metabolic acidosis increases ∼4-fold the amount of Rhcg present in the apical plasma membrane in both intercalated cells and principal cells in the inner stripe of the outer medulla. Increased apical plasma membrane expression can result from either an increased proportion of total Rhcg present in the apical membrane, i.e., changes in subcellular distribution, or from increased total cellular Rhcg. Middle: proportion of total cellular Rhcg present in the apical plasma membrane in both intercalated and principal cells in response to metabolic acidosis. In both cell types, there is a significant increase in the proportion of total cellular Rhcg present in the apical plasma membrane, but the relative increase is substantially greater in the intercalated cell. Bottom: effect of metabolic acidosis on total cellular Rhcg expression. In both intercalated cells and principal cells, there is a significant increase in total cellular Rhcg, but the relative increase is substantially greater in the principal cell. In top and bottom panels, separate scales are used on the right, for the principal cell, because of differences in absolute level of Rhcg expression. Data are from Ref. 85.
Basolateral Rhcg expression in principal cells is also regulated by metabolic acidosis. Immunogold electron microscopy shows that metabolic acidosis in rats increases the amount of Rhcg in the principal cell basolateral plasma membrane (85). The proportion of total cellular Rhcg in the basolateral plasma membrane does not change significantly, indicating that the increase in basolateral plasma membrane Rhcg is not due to changes in protein trafficking.
Several gene-deletion studies have confirmed the critical role of Rhcg expression in the response to metabolic acidosis. Global Rhcg deletion, haploinsufficiency of Rhcg, collecting duct-specific deletion, and intercalated cell-specific deletion each substantially impaired the expected metabolic acidosis-induced increase in urinary ammonia excretion (10, 11, 54, 57). Studies using in vitro microperfusion of CCD and OMCD segments from acid-loaded animals showed that Rhcg deletion decreases transepithelial ammonia permeability and both apical and basolateral plasma membrane NH3 permeability significantly (10, 11). Thus Rhcg-mediated NH3 transport across both the apical and basolateral plasma membrane is necessary for the normal response to metabolic acidosis.
Studies using intercalated cell-specific Rhcg deletion show important differences from collecting duct-specific, i.e., intercalated and principal cell, Rhcg deletion (57). Intercalated cell-specific Rhcg deletion significantly impairs ammonia excretion in response to metabolic acidosis, but the inhibition is substantially less than in mice with Rhcg deleted from the entire collecting duct (54). Thus both intercalated cell and principal cell Rhcg expression are necessary for acidosis-stimulated changes in ammonia excretion.
RHBG.
Metabolic acidosis also enhances Rhbg expression. In studies in the mouse, HCl-induced metabolic acidosis increases Rhbg expression in the cortex and the outer medulla (9). By immunohistochemistry, increased expression was evident in both principal and intercalated cells in the CCD and OMCD. In contrast, studies performed in the rat examining the effect of metabolic acidosis did not show changes in Rhbg expression (84). The reason for this species-specific difference is not clear.
Multiple studies have examined the necessity of Rhbg expression in the renal response to metabolic acidosis. One study, using ammonium chloride addition to drinking water to induce metabolic acidosis, found that global Rhbg deletion did not alter changes in ammonia excretion (15). A second study added HCl to powdered chow to induce metabolic acidosis and found that Rhbg deletion only from intercalated cells significantly inhibited changes in urinary ammonia excretion (9). One possible explanation for these differing results is that metabolic acidosis induced by different acid sources and delivery techniques may involve different response mechanisms (80). However, an alternative explanation is based upon the observation that the increase in ammonia excretion was substantially greater in the study that found a role for Rhbg than in the study that did not find a role for Rhbg. It is possible that the adaptive responses resulting from Rhbg deletion can compensate when there is a relatively small increase in ammonia excretion, but not when the stimulus is greater. Supporting the latter interpretation is the finding in two other studies which showed that deletion of Rhcg from either the collecting duct or from intercalated cells of acid-loaded mice increased Rhbg expression (54, 57), a result suggesting that increased Rhbg expression can partially compensate for the lack of basolateral Rhcg-mediated ammonia transport and that Rhbg has an important role in transepithelial ammonia transport during acidosis.
Hypokalemia.
In hypokalemia, renal ammonia excretion increases and may contribute to the development of the metabolic alkalosis which is observed commonly. The mechanisms underlying increased ammoniagenesis are similar and involve increased expression of the proximal tubule glutamine transporter SN1/SNAT3, increased expression of multiple enzymes involved in ammoniagenesis, including phosphate-dependent glutaminase, glutamate dehydrogenase and phosphoenolpyruvate carboxykinase (14, 39), and decreased expression of proximal tubule glutamine synthetase (95). However, in metabolic acidosis urine pH decreases, whereas it increases in hypokalemia, suggesting that different mechanisms may underlie changes in collecting duct ammonia transport.
RHCG.
Hypokalemia increases Rhcg expression, consistent with Rhcg having an important role in the increased ammonia excretion. However, the pattern of changes differs from those seen with metabolic acidosis (8, 34, 58). Rhcg expression increases in OMCD intercalated cells, where there is more discrete apical Rhcg expression in intercalated cells and a marked increase in apical plasma membrane immunolabel compared with control animals (34). In OMCD principal cells, hypokalemia increases both apical and basolateral Rhcg intensity; the increase in principal cell apical Rhcg expression results in apical label intensity almost equivalent to that in intercalated cells. Basolateral Rhcg expression also increases in principal cells to the extent that it is of almost equal immunolabel intensity as in intercalated cells. Thus, although hypokalemia increases Rhcg expression in the OMCD in both intercalated and principal cells, the increase appears to be substantially more in principal cells than in intercalated cells.
The role of Rhcg in the response to hypokalemia has been examined further using Rhcg gene deletion. Although collecting duct-specific Rhcg deletion does not alter hypokalemia-induced changes in urinary ammonia excretion (58), adaptive responses in other proteins involved in renal ammonia metabolism occur, which may have compensated for the lack of Rhcg-mediated ammonia transport (58). In particular, phosphate-dependent glutaminase, a major enzyme involved in renal ammoniagenesis, is expressed at higher levels in the outer medulla in hypokalemic mice with collecting duct Rhcg deletion compared with hypokalemic mice with intact collecting duct Rhcg expression. This increased glutaminase expression is likely to increase ammoniagenesis, thereby increasing interstitial ammonia concentration, which would enhance collecting duct Rhcg-independent ammonia secretion. Importantly, the finding that adaptive responses to Rhcg deletion can compensate for its absence in hypokalemia, but not in metabolic acidosis, suggests that the role of Rhcg in the renal response to hypokalemia may be less than its role in response to metabolic acidosis.
RHBG.
Two studies have addressed Rhbg's role in the response to hypokalemia. Rhbg expression increases in response to hypokalemia in studies performed in the mouse kidney (8). Immunoblot analysis combined with immunohistochemistry demonstrated increased basolateral Rhbg expression in intercalated cells in both the CCD and OMCD. Studies in the rat kidney, in contrast, demonstrated no changes in Rhbg expression (34). This difference could be due either to differences in hypokalemic models examined or to species-specific differences in the regulation of Rhbg expression.
In mice, Rhbg deletion only from intercalated cells significantly inhibited hypokalemia-induced increases in total urinary ammonia excretion and completely prevented increases in urinary ammonia concentration (8). These findings show the importance of Rhbg in renal ammonia metabolism and the critical role of Rhbg in the renal response to hypokalemia.
Comparison of Rhbg and Rhcg's roles in metabolic acidosis and hypokalemia.
The availability of studies utilizing cell-specific deletion of Rhbg and Rhcg in two different models of increased ammonia excretion, metabolic acidosis, and hypokalemia, enables comparison of the role of these Rhesus glycoproteins in different conditions. Both proteins appear to be important, but quantitatively, as assessed by the effect of cell-specific deletion, Rhcg may be somewhat more important in metabolic acidosis, whereas Rhbg appears somewhat more important in hypokalemia. These observations suggest Rhbg and Rhcg have different functional roles in renal ammonia metabolism.
Reduced renal mass.
Kidneys are able to maintain acid-base homeostasis even in the presence of substantial decreases in functional renal parenchyma. To do so requires adaptive increases in single-nephron ammonia metabolism (13). To determine the roles of Rhbg and Rhcg in this response, normal Sprague-Dawley rats underwent a ⅚ ablation-infarction model of reduced renal mass and were studied 1 wk later, before development of interstitial fibrosis (47). Total urinary ammonia excretion was unchanged, but ammonia excretion adjusted for creatinine clearance, a measure of single-nephron ammonia metabolism, increased substantially. Although steady-state total Rhcg and Rhbg protein expression did not change, there was increased apical and basolateral Rhcg polarization (47). The redistribution of Rhcg to the plasma membrane compartments without an increase in total renal Rhcg could enhance ammonia secretion and thereby contribute to adaptive increases in single-nephron ammonia metabolism and thus the maintenance of acid-base homeostasis despite severely reduced renal mass.
Cyclosporine A.
The calcineurin inhibitor cyclosporine A has a number of adverse effects on renal function, one of which is induction of renal tubular acidosis (38, 89). In rats treated with cyclosporine A for 4 wks, mild metabolic acidosis developed in conjunction with decreased urinary ammonia excretion (60). The decreased ammonia excretion suggested that impaired renal ammonia excretion contributed to the development of metabolic acidosis. Rhcg protein expression was significantly reduced in both the cortex and outer medulla in cyclosporine A-treated rats (60) although Rhcg mRNA was not altered, suggesting that changes in steady-state protein expression occurred through posttranscriptional regulatory mechanisms. Decreased renal Rhcg protein is likely to mediate, at least in part, cyclosporine A-induced renal tubular acidosis (60).
Mineralocorticoids.
Mineralocorticoids play an important role in both basal and acidosis-stimulated ammonia excretion (41, 42). Recent studies show that administration of the mineralocorticoid analog fludrocortisone increases urinary ammonia excretion and increases Rhcg protein expression (43). Thus mineralocorticoid-stimulated increases in ammonia excretion are likely to involve increases in Rhcg-mediated ammonia transport.
Vasopressin V1a receptors.
Genetic deletion of the vasopressin V1a receptor results in generation of type IV renal tubular acidosis, associated with mild increases in serum potassium and the development of metabolic acidosis with impaired ammonia excretion, but not impairment of urinary pH (43). This impairment of ammonia excretion is at least partly due to decreased Rhcg expression (43).
Molecular Ammonia Species, NH3 vs. NH4+, Transported by Rh Glycoproteins
Ammonia exists in two molecular forms, NH3 and NH4+, which are in equilibrium with each other according to the reaction, NH3 + H+ ↔ NH4+. Transport of these two molecular species is very different (106, 109). NH3 is a strong base, hence it alkalinizes the compartment into which it is transported. Because NH3 is uncharged, its movement across the plasma membrane is unaffected by membrane voltage, and it moves down its concentration gradient, from areas of higher to lower NH3 concentration. At present, no known proteins, including Rh glycoproteins, have been found that can transport NH3 against its concentration gradient. In contrast, NH4+ is a weak acid, so its secretion into a fluid causes mild acidification of that fluid. Moreover, because NH4+ is a cation, its transport will be affected by transmembrane voltage. Because the cell cytoplasm is negatively charged relative to extracellular compartments, electrogenic NH4+ transport generally results in NH4+ transport into cells and can enable transport from compartments of low NH4+ concentration to high NH4+ concentration. Figure 7 summarizes the effects of electrochemical gradients on the relative directions of NH3 and NH4+ movement across the apical and basolateral plasma membranes of collecting duct cells.
Fig. 7.
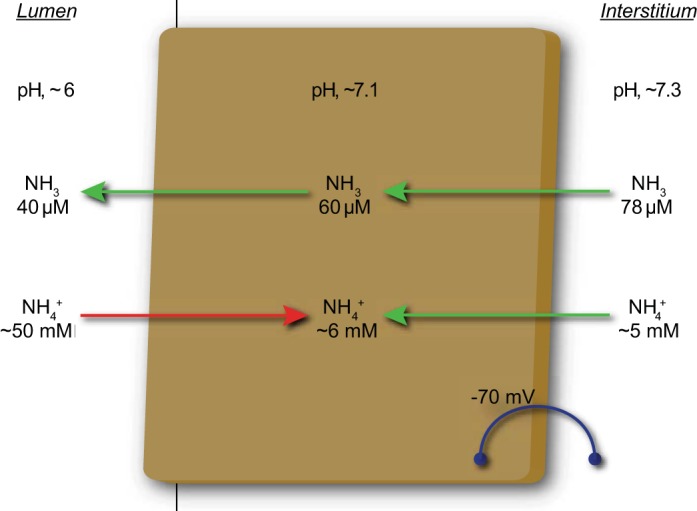
Directions of NH3 and NH4+ transport across the apical and basolateral plasma membrane of collecting duct cells. Model shows electrochemical gradients for NH3 and NH4+ movement across apical and basolateral plasma membranes of collecting duct cells. Because NH3 is uncharged, membrane voltage does not directly affect NH3 transport. As a result, NH3 movement from the interstitium into the cell cytoplasm and across the apical plasma membrane is driven solely by the chemical concentration gradients. For NH4+, electrochemical gradients, driven largely by intracellular electronegativity, favor NH4+ uptake across the basolateral plasma membrane. Across the apical plasma membrane, intracellular electronegativity would facilitate lumen-to-cytoplasm NH4+ movement. Consequently, an electrogenic NH4+ transport mechanism is highly unlikely to be able to contribute to collecting duct ammonia secretion. Arrows show likely movements of NH3 and NH4+ transport across apical and basolateral plasma membranes given typical total ammonia concentrations and pH in interstitium, cytoplasm, and lumen and cell membrane voltage. Arrows in green shown transport consistent with known ammonia movement, and arrow in red, showing luminal NH4+ reabsorption, shows transport unlikely to contribute to ammonia secretion.
A number of studies have addressed the specific molecular ammonia species transported by Rhbg and Rhcg. The results of these studies are shown in Table 2. As can be seen, different studies, using different experimental approaches, have reached different conclusions regarding the molecular ammonia species transported by Rhbg and Rhcg. At present, the explanation for the apparent discrepancies is not clear.
Table 2.
Transport characteristics for NH3 and NH4+ in heterologous expression systems
| Rhbg | |||||
|---|---|---|---|---|---|
| Electroneutral |
Electrogenic |
||||
| Expression system | NH3 transport | CH3NH2 transport | NH4+ transport | CH3NH3+ transport | Citation, Year |
| Murine Rhbg expressed in Xenopus oocyte | Absent | Present | (78), 2004 | ||
| Human RhBG in Xenopus oocyte | Present | Absent | (64), 2004 | ||
| Human RhBG in HEK293 and MDCK cells | Present | Absent | (114), 2005 | ||
| Murine Rhbg | Present | Absent | (66), 2006 | ||
| Murine Rhbg in Xenopus oocyte | Present | Present | Present | (76), 2010 | |
| Murine Rhbg in Xenopus oocyte | Absent | Present | Present | Present | (77), 2010 |
| Human RhBG in Xenopus oocyte | Present | (25), 2013 | |||
| Rhcg | |||||
| Human RhCG expressed in Xenopus oocyte | Present | Present | (7), 2004 | ||
| Human RhCG in HEK293 and MDCK cells | Present | Absent | (114), 2005 | ||
| Murine Rhcg in Xenopus oocyte | Present | Absent | (66), 2006 | ||
| Human RhCG in Xenopus oocyte and in S. cerevisiae | Present | Absent | (71), 2006 | ||
| Rhcg gene deletion, perfused collecting duct segment, apical transport | Present | Absent | (10), 2008 | ||
| Purified RhCG-HA reconstituted in liposomes | Present | Absent | (74), 2010 | ||
| Human RhCG reconstituted in liposomes | Present | Absent | (26), 2010 | ||
| Rhcg gene deletion, perfused collecting duct segment, basolateral transport | Present | Absent | (11), 2012 | ||
| Human RhCG in Xenopus oocyte | Present | (25), 2013 | |||
MDCK, Madin-Darby canine kidney; HA, hemagglutinin.
If neither “present” nor “absent” is noted for a specific transport modality, then that transport modality was not explicitly examined.
It is important to note that the majority of studies have found that Rhcg transports NH3, not NH4+. This finding is critical for Rhcg to contribute to ammonia transport across the apical plasma membrane of collecting duct cells. Because urine pH is generally lower than cytoplasmic pH, luminal NH3 concentrations are less than cytoplasmic NH3. Thus the cytoplasm-to-lumen NH3 gradient can enable apical Rhcg-mediated electroneutral NH3 secretion. If apical Rhcg functioned as an electrogenic NH4+ transporter, it would be unlikely to contribute to collecting duct ammonia secretion. This is because luminal NH4+ concentrations are typically higher than cytoplasmic NH4+ concentrations, and this, in conjunction with intracellular electronegativity, would favor NH4+ reabsorption, not secretion, across the apical plasma membrane.
The conditions for ammonia transport across basolateral plasma membranes are more complex. Both Rhcg and Rhbg are present in the basolateral plasma membrane, and several studies have shown that Rhbg can transport NH4+ as well as NH3. Furthermore, the net electrochemical gradient for NH3 and NH4+ transport differs from that at the apical plasma membrane. Interstitial NH3 concentrations are believed generally to be higher than intracellular NH3 concentrations, indicating that electroneutral NH3 transport could enable cellular ammonia uptake across the basolateral membrane. Intracellular electronegativity would provide a sufficient gradient for electrogenic NH4+ uptake. Thus Rhbg would be predicted to result in basolateral ammonia uptake regardless of whether NH3 or NH4+ is transported. Basolateral Rhcg, because most studies suggest that it transports NH3, also contributes to basolateral ammonia uptake.
Many studies examining transport mediated by Rhbg and Rhcg have utilized the methyl derivative of ammonia, methylammonia3. In large part, this is because methylammonia can be obtained in a radioactive form, 14C-methylammonia, which facilitates quantification of cellular uptake. However, there may be important differences in the relative transport of the different molecular forms of ammonia and methylammonia (77). In particular, carefully performed studies have shown that exposing Rhbg-expressing Xenopus oocytes to ammonia causes intracellular acidification and increases in electric current, indicating substantial NH4+ transport. In contrast, methylammonia exposure causes intracellular alkalinization and does not induce a significant electric current, suggesting predominant transport of the electroneutral species, CH3NH2 (77). These findings indicate that although ammonia and methylammonia can both be transported by Rhbg and Rhcg, the preferential transport of the different molecular forms of ammonia (NH3 vs. NH4+) and methylammonia (CH3NH2 and CH3NH3+) may differ.
Mechanism of Transport by Rhbg and Rhcg
A variety of experimental approaches, including amino acid substitution analysis, X-ray crystallography, in silico computational analysis, and molecular dynamic simulations, have provided important information regarding the putative mechanisms by which Rhesus glycoproteins transport ammonia. Mammalian Rhesus glycoproteins are integral membrane proteins, with 12 transmembrane-spanning segments. Both the carboxyl and amino termini are present in cytoplasmic compartments. X-ray crystallography studies of human Rhcg, related Rhesus glycoproteins, and bacterial AMT orthologs consistently show that Rhesus glycoproteins exist in a homotrimeric form (26, 45, 65, 113). There are both a cytoplasmic and external vestibule and a narrow, hydrophobic, ∼20-Å-long central channel. A number of X-ray crystallography and molecular dynamics simulation studies suggest stabilization of NH3 through this hydrophobic channel through interaction with two in-line histidines. In addition, these in-line histidines enable selectivity of the Rhesus glycoproteins for ammonia, but not cation, transport (28). A phenylalanine ring interrupts the communication between the extracellular vestibule and the hydrophobic core and may regulate transport. Both the cytoplasmic and external vestibule contain specific acidic amino acid residues. These are likely to facilitate electrogenic interaction of NH4+ with these vestibules. Figure 8 summarizes this model. In addition, in these vestibules a change in the pKa of the NH3-NH4+ buffer reaction may occur (45), facilitating dissociation of NH4+ into NH3 and H+. This generation of a locally high NH3 concentration optimizes NH3 transport through the central channel.
Fig. 8.

Proposed mechanism of NH3 transport by human Rhcg. The NH3 secretory mechanism is shown, but transport appears to be bidirectional and reversible based upon the net NH3 gradient. Acidic residues in the cytoplasmic vestibule result in NH4+ concentration near the cytoplasmic opening of a 20-Å-long, narrow central pore. Change in the pKa of the NH4+/NH3 buffer reaction results in increased local NH3 concentrations. NH3 can then transit the central pore, with weak stabilization mediated by 2 in-line histidine residues. A phenylalanine residue gates the extracellular vestibule and limits transit through Rhcg. Once in the extracellular vestibule, NH3 is protonated, reforming NH4+. Acidic residues, such as glutamate-166, in the extracellular vestibule provide weak interaction with NH4+. A movie showing these critical amino acid residues in 3-dimensional format is provided in the supplementary materials (all supplementary material for this article is accessible on the journal Web site).
An unexpected finding from the crystal structure of human Rhcg was the presence of a shunt pathway or pocket (26). This pocket extends from the cytosolic aperture to the lateral exterior surface of Rhcg which is in contact with the lipid bilayer. The amino acids lining this shunt are conserved among human Rh glycoproteins, and this shunt is also seen in the structure of the bacterial Rh glycoprotein ortholog, NeRh, suggesting that it is common to the Rh subfamily. While the functional role of this shunt pathway has not been experimentally determined, it is possible that it represents an alternative path for NH3 transport that bypasses the polar head groups of the cytoplasmic leaflet (26).
The molecular mechanism(s) through which Rhesus glycoproteins could mediate electrogenic NH4+ transport is incompletely understood at present. In particular, whether transports occurs as NH4+ transport or as cotransport of NH3 with H+ has been the subject of several investigations. Multiple molecular dynamics simulation studies suggest that transport of NH4+ through the central channel is highly energetically unfavorable (81, 102), and instead suggest that electrogenic NH4+ transport is more likely to occur through an NH3-H+ cotransport mechanism. One possible mechanism involves the suggestion that water molecules may exist in the central pore (6), which could enable H+ transport through a water chain. This could enable NH3-H+ cotransport, with NH3 transport through mechanisms described in the previous paragraph. Another possibility involves H+ transport involving the twin-inline histidines (102). At present, these interpretations are based upon molecular dynamic simulations of the bacterial ortholog AmtB and have not been examined experimentally in mammalian Rh glycoproteins.
CO2 Transport by Rh Glycoproteins
The discovery that Rhesus glycoproteins transport the gas molecule NH3 led to a number of investigations as to whether Rhesus glycoproteins might transport other gas molecules. Initial studies examining the green algae Chlamydomonas rheinhardtii found that the Rhesus glycoprotein ortholog Rh1 was necessary for the normal responses to CO2, suggesting Rh1 can transport CO2 (87, 88). Quantitative studies using human erythrocytes deficient in RhAG show that the absence of RhAG decreases CO2 transport (20, 21). More recently, studies involving heterologous expression in Xenopus oocytes have shown that all three mammalian Rhesus glycoproteins, Rhag, Rhbg, and Rhcg, can transport CO2 (25, 75).
A physiological role for Rhbg- or Rhcg-mediated CO2 transport in the kidney is not clear. Intercalated cells use cytoplasmic CO2 to generate the intracellular H+ used for urinary acidification, through a carbonic anhydrase-catalyzed process. Several studies using deletion of either Rhbg or Rhcg, or of both simultaneously, show that Rhbg and Rhcg expression are not necessary for urine acidification (9, 10, 15, 54, 57, 59). However, these studies cannot exclude the possibility of either altered intrarenal CO2 concentrations, which might enhance diffusive CO2 movement in the absence of Rhbg and Rhcg, or adaptive changes in other CO2 transport mechanisms.
Summary
The identification of Rhesus glycoproteins has led to dramatic alterations in understanding of renal and extrarenal ammonia metabolism. The nonerythroid Rhesus glycoproteins Rhbg and Rhcg are expressed in specific cells in the kidney in specific subcellular locations, as well as in many nonrenal tissues involved in ammonia metabolism. Rhbg and Rhcg protein expression is regulated in parallel with renal ammonia excretion in a wide variety of conditions, and a number of gene deletion studies of Rhbg and Rhcg confirm their critical roles in ammonia metabolism.
Despite the important advances in our understanding of the role of Rh glycoproteins in renal ammonia transport, there are many important questions that remain incompletely understood. These involve issues such as the specific molecular form of ammonia transported; if this form is NH4+, then whether this involves NH4+ transport or NH3-H+ cotransport; if NH3-H+ cotransport, then what is the mechanism through which the H+ is transported; whether there are posttranslational modifications of Rh glycoproteins that affect their transport activity; what is the mechanism regulating changes in the subcellular distribution of Rhcg in the apical region of collecting duct cells; why does the relative role of total protein expression and changes in subcellular distribution in the regulation of apical Rhcg expression differ in intercalated cells and principal cells; and, which hormones alter Rhbg and Rhcg expression and what is the mechanism through which they do so. Clearly, our studies of the mechanisms through which Rh glycoproteins play critical roles in renal transport are only beginning.
GRANTS
This work was supported by funds from the National Institutes of Health (R01-DK045788) and the Department of Veterans Affairs (1I01BX000818-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: I.D.W. and J.W.V. provided conception and design of research; I.D.W. and J.W.V. performed experiments; I.D.W. and J.W.V. analyzed data; I.D.W. and J.W.V. interpreted results of experiments; I.D.W. and J.W.V. prepared figures; I.D.W. drafted manuscript; I.D.W. and J.W.V. edited and revised manuscript; I.D.W. and J.W.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the numerous highly talented investigators with whom we have been fortunate to work on our studies investigating Rh glycoprotein-mediated ammonia metabolism.
Footnotes
1Ammonia exists in two molecular forms, NH3 and NH4+, which are in equilibrium with each other. When referring to a specific molecular form, we specifically state either “NH3” or “NH4+.” When referring to the combination of both molecular forms, we use the term “ammonia.”
2Because most yeast ammonia transport studies utilized measurement of radiolabeled methanol ammonia uptake, the transport activity was referred to as a “methylammonia permease.” Accordingly, the first protein identified which could mediate this transport activity was named Mep1.
3Similar to ammonia, methylammonia exists in two molecular forms, CH3NH2 and CH3 NH3+, which are in equilibrium with each other. We use the term methylammonia to refer to the combination of these two molecular forms. When referring to one specific molecular form, we specifically state either, “CH3NH2” or “CH3NH3+.”
REFERENCES
- 1.Adrogué HJ, Madias NE. Medical progress: management of life-threatening acid-base disorders. N Engl J Med 338: 26–34, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Adrogué HJ, Madias NE. Medical progress: management of life-threatening acid-base disorders. Second of two parts. N Engl J Med 338: 107–111, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Arruda JA, Dytko G, Withers L. Ammonia transport by the turtle urinary bladder. Am J Physiol Renal Fluid Electrolyte Physiol 246: F635–F647, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Avent N, Judson PA, Parsons SF, Mallinson G, Anstee DJ, Tanner MJ, Evans PR, Hodges E, Maciver AG, Holmes C. Monoclonal antibodies that recognize different membrane proteins that are deficient in Rhnull human erythrocytes. One group of antibodies reacts with a variety of cells and tissues whereas the other group is erythroid-specific. Biochem J 251: 499–505, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avent ND, Reid ME. The Rh blood group system: a review. Blood 95: 375–387, 2000 [PubMed] [Google Scholar]
- 6.Baday S, Wang S, Lamoureux G, Bernèche S. Different hydration patterns in the pores of AmtB and RhCG could determine their transport mechanisms. Biochemistry 52: 7091–7098, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem 279: 15975–15983, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bishop JM, Lee HW, Handlogten ME, Han KH, Verlander JW, Weiner ID. Intercalated cell-specific Rh B Glycoprotein deletion diminishes renal ammonia excretion response to hypokalemia. Am J Physiol Renal Physiol 304: F422–F431, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID. Role of the Rhesus glycoprotein, Rh B Glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299: F1065–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Bourgeois S, Bounoure L, Christensen EI, Ramakrishnan SK, Houillier P, Devuyst O, Wagner CA. Haploinsufficiency of the ammonia transporter Rhcg predisposes to chronic acidosis: Rhcg is critical for apical and basolateral ammonia transport in the mouse collecting duct. J Biol Chem 288: 5518–5529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown ACN, Hallouane D, Mawby WJ, Karet FE, Saleem MA, Howie AJ, Toye AM. RhCG is the major putative ammonia transporter expressed in human kidney and RhBG is not expressed at detectable levels. Am J Physiol Renal Physiol 296: F1279–F1290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buerkert J, Martin D, Trigg D, Simon E. Effect of reduced renal mass on ammonium handling and net acid formation by the superficial and juxtamedullary nephron of the rat. Evidence for impaired reentrapment rather than decreased production of ammonium in the acidosis of uremia. J Clin Invest 71: 1661–1675, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busque SM, Wagner CA. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am J Physiol Renal Physiol 297: F440–F450, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Dubois E, Grenson M. Methylamine/ammonia uptake systems in Saocharomyces cerevisiae: multiplicity and regulation. Mol Gen Genet 175: 67–76, 1979 [DOI] [PubMed] [Google Scholar]
- 17.DuBose TD, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol 1: 1193–1203, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Elkinton JR, Huth EJ, Webster GD, Jr, RA McCance. The renal excretion of hydrogen ion in renal tubular acidosis. Am J Med 36: 554–575, 1960 [DOI] [PubMed] [Google Scholar]
- 20.Endeward V, Cartron JP, Ripoche P, Gros G. Red cell membrane CO2 permeability in normal human blood and in blood deficient in various blood groups, and effect of DIDS. Transfus Clin Biol 13: 123–127, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22: 64–73, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Flessner MF, Wall SM, Knepper MA. Ammonium and bicarbonate transport in rat outer medullary collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 262: F1–F7, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Fried M, Zsoldos F, Vose PB, Shatokaim IL. Characterizing the NO3 and NH4+ uptake rice roots by use of 15N labelled NH4NO3. Physiol Plant 18: 313–320, 1965 [Google Scholar]
- 24.Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, von Wiren N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 11: 937–948, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geyer RR, Parker MD, Toye AM, Boron WF, Musa-Aziz R. Relative CO2/NH3 permeabilities of human RhAG, RhBG and RhCG. J Membr Biol 246: 915–926, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, Sali A, Westhoff CM, Stroud RM. Function of human Rh based on structure of RhCG at 2.1 Ä. Proc Natl Acad Sci USA 107: 9638–9643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackette SL, Skye GE, Burton C, Segel IH. Characterization of an ammonium transport system in filamentous fungi with methylammonium-14C as the substrate. J Biol Chem 245: 4241–4250, 1970 [PubMed] [Google Scholar]
- 28.Hall JA, Yan D. The molecular basis of K+ exclusion by the Escherichia coli ammonium channel AmtB. J Biol Chem 288: 14080–14086, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F595–F605, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Hamm LL, Trigg D, Martin D, Gillespie C, Buerkert J. Transport of ammonia in the rabbit cortical collecting tubule. J Clin Invest 75: 478–485, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C Glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han KH, Mekala K, Babida V, Kim HY, Handlogten ME, Verlander JW, Weiner ID. Expression of the gas transporting proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the murine lung. Am J Physiol Lung Cell Mol Physiol 297: L153–L163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han KH, Lee HW, Handlogten ME, Whitehill FM, Croker BP, Clapp W, Verlander JW, Weiner ID. Expression of the ammonia transporter family member, Rh B Glycoprotein, in the human kidney. Am J Physiol Renal Physiol 304: F972–F981, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han KH, Lee HW, Handlogten ME, Bishop JM, Levi M, Kim J, Verlander JW, Weiner ID. Effect of hypokalemia on renal expression of the ammonia transporter family members, Rh B Glycoprotein and Rh C Glycoprotein, in the rat kidney. Am J Physiol Renal Physiol 301: F823–F832, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 287: F628–F638, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the intestinal tract. Am J Physiol Gastrointest Liver Physiol 288: G1036–G1047, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Heering P, Grabensee B. Influence of ciclosporin A on renal tubular function after kidney transplantation. Nephron 59: 66–70, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Hossain SA, Chaudhry FA, Zahedi K, Siddiqui F, Amlal H. Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal Physiol 301: F969–F978, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Huang CH. The human Rh50 glycoprotein gene. Structural organization and associated splicing defect resulting in Rhnull disease. J Biol Chem 273: 2207–2213, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Hulter HN, Licht JH, Glynn RD, Sebastian A. Renal acidosis in mineralocorticoid deficiency is not dependent on NaCl depletion or hyperkalemia. Am J Physiol Renal Fluid Electrolyte Physiol 236: F283–F294, 1979 [DOI] [PubMed] [Google Scholar]
- 42.Hulter HN, Ilnicki LP, Harbottle JA, Sebastian A. Impaired renal H+ secretion and NH3 production in mineralocorticoid- deficient glucocorticoid-replete dogs. Am J Physiol Renal Fluid Electrolyte Physiol 232: F136–F146, 1977 [DOI] [PubMed] [Google Scholar]
- 43.Izumi Y, Hori K, Nakayama Y, Kimura M, Hasuike Y, Nanami M, Kohda Y, Otaki Y, Kuragano T, Obinata M, Kawahara K, Tanoue A, Tomita K, Nakanishi T, Nonoguchi H. Aldosterone requires vasopressin V1a receptors on intercalated cells to mediate acid-base homeostasis. J Am Soc Nephrol 22: 673–680, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8: 275–282, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Khademi S, O'Connell J, III, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 A. Science 305: 1587–1594, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Kim HY, Handlogten ME, Cain B, Guo H, Han KH, Verlander JW. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney (Abstract). J Am Soc Nephrol 18: 589A, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleiner D. The transport of NH3 and NH4+ across biological membranes. Biochim Biophys Acta 639: 41–52, 1981 [DOI] [PubMed] [Google Scholar]
- 50.Knepper MA. NH4+ transport in the kidney. Kidney Int 40: S95–S102, 1991 [PubMed] [Google Scholar]
- 51.Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 247: F729–F738, 1984 [DOI] [PubMed] [Google Scholar]
- 52.Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 249: F870–F877, 1985 [DOI] [PubMed] [Google Scholar]
- 53.Lapsia VH, Weiner ID. Acid-base disorders. In: Principles and Practices of Hospital Medicine, edited by McKean SC, Ross JJ, Dressler DD, Brotman DJ, Ginsberg JS. New York: McGraw Hill Medical, 2012, p. 2051–2057 [Google Scholar]
- 54.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C Glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HW, Verlander JW, Handlogten ME, Han KH, Cooke PS, Weiner ID. Expression of the rhesus glycoproteins, ammonia transporter family members, Rhcg and Rhbg, in male reproductive organs. J Reprod Fertil 146: 283–296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee HW, Verlander JW, Bishop JM, Handlogten ME, Weiner ID. Effect of collecting duct-specific deletion of both Rh B Glycoprotein (Rhbg) and Rh C Glycoprotein (Rhcg) on renal response to metabolic acidosis (Abstract). J Am Soc Nephrol 23: 31A, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C Glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee HW, Verlander JW, Bishop JM, Handlogten ME, Han KH, Weiner ID. Renal ammonia excretion in response to hypokalemia: effects of collecting duct-specific Rh C Glycoprotein deletion. Am J Physiol Renal Physiol 304: F410–F421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HW, Verlander JW, Handlogten ME, Han KH, Weiner ID. Effect of collecting duct-specific deletion of both Rh B Glycoprotein (Rhbg) and Rh C Glycoprotein (Rhcg) on renal response to metabolic acidosis. Am J Physiol Renal Physiol 306: F389–F400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim SW, Ahn KO, Kim WY, Han DH, Li C, Ghee JY, Han KH, Kim HY, Handlogten ME, Kim J, Yang CW, Weiner ID. Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats. Nephron Exp Nephrol 110: e49–e58, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z, Chen Y, Mo R, Hui C, Cheng JF, Mohandas N, Huang CH. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J Biol Chem 275: 25641–25651, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Lopez C, Metral S, Eladari D, Drevensek S, Gane P, Chambrey R, Bennett V, Cartron JP, Van Kim C, Colin Y. The ammonium transporter RhBG: requirement of a tyrosine-based signal and ankyrin-G for basolateral targeting and membrane anchorage in polarized kidney epithelial cells. J Biol Chem 280: 8221–8228, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Ludewig U. Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J Physiol 559: 751–759, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lupo D, Li XD, Durand A, Tomizaki T, Cherif-Zahar B, Matassi G, Merrick M, Winkler FK. The 1.3-A resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc Natl Acad Sci USA 104: 19303–19308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet 26: 341–344, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Marini AM, Soussi-Boudekou S, Vissers S, Andre B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol 17: 4282–4293, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marini AM, Urrestarazu A, Beauwens R, Andre B. The Rh (rhesus) blood group polypeptides are related to NH4+ transporters. Trends Biochem Sci 22: 460–461, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Marini AM, Vissers S, Urrestarazu A, Andre B. Cloning and expression of the MEP1 gene encoding an ammonium transporter in Saccharomyces cerevisiae. EMBO J 13: 3456–3463, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayer M, Schaaf G, Mouro I, Lopez C, Colin Y, Neumann P, Cartron JP, Ludewig U. Different transport mechanisms in plant and human AMT/Rh-type ammonium transporters. J Gen Physiol 127: 133–144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montesinos ML, Muro-Pastor AM, Herrero A, Flores E. Ammonium/methylammonium permeases of a Cyanobacterium. Identification and analysis of three nitrogen-regulated amt genes in synechocystis sp. PCC 6803. J Biol Chem 273: 31463–31470, 1998 [DOI] [PubMed] [Google Scholar]
- 73.Moore S, Green C. The identification of specific Rhesus-polypeptide-blood-group-ABH-active-glycoprotein complexes in the human red-cell membrane. Biochem J 244: 735–741, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mouro-Chanteloup I, Cochet S, Chami M, Genetet S, Zidi-Yahiaoui N, Engel A, Colin Y, Bertrand O, Ripoche P. Functional reconstitution into liposomes of purified human RhCG ammonia channel. PLoS ONE 5: e8921, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakhoul NL, Abdulnour-Nakhoul SM, Schmidt E, Doetjes R, Rabon E, Hamm LL. pH sensitivity of ammonium transport by Rhbg. Am J Physiol Cell Physiol 299: C1386–C1397, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakhoul NL, Abdulnour-Nakhoul SM, Boulpaep EL, Rabon E, Schmidt E, Hamm LL. Substrate specificity of Rhbg: ammonium and methyl ammonium transport. Am J Physiol Cell Physiol 299: C695–C705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Ninnemann O, Jauniaux JC, Frommer WB. Identification of a high affinity NH4+ transporter from plants. EMBO J 13: 3464–3471, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nowik M, Kampik NB, Mihailova M, Eladari D, Wagner CA. Induction of metabolic acidosis with ammonium chloride (NH4Cl) in mice and rats—species differences and technical considerations. Cell Physiol Biochem 26: 1059–1072, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Nygaard TP, Alfonso-Prieto M, Peters GH, Jensen MO, Rovira C. Substrate recognition in the Escherichia coli ammonia channel AmtB: a QM/MM investigation. J Phys Chemy B 114: 11859–11865, 2010 [DOI] [PubMed] [Google Scholar]
- 82.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 83.Ridgwell K, Spurr NK, Laguda B, MacGeoch C, Avent ND, Tanner MJ. Isolation of cDNA clones for a 50 kDa glycoprotein of the human erythrocyte membrane associated with Rh (rhesus) blood-group antigen expression. Biochem J 287 (Pt 1): 223–228, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Sohet F, Colin Y, Genetet S, Ripoche P, Metral S, Le Van Kim C, Lopez C. Phosphorylation and ankyrin-G binding of the C-terminal domain regulate targeting and function of the ammonium transporter RhBG. J Biol Chem 283: 26557–26567, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soupene E, Inwood W, Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc Natl Acad Sci USA 101: 7787–7792, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soupene E, King N, Feild E, Liu P, Niyogi KK, Huang CH, Kustu S. Rhesus expression in a green alga is regulated by CO2. Proc Natl Acad Sci USA 99: 7769–7773, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stahl RA, Kanz L, Maier B, Schollmeyer P. Hyperchloremic metabolic acidosis with high serum potassium in renal transplant recipients: a cyclosporine A associated side effect. Clin Nephrol 25: 245–248, 1986 [PubMed] [Google Scholar]
- 90.Star RA, Burg MB, Knepper MA. Luminal disequilibrium pH and ammonia transport in outer medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 252: F1148–F1157, 1987 [DOI] [PubMed] [Google Scholar]
- 91.Star RA, Kurtz I, Mejia R, Burg MB, Knepper MA. Disequilibrium pH and ammonia transport in isolated perfused cortical collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 253: F1232–F1242, 1987 [DOI] [PubMed] [Google Scholar]
- 92.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ullrich WR, Larsson M, Larsson CM, Lesch S, Novacky A. Ammonium uptake in Lemna gibba G 1, related membrane potential changes, and inhibition of anion uptake. Physiol Plant 61: 369–376, 1984 [Google Scholar]
- 94.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 95.Verlander JW, Chu D, Lee HW, Handlogten ME, Weiner ID. Expression of glutamine synthetase in the mouse kidney: localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal Physiol 305: F701–F713, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.von Wiren N, Gazzarrini S, Gojon A, Frommer WB. The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol 3: 254–261, 2000 [PubMed] [Google Scholar]
- 97.Wall SM. NH4+ augments net acid-secretion by a ouabain-sensitive mechanism in isolated-perfused inner medullary collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 270: F432–F439, 1996 [DOI] [PubMed] [Google Scholar]
- 98.Wall SM, Fischer MP. Contribution of the Na+-K+-2Cl− cotransporter (NKCC1) to transepithelial transport of H+, NH4+, K+, and Na+ in rat outer medullary collecting duct. J Am Soc Nephrol 13: 827–835, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Wall SM, Koger LM. NH4+ transport mediated by Na+-K+-ATPase in rat inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 267: F660–F670, 1994 [DOI] [PubMed] [Google Scholar]
- 100.Wang MY, Glass ADM, Shaff JE, Kochian LV. Ammonium uptake by rice roots. III. Electrophysiology. Physiol Plant 104: 899–906, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang MY, Siddiqi MY, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. II. Kinetics of 13NH4+ influx across the plasmalemma. Physiol Plant 103: 1259–1267, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang S, Orabi EA, Baday S, Berneche S, Lamoureux G. Ammonium transporters achieve charge transfer by fragmenting their substrate. J Am Chem Soc 134: 10419–10427, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Weiner ID. The Rh gene family and renal ammonium transport. Curr Opin Nephrol Hypertens 13: 533–540, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B Glycoprotein and Rh C Glycoprotein in the mouse liver. Gastroenterology 124: 1432–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weiner ID, Verlander JW. Renal acidification mechanisms. In: Brenner and Rector's The Kidney, edited by Taal M, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM. Philadelphia, PA: Elsevier, 2012, p. 293–325 [Google Scholar]
- 108.Weiner ID, Verlander JW. Molecular physiology of the Rh ammonia transport proteins. Curr Opin Nephrol Hypertens 19: 471–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weiner ID, Verlander JW. Renal ammonia metabolism and transport. Compr Physiol 3: 201–220, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weinstein AM. A mathematical model of rat ascending Henle limb. III. Tubular function. Am J Physiol Renal Physiol 298: F543–F556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Westhoff CM. The structure and function of the Rh antigen complex. Semin Hematol 44: 42–50, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yip KP, Kurtz I. NH3 permeability of principal cells and intercalated cells measured by confocal fluorescence imaging. Am J Physiol Renal Fluid Electrolyte Physiol 269: F545–F550, 1995 [DOI] [PubMed] [Google Scholar]
- 113.Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci USA 101: 17090–17095, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, Lopez C, Gane P, Kim CVAN, Cartron JP, Colin Y, Ripoche P. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J 391: 33–40, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]