Abstract
During the first trimester of human pregnancy, the maternal systemic circulation undergoes remarkable vasodilation. The kidneys participate in this vasodilatory response resulting in marked increases in renal plasma flow (RPF) and glomerular filtration rate (GFR). Comparable circulatory adaptations are observed in conscious gravid rats. Administration of the corpus luteal hormone relaxin (RLN) to nonpregnant rats and humans elicits vasodilatory changes like those of pregnancy. Systemic and renal vasodilation are compromised in midterm pregnant rats by neutralization or elimination of circulating RLN and in women conceiving with donor eggs who lack a corpus luteum and circulating RLN. Although RLN exerts both rapid (minutes) and sustained (hours to days) vasodilatory actions through different molecular mechanisms, a final common pathway is endothelial nitric oxide. In preeclampsia (PE), maternal systemic and renal vasoconstriction leads to hypertension and modest reduction in GFR exceeding that of RPF. Elevated level of circulating soluble vascular endothelial growth factor receptor-1 arising from the placenta is implicated in the hypertension and disruption of glomerular fenestrae and barrier function, the former causing reduced Kf and the latter proteinuria. Additional pathogenic factors are discussed. Last, potential clinical ramifications include RLN replacement in women conceiving with donor eggs and its therapeutic use in PE. Another goal has been to apply knowledge gained from investigating circulatory adaptations in pregnancy toward identifying and developing novel therapeutic strategies for renal and cardiovascular disease in the nonpregnant population. So far, one candidate to emerge is RLN and its potential therapeutic use in heart failure.
Keywords: renal hemodynamics, glomerular filtration, osmoregulation, relaxin, nitric oxide, assisted reproductive technology, glomerular endotheliosis, soluble vascular endothelial growth factor receptor-1, heart failure
Renal Plasma Flow and Glomerular Filtration in Normal Pregnancy
marked vasodilation of the maternal circulation occurs in the first trimester of human pregnancy. Consequently, there is a precipitous and profound decline in systemic vascular resistance (SVR) that in turn abets a reciprocal increase in cardiac output (CO) of ∼40% or 2 l/min lasting throughout pregnancy (23, 163). Vasodilation of nonreproductive organs like the kidneys accounts mostly for the early gestational fall in SVR and rise in CO. The fall in SVR is nearly offset by the rise in CO; hence, mean arterial pressure (MAP) declines, but only modestly, by 5–10 mmHg (23, 38, 163). Although counterintuitive, this metamorphosis of the maternal circulation is virtually complete by the end of the first or beginning of the second trimester, when fetal crown rump length and placental diameter are only ∼6 cm each (15) and uterine blood flow is ∼0.25 l/min (cf. late pregnancy, ∼0.75 l/min) (60). Thus, these circulatory changes are apparently preparatory events in anticipation of the rapid growth phase of the fetus and placenta in the second half of gestation, when oxygen and nutrient demands accelerate, rising exponentially. The anticipatory nature of these gestational adaptations in the maternal circulation is underscored by the difference in arterial-mixed venous oxygen content, which narrows in early pregnancy, indicating that oxygen supply exceeds demands (10, 84, 156). As shown in Fig. 1, the systemic circulatory adaptations of pregnancy in women have been modeled in the conscious gravid rat, including reduction in SVR and increases in CO and global arterial compliance (84, 176), thus affording the opportunity to explore potential hormonal and molecular mechanisms, as described below.
Fig. 1.
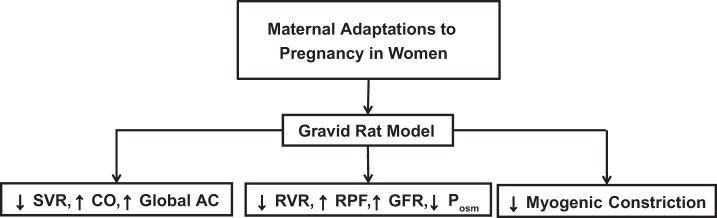
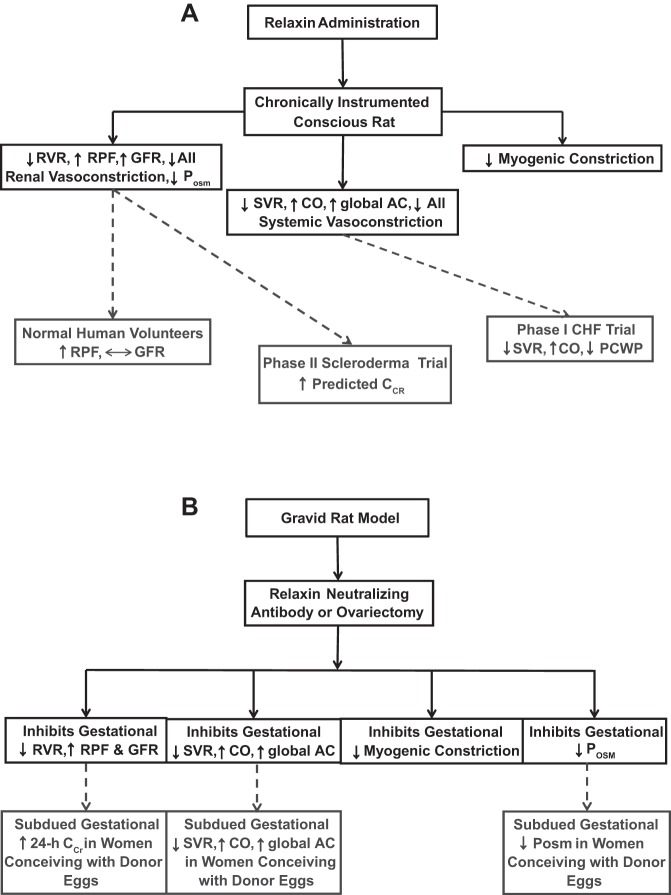
Maternal circulatory and osmoregulatory adaptations to pregnancy in women are recapitulated in gravid rats. SVR, systemic vascular resistance; CO, cardiac output; AC, arterial compliance; RVR, renal vascular resistance; RPF, renal plasma flow; GFR, glomerular filtration rate; Posm, plasma osmolality. ↓Myogenic Constriction, inhibition of myogenic constriction of small renal and mesenteric arteries harvested from gravid rats. See text for details and citations.
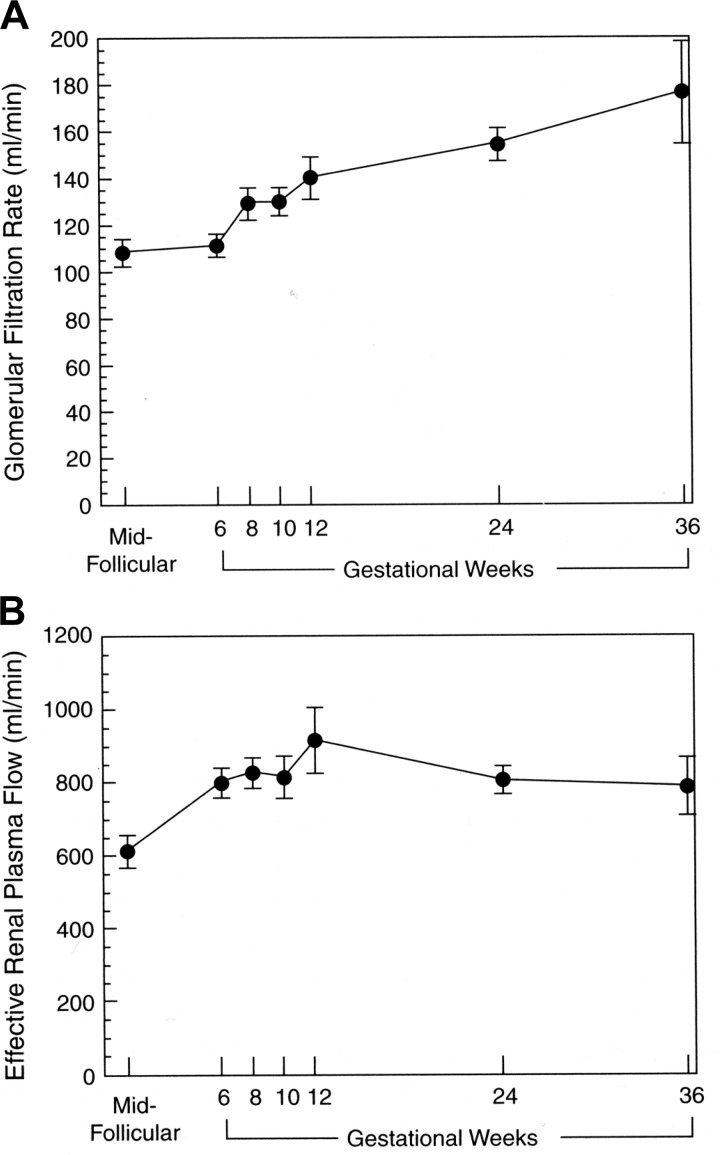
Renal vasodilation underlies the massive increase in effective renal plasma flow (ERPF) and glomerular filtration rate (GFR) during early gestation, as measured by the renal clearances of para-aminohippurate and inulin, respectively. RPF and GFR increase by 40–65 and 50–85%, respectively, during human pregnancy (reviewed in Refs. 35 and 38). This dramatic rise in RPF and GFR during early pregnancy is exemplified by the meticulous investigation of Chapman et al. (23) illustrated in Fig. 2, which corroborates one of the earliest reports of GFR measured in the first trimester by the 24-h endogenous creatinine clearance, as shown in Fig. 3 (54). As further shown in Fig. 1, the renal hemodynamic changes of human pregnancy have also been observed in the conscious gravid rat, including the reduction in RVR and elevations in RPF and GFR (29), again affording the opportunity to explore potential hormonal and molecular mechanisms. Interestingly, plasma osmolality is similarly reduced during pregnancy in both species, too (Fig. 1) (118). Investigations in gravid rats and women show that the rise in GFR is due largely to increases in RPF without elevation in glomerular hydrostatic pressure as a consequence of parallel decreases in both afferent and efferent arteriolar resistances (14, 132, 134, 161). In mothers past their child-bearing years (∼52 yr of age), estimated GFR as calculated by the Modification of Diet in Renal Disease formula was not reduced by multiparity, nor was there an elevation in urinary protein excretion (87). These observations are consistent with the concept that there is glomerular hyperfiltration but not glomerular hypertension during normal pregnancy. The decrease in plasma oncotic pressure and increase in Kf also contribute to the rise in GFR (108, 132, 134, 161). As a consequence of the elevation in GFR, both serum creatinine and urea concentrations reset to lower values of ∼0.5 and 9.0 mg/dl, respectively (38).
Fig. 2.
A: serial study of glomerular filtration rate before pregnancy in the midfollicular phase of the menstrual cycle and then throughout pregnancy in 11 women. B: serial study of effective renal plasma flow in the same women. All values during pregnancy are significantly different from those obtained during the midfollicular phase of the menstrual cycle before pregnancy. Redrawn from Ref. 23 with permission.
Fig. 3.
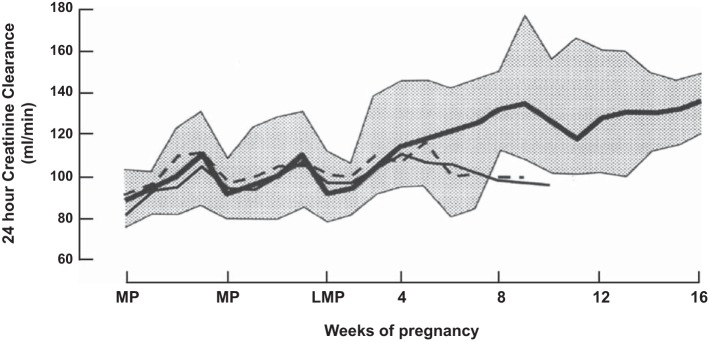
Alterations in the 24-h renal creatinine clearance evaluated weekly before conception and throughout early pregnancy in 11 women. Solid line, mean; stippled area, range for 9 women with normal pregnancy outcome. Two women designated by the thin and dashed lines had uncomplicated spontaneous abortions. MP, menstrual period; LMP, last menstrual period. Adapted from Ref. 54 with permission.
Hormonal Mechanisms of Renal and Systemic Vasodilation in Pregnancy
Whatever the dilatory mechanism(s) of pregnancy, it must be potent, because despite compensatory structural and functional hypertrophy, the renal allograft and single kidney can adapt even further, displaying gestational hyperfiltration (51, 52). Of note, both the renal and systemic hemodynamic changes of pregnancy are anticipated in the luteal phase of the menstrual cycle when RPF, GFR, and CO increase, albeit to a lesser degree (24), thus implicating a role for corpus luteal (CL) hormone(s). Although estrogen has little or no effect on the renal circulation, progesterone can increase RPF and GFR, but probably not to the same extent as observed in pregnancy (reviewed in Refs. 35 and 38).
The 6-KDa protein, relaxin (RLN) is secreted by the CL, circulates during the late luteal phase, markedly increases after conception, reaching a peak at the end of the first trimester, and falls to intermediate levels thereafter for the duration of pregnancy (172). Taking a pharmacological approach, as shown in Fig. 4A, both short- (within 1–2 h) and long-term (hours to days) RLN administration elicited renal vasodilation, increasing RPF and GFR in conscious nonpregnant, intact, or ovariectomized female and male rats analogous to the gravid state (29, 46–48, 130). Long-term RLN administration also mimicked the gestational changes in systemic hemodynamics, decreasing SVR and increasing CO and global arterial compliance in normotensive and hypertensive female and male conscious rats (34, 55, 56). However, RLN vasodilation of the systemic circulation in normotensive rats may be of slower onset than in the renal circulation (46, 56). Analogous to pregnancy (Fig. 1) (75), RLN administration also inhibited myogenic constriction of rat small renal (and mesenteric) arteries ex vivo (Fig. 4A) (147).
Fig. 4.
A: administration of relaxin to nonpregnant rats reproduces maternal circulatory and osmoregulatory changes of pregnancy. Although there are fewer investigations to date in humans, similar, but not identical, responses to relaxin administration have been observed. B: neutralization or elimination of circulating relaxin in midterm pregnant rats precludes the gestational changes in the maternal circulation and in osmoregulation. Emerging evidence suggests that women conceiving with donor eggs who lack a corpus luteum and circulating relaxin may have attenuated gestational circulatory changes and decreases in plasma osmolality. AII, angiotensin II; CCr, creatinine clearance; PCWP, pulmonary capillary wedge pressure. See text for details and citations.
As further illustrated in Fig. 4A, comparable circulatory changes in response to RLN appear to occur in men and women, although the number of studies is limited. In one study, short-term RLN infusion (30 min to 5 h) in healthy female and male volunteers led to a 60% increase in RPF but, surprisingly, no rise in GFR (178). In contrast, long-term administration for weeks in patients with mild scleroderma produced a 15–20% increase in predicted creatinine clearance (65). In a phase 1 trial of RLN in stable congestive heart failure, the hormone reduced SVR, pulmonary capillary wedge pressure, serum creatinine, and BUN and increased CO (62). In a recently conducted evaluation of RLN administration in acute heart failure (158), the hormone reduced SVR, arterial pressure, pulmonary vascular resistance, pulmonary capillary wedge pressure, pulmonary artery pressure, and right atrial pressure and increased 24-h endogenous creatinine clearance. There was no significant increase in CO. On balance and perhaps of little surprise, the circulatory effects of RLN in human heart failure appear to be less pronounced than in healthy conscious rats.
Taking a physiological approach, as shown in Fig. 4B, RLN neutralization with specific antibodies prevented the gestational changes in the renal circulation of conscious, midterm pregnant rats and abrogated the inhibition of myogenic constriction in small renal arteries ex vivo. Comparable results were observed in ovariectomized, midterm pregnant rats, which lacked circulating RLN. Parenthetically, the gestational reduction in plasma osmolality was also inhibited by RLN-neutralizing antibodies or ovariectomy (Fig. 4B) (144). As illustrated further in Fig. 4B, the rise in CO and global AC and reduction in SVR during midterm pregnancy were also inhibited by RLN-neutralizing antibodies (58). In women with ovarian failure who conceive using donor eggs, in vitro fertilization, and embryo transfer, circulating CL factors, including RLN, are absent (estradiol and progesterone are given for luteal support). These women demonstrated a markedly subdued first-trimester increase in GFR, and the gestational decline in plasma osmolality was attenuated (later time points were not investigated; Fig. 4B) (179). The former is consistent with the absence of a circulating renal vasodilator like RLN, thereby attenuating the increase in RPF and hence, of GFR. A logical extension is that deficient renal vasodilation (and perhaps vasodilation of other maternal organs as well) would compromise the gestational decline in SVR and thus rise in CO (30). Indeed, preliminary findings from an ongoing investigation of infertile women conceiving with donor eggs support this concept although further study is needed (Fig. 4B) (41). In contrast, Lafayette et al. (107) recently reported no significant correlation between serum RLN concentration and renal function in normal pregnant or preeclamptic women in the early postpartum period. One possible explanation for this apparent discrepancy is that RLN may be critical to the development of renal and systemic vasodilation in the first half of pregnancy, but as the placenta matures, placental vasodilators contribute to the maintenance of the vasodilatory state thereafter (30). However, there may be methodological concerns with the investigation of Lafayette et al., as described elsewhere (133, 134), and the timing of the study was not ideal being post- rather than prepartum.
Another classic phenotype of normal pregnancy in animals and humans is the attenuation of angiotensin II (AII) vasoconstriction. This phenomenon is manifested in both the systemic (33, 76) and renal (33, 44, 148) circulations. It can be duplicated in conscious, nonpregnant rats by administrating RLN (48, 56). Vascular refractoriness to AII during pregnancy may also be shared by other vasoconstrictors, such as α-adrenergic agonists (33, 142) as well as arginine vasopressin (151), and likely abets the gestational decline in RVR and SVR.
Molecular Mechanisms of Renal Vasodilation in Pregnancy
Early investigations tested the hypothesis that prostaglandins (PG) are the vasodilators of pregnancy; however, on balance, a major role for these autocoids was not revealed either in women or animal models (reviewed in Ref. 38). In contrast, endothelium-derived hyperpolarizing factor (83, 86, 114, 123, 124, 126, 164, 175), KATP+ channel activation (100) and endothelium-derived relaxing factor (EDRF) or nitric oxide (NO) (175) may be more important players. The first clue about a possible role for EDRF (subsequently identified as NO; Ref. 152) emerged with the finding that plasma level, urinary excretion, and metabolic production of cyclic guanosine-3′,5′-monophosphate (cGMP) rises during pregnancy in rats. Insofar as cGMP is a major second messenger of EDRF or NO, this autocoid was implicated in the vasodilatory phenomena of pregnancy (28, 43). Later, evidence for increased NO biosynthesis was reported in gravid rats (37). Although comparable findings for cGMP were observed in pregnant women (39, 103, 165), whether NO biosynthesis increases remains unclear at least when estimated by major metabolites in the circulation or urine (39, 175).
To test directly for a functional role of NO in the renal vasodilation and hyperfiltration of pregnancy, l-arginine analogs that inhibit NO synthase were employed as illustrated in Fig. 5. Acute infusion of nitro-l-arginine methyl ester (l-NAME) or NG-monomethyl-l-arginine (l-NMMA) in conscious, midterm pregnant rats abolished the renal hemodynamic changes of pregnancy (44). Likewise, inhibition of myogenic constriction in small renal arteries isolated from midterm pregnant rats was restored to the robust phenotype of the nonpregnant condition by endothelial removal or addition of l-arginine analogs to the vessel bath (75). Chronic administration of l-arginine analogs to gravid rats also inhibited renal vasodilation and hyperfiltration in some (13, 20) but not all studies (45); in the latter, vasodilatory PG were recruited in the setting of chronic NO synthase blockade, serving a compensatory role (45). Discrepancies between these studies might stem from the different doses of NOS inhibitor used or differences in the comparison (control) group of animals employed. An essential role for NO in renal vasodilation, hyperfiltration, and inhibited myogenic constriction of small renal arteries ex vivo was also revealed for RLN-treated nonpregnant rats again by employing l-arginine analogs (48, 147). Whether expression of endothelial NO synthase or inducible NO synthase increases in maternal kidneys or arteries during pregnancy is controversial (6, 85, 146, 177, 205). Recent evidence suggests that nueronal NO synthase (nNOS)β is elevated in the kidneys of gravid rats (177); to our knowledge, however, whether nueronal nNOSβ mediates the gestational increase in GFR and RPF has not been tested. Another group of investigators reported that short-term infusion of 7-nitroindazole inhibited gestational renal vasodilation and hyperfiltration, thereby implicating a role for nNOS (2). However, the selectivity of the inhibitor for nNOS was not verified in vivo.
Fig. 5.
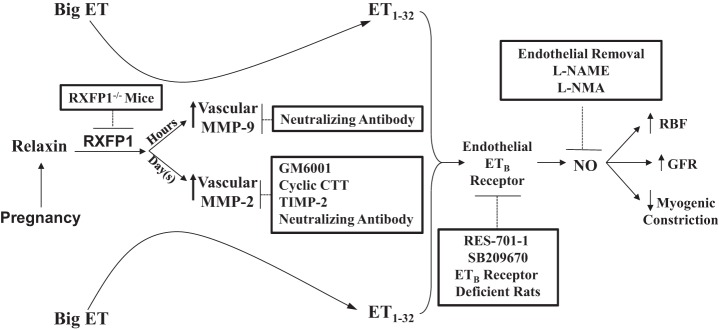
Working model for the sustained vasodilatory actions of relaxin. Not depicted in this schema are the essential roles of vascular endothelial and placental growth factors in mediating the sustained vasodilatory actions of relaxin. Inhibitors of relaxin-induced vasodilation are shown in the boxes. ET, endothelin; MMP, matrix metalloproteinase; RBF, renal blood flow; GFR, glomerular filtration rate; GM6001, a general MMP inhibitor; cyclic CTT, a specific peptide inhibitor of MMP-2; TIMP-2, tissue inhibitor of matrix metalloproteinase; RES-701-1, a specific ETB receptor antagonist; SB209670, a mixed ETA and ETB receptor antagonist; l-NAME, nitro-l-arginine methyl ester; l-NMMA, NG-monomethyl-l-arginine. Note that phosphoramidon (an inhibitor of the classical big endothelin-converting enzyme), STT (control peptide for cyclic CTT), heat-inactivated TIMP-2, BQ-123 (a specific ETA receptor antagonist), d-NAME, and IgGs (control antibodies for MMP-neutralizing antibodies) did not affect the slow vasodilatory responses of relaxin. See text for further details and citations.
As shown further in Fig. 5, subsequent studies revealed a critical role for the endothelial (endothelin) ETB receptor. In the endothelium, signaling by the ETB receptor involves NO synthase and other vasodilatory pathways (88, 89, 192). Previous work revealed an important role for the endothelial ETB receptor in maintaining the physiologically low vascular resistance of the renal circulation in conscious male rats (77, 78). Short-term administration of an ETB or mixed ETB/ETA receptor antagonist in conscious midterm pregnant rats or in nonpregnant rats chronically treated with RLN inhibited renal vasodilation and hyperfiltration (36, 47) (comparable with NO synthase blockade, vide supra). Furthermore, addition of an ETB or mixed ETB/ETA but not ETA receptor antagonist to the vessel bath reversed the inhibition of myogenic constriction in small renal arteries isolated from midterm pregnant or RLN-treated nonpregnant rats (75, 147). Consistent with these findings using ETB receptor antagonists, inhibited myogenic constriction was not observed in small renal arteries isolated from gravid ETB receptor-deficient rats or from nonpregnant ETB receptor-deficient rats administered RLN (96, 143). Although an appealing hypothesis, neither RLN administration nor pregnancy increased endothelial ETB receptor expression (99), but not all agree (61).
The enzymatic source of ET was explored next. Unexpectedly, short-term administration of phosphoramidon, a classic big ET-converting enzyme inhibitor, at rates sufficient to block the slow rise of MAP in response to a large bolus of big ET-1 did not inhibit renal vasodilation and hyperfiltration elicited by long-term RLN treatment in conscious female rats (96). Nor was the inhibited myogenic constriction of small renal arteries isolated from RLN-treated nonpregnant or midterm pregnant rats restored to the nonpregnant phenotype of robust myogenic constriction by addition of phosphoramidon to the vessel bath (70, 96). Contemporaneously, Fernandez-Patron et al. (70, 71) reported that matrix metalloproteinase (MMP)-2 can hydrolyze big ET-1 at a Gly-Leu bond, producing a novel ET, ET1–32, that is fully active on ET receptors. Exploiting this new knowledge, short-term infusion of either a general MMP inhibitor or a specific gelatinase inhibitor blocked renal vasodilation and hyperfiltration produced by chronic (several days) RLN administration in conscious female rats (96). Consistent with these in vivo observations, a variety of MMP inhibitors added either to the artery bath or lumen restored robust myogenic constriction in small renal arteries isolated from rats administered RLN or from midterm pregnant rats (96). Small renal and mesenteric arteries, as well as aorta isolated from pregnant or RLN-treated nonpregnant rats showed increased activity, protein, and mRNA for MMP-2 and -9 (49, 95–98, 130, 194). Small renal arteries harvested from RLN-treated, ETB receptor-deficient rats showed upregulation of vascular MMP-2 activity but failed to show inhibited myogenic constriction (96). This dissociation of the biochemical and functional responses to RLN administration in the ETB receptor-deficient rat suggests that vascular gelatinase is in series with and proximal to the endothelial ETB receptor/NO signaling pathway. In summary, the endothelial ETB receptor/NO vasodilatory pathway is activated by RLN administration or pregnancy through upregulation of vascular gelatinase(s) expression and activity. Figure 5 depicts the role of vascular gelatinase in the so-called “sustained” vasodilatory actions of the hormone (i.e., after hours to days of RLN exposure).
Interestingly, MMP-9, but not MMP-2, was upregulated in small renal arteries isolated from nonpregnant rats treated with RLN for only 4–6 h, and by using specific MMP-2- and MMP-9-neutralizing antibodies, MMP-9 was shown to specifically mediate the inhibition of myogenic constriction (97). In contrast, both MMP-2 and -9 were increased in small renal arteries isolated from nonpregnant rats administered RLN for several days, although MMP-2 activity greatly exceeded that of MMP-9. In this case, again by using a specific neutralizing antibody, MMP-2 was shown to mediate the inhibition of myogenic constriction (96). Thus, the vascular gelatinase of functional relevance to vasodilation is determined by the duration of exposure to RLN (Fig. 5).
Obviously, because of ethical reasons, the molecular mechanisms of renal vasodilation and hyperfiltration in pregnancy or by chronic RLN administration in humans cannot be tested in vivo. However, a bioassay of the in vivo vasodilatory phenotype produced by pregnancy or RLN administration is inhibition of myogenic constriction of small arteries ex vivo (vide supra). Thus, we sought to determine whether incubation of rat and mouse small arteries in vitro with RLN would inhibit myogenic constriction, and if so, then this experimental approach could be applied to human arteries. Indeed, incubation of rat and mouse small renal as well as human subcutaneous (sc) arteries with RLN in vitro for 3 h inhibited myogenic constriction (a shorter incubation time has not been evaluated for myogenic constriction) (130). The inhibition of myogenic constriction was abolished by subsequently adding to the bath or artery lumen l- but not d-arginine analogs, ETB but not ETA receptor antagonists, or general and specific inhibitors of MMPs, including neutralizing antibodies and TIMP-2, but not by phosphoramidon (130). In addition, vascular endothelial growth factor (VEGF) receptor tyrosine kinase activity was implicated in the sustained RLN vasodilatory pathway, insofar as daily subcutaneous (sc) injections of the VEGF RTK inhibitor SU5416 before and during 5-day RLN administration prevented the reduction in renal vascular resistance and increases in RPF and GFR in conscious rats (130). Moreover, pretreatment of small renal arteries from rats and mice and human sc arteries with SU5416 and placental growth factor (PlGF)- or VEGF-neutralizing antibodies, followed by a 3-h incubation with RLN, prevented the inhibition of myogenic constriction by the hormone (130). Small sc arteries isolated from late pregnant women also show inhibited myogenic constriction (Debrah JE, Novak J, and Conrad KP, unpublished observations); however, whether the vasodilatory pathway outlined above is involved has not yet been tested. Investigation of accessible arteries isolated from humans is one way to translate the discoveries of the molecular pathways of vasodilation by pregnancy and RLN in animals to humans.
RLN also elicited “rapid” relaxation (within minutes) of preconstricted rat and mouse small renal and human sc arteries in vitro (131). The signaling pathway involves Gαi/o protein coupled to phosphoinositide 3-kinase, protein kinase B, and endothelial NO synthase but not VEGF or increases in endothelial cytosolic calcium (131). Because arteries express a local RLN ligand receptor system (57, 145), this signaling pathway may be activated on a minute-to-minute basis, thus fine-tuning arterial relaxation. It is possible that this signaling pathway is also engaged immediately upon RLN administration, contributing to the rapid increases of RPF and GFR in conscious rats (within 1–2 h) and the rapid rise of RPF in humans (within 30 min). On the other hand, tonic activation of this arterial RLN ligand receptor system opposes myogenic constriction (sustained vasodilation, vide supra) and arterial stiffness (57, 145). Further investigation is needed to determine which factors regulate the arterial expression of RLN and its receptor RXFP1 and to define the degree of overlap and interaction among the molecular players underlying rapid and sustained vasodilation by RLN.
The molecular mechanisms underlying the refractory vasoconstrictor responses in pregnancy or during RLN administration are not entirely clear but may involve both endothelium-dependent and -independent factors. Whether vasodilatory PG play a role is disputed (33, 67, 94, 175), although NO is likely to contribute (3, 175). Renal mesangial cells prepared from gravid rats demonstrated an attenuated rise in intracellular calcium in response to AII (72). A comparable attenuation of increases in cytosolic calcium by AII was observed in mesangial cells prepared from nonpregnant rats and incubated with RLN for 24 h (22). These results implicate a direct effect of pregnancy and RLN on contractile cells independent of the endothelium, an effect that is perhaps mediated through attenuation of phosphoinositide turnover (31).
In addition to RLN, other factors likely participate in the gestational increases in GFR and RPF. PlGF may play a role especially after the first trimester, when serum concentration begins to rise as the placenta grows and matures (115). Another candidate is calcitonin gene-related peptide (CGRP), which increases in the blood during early pregnancy in women (183). CGRP is a potent vasodilator (187), and therefore, it may contribute to systemic and renal vasodilation of pregnancy. To our knowledge, however, a potential role for CGRP has not been tested, e.g., by administration of CGRP antagonists in gravid animal models. Recent evidence supports an important contribution of the AT2 receptor in mediating the midterm decline in systolic blood pressure in mice (21, 25) and in attenuating constrictor responses to phenylephrine in the aorta from gravid rats (182), thus suggesting a potential role for AT2 receptor activation in the renal vasodilation of pregnancy. Intriguingly, both histidine decarboxylase and its enzymatic product histamine, a potent vasodilator, were reported to be increased in the superficial cortex of gravid mice (135). Finally, renal production of epoxyeicosatrienoic acid may also contribute to renal vasodilation and hyperfiltration of pregnancy (91). To what degree, if any, these vasodilatory mechanisms may be activated by RLN in pregnancy is unknown.
Renal Plasma Flow and Glomerular Filtration in Preeclampsia
Depending on severity, preeclampsia (PE) can be a volatile and dangerous life-threatening disease. PE occurs in 3–5% of pregnancies, typically arising during the third trimester, and is a leading cause of maternal, fetal, and neonatal morbidity and mortality (66, 80, 101, 104, 141, 174, 199). In developed countries, PE can lead to iatrogenic premature delivery, which is frequently associated with major morbidities for the neonate immediately after delivery and long term. In the developing world, where (timely) access to health care is frequently lacking, PE can result in maternal, fetal, and neonatal death. Of note is that specific therapies for PE, which counteract pathogenic mechanisms, are currently lacking. Rather, “palliative” measures are implemented to control blood pressure and prevent seizures, and delivery (of the placenta) remains the only known cure.
In brief, preeclampsia has been defined as onset of sustained hypertension ≥140 mmHg systolic or ≥90 mmHg diastolic, with development of proteinuria of at least 1+ on dipstick or ≥300 mg/24 h after 20 wk of gestation. Severe disease is defined as hypertension ≥160 mmHg systolic or ≥110 mmHg diastolic, proteinuria >5 g/24 h, neurological symptoms such as seizures, pulmonary edema, hepatic or renal dysfunction, thrombocytopenia, or fetal growth restriction.1 Although differential diagnosis is beyond the scope of this review and has been detailed previously (160), it should be pointed out that many patients diagnosed as preeclamptic by clinical criteria alone are often misclassified. Such misclassification may pertain particularly to multiparous women diagnosed with PE (73). In the future, a redefining or reclassification of PE will most likely include pathologically relevant biomarkers, in addition to clinical criteria, maternal history, demographic information, and standard biochemical analyses, to guide diagnosis, management, prediction, and ultimately prevention (181).
During active disease, PE is characterized by a low-cardiac output/high-systemic vascular resistance state with compromised organ perfusion, including the kidneys (40). An exhaustive review of 23 reports in the older literature on renal function in PE shows that GFR and ERPF are modestly reduced, on average by 32 and 24%, respectively, from normal, late pregnant values (Ref. 35 and citations therein). GFR and ERPF may also be decreased in PE compared with nonpregnant levels, but to a lesser degree (35). Although the criteria used for the diagnosis of PE in these studies were often not presented or failed to meet current standards, the work of Assali et al. (9) is exceptional because of rigorous diagnosis. In this report, GFR and ERPF were decreased by 29% and 20%, respectively, relative to normal pregnancy. In the studies by McCartney et al. (129) and Sarles et al. (168) the clinical diagnosis of PE was corroborated by renal histology, i.e., the presence of glomerular endotheliosis (discussed below). In these reports, GFR and/or ERPF were also modestly reduced in women with PE compared with normal pregnant women. Several investigators calculated renal segmental vascular resistances, and only preglomerular arteriolar resistance was increased in the disease; no alterations in post-glomerular arteriolar resistances were noted (Table 1) (35). The combination of findings that reduction in GFR generally exceeded that of ERPF accompanied by an increase in pre-, but not post-glomerular arteriolar resistance suggests a concomitant reduction in the glomerular ultrafiltration coefficient during PE (35, 134). Interestingly, GFR appears to rapidly recover during the first week or so after delivery, more or less coinciding with recovery of the glomerular structural abnormalities observed on renal histology (discussed later). On the other hand, ERPF may recover more slowly (35).
Table 1.
Segmental renal vascular resistances in normal pregnancy and preeclampsia
| Segmental Renal Vascular Resistances (dyne·s−1· cm−5)a |
|||||
|---|---|---|---|---|---|
| Diagnosis | No. of Subjects | Total | Afferent | Efferent | Venular |
| Normal pregnancy | 97 | 6,534 | 2,524 | 1,877 | 2,090 |
| Preeclampsia | 65 | 15,994 | 11,296 | 1,779 | 2,976 |
Grand means for renal vascular resistances are shown. They were calculated from mean values provided in 5 studies involving a total of 97 normal and 65 preeclamptic subjects. Segmental renal vascular resistances were estimated in these publications according to the calculations of Gomez DM (86a).
More recently, Irons et al. (93) reported an ERPF and GFR of 766 ± 52 and 153 ± 13 ml/min, respectively, in normal pregnant women. In primiparous preeclamptic women, ERPF and GFR were 609 ± 24 and 97 ± 7 ml/min, respectively (92). Thus, in agreement with the earlier studies, both ERPF and GFR were reduced in PE, the latter to a greater degree. The same group reported comparable findings in a subsequent study, but simultaneous analyses of the renal clearances of inulin, para-aminohippurate, and dextrans allowed for estimation of Kf, which was ∼50% lower in PE compared with normal pregnancy (133). Using different methodologies, Lafayette et al. (106) and Hladunewich et al. (90) also concluded that there was ∼50% reduction of Kf in women with PE compared with control pregnant subjects 1 day after delivery, which fully accounted for the comparable percentage decrease in GFR in their study because, in contrast to the majority of other investigations, they did not observe impairment of RPF in PE. In the aforementioned studies, impaired Kf in PE women returned to the normal range by 5 wk postpartum (90) or by ≥5 mo after delivery (133).
Clinical studies reveal that proteinuria and hypertension may take ≤2 yr to disappear, and the time interval between diagnosis and delivery is associated with postpartum time to resolution of proteinuria and hypertension, implying that duration of exposure to endothelial injury may be important (18). Thus, although renal changes have been assumed to resolve fairly quickly and completely after delivery (“delivery cures the disease”), there is the distinct possibility that PE may inflict permanent renal impairment or add further to the deficit of already damaged kidneys in some women (136, 196). This damage may be indirect or direct via hypertension and/or widespread endothelial dysfunction. After PE, women have three- to eightfold increased risk of cardiovascular disease (including ischemic heart disease, hypertension, and stroke), obesity, dyslipidemia, and end-stage renal disease (196, 203). Because PE and cardiovascular disease share risk factors such as hypertension, obesity, diabetes, and hypercholesterolemia, PE is certainly a marker for cardiovascular risk, but it is not yet definitely known whether PE per se adds to the risk (17, 63, 116). If so, then PE would be an independent risk factor and not just a marker. The remote risks are apparently greatest in those who also had preterm birth, fetal growth restriction, and/or recurrent PE (140). Furthermore, the offspring of PE mothers are more likely to have a higher blood pressure and BMI from childhood and cardiovascular disease in later life (8, 50). Low birth weight is not an uncommon neonatal outcome of PE (139) that is independently associated with increased risk of hypertension and ischemic heart disease (among other pathologies) in adulthood (11, 12). Thus there is a need to elucidate the underlying biological factors that underpin the association between PE and disease later in life.
Possible Mechanisms of Renal Impairment in Preeclampsia
The factor(s) underlying compromised GFR and ERPF in PE is unknown but may relate to the generalized “endothelial dysfunction” believed to account for widespread vasospasm and organ hypoperfusion in the disease (27, 159). Specific mechanisms have been interrogated in animal models of PE, including the RUPP (reduced uterine perfusion pressure) model in gravid rats, which produces modest hypertension relative to late pregnant control rats with increased SVR and decreased CO, proteinuria (in some but not all reports), and reduced renal function (5, 173). The hypertension and/or renal dysfunction in this animal model were not improved by angiotensin-converting enzyme (4) or thromboxane synthase (120) inhibition. In contrast, inhibition of cytochrome P450 with 1-aminobenzotriazol, which reduced renal cortical 20-HETE and CYP4A but not epoxygenase activity, normalized MAP and renal function, thus implicating overproduction of 20-HETE in the hypertension and renal dysfunction in RUPP rats (119).
Taking the lead from emerging literature on the pathogenesis of PE in women (32, 128, 195, 200, 204), potential pathogenic roles for the antiangiogenic factors soluble vascular endothelial growth factor receptor-1 (also known as sFlt1) and soluble endoglin (81, 82,137, 138, 154), the cytokines IL-6 and TNFα (74, 111–113, 138, 154), and AT1 receptor autoantibodies (111, 154, 155) were explored in the RUPP model of PE. Administration of VEGF121 ameliorated hypertension and restored renal function to normal, thus implicating a major pathophysiological role for sFlt1 (82). In contrast, although etanercept [a dimeric fusion protein consisting of the extracellular ligand-binding portion of the human 75-kDa (p75) tumor necrosis factor receptor linked to the Fc portion of human IgG1] blocked the elevation of plasma TNFα and sFlt1 in RUPP rats, the hypertension persisted (138). In subsequent reports, preproET-1 mRNA expression was increased in the kidneys of RUPP rats or normal pregnant rats administered TNFα or sFlt1, implicating ETA receptor activation as a major downstream mechanism (79, 113, 137). Furthermore, the ETA receptor antagonist ABT 627 abolished the increase in blood pressure in RUPP rats and normal pregnant rats administered TNFα or sFlt1 (7, 113, 137, 189).
Autoantibodies to the angiotensin II type I receptor (AT1-AA) have been implicated in the pathogenesis of PE (200), as reviewed recently (204). In support of their pathophysiological role, circulating AT1-AA increased in RUPP rats, and AT1 receptor blockade with losartan or depletion of B-lymphocytes with Rituximab ameliorated hypertension (110, 111). AT1-AA administration increased renal preproET-1 and MAP in late pregnant rats, which was prevented by losartan (109). ABT 627 also prevented the development of hypertension in this setting (109). Interestingly, administration of activated splenocytes with a Th1 profile to pregnant mice elicited a PE-like syndrome (206). Given the importance of CD4+T helper cells in the production of antibodies by B-lymphocytes, CD4+T-splenocytes isolated from RUPP rats were injected into normal pregnant rats, which increased circulating AT1-AA, TNF-1, sFlt1, and MAP and reduced GFR (149, 198). Administration of either losartan or ABT 627 or depletion of B-lymphocytes prevented the increase in MAP, and the latter maneuver also prevented the increase in circulating AT1-AA (149, 197). Finally, recent evidence suggests an imbalance between T regulatory and IL-17-expressing CD4+T cells in preeclampsia (167). Administration of IL-17 to pregnant rats increased circulating AT1-AA and MAP, the latter blocked by losartan (59).
Several of these putative pathogenic factors have been investigated in other animal models of PE. Uteroplacental ischemia produced hypertension and proteinuria as well as elevation in circulating sFlt1 in pregnant baboons (Papio hamadryas; see Ref. 125). In the same species, administration of TNFα elicited PE manifestations (188). Furthermore, overexpression of sFlt1 in gravid mice also produced features of PE (16, 105, 122).
A noteworthy caveat to these animal studies is that, in most instances, MAP and not renal function was the measured experimental endpoint; thus, we can infer only that the mechanisms shown to be involved in hypertension also contributed to reductions of RPF and GFR in the RUPP model. Nevertheless, sFlt1 could conceivably restrain the physiological processes of normal pregnancy, e.g., renal vasodilation by RLN through competing for local, arterial-derived PlGF and VEGF, which participate in the RLN vasodilatory pathway (supra vide; see Ref. 130). Another possibility is that pathophysiological levels of ET-11–32 (or ET-11–21) and/or heightened expression of ETA receptors on vascular smooth muscle lead to overactivation of ETA receptors, thus overwhelming the physiological activation of the endothelial ETB-NO vasodilatory pathway by RLN, leading to renal vasoconstriction. Circulating AT1-AA could directly agonize the AT1 receptor on the afferent arteriole and synergize with circulating AII, thereby impairing renal function (19). Furthermore, this synergy may contribute to the increase in systemic AII pressor response in PE (76, 201). Although these exciting and provocative data emerging from PE animal models provide much-needed proof of concept that angiogenic growth factors, proinflammatory cytokines, and AT1 autoantibodies are indeed likely to be mediators of disease pathogenesis, the precise interaction among these various inciting agents and the chain of events leading to reduced perfusion of maternal organs, including the kidneys and hypertension in PE, require further clarification. Based on the pleotropic actions of relaxin, as described previously, including renal vasodilation, antagonism of AII vasoconstriction, and possible augmentation of VEGF/PlGF activity in the vascular wall, a logical next step is to test relaxin as a potential therapeutic in animal models of PE as proof of principle that, if promising, could pave the way for clinical trials (53).
Glomerular Lesion in Preeclampsia
Swelling and vacuolization of glomerular endothelial cells that can lead to obliteration of the capillary lumen occurs in preeclampsia and is called “glomerular endotheliosis” (Ref. 35 and citations therein). This typical lesion inspired the term “bloodless glomeruli.” In addition, endothelial fenestrae disappear, and the endothelial swelling can be so severe that the glomerular tuft herniates into the proximal tubule, so-called “pouting.” In 1924, using light microscopy of that era, Mayer may have been one of the first to suggest glomerular endothelial swelling in eclampsia (127), providing the first clue that endothelial dysfunction may figure prominently in disease pathophysiology. The lesion was definitively described when electron microscopy became available (69, 180), as well as with thin sectioning for light microscopy (171).
Perhaps surprisingly, there are no consistent microscopic abnormalities noted in the other glomerular structures [however, some have claimed that glomerular sclerosis can occur, whereas others have suggested that these are unrecognized lesions present before conception (35)]. That is, the basement membrane, podocyte, and foot processes usually appear unaffected and intact, although some investigators have reported swollen mesangial cells and expanded mesangial matrix. Moreover, interstitial and tubular structural abnormalities are absent. Glomerular subendothelial fibrin deposition can be seen particularly in intrapartum biopsies resolving quickly postpartum. Immunoglobulin deposits (IgM and IgG) are infrequent and of low intensity, suggesting nonspecific trapping rather than primary autoimmune disorder. Although glomerular endotheliosis is believed to be a renal lesion characteristic of preeclampsia, it has also been reported in women with placental abruption (121, 162). A recent publication suggested that near term, mild glomerular endotheliosis may be observed in normal pregnancy, but the cases described did not have associated enlarged glomeruli (185, 186, 202). The significance of this claim has been disputed (35). (A discussion of the ethics of these studies by Stevens and colleagues, specifically rensl biopsy of normal pregnant volunteers, is discussed in a correspondence exchange. Br J Obstet Gynaecol 111: 191–195, 2004.) However, if true, it may be related to the physiological elevation of sFlt1 during late pregnancy, which is highest at term (115).
In rats and mice with elevated circulating sFlt1 achieved by infection with recombinant adenovirus, hypertension, proteinuria, and glomerular endotheliosis were observed (105, 117, 122, 128). Comparable glomerular lesions and proteinuria were reported in mutant mice lacking podocyte expression of VEGF (64). In patients receiving antiangiogenic therapy for cancer, hypertension, and proteinuria have been reported (184). On balance, these findings support the concept that in PE high circulating levels of sFlt1 neutralize podocyte VEGF, thereby depriving the glomerular endothelium of this essential growth factor. In addition to disrupting the glomerular barrier function leading to proteinuria, elevated circulating sFlt1 may ultimately be one factor contributing to the reduced Kf in PE by compromising the density and size of endothelial fenestrae and hence, glomerular hydraulic conductivity (106). Another factor may be circulating AT1-AA that could conceivably cause mesangial cell contraction, thereby reducing glomerular capillary surface area for filtration. The overall consensus is that these lesions of preeclampsia resolve completely after delivery, with regression seen as early as the first week postpartum (68, 102, 150,s 157). Nevertheless, as discussed above, hypertension and proteinuria may persist ≤2 yr postpartum.
Perspectives
The maternal systemic and renal circulations undergo a truly remarkable transformation during pregnancy that is epitomized by massive vasodilation. Over the past decade, the corpus luteal hormone RLN has emerged as an important player in the renal vasodilation and hyperfiltration of pregnancy, as first revealed using the conscious gravid rat model. In the renal circulation of conscious rats and small renal arteries isolated therefrom, the molecular mechanisms of vasodilation vary according to the duration of exposure to RLN, but the final common pathway is endothelial production of nitric oxide. These discoveries derived from rats appear to be translating to humans, insofar as women conceiving with donor eggs who lack a corpus luteum and circulating RLN show markedly subdued maternal circulatory adaptations, especially during early pregnancy (41, 179). Moreover, subcutaneous arteries isolated from humans manifest both the rapid and sustained vasodilatory responses to RLN, as originally described in small renal arteries from rats and mice, and they are also mediated through the same molecular mechanisms, culminating ultimately in nitric oxide production.
A reclassification of preeclampsia based in part on pathologically relevant biomarkers would improve the likelihood of making the correct diagnosis (181). This eventuality might have profound implications for healthcare, because it could lead to the early identification of a group of women at risk for adverse health outcomes later in life, and may further allow for early preventative measures to be instituted. Tremendous advances in understanding the pathophysiology of preeclampsia have transpired over the last decade. Many circulating injurious agents, including antiangiogenic factors, syncytiotrophoblast microparticles, proinflammatory cytokines, and AII type 1 receptor agonist autoantibodies, were identified in preeclamptic women, and the ability of many of these factors to produce preeclamptic disease manifestations, including compromised renal function, has been verified in animal models. Taken altogether, it seems an opportune time to capitalize upon and synthesize our newly gained knowledge of the physiological and pathophysiological mechanisms in normal pregnancy and preeclampsia, respectively, to develop specific therapeutic approaches for preeclampsia, which are presently lacking.
The investigation of the hormonal and molecular mechanisms of circulatory changes in gestation could potentially have far-reaching clinical implications in both the pregnant and nonpregnant populations. First, incorporation of RLN into the medical regimen of women conceiving with donor eggs might be considered to restore and/or augment maternal circulatory adaptations, which may be deficient, particularly if these pregnancies are at increased risk for adverse outcomes, including preeclampsia, as suggested by recent reports in the literature (reviewed in Ref. 30). Second, there are a number of pathological mechanisms in preeclampsia, many of which might be particularly amenable to treatment by RLN administration, including maternal vasoconstriction and reduced organ perfusion (27, 130). Finally, a long-term goal of investigating circulatory adaptations in pregnancy has been to reveal possible novel therapeutic opportunities for cardiovascular and renal disease in the nonpregnant population. Fortuitously and unexpectedly, in the case of RLN, this long-term goal may apply to both sexes, because administration of RLN is equally efficacious in the circulation of males most likely because of the existence of a local, arterial-derived RLN ligand-receptor system (57, 145) (lacking a corpus luteum, endogenous RLN is unlikely to circulate in males; moreover, RLN is not feminizing). To this end, the vasodilatory attributes of RLN in the renal circulation (48), as well as its matrix-degrading attributes (193), have inspired numerous investigations of its potential therapeutic efficacy in various animal models of renal disease with remarkable success (e.g., see Refs. 26, 49, and 169). Moreover, the vasodilatory attributes of RLN, particularly in the renal circulation, as detailed in this review, as well as other salutary properties, including the potential to improve arterial stiffness (34, 42), suggested the therapeutic use of RLN in heart failure (34, 191). In further support of this idea are more recent findings that RLN improves cardiac fibrosis (153, 166) and mobilizes and increases the activity of bone marrow angiogenic progenitor cells (170). Indeed, a recent phase III study of RLN in acute heart failure appears promising (190).
GRANTS
The work in K.P. Conrad's laboratory was supported by National Institutes of Health Grants K11-HD-00662, RO1-HL-038076, RO1-HD-030325, RO1-DK-063321, RO1-HL-067937, R21-HL-093605, and PO1-HD-065647, the American Heart Association (Flinn Newly Independent Investigator Award), a Grant-in-Aid from the American Heart Association (No. 0855090E), and the 8th Mallinckrodt Scholar Award.
DISCLOSURES
J. M. Davison's research was sponsored by the UK Medical Research Council, the Northern Counties Kidney Research Fund, The Royal Society, and WellBeing. K. P. Conrad is an inventor or coinventor of use patents for relaxin and has served as a paid or unpaid consultant for Connetics, BAS Medical, Corthera, Novartis, and other companies concerning the use of relaxin. J. M. Davison reports no affiliations that might be perceived as influencing the objectivity of this review.
AUTHOR CONTRIBUTIONS
K.P.C. prepared figures; K.P.C. and J.M.D. drafted manuscript; K.P.C. and J.M.D. edited and revised manuscript; K.P.C. and J.M.D. approved final version of manuscript.
ACKNOWLEDGMENTS
K. P. Conrad gratefully acknowledges the invaluable contributions of many colleagues over the years, particularly Drs. Lee A. Danielson, Arun Jeyabalan, Jonathan T. McGuane, Jacqueline Novak, Laura J. Parry, Mark S. Segal, and Sanjeev G. Shroff. The assistance of Ellie Bushhousen of the University of Florida Health Sciences Center Libraries in conducting systematic literature searches for this review is also gratefully acknowledged. J. M. Davison gratefully recognizes the essential contributions of Drs. Duncan W. Irons, Jane E. C. Milne, Paul Moran, Mark Roberts, and Marie C. Smith. We both thank Drs. Marshall D. Lindheimer and Christine Baylis for their constant support and valuable insights through the years, as well as Drs. O. David Sherwood and Elaine Unemori (BAS Medical and Corthera) for contributing valuable reagents, without which much of the work in our laboratories would not have been possible.
Footnotes
Finally, it should be noted that the diagnosis of preeclampsia was recently revised to exclude the requirement for proteinuria. Thus, hypertension in the absence of proteinuria but accompanied by thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and cerebral or visual symptoms fulfills the diagnosis (1).
REFERENCES
- 1.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Abram SR, Alexander BT, Bennett WA, Granger JP. Role of neuronal nitric oxide synthase in mediating renal hemodynamic changes during pregnancy. Am J Physiol Regul Integr Comp Physiol 281: R1390–R1393, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Ahokas RA, Sibai BM. Endothelium-derived relaxing factor inhibition augments vascular angiotensin II reactivity in the pregnant rat hind limb. Am J Obstet Gynecol 167: 1053–1058, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension 38: 742–745, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Alexander BT, Miller MT, Kassab S, Novak J, Reckelhoff JF, Kruckeberg WC, Granger JP. Differential expression of renal nitric oxide synthase isoforms during pregnancy in rats. Hypertension 33: 435–439, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37: 485–489, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Anderson CM. Preeclampsia: exposing future cardiovascular risk in mothers and their children. J Obstet Gynecol Neonatal Nurs 36: 3–8, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Assali NS, Kaplan SA, Fomon SJ, Douglass RA., Jr Renal function studies in toxemia of pregnancy; excretion of solutes and renal hemodynamics during osmotic diuresis in hydropenia. J Clin Invest 32: 44–51, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bader RA, Bader ME, Rose DF, Braunwald E. Hemodynamics at rest and during exercise in normal pregnancy as studies by cardiac catheterization. J Clin Invest 34: 1524–1536, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 301: 259–262, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 2: 577–580, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Baylis C. Cyclooxygenase products do not contribute to the gestational renal vasodilation in the nitric oxide synthase inhibited pregnant rat. Hypertens Pregnancy 21: 109–114, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Baylis C. The mechanism of the increase in glomerular filtration rate in the twelve-day pregnant rat. J Physiol 305: 405–414, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benirschke K, Kaufmann P. Pathology of the Human Placenta. New York: Springer-Verlag, 2000 [Google Scholar]
- 16.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves A, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med 14: 1857–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berks D, Hoedjes M, Raat H, Duvekot JJ, Steegers EA, Habbema JD. Risk of cardiovascular disease after pre-eclampsia and the effect of lifestyle interventions: a literature-based study. BJOG 120: 924–931, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Berks D, Steegers EA, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 114: 1307–1314, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Brewer J, Liu R, Lu Y, Scott J, Wallace K, Wallukat G, Moseley J, Herse F, Dechend R, Martin JN, Jr, Lamarca B. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension 62: 886–892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cadnapaphornchai MA, Ohara M, Morris KG, Jr, Knotek M, Rogachev B, Ladtkow T, Carter EP, Schrier RW. Chronic NOS inhibition reverses systemic vasodilation and glomerular hyperfiltration in pregnancy. Am J Physiol Renal Physiol 280: F592–F598, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Carey LC, Rose JC. The midgestational maternal blood pressure decline is absent in mice lacking expression of the angiotensin II AT2 receptor. J Renin Angiotensin Aldosterone Syst 12: 29–35, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Carvalho LN, Cristovam PC, Passos CS, Boim MA. Mesangial cells cultured from pregnant rats display reduced reactivity to angiotensin II: the role of relaxin, nitric oxide and AT2 receptor. Cell Physiol Biochem 30: 1456–1464, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54: 2056–2063, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT, Schrier RW. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol Renal Physiol 273: F777–F782, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Merrill DC, Rose JC. The importance of angiotensin II subtype receptors for blood pressure control during mouse pregnancy. Reprod Sci 14: 694–704, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Collino M, Rogazzo M, Pini A, Benetti E, Rosa AC, Chiazza F, Fantozzi R, Bani D, Masini E. Acute treatment with relaxin protects the kidney against ischaemia/reperfusion injury. J Cell Mol Med 17: 1494–1505, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conrad KP. Emerging role of relaxin in the maternal adapations to normal pregnancy: implications for preeclampsia. Semin Nephrol 31: 15–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conrad KP. Possible mechanisms for changes in renal hemodynamics during pregnancy: studies from animal models. Am J Kidney Dis 9: 253–259, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Conrad KP. Renal hemodynamics during pregnancy in chronically catheterized, conscious rats. Kidney Int 26: 24–29, 1984 [DOI] [PubMed] [Google Scholar]
- 30.Conrad KP, Baker VL. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol 304: R69–R72, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conrad KP, Barrera SA, Friedman PA, Schmidt VM. Evidence for attenuation of myo-inositol uptake, phosphoinositide turnover and inositol phosphate production in aortic vasculature of rats during pregnancy. J Clin Invest 87: 1700–1709, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 37: 240–249, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Conrad KP, Colpoys MC. Evidence against the hypothesis that prostaglandins are the vasodepressor agents of pregnancy. Serial studies in chronically instrumented, conscious rats. J Clin Invest 77: 236–245, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conrad KP, Debrah DO, Novak J, Danielson LA, Shroff SG. Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology 145: 3289–3296, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Conrad KP, Gaber LW, Lindheimer MD. The kidney in normal pregnancy and preeclampsia. In: Chesley's Hypertensive Disorders in Pregnancy, edited by Lindheimer MD, Roberts JM, Cunningham FG. San Diego, CA: Academic, 2009, p. 297–334 [Google Scholar]
- 36.Conrad KP, Gandley RE, Ogawa T, Nakanishi S, Danielson LA. Endothelin mediates renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. Am J Physiol Renal Physiol 276: F767–F776, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Chavez JE, Mosher MD. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J 7: 566–571, 1993 [PubMed] [Google Scholar]
- 38.Conrad KP, Karumanchi SA. Renal physiology and disease in pregnancy. In: Seldin and Giebisch's The Kidney, edited by Alpern RJ, Caplan MJ, Moe OW. San Diego, CA: Academic, 2013, p. 2689–2761 [Google Scholar]
- 39.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NOx and cGMP during normal pregnancy and preeclampsia in women on a reduced NOx diet. Am J Physiol Renal Physiol 277: F48–F57, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Conrad KP, Lindheimer M. Renal and cardiovascular alterations in normal and preeclamptic pregnancy. In: Chesley's Hypertensive Disorders in Pregnancy (2nd ed.), edited by Lindheimer MD, Cunningham FG, Roberts JM. Stamford, CT: Appleton & Lange, 1999, p. 263–326 [Google Scholar]
- 41.Conrad KP, Segal MS, Keller-Wood M, Chi YY, Hamilton KK, Williams RS, Rhoton-Vlasak A, Nichols WW, Liu J. Evidence for impaired maternal circulatory adaptations in pregnancies conceived using donor eggs (Abstract). Reproductive Sci 21: 282A, 2014 [Google Scholar]
- 42.Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 13: 409–420, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Conrad KP, Vernier KA. Plasma level, urinary excretion, and metabolic production of cGMP during gestation in rats. Am J Physiol Regul Integr Comp Physiol 257: R847–R853, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest 96: 482–490, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danielson LA, Conrad KP. Prostaglandins maintain renal vasodilation and hyperfiltration during chronic nitric oxide synthase blockade in conscious pregnant rats. Circ Res 79: 1161–1166, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Danielson LA, Conrad KP. Time course and dose response of relaxin-mediated renal vasodilation, hyperfiltration, and changes in plasma osmolality in conscious rats. J Appl Physiol 95: 1509–1514, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Danielson LA, Kercher LJ, Conrad KP. Impact of gender and endothelin on renal vasodilation and hyperfiltration induced by relaxin in conscious rats. Am J Physiol Regul Integr Comp Physiol 279: R1298–R1304, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Danielson LA, Sherwood OD, Conrad KP. Relaxin is a potent renal vasodilator in conscious rats. J Clin Invest 103: 525–533, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danielson LA, Welford A, Harris A. Relaxin improves renal function and histology in aging Munich Wistar rats. J Am Soc Nephrol 17: 1325–1333, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 129: e1552–e1561, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Davison JM. Changes in renal function in early pregnancy in women with one kidney. Yale J Biol Med 51: 347–349, 1978 [PMC free article] [PubMed] [Google Scholar]
- 52.Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int 27: 74–79, 1985 [DOI] [PubMed] [Google Scholar]
- 53.Davison JM, Homuth V, Jeyabalan A, Conrad KP, Karumanchi SA, Quaggin S, Dechend R, Luft FC. New aspects in the pathophysiology of preeclampsia. J Am Soc Nephrol 15: 2440–2448, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol 88: 10–17, 1981 [DOI] [PubMed] [Google Scholar]
- 55.Debrah DO, Conrad KP, Danielson LA, Shroff SG. Effects of relaxin on systemic arterial hemodynamics and mechanical properties in conscious rats: sex dependency and dose response. J Appl Physiol 98: 1013–1020, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Debrah DO, Conrad KP, Jeyabalan A, Danielson LA, Shroff SG. Relaxin increases cardiac output and reduces systemic arterial load in hypertensive rats. Hypertension 46: 745–750, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Debrah DO, Debrah JE, Haney JL, McGuane JT, Sacks MS, Conrad KP, Shroff SG. Relaxin regulates vascular wall remodeling and passive mechanical properties in mice. J Appl Physiol 111: 260–271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 147: 5126–5131, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Dhillion P, Wallace K, Herse F, Scott J, Wallukat G, Heath J, Mosely J, Martin JN, Jr, Dechend R, LaMarca B. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol 303: R353–R358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dickey RP, Hower JF. Ultrasonographic features of uterine blood flow during the first 16 weeks of pregnancy. Hum Reprod 10: 2448–2452, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Dschietzig T, Bartsch C, Richter C, Laule M, Baumann G, Stangl K. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist: attenuation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-kappaB. Circ Res 92: 32–40, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, Baumann G, Stangl K. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail 15: 182–190, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Epstein FH. Late vascular effects of toxemia of pregnancy. N Engl J Med 271: 391–395, 1964 [DOI] [PubMed] [Google Scholar]
- 64.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erikson MS, Unemori EN. Relaxin clinical trials in systemic sclerosis. In: Proceedings of the Third International Conference on Relaxin and Related Peptides, edited by Tregear GW, Ivell R, Bathgate RA, Wade JD. Broome, Australia: Kluwer Academic Publishers, 2000, p. 373–381. [Google Scholar]
- 66.Eskenazi B, Fenster L, Sidney S, Elkin EP. Fetal growth retardation in infants of multiparous and nulliparous women with preeclampsia. Am J Obstet Gynecol 169: 1112–1118, 1993 [DOI] [PubMed] [Google Scholar]
- 67.Everett RB, Worley RJ, MacDonald PC, Gant NF. Effect of prostaglandin synthetase inhibitors on pressor response to angiotensin II in human pregnancy. J Clin Endocrinol Metab 46: 1007–1010, 1978 [DOI] [PubMed] [Google Scholar]
- 68.Fadel HE, Sabour MS, Mahran M, Seif el-Din D, el-Mahallawi MN. Reversibility of the renal lesion and functional impairment in preeclampsia diagnosed by renal biopsy. Obstet Gynecol 33: 528–534, 1969 [PubMed] [Google Scholar]
- 69.Farquar M. Review of Normal and Pathologic Glomerular Ultrastructure. Proceedings of the 10th Annual Conference of Nephritic Syndrome. New York: National Kidney Foundation, 1959 [Google Scholar]
- 70.Fernandez-Patron C, Radomski MW, Davidge ST. Role of matrix metalloproteinase-2 in thrombin-induced vasorelaxation of rat mesenteric arteries. Am J Physiol Heart Circ Physiol 278: H1473–H1479, 2000 [DOI] [PubMed] [Google Scholar]
- 71.Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res 85: 906–911, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Ferreira VM, Gomes TS, Reis LA, Ferreira AT, Razvickas CV, Schor N, Boim MA. Receptor-induced dilatation in the systemic and intrarenal adaptation to pregnancy in rats. PLoS One 4: e4845, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fisher K, Luger A, Spargo B, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine (Baltimore) 60: 267–276, 1981 [PubMed] [Google Scholar]
- 74.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48: 711–716, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Gandley RE, Conrad KP, McLaughlin MK. Endothelin and nitric oxide mediate reduced myogenic reactivity of small renal arteries from pregnant rats. Am J Physiol Regul Integr Comp Physiol 280: R1–R7, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gellai M. Physiological role of endothelin in cardiovascular and renal hemodynamics: studies in animals. Curr Opin Nephrol Hypertens 6: 64–68, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Gellai M, Fletcher T, Pullen M, Nambi P. Evidence for the existence of endothelin-B receptor subtypes and their physiological roles in the rat. Am J Physiol Regul Integr Comp Physiol 271: R254–R261, 1996 [DOI] [PubMed] [Google Scholar]
- 79.George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57: 941–948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 36: 56–59, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Gilbert JS, Gilbert SA, Arany M, Granger JP. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gillham JC, Kenny LC, Baker PN. An overview of endothelium-derived hyperpolarising factor (EDHF) in normal and compromised pregnancies. Eur J Obstet Gynecol Reprod Biol 109: 2–7, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Gilson GJ, Mosher MD, Conrad KP. Systemic hemodynamics and oxygen transport during pregnancy in chronically instrumented, conscious rats. Am J Physiol Heart Circ Physiol 263: H1911–H1918, 1992 [DOI] [PubMed] [Google Scholar]
- 85.Goetz RM, Morano I, Calovini T, Studer R, Holtz J. Increased expression of endothelial constitutive nitric oxide synthase in rat aorta during pregnancy. Biochem Biophys Res Commun 205: 905–910, 1994 [DOI] [PubMed] [Google Scholar]
- 86.Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol 299: H1642–H1652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86a.Gomez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest 30: 1143–1155, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gumus II, Uz E, Bavbek N, Kargili A, Yanik B, Turgut FH, Akcay A, Turhan NO. Does glomerular hyperfiltration in pregnancy damage the kidney in women with more parities? Int Urol Nephrol 41: 927–932, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest 91: 1367–1373, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirata Y, Hayakawa H, Suzuki E, Kimura K, Kikuchi K, Nagano T, Hirobe M, Omata M. Direct measurements of endothelium-derived nitric oxide release by stimulation of endothelin receptors in rat kidney and its alteration in salt-induced hypertension. Circulation 91: 1229–1235, 1995 [DOI] [PubMed] [Google Scholar]
- 90.Hladunewich MA, Myers BD, Derby GC, Blouch KL, Druzin ML, Deen WM, Naimark DM, Lafayette RA. Course of preeclamptic glomerular injury after delivery. Am J Physiol Renal Physiol 294: F614–F620, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Huang H, Chang HH, Xu Y, Reddy DS, Du J, Zhou Y, Dong Z, Falck JR, Wang MH. Epoxyeicosatrienoic Acid inhibition alters renal hemodynamics during pregnancy. Exp Biol Med (Maywood) 231: 1744–1752, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Irons DW, Baylis PH, Butler TJ, Davison JM. Atrial natriuretic peptide in preeclampsia: metabolic clearance, sodium excretion and renal hemodynamics. Am J Physiol Renal Physiol 273: F483–F487, 1997 [DOI] [PubMed] [Google Scholar]
- 93.Irons DW, Baylis PH, Davison JM. Effect of atrial natriuretic peptide on renal hemodynamics and sodium excretion during human pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 271: F239–F242, 1996 [DOI] [PubMed] [Google Scholar]
- 94.Jaspers WJ, De Jong PA, Mulder AW. Angiotensin-II sensitivity and prostaglandin-synthetase inhibition in pregnancy. Eur J Obstet Gynecol Reprod Biol 11: 379–384, 1981 [DOI] [PubMed] [Google Scholar]
- 95.Jeyabalan A, Kerchner LJ, Fisher MC, McGuane JT, Doty KD, Conrad KP. Matrix metalloproteinase-2 activity, protein, mRNA, and tissue inhibitors in small arteries from pregnant and relaxin-treated nonpregnant rats. J Appl Physiol 100: 1955–1963, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res 93: 1249–1257, 2003 [DOI] [PubMed] [Google Scholar]
- 97.Jeyabalan A, Novak J, Doty KD, Matthews J, Fisher MC, Kerchner LJ, Conrad KP. Vascular matrix metalloproteinase-9 mediates the inhibition of myogenic reactivity in small arteries isolated from rats after short-term administration of relaxin. Endocrinology 148: 189–197, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Kelly BA, Bond BC, Poston L. Aortic adaptation to pregnancy: elevated expression of matrix metalloproteinases-2 and -3 in rat gestation. Mol Hum Reprod 10: 331–337, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Kerchner LJ, Novak J, Hanley-Yanez K, Doty KD, Danielson LA, Conrad KP. Evidence against the hypothesis that endothelial endothelin B receptor expression is regulated by relaxin and pregnancy. Endocrinology 146: 2791–2797, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Keyes L, Rodman DM, Curran-Everett D, Morris K, Moore LG. Effect of KATP+ channel inhibition on total and regional vascular resistance in guinea pig pregnancy. Am J Physiol Heart Circ Physiol 275: H680–H688, 1998 [DOI] [PubMed] [Google Scholar]
- 101.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 367: 1066–1074, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Kincaid-Smith P. The renal lesion of preeclampsia revisited. Am J Kidney Dis 17: 144–148, 1991 [DOI] [PubMed] [Google Scholar]
- 103.Kopp L, Paradiz G, Tucci J. Urinary excretion of cyclic 3′,5′-adenosine monophosphate and cyclic 3′,5′-guanosine monophosphate during and after pregnancy. J Clin Endocrinol Metab 44: 590–594, 1977 [DOI] [PubMed] [Google Scholar]
- 104.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 113: 1299–1306, 2009 [DOI] [PubMed] [Google Scholar]
- 105.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA 108: 1451–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lafayette RA, Druzin M, Sibley R, Derby G, Malik T, Huie P, Polhemus C, Deen WM, Myers BD. Nature of glomerular dysfunction in pre-eclampsia. Kidney Int 54: 1240–1249, 1998 [DOI] [PubMed] [Google Scholar]
- 107.Lafayette RA, Hladunewich MA, Derby G, Blouch K, Druzin ML, Myers BD. Serum relaxin levels and kidney function in late pregnancy with or without preeclampsia. Clin Nephrol 75: 226–232 [DOI] [PubMed] [Google Scholar]
- 108.Lafayette RA, Malik T, Druzin M, Derby G, Myers BD. The dynamics of glomerular filtration after Caesarean section. J Am Soc Nephrol 10: 1561–1565, 1999 [DOI] [PubMed] [Google Scholar]
- 109.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54: 905–909, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN, Jr, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 57: 865–871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 52: 1168–1172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension 46: 1022–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 113.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Lang NN, Luksha L, Newby DE, Kublickiene K. Connexin 43 mediates endothelium-derived hyperpolarizing factor-induced vasodilatation in subcutaneous resistance arteries from healthy pregnant women. Am J Physiol Heart Circ Physiol 292: H1026–H1032, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 116.Levine RJ, Vatten LJ, Horowitz GL, Qian C, Romundstad PR, Yu KF, Hollenberg AN, Hellevik AI, Asvold BO, Karumanchi SA. Pre-eclampsia, soluble fms-like tyrosine kinase 1, and the risk of reduced thyroid function: nested case-control and population based study. BMJ 339: b4336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O'Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50: 686–692, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Lindheimer MD, Barron W, Davison J. Osmoregulation of thirst and vasopressin release in pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 257: F159–F169, 1989 [DOI] [PubMed] [Google Scholar]
- 119.Llinas MT, Alexander BT, Capparelli MF, Carroll MA, Granger JP. Cytochrome P-450 inhibition attenuates hypertension induced by reductions in uterine perfusion pressure in pregnant rats. Hypertension 43: 623–628, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Llinas MT, Alexander BT, Seedek M, Abram SR, Crell A, Granger JP. Enhanced thromboxane synthesis during chronic reductions in uterine perfusion pressure in pregnant rats. Am J Hypertens 15: 793–797, 2002 [DOI] [PubMed] [Google Scholar]
- 121.Lopez-Lera M, Rubio G. Severe abruption placentae, toxemia of pregnancy and renal biopsy. Am J Obstet Gynecol 93: 1144–1150, 1965 [DOI] [PubMed] [Google Scholar]
- 122.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol 196: 396.e1–396.e7; discussion 396.e7, 2007 [DOI] [PubMed] [Google Scholar]
- 123.Luksha L, Luksha N, Kublickas M, Nisell H, Kublickiene K. Diverse mechanisms of endothelium-derived hyperpolarizing factor-mediated dilatation in small myometrial arteries in normal human pregnancy and preeclampsia. Biol Reprod 83: 728–735, 2010 [DOI] [PubMed] [Google Scholar]
- 124.Luksha L, Nisell H, Kublickiene K. The mechanism of EDHF-mediated responses in subcutaneous small arteries from healthy pregnant women. Am J Physiol Regul Integr Comp Physiol 286: R1102–R1109, 2004 [DOI] [PubMed] [Google Scholar]
- 125.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 71: 977–984, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Mandala M, Gokina N, Barron C, Osol G. Endothelial-derived hyperpolarization factor (EDHF) contributes to PlGF-induced dilation of mesenteric resistance arteries from pregnant rats. J Vasc Res 49: 43–49, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mayer A. Changes in the endothelium during eclampsia and their significance (translation from German). Klin Wochenzetschrift: H27, 1924 [Google Scholar]
- 128.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCartney CP, Spargo B, Lorincz AB, Lefebvre Y, Newton RE. Renal structure and function in pregnant patients with acute hypertension; osmolar concentration. Am J Obstet Gynecol 90: 579–592, 1964 [DOI] [PubMed] [Google Scholar]
- 130.McGuane JT, Danielson LA, Debrah JE, Rubin JP, Novak J, Conrad KP. Angiogenic growth factors are new and essential players in the sustained relaxin vasodilatory pathway in rodents and humans. Hypertension 57: 1151–1160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McGuane JT, Debrah JE, Sautina L, Jarajapu YP, Novak J, Rubin JP, Grant MB, Segal M, Conrad KP. Relaxin induces rapid dilation of rodent small renal and human subcutaneous arteries via PI3 kinase and nitric oxide. Endocrinology 152: 2786–2796, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milne JE, Lindheimer MD, Davison JM. Glomerular heteroporous membrane modeling in third trimester and postpartum before and during amino acid infusion. Am J Physiol Renal Physiol 282: F170–F175, 2002 [DOI] [PubMed] [Google Scholar]
- 133.Moran P, Baylis PH, Lindheimer MD, Davison JM. Glomerular ultrafiltration in normal and preeclamptic pregnancy. J Am Soc Nephrol 14: 648–652, 2003 [DOI] [PubMed] [Google Scholar]
- 134.Moran P, Lindheimer MD, Davison JM. The renal response to preeclampsia. Semin Nephrol 24: 588–595, 2004 [DOI] [PubMed] [Google Scholar]
- 135.Morgan TK, Montgomery K, Mason V, West RB, Wang L, van de Rijn M, Higgins JP. Upregulation of histidine decarboxylase expression in superficial cortical nephrons during pregnancy in mice and women. Kidney Int 70: 306–314, 2006 [DOI] [PubMed] [Google Scholar]
- 136.Munkhaugen J, Vikse BE. New aspects of pre-eclampsia: lessons for the nephrologist. Nephrol Dial Transplant 24: 2964–2967, 2009 [DOI] [PubMed] [Google Scholar]
- 137.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55: 394–398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-α. Am J Physiol Regul Integr Comp Physiol 304: R130–R135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ness RB, Roberts JM. Epidemiology of pregnancy-related hypertension. In: Chesley's Hypertensive Disorders in Pregnancy, edited by Lindheimer MD, Roberts JM, Cunningham FG. San Diego, CA: Academic, 2009 [Google Scholar]
- 140.Newstead J, von Dadelszen P, Magee LA. Preeclampsia and future cardiovascular risk. Expert Rev Cardiovasc Ther 5: 283–294, 2007 [DOI] [PubMed] [Google Scholar]
- 141.Ngoc NT, Merialdi M, Abdel-Aleem H, Carroli G, Purwar M, Zavaleta N, Campodonico L, Ali MM, Hofmeyr GJ, Mathai M, Lincetto O, Villar J. Causes of stillbirths and early neonatal deaths: data from 7993 pregnancies in six developing countries. Bull World Health Organ 84: 699–705, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nisell H, Hjemdahl P, Linde B. Cardiovascular responses to circulating catecholamines in normal pregnancy and in pregnancy-induced hypertension. Clin Physiol 5: 479–493, 1985 [DOI] [PubMed] [Google Scholar]
- 143.Novak J, Conrad KP. Small renal arteries isolated from ETB receptor deficient rats fail to exhibit the normal maternal adaptation to pregnancy (Abstract). FASEB J 18: 32, 2004 [Google Scholar]
- 144.Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, Moalli PA, Conrad KP. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest 107: 1469–1475, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, Doty KD, Debrah DO, Shroff SG, Conrad KP. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J 20: 2352–2362, 2006 [DOI] [PubMed] [Google Scholar]
- 146.Novak J, Rajakumar A, Miles TM, Conrad KP. Nitric oxide synthase isoforms in the rat kidney during pregnancy. J Soc Gynecol Investig 11: 280–288, 2004 [DOI] [PubMed] [Google Scholar]
- 147.Novak J, Ramirez RJ, Gandley RE, Sherwood OD, Conrad KP. Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am J Physiol Regul Integr Comp Physiol 283: R349–R355, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Novak J, Reckelhoff J, Bumgarner L, Cockrell K, Kassab S, Granger JP. Reduced sensitivity of the renal circulation to angiotensin II in pregnant rats. Hypertension 30: 580–584, 1997 [DOI] [PubMed] [Google Scholar]
- 149.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN, Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Regul Integr Comp Physiol 302: R1197–R1201, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oe PL, Ooms EC, Uttendorfsky OT, Stolte LA, van Delden L, Graaff P. Postpartum resolution of glomerular changes in edema-proteinuria-hypertension gestosis. Renal Physiol 3: 375–379, 1980 [DOI] [PubMed] [Google Scholar]
- 151.Paller MS. Decreased pressor responsiveness in pregnancy: studies in experimental animals. Am J Kidney Dis 9: 308–311, 1987 [DOI] [PubMed] [Google Scholar]
- 152.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 153.Parikh A, Patel D, McTiernan CF, Xiang W, Haney J, Yang L, Lin B, Kaplan AD, Bett GC, Rasmusson RL, Shroff SG, Schwartzman D, Salama G. Relaxin suppresses atrial fibrillation by reversing fibrosis and myocyte hypertrophy and increasing conduction velocity and sodium current in spontaneously hypertensive rat hearts. Circ Res 113: 313–321, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 23: 911–916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, Wallukat G, Ray LF, Arany M, Cockrell K, Martin JN, Dechend R, Lamarca B. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am J Hypertens 24: 835–840, 2011 [DOI] [PubMed] [Google Scholar]
- 156.Pivarnik JM, Lee W, Clark SL, Cotton DB, Spillman HT, Miller JF. Cardiac output responses of primigravid women during exercise determined by the direct Fick technique. Obstet Gynecol 75: 954–959, 1990 [PubMed] [Google Scholar]
- 157.Pollak VE, Pirani CL, Kark RM, Muehrcke RC, Freda VC, Nettles JB. Reversible glomerular lesions in toxaemia of pregnancy. Lancet 271: 59–62, 1956 [DOI] [PubMed] [Google Scholar]
- 158.Ponikowski P, Mitrovic V, Ruda M, Fernandez A, Voors AA, Vishnevsky A, Cotter G, Milo O, Laessing U, Zhang Y, Dahlke M, Zymlinski R, Metra M. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J 35: 431–441, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 308: 1592–1594, 2005 [DOI] [PubMed] [Google Scholar]
- 160.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension 41: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 161.Roberts M, Lindheimer MD, Davison JM. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol Renal Fluid Electrolyte Physiol 270: F338–F343, 1996 [DOI] [PubMed] [Google Scholar]
- 162.Robson JS. Proteinuria and the renal lesion in preeclampsia and abruptio placentae. Perspect Nephrol Hypertens 5: 61–73, 1976 [PubMed] [Google Scholar]
- 163.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol Heart Circ Physiol 256: H1060–H1065, 1989 [DOI] [PubMed] [Google Scholar]
- 164.Rosenfeld CR, Liu XT, DeSpain K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol 296: H1878–H1887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sala C, Campise M, Ambroso G, Motta T, Zanchetti A, Morganti A. Atrial natriuretic peptide and hemodynamics changes during normal human pregnancy. Hypertension 25: 631–636, 1995 [DOI] [PubMed] [Google Scholar]
- 166.Samuel CS, Cendrawan S, Gao XM, Ming Z, Zhao C, Kiriazis H, Xu Q, Tregear GW, Bathgate RA, Du XJ. Relaxin remodels fibrotic healing following myocardial infarction. Lab Invest 91: 675–690, 2011 [DOI] [PubMed] [Google Scholar]
- 167.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de, St Groth B, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 183: 7023–7030, 2009 [DOI] [PubMed] [Google Scholar]
- 168.Sarles HE, Hil SS, LeBlanc AL, Smith GH, Canales CO, Remmers AR., Jr Sodium excretion patterns during and following intravenous sodium chloride loads in normal and hypertensive pregnancies. Am J Obstet Gynecol 102: 1–7, 1968 [DOI] [PubMed] [Google Scholar]
- 169.Sasser JM, Molnar M, Baylis C. Relaxin ameliorates hypertension and increases nitric oxide metabolite excretion in angiotensin II but not N(ω)-nitro-l-arginine methyl ester hypertensive rats. Hypertension 58: 197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Segal MS, Sautina L, Li S, Diao Y, Agoulnik AI, Kielczewski J, McGuane JT, Grant MB, Conrad KP. Relaxin increases human endothelial progenitor cell NO and migration and vasculogenesis in mice. Blood 119: 629–636, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sheehan HL. Renal morphology in preeclampsia. Kidney Int 18: 241–252, 1980 [DOI] [PubMed] [Google Scholar]
- 172.Sherwood OD. Relaxin. In: The Physiology of Reproduction, edited by Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW. New York: Raven, 1994, p. 861–1008 [Google Scholar]
- 173.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007 [DOI] [PubMed] [Google Scholar]
- 174.Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin Perinatol 30: 16–19, 2006 [DOI] [PubMed] [Google Scholar]
- 175.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol Regul Integr Comp Physiol 272: R441–R463, 1997 [DOI] [PubMed] [Google Scholar]
- 176.Slangen BF, Out IC, Verkeste CM, Peeters LL. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am J Physiol Heart Circ Physiol 270: H1779–H1784, 1996 [DOI] [PubMed] [Google Scholar]
- 177.Smith CA, Santymire B, Erdely A, Venkat V, Losonczy G, Baylis C. Renal nitric oxide production in rat pregnancy: role of constitutive nitric oxide synthases. Am J Physiol Renal Physiol 299: F830–F836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Smith MC, Danielson LA, Conrad KP, Davison JM. Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol 17: 3192–3197, 2006 [DOI] [PubMed] [Google Scholar]
- 179.Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM. Relaxin has a role in establishing a renal response in pregnancy. Fertil Steril 86: 253–255, 2006 [DOI] [PubMed] [Google Scholar]
- 180.Spargo B, McCartney CP, Winemiller R. Glomerular capillary endotheliosis in toxemia of pregnancy. Arch Pathol 68: 593–599, 1959 [PubMed] [Google Scholar]
- 181.Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, Charnock-Jones DS, Redman CW. Redefining preeclampsia using placenta-derived biomarkers. Hypertension 61: 932–942, 2013 [DOI] [PubMed] [Google Scholar]
- 182.Stennett AK, Qiao X, Falone AE, Koledova VV, Khalil RA. Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am J Physiol Heart Circ Physiol 296: H745–H755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stevenson JC, Macdonald DW, Warren RC, Booker MW, Whitehead MI. Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed) 293: 1329–1330, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol 18: 2281–2284, 2007 [DOI] [PubMed] [Google Scholar]
- 185.Strevens H, Wide-Swensson D, Grubb A, Hansen A, Horn T, Ingemarsson I, Larsen S, Nyengaard JR, Torffvit O, Willner J, Olsen S. Serum cystatin C reflects glomerular endotheliosis in normal, hypertensive and pre-eclamptic pregnancies. BJOG 110: 825–830, 2003 [PubMed] [Google Scholar]
- 186.Strevens H, Wide-Swensson D, Hansen A, Horn T, Ingemarsson I, Larsen S, Willner J, Olsen S. Glomerular endotheliosis in normal pregnancy and pre-eclampsia. BJOG 110: 831–836, 2003 [PubMed] [Google Scholar]
- 187.Struthers AD, Brown MJ, Macdonald DW, Beacham JL, Stevenson JC, Morris HR, MacIntyre I. Human calcitonin gene related peptide: a potent endogenous vasodilator in man. Clin Sci (Lond) 70: 389–393, 1986 [DOI] [PubMed] [Google Scholar]
- 188.Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 56: 192–199, 2011 [DOI] [PubMed] [Google Scholar]
- 189.Tam Tam KB, George E, Cockrell K, Arany M, Speed J, Martin JN, Jr, Lamarca B, Granger JP. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol 204: 330.e1–330.e4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 381: 29–39, 2013 [DOI] [PubMed] [Google Scholar]
- 191.Teichman SL, Unemori E, Dschietzig T, Conrad K, Voors AA, Teerlink JR, Felker GM, Metra M, Cotter G. Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev 14: 321–329, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tsukahara H, Ende H, Magazine HI, Bahou WF, Goligorsky MS. Molecular and functional characterization of the non-isopeptide-selective ETB receptor in endothelial cells. Receptor coupling to nitric oxide synthase. J Biol Chem 269: 21778–21785, 1994 [PubMed] [Google Scholar]
- 193.Unemori EN, Pickford LB, Salles AL, Piercy CE, Grove BH, Erikson ME, Amento EP. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest 98: 2739–2745, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.van Eijndhoven HW, Janssen GM, Aardenburg R, Spaanderman ME, Peeters LL, De Mey JG. Mechanisms leading to increased vasodilator responses to calcitonin-gene-related peptide in mesenteric resistance arteries of early pregnant rats. J Vasc Res 45: 350–356, 2008 [DOI] [PubMed] [Google Scholar]
- 195.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 12: 642–649, 2006 [DOI] [PubMed] [Google Scholar]
- 196.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809, 2008 [DOI] [PubMed] [Google Scholar]
- 197.Wallace K, Novotny S, Heath J, Moseley J, Martin JN, Jr, Owens MY, LaMarca B. Hypertension in response to CD4+ T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol 303: R144–R149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN, Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 21: 521–526, 2008 [DOI] [PubMed] [Google Scholar]
- 200.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 103: 945–952, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 58: 77–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wide-Swensson D, Strevens H, Willner J. Antepartum percutaneous renal biopsy. Int J Gynaecol Obstet 98: 88–92, 2007 [DOI] [PubMed] [Google Scholar]
- 203.Williams D. Long-term complications of preeclampsia. Semin Nephrol 31: 111–122, 2011 [DOI] [PubMed] [Google Scholar]
- 204.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res 113: 78–87, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu DL, Martin PY, St John J, Tsai P, Summer SN, Ohara M, Kim JK, Schrier RW. Upregulation of endothelial and neuronal constitutive nitric oxide synthase in pregnant rats. Am J Physiol Regul Integr Comp Physiol 271: R1739–R1745, 1996 [DOI] [PubMed] [Google Scholar]
- 206.Zenclussen AC, Fest S, Joachim R, Klapp BF, Arck PC. Introducing a mouse model for pre-eclampsia: adoptive transfer of activated Th1 cells leads to pre-eclampsia-like symptoms exclusively in pregnant mice. Eur J Immunol 34: 377–387, 2004 [DOI] [PubMed] [Google Scholar]