Abstract
Tuberculosis (TB) has been a human disease for centuries. Its frequency is increased manyfold in patients with liver cirrhosis. The gold standard of TB management is a 6-mo course of isoniazid, rifampicin, pyrazinamide and ethambutol. Although good results are seen with this treatment in general, the management of patients with underlying cirrhosis is a challenge. The underlying depressed immune response results in alterations in many diagnostic tests. The tests used for latent TB have many flaws in this group of patients. Three of four first-line antitubercular drugs are hepatotoxic and baseline liver function is often disrupted in patients with underlying cirrhosis. Frequency of hepatotoxicity is increased in patients with liver cirrhosis, frequently leading to severe liver failure. There are no established guidelines for the treatment of TB in relation to the severity of liver disease. There is no consensus on the frequency of liver function tests required or the cut-off used to define hepatotoxicity. No specific treatment exists for prevention or treatment of hepatotoxicity, making monitoring even more important. A high risk of multidrug-resistant TB is another major worry due to prolonged and interrupted treatment.
Keywords: Antitubercular therapy, Drug hepatotoxicity, Multidrug-resistant tuberculosis, Immune dysfunction
Core tip: Treatment of tuberculosis (TB) in patients with underlying cirrhosis is a challenge because of the compromised liver functions and high risk of hepatotoxicity. There is no consensus regarding the treatment and monitoring of TB in this group of patients. This paper reviews the differences in diagnosis, treatment, monitoring, hepatotoxicity and other issues in treatment of TB in patients with cirrhosis. Suggestions for treatment of TB in patients with different grades of cirrhosis, as well as monitoring guidelines, are provided. Finally, issues such as liver transplantation, multidrug-resistant TB and reactivation of TB by interferon are briefly reviewed.
INTRODUCTION
Tuberculosis (TB) has afflicted humans for many centuries[1]. About one-third of the world’s population is infected with Mycobacterium tuberculosis (M. tuberculosis). TB is widely prevalent worldwide, especially in the developing countries in Africa and Asia, with an estimated 40%-50% of the adult population being infected[2]. India has the highest TB burden in the world according to World Health Organization (WHO) statistics for 2011, giving an estimated incidence of 2.2 million cases in India out of a global incidence of 8.7 million cases[3]. Primary infection with M. tuberculosis leads to clinical disease in only approximately 10% of individuals and in the rest, latent TB infection develops. In 5%-10% of latently infected persons, the infection reactivates and causes active TB[4]. The progression from latent infection to active disease depends on a number of factors, of which the most important is the presence of an underlying immunodeficient state[5]. Cirrhosis is a widely prevalent disease that leads to immunosuppression and a higher prevalence of TB than in the general population[6]. However, treatment in patients with underlying cirrhosis is complicated by poor tolerance, higher incidence of hepatotoxicity, no consensus regarding monitoring and treatment regimens, and higher chances of multidrug-resistant (MDR) TB. This paper reviews the differences in diagnosis, treatment, monitoring, hepatotoxicity and other issues in treatment of TB in patients with cirrhosis.
CIRRHOSIS AND TB
Prevalence and relationship
Liver cirrhosis is also a relatively common condition with autopsy studies showing a prevalence of 5%-10%[6]. Evidence suggests a higher prevalence of TB in patients with cirrhosis as compared to the general population. The high incidence of TB in patients with cirrhosis has been ascribed mainly to immune dysfunction with associated higher virulence as compared to the general population[7]. In a cohort study of patients with liver cirrhosis from Denmark (1977-1993), the incidence of TB was 168.6 per 100000. It was highest in men aged > 65 years, with an incidence of 246 per 100000[8]. Furthermore, patients with cirrhosis who acquired TB had a poor prognosis in that study. A study conducted in Western India showed that the prevalence rate was 15 times higher than in the general population[9]. Another study from India showed that there is nearly five times higher prevalence of TB in cirrhosis patients (8.1%) compared to the general population (1.6%), with pulmonary TB being the commonest form[10].
Pulmonary TB is generally responsible for 80%-85% of all cases of TB reported[11]. Cirrhosis has been suggested as a risk factor for extrapulmonary TB in a previous study[12]. In a Korean study, 31% patients with cirrhosis had extrapulmonary TB, as compared to 12% in the non-cirrhosis control group with a predominance of peritoneal TB[7]. There are several reports of unusual manifestation of TB in patients with cirrhosis[13]. Little is known about the immunopathogenesis of TB in such clinical conditions. Although most of the host defense systems, especially the clearance capacity of the reticuloendothelial system, are thought to be diminished in patients with cirrhosis, there is no simple explanation as to how this immune dysfunction results in patients being more likely to develop extrapulmonary TB than pulmonary TB.
Cirrhosis-associated immune dysfunction
Cirrhosis-associated immune dysfunction syndrome is a multifactorial process in which the ability to clear cytokines, bacteria and endotoxins from the circulation is decreased[14]. The liver is the major organ of the reticuloendothelial system and contains 90% of the cells of the reticuloendothelial system that are central to clearing bacteria, such as Kupffer cells and sinusoidal endothelial cells[14]. There is reticuloendothelial system dysfunction in patients with cirrhosis, which leads to significantly reduced monocyte spreading, chemotaxis, bacterial phagocytosis, and bacterial killing in cirrhosis compared with controls, and hence compromised innate immunity[14]. These patients also have decreased neutrophil mobilization and phagocytic activity with reduced oxidative bursts; a phenomenon that has been shown to correlate with the severity of liver disease. Hyperammonemia and hyponatremia have been shown to lead to reduced neutrophil function and impaired phagocytosis[15]. Furthermore, specific etiology of liver disease, such as alcohol and hepatitis B and C, have been shown to be associated with additional impairment in immune function and/or increase in proinflammatory cytokines[16].
Toll-like receptors (TLRs) are encoded pattern recognition receptors that play a central role in host cell recognition and responses to microbial pathogens. About 10 functional human TLRs (TLR1-10) have been described; each one being involved in the sensing of distinct microbial products[17]. TLR-2 is capable of recognizing pathogen-associated molecular patterns expressed by M. tuberculosis, such as a 19-kDa lipoprotein, lipoarabinomannan, and soluble TB factor[18]. Immune evasion allows M. tuberculosis to establish persistent or latent infection in macrophages and results in TLR-2-dependent inhibition of MHC class II transactivator expression, MHC class II molecule expression, and antigen presentation[19]. TLR-2 genetic polymorphisms have been shown to influence susceptibility to pulmonary TB. TLR-2 variants play a role in the development of TB phenotypes, probably by controlling the expansion of natural killer cells[20]. Patients with stable alcoholic chronic liver disease show an attenuated TLR-2-mediated innate immune response[21]. The extent to which cirrhosis interacts with TLR polymorphism in promoting mycobacterial immune evasion is not known.
Diagnosis of TB in cirrhosis
Diagnosis of latent as well as clinical TB can be challenging in the setting of cirrhosis. There can be overlap between the symptoms of TB and decompensation of cirrhosis leading to delay in diagnosis. These patients demonstrate impaired delayed-type hypersensitivity; hence, there is a higher likelihood of false-negative tuberculin test results[22]. The exact mechanism of anergy to skin testing is not well known. Schirren et al[23] have shown that, although in patients with alcoholic liver cirrhosis, T-cell-dependent functions are impaired in vivo, T-cell-activation pathways are not responsible for the observed immune defect. A strong association was observed between increased soluble intercellular adhesion molecule (ICAM)-1 concentrations and impairment of delayed-type hypersensitivity skin tests, suggesting that soluble ICAM-1 may be implicated in the immune depression seen in patients with chronic liver disease[24]. In the same study, serum alkaline phosphatase levels were also correlated with the impaired delayed-type hypersensitivity skin test[24]. The tuberculin skin test (TST) is further confounded by the etiology of cirrhosis. A recent study by Çelikbilek et al[25] showed that TST findings were more often falsely positive in the end-stage liver disease caused by viral as compared to nonviral etiology.
Interferon (IFN)-γ release assay(IGRA) is an alternative to purified protein derivative (PPD) testing. The test requires only a single contact with a patient. In addition, unlike the PPD, which is subject to interpretation bias, IFN-γ release assays are machine read and have single cut-offs. Thus, there is little subjectivity to the reading of results. IFN-γ-release assays have been tested and found to perform reasonably well in healthy populations as well as in patients with end-stage liver disease[26]. Several controversies still exist regarding their operational value, such as their discordance with the TST, role in immunocompromised subgroups, role in healthcare workers, role of serial testing, and ability to identify people who are likely to progress to active[27]. In high-burden settings, IFN-γ release assays tend to have decreased sensitivity because of the confounding effects of malnutrition, nontuberculous mycobacterium (NTM) exposure (especially Mycobacterium kansasii and Mycobacterium marinum), leprosy, and parasitic and other tropical infections that may alter the host T helper 1/T helper 2 cell balance[28].
Great efforts have been made globally to accelerate the development and expansion of new diagnostic technologies. However, pulmonary TB case detection remains dependent upon sputum smear and culture, radiography and clinical symptomatology. The role of sputum smear and radiography in the presence of cirrhosis is similar to that in patients without underlying cirrhosis. The M. tuberculosis-specific nucleic acid amplification tests (NAAT) performed on bronchopulmonary specimens is the most frequently used molecular test for laboratory diagnosis of pulmonary TB. NAAT results can be available to the clinician within 1 d after obtaining sputum or bronchoalveolar lavage (BAL) fluid and can have important implications for the management of patients. Unfortunately, NAAT amplification targets are not standardized and the diagnostic accuracy of the tests is highly heterogeneous[28,29]. In individuals with positive acid-fast bacillus (AFB) sputum smears, the sensitivity of NAAT to detect M. tuberculosis nucleic acid on these specimens is > 95%[29]. When AFBs are found on sputum or BAL smears, the presumptive diagnosis of TB can thus be rapidly confirmed. Apart from rare exceptions, a negative NAAT result in this situation strongly indicates the presence of an NTM species. Currently available serological tests cannot be recommended for the diagnosis of TB because of poor sensitivity and specificity. Recently, Steingart and colleagues conducted a meta-analysis of the published studies of distinct single antigens and multiple-antigen combinations in terms of their performance in diagnosing pulmonary TB[30]. The authors concluded that none of the antigen sensitivity was high enough to replace sputum smear microscopy. A recent test has been approved by the FDA (MTB/RIF test), which provides sensitive detection of TB and rifampin resistance directly from untreated sputum in < 2 h with minimal hands-on time. The role of this test in cirrhosis needs to be evaluated as the proportion of patients with pulmonary TB is much lower than in the general population[31].
The diagnosis of extrapulmonary TB in cirrhosis is similar to the disease in the general population. TB peritonitis possibly mimics spontaneous bacterial peritonitis. TB peritonitis occurs in less-advanced cirrhosis and ascitic fluid analysis usually shows lower white blood cell counts, higher proportions of mononuclear cells, and higher levels of protein and adenosine deaminase (ADA)[32]. In developed countries where TB peritonitis is uncommon, the diagnosis of TB peritonitis should prompt a workup for cirrhosis. In a study from the United States, > 50% of TB peritonitis cases had underlying cirrhosis, predominantly alcohol-related[33]. Although ADA level is generally helpful in the detection of TB peritonitis, the presence of cirrhosis may reduce its sensitivity to 30%[33-35]. In addition, abdominal TB is a paucibacillary disease and AFB smears are generally negative in such patients. Sometimes the TB manifestation in cirrhosis could be just the worsening of the liver function.
Drugs used in tuberculosis
There is no consensus regarding the use of antitubercular drugs in patients with cirrhosis. The potential hepatotoxicity of antitubercular drugs is a major concern. First, in the setting of pre-existing liver disease, the likelihood of developing drug-induced hepatitis may be higher. Second, the outcome of drug-induced hepatitis in patients with compromised liver function may be poor. Third, monitoring of drug-induced hepatitis may be confounded in the presence of underlying liver disease due to fluctuating liver function tests related to the pre-existing liver disease[36-38].
First-line drugs
Isoniazid: Isoniazid is a synthetic analog of pyridoxine and the most potent tuberculocidal drug[39]. It is an essential component of all regimens. Isoniazid is effective against both intra- and extracellular organisms because it inhibits the synthesis of mycolic acids in the bacterial cell wall[39]. Isoniazid is metabolized in the liver through two main pathways. Acetyl hydrazine, a nontoxic metabolite, is formed when metabolism proceeds along the N-acetyltransferase (NAT) 2 pathway, while hydrazine, the toxic metabolite, is formed when it proceeds along the amidase pathway[40]. Most previous research had identified acetyl hydrazine as the toxic metabolite of isoniazid[41,42]. Later studies, however, suggested that hydrazine, and not isoniazid or acetyl hydrazine, was most likely to be the cause of isoniazid-induced hepatotoxicity[43].
An asymptomatic, self-limited increase in aminotransferase levels is observed in the majority of patients treated with isoniazid, which does not progress to more serious forms of liver injury[44]. Frequency of liver damage increases with age and in general is < 2%. A meta-analysis of six studies estimated the rate of clinical hepatitis in patients given isoniazid alone to be 0.6%[45]. Hepatotoxicity due to isoniazid therapy seems to be idiosyncratic in most patients and does not recur with rechallenge, hence, it can be reintroduced after complete clinical recovery[46].
Rifampicin: Rifampicin is a bactericidal agent that inhibits mycobacterium DNA-dependent RNA polymerase. It has profound early bactericidal activity against rapidly dividing cells and also against semidormant bacterial populations[47]. Transient elevation of hepatitis enzymes are however routinely observed in these patients. However, they return to normal on continuation of therapy. Yee et al[48] reported a rate of 0.05 per 100 person-months for hepatitis caused by rifampicin. Conjugated hyperbilirubinemia probably results from rifampicin inhibiting the major bile salt exporter pump, impeding secretion of conjugated bilirubin at the canalicular level[49]. Rifampicin can cause hepatocellular changes such as centrilobular necrosis, associated with cholestasis[37].
Pyrazinamide: Pyrazinamide is a weak bactericidal drug. Its active form, pyrazinoic acid, disrupts the bacterial membrane and inhibits membrane transport functions. It exerts greatest activity against the population of dormant or semidormant organisms contained within macrophages or the acidic environment of caseous foci[50]. Historically, it was considered the most hepatotoxic antitubercular drug. When the drug was first introduced in the 1950s, a high incidence of hepatotoxicity was reported and the drug was nearly abandoned[51]. This appeared to be related to the high dosage of 40-70 mg/kg used at that time. Toxicity is rare when pyrazinamide is used at a daily dose of < 35 mg/kg[52]. In murine models, pyrazinamide inhibits CYP45058 activity and NAD59 levels are altered in association with free-radical-species-mediated hepatotoxicity[53]. Bridging necrosis, lymphocytic infiltration, focal cholestasis, increased fibrosis, and micronodular cirrhosis have been observed in the liver of a patient who died of rifampicin- and pyrazinamide-induced hepatotoxicity[54]. The rate of hepatotoxicity of pyrazinamide monotherapy in its currently used dose is unknown. However, more data on the safety of pyrazinamide are needed to clarify its use in patients with cirrhosis.
Ethambutol: Ethambutol is a bacteriostatic antibiotic approved for the treatment of TB. It works by preventing the formation of the bacterial cell wall. Hepatotoxic effects of this agent are not clinically significant[55].
Second-line antitubercular drugs
The second-line drugs are considered as the reserved therapy for TB. These drugs are often used in special conditions. When situations like resistance to first-line therapy, extensively drug-resistant tuberculosis (XDR-TB) or MDR-TB arise, the second-line drugs are implemented[56]. These include: (1) aminoglycosides such as amikacin and kanamycin; (2) polypeptides such as capreomycin, viomycin and enviomycin; (3) fluroquinolones such as ciprofloxacin, levofloxacin and moxifloxacin; (4) thioamides such as ethionamide and prothionamide; (5) cycloserine; and (6) terizidone.
Third-line antitubercular drugs
Third-line antitubercular agents include rifabutin, macrolides (clarithromycin), linezolid, thioacetazone, thioridazine, arginine, and vitamin D. These drugs may be considered third-line because they are not very effective (e.g., clarithromycin) or because their efficacy has not been proven (e.g., linezolid)[57].
ANTITUBERCULAR THERAPY IN CIRRHOSIS: THE CHALLENGES
Challenges in the treatment of TB in patients with cirrhosis arise because three of the first-line antitubercular drugs are potentially hepatotoxic. The administration of these drugs can lead to worsening liver function with decompensation of stable cirrhosis and sometimes cause fulminant hepatic failure, with a high mortality. There is no consensus on the drugs to be given for different grades of liver injury, although the WHO guidelines mention that the more unstable or severe the liver disease is, the fewer hepatotoxic drugs should be used[58].
Incidence of antitubercular drug hepatotoxicity
There is a high incidence of hepatotoxicity ranging from 2% to 28%. TB is usually treated with multiple drugs to prevent emergence of MDR strains. This makes the determination of the exact drug responsible for hepatotoxicity difficult. Temporal data are sometimes helpful in providing evidence for hepatotoxicity of particular drugs. Therefore, there are limited data on toxicity rates of individual antitubercular drugs, except for isoniazid, which has been widely used as prophylactic monotherapy for latent TB infection. A meta-analysis of development of toxic hepatitis with isoniazid and rifampicin alone and in combination was done by Steele et al[59]; a summary of which is provided in Table 1.
Table 1.
Incidence of hepatotoxicity of isoniazid and rifampicin individually, and in combination[49]
| Drugs used | Total no. of patients | Patients with hepatotoxicity | Incidence of hepatotoxicity |
| INH | 38257 | 210 | 0.6% |
| RIF | NA | NA | NA |
| INH + RIF | 6155 | 168 | 2.73% |
| INH + other drugs | 2053 | 33 | 1.6% |
| RIF + other drugs | 1264 | 14 | 1.1% |
INH: Isoniazid; RIF: Rifampicin.
An asymptomatic, self-limited increase in aminotransferase levels was observed in most patients treated with isoniazid. Approximately 0.5% of all patients treated with isoniazid monotherapy for latent TB developed clinically important increases in aminotransferase levels in a large study. The percentage was higher in combination therapy[59]. Isoniazid-induced hepatotoxicity is seen mainly as hepatocellular steatosis and necrosis, and it has been suggested that toxic drug metabolites may bind covalently to cell macromolecules[60].
Hepatotoxicity associated with rifampicin is usually idiosyncratic. Rifampicin may occasionally cause dose-dependent interference with bilirubin uptake due to competition with bilirubin for clearance at the sinusoidal membrane, resulting in mild, asymptomatic unconjugated hyperbilirubinemia or jaundice without hepatocellular damage. Occasionally, rifampicin can cause hepatocellular injury and can potentiate hepatotoxicity of other antitubercular drugs[49].
Hepatotoxicity is a major toxic effect of pyrazinamide. Previously reported studies have shown high rates of hepatotoxicity with high doses of pyrazinamide. Doses used currently (< 35 mg/kg per day) are considered much safer[60].
A study by Park et al[38] in patients with chronic liver disease and TB found that the incidence of hepatotoxicity was 17%, with no difference in patients with or without cirrhosis. The incidence of antitubercular-drug-induced hepatotoxicity when used as part of combination regimens in various studies is shown in Table 2[38,61-75].
Table 2.
Studies on hepatotoxicity of antitubercular drugs in combination therapy
| Ref. | Definition of hepatotoxicity | Incidence | Risk factors |
| Døssing et al[61] 1996 | AST > 6 × ULN and confirmation by re-challenge | 2.0 | Female sex, advanced age |
| Ormerod et al[62] 1996 | ALT > 5 × pre-treatment level | 2.3 | Advanced age |
| Tost et al[63] 2005 | ALT/AST > 10 × ULN | 2.6 | Alcoholism, hepatitis B carrier state, other |
| hepatotoxic drugs | |||
| Yee et al[48] 2003 | ALT > 3 × ULN | 3.0 | Advanced age, female sex, Asian, HIV positive |
| Van Hest et al[64] 2004 | ALT > 5 × ULN | 3.4 | Female gender |
| Teleman et al[65] 2002 | ALT/AST > 3 × ULN | 5.3 | Abnormal baseline values, female sex, advanced age |
| Fernández-Villar et al[66] 2004 | ALT/AST > 5 × ULN | 8.1 | Abnormal baseline liver function, low BMI, |
| hepatitis B/C, other drugs | |||
| Pukenyte et al[67] 2007 | ALT > 5 × ULN | 10.7 | Baseline CD4 < 100 cells/mL, bilirubin > 13 mmol/L or ALT > 51 U/L |
| Schaberg et al[68] 1996 | ALT/AST > 3 × ULN | 11.0 | Advanced age, past history of hepatitis, female sex |
| Saigal et al[69] 2001 | AST/ALT > 5ULN or > 400 IU/mL | 12.9 | Advanced child status |
| Bilirubin rise > 2.5 mg/dL | |||
| Breen et al[70] 2006 | ALT/AST > 5 × ULN | 13.0 | HIV infection, Asian |
| Huang et al[71] 2003 | ALT > 3 × ULN | 15.0 | Advanced age, low BMI, slow acetylator |
| status, CYP2E1 c1/c1 genotype | |||
| Sharma et al[72] 2002 | ALT/AST > 5 × ULN, or any increase + symptoms | 16.1 | Advanced age |
| Park et al[38] 2010 | ALT > 3 × ULN | 17.0 | Female sex, total no. of hepatotoxic drugs administered and baseline ALP levels |
| Ungo et al[73] 1998 | ALT/AST > 3 × ULN | 19.0 | HIV or hepatitis C infection |
| Sharifzadeh et al[74] 2005 | ALT > 3 × ULN with or > 5 × ULN without symptoms | 27.7 | No significant risk factors |
| Pande et al[75] 1996 | AST > 3 × ULN | ND | Advanced age, high alcohol intake, slow acetylators |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; ULN: Upper limit of normal; BMI: Body mass index; HIV: Human immunodeficiency virus.
Treatment of TB in compensated cirrhosis
Due to better functional reserve, patient with compensated cirrhosis have more treatment options and better tolerability. There has been no study to date comparing the full antitubercular therapy course with regimens containing only two potentially hepatotoxic drugs. Some authors do not favor the use of pyrazinamide, but at currently used doses, pyrazinamide has not been shown to be more hepatotoxic as compared to isoniazid or rifampicin[60]. Pyrazinamide is generally substituted with a fluoroquinolone or an aminoglycoside as per the clinician preference. It is prudent to use only two hepatotoxic drugs in treating compensated cirrhosis until a randomized controlled trial (RCT) proves the safety of low-dose pyrazinamide-containing combinations of three potentially hepatotoxic drugs. Proposed regimens are: (1) rifampicin, isoniazid, pyrazinamide and ethambutol for 2 mo followed by 4 mo rifampicin and isoniazid; (2) rifampicin, isoniazid, fluoroquinolone/aminoglycoside and ethambutol for 2 mo followed by 4 mo rifampicin and isoniazid; and (3) rifampicin, isoniazid, and ethambutol for 2 mo followed by 7 mo rifampicin and isoniazid.
Treatment in decompensated cirrhosis
Treatment of TB in decompensated cirrhosis is challenging because treatment is a double-edged sword. Treatment may lead to hepatotoxicity and progressive TB may lead to liver decompensation. Treatment regimens should ideally contain one of either isoniazid or rifampicin because they are the most potent antitubercular drugs. Currently, rifampicin is generally the preferred single hepatotoxic agent due to its potentially lower hepatotoxicity, although this has not been proven in an RCT. The high efficacy of isoniazid against mycobacteria warrants a head to head comparison between isoniazid and rifampicin when only one agent can be used. Other agents that are combined in regimens with single hepatotoxic agents include ethambutol, fluoroquinolone, injectable aminoglycoside, and cycloserine. No data on duration of therapy are available but treatment duration usually exceeds 12 mo, depending upon the site and extent of the disease.
In patients with advanced liver disease with complications of cirrhosis and signs of liver failure, it may not be possible to use even a single hepatotoxic drug. The presence of hepatorenal syndrome or other renal dysfunction further complicates the situation, limiting the use of aminoglycosides. Altered mental status may also hamper administration of oral drugs. The outcome in such group patients is poor, with high mortality due to the underlying poor hepatic function. There are no data to guide the choice of agents or the duration of treatment, or that indicate the effectiveness of such a regimen. Expert opinion suggests that a regimen of this sort should be given for at least 18-24 mo[58]. The American Thoracic Society (ATS) guidelines advise the use of ethambutol with fluoroquinolone, cycloserine and capreomycin or aminoglycoside for 18-24 mo if the patient has liver cirrhosis with encephalopathy[45]. Proposed regimens are: (1) rifampicin, ethambutol, fluoroquinolone with/without aminoglycoside for 9-12 mo; (2) isoniazid, ethambutol, fluoroquinolone with/without aminoglycoside for 9-12 mo; and (3) ethambutol, fluoroquinolone with/without aminoglycoside for 12-24 mo.
We propose treatment options according to Child’s class as shown in Table 3. Studies are needed in this grey zone. It would be interesting to evaluate the safety and efficacy of low-dose isoniazid and rifampicin in advanced decompensated cirrhosis.
Table 3.
Proposed treatment according to stage of liver disease
| Child’s status | Treatment |
| A | Two hepatotoxic drugs can be used namely isoniazid and rifampicin with/without pyrazinamide (low dose). Duration 6-9 mo |
| B | Ideally one hepatotoxic drug is used in combination. Pyrazinamide generally avoided Duration generally 9-12 mo |
| C | No hepatotoxic drugs to be used. Can use second-line drugs like streptomycin, ethambutol, fluoroquinolones, amikacin, kanamycin for extended duration of 12 mo or more. Role of aminoglycosides may be limited due to reduced renal reserve in these patients |
There is generally no difference in treatment of pulmonary or extrapulmonary TB but there could be a need for prolongation of antitubercular therapy in cases of central nervous system or skeletal TB. Bone infections have always been difficult to eradicate, which is why prolonged antitubercular therapy (9-18 mo) is routinely prescribed in endemic countries such as India[76]. No consensus or data on the duration of antitubercular therapy in these conditions with concomitant cirrhosis is available.
Monitoring for development of hepatotoxicity
Drug induced liver injury usually occurs in the first 2 mo of treatment. Clinical, biochemical and histological features of drug hepatotoxicity are hard to distinguish from viral hepatitis[44,77]. The signs and symptoms of liver injury include but are not limited to jaundice, abdominal pain, nausea, vomiting and asthenia[78]. Antitubercular treatment drug hepatotoxicity (ATDH) is usually reversible on withdrawal of the offending drug. Monitoring liver function tests more frequently at the start of therapy is a reasonable way to identify these patients. No recommendation for monitoring interval duration exists but once weekly liver function test for the initial 2 mo followed by once monthly should be reasonable. It should be supplemented by liver function tests in between if clinically warranted (Figure 1).
Figure 1.
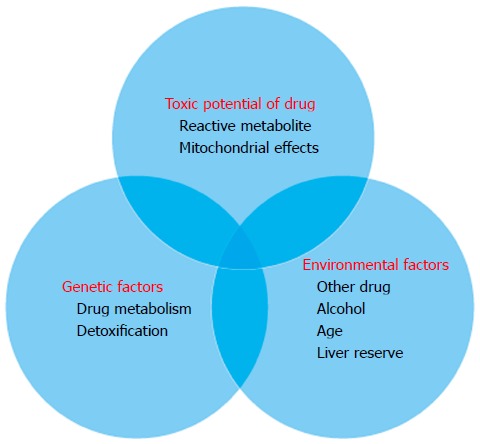
Interaction of factors to produce hepatotoxicity in cirrhosis.
Diagnosis of hepatotoxicity
The definition of hepatotoxicity in patients with previous liver diseases is controversial, because of difficulty in defining the influence of the natural evolution of the underlying liver disease. There is a need to define better the level of aspartate aminotransferase (AST)/alanine aminotransferase (ALT) and serum bilirubin at which to consider hepatotoxicity to avoid unnecessary treatment withdrawal and to avoid dangerous continuation of antitubercular therapy when hepatotoxicity has set in. The baseline AST/ALT and serum bilirubin are already elevated prior to the institution of antitubercular therapy. Although it is generally recommended that therapy be interrupted when transaminase levels increase to 3-5 times the upper limit of normal, this limit has not been defined in patients with transaminase values already elevated before starting therapy[79]. Schenker et al[80] reported that elevations in the ALT and/or AST levels to 50-100 IU/ L more than the baseline levels might define toxicity. In a study by Saigal et al[69], hepatotoxicity was diagnosed if ALT/AST levels increased to more than fivefold of the baseline level, or to more than 400 IU/L, or if the bilirubin increased by 2.5 mg/dL after exclusion of superimposed acute hepatitis. The role of fibroscan and other newer blood test needs to be evaluated in early detection of hepatotoxicity and for differentiation of hepatic adaptation from toxicity.
REINSTITUTION OF ANTITUBERCULAR DRUGS
Guidelines for management of ATDH have been published by the ATS, British Thoracic Society (BTS), Task Force of the European Respiratory Society, WHO and International Union against Tuberculosis and Lung Disease[81-83]. No universally accepted consensus on management is available. All confounding factors like superimposed acute viral hepatitis and recidivism towards alcohol should be investigated. Usually, asymptomatic transaminase elevation resolves spontaneously. When the initial antitubercular regimen has been interrupted due to hepatotoxicity, it is reasonable to maintain at least 3 nonhepatotoxic drugs if possible. These generally include ethambutol, a fluoroquinolone and an aminoglycoside.
After TB treatment has been stopped because of hepatotoxicity, both the BTS and ATS advise restarting the antitubercular drugs one at a time. The Task Force advises restarting all the drugs simultaneously; after a second episode of hepatotoxicity the drugs need to be reintroduced consecutively. These recommendations are in general and not specific to groups of patients with underlying cirrhosis. It is more prudent to start one drug at a time after the serum bilirubin and AST/ALT levels have returned to near the baseline. After bilirubin and AST/ALT levels return to baseline, rifampicin may be restarted first at a reduced dose of 150 mg/d and increased every 3 d with simultaneous liver function test monitoring to the full dose. After successful reintroduction of one hepatotoxic drug, the second agent isoniazid may be restarted at a reduced dose of 50 mg/d and increased slowly every 3-4 d like rifampicin. Rifampicin is generally restarted first because it is thought to be less likely to cause hepatotoxicity than isoniazid. There is no data on reintroduction of pyrazinamide after development of hepatotoxicity episode. The rationale for reintroduction is that majority of hepatotoxicity episodes are hepatic adaptation and it is likely that rechallenge in a gradual manner may be easily tolerated without any evidence of hepatotoxicity. If any single drug is implicated as the cause, it is permanently eliminated from the regimen. If a second episode of hepatotoxicity occurs after full institution of antitubercular therapy, all hepatotoxic drugs should be stopped and extended duration antitubercular therapy with no potentially hepatotoxic drugs should be provided (Figure 2).
Figure 2.
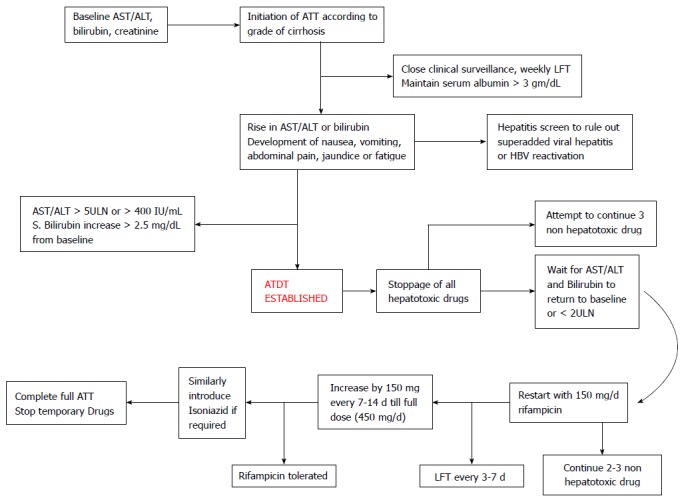
Proposed algorithm for monitoring and management of Antitubercular treatment drug hepatotoxicity. ALT: Alanine aminotransferase; ATDH: Antitubercular treatment drug hepatotoxicity; AST: Aspartate aminotransferase; ULN: Upper limit of normal; ATT: Antitubercular therapy; LFT: Liver function test.
Liver transplantation
ATDH can worsen the liver function in patients with cirrhosis and lead to drug withdrawal. This makes the situation difficult because ongoing infection is generally considered as a contraindication for liver transplantation. In these cases, the strategy for the treatment of TB is poorly defined. In patients with acute decompensation and/or intolerance of antitubercular drugs, liver transplantation has been performed on an urgent basis[84]. In such cases in a post-transplantation setting, rifampicin should be used carefully because drug interactions may change the drug levels significantly and switching to rifabutin may be beneficial[85]. There is also a risk of graft rejection by rifampicin-induced reduction in the level of immunosuppressant because rifampicin is a strong enzyme inducer.
Special situations
Hepatitis B and/or C infections are common causes of the chronic liver disease that is frequently seen in populations at risk for TB infection, and these patients have increased risk of ATDH. In a study from Korea, amongst 110 inactive hepatitis B surface antigen (HBsAg) carriers and 97 controls without hepatitis B infection, 38 inactive HBsAg carriers (35%) and 19 controls (20%) developed elevated liver enzyme levels during antitubercular therapy (P = 0.016). A higher proportion of inactive HBsAg carriers who received antitubercular therapy experienced moderate-to-severe drug-induced hepatotoxicity when compared with the controls (8% vs 2%, P < 0.05)[86]. Ungo et al[73] showed that the relative risk of developing hepatotoxicity if the patient had hepatitis C or was HIV positive was fivefold and fourfold, respectively (P < 0.05). If a patient was co-infected with hepatitis C and HIV, the relative risk of developing drug-induced hepatitis was increased by 14.4-fold (P < 0.002). Alcoholism is associated with a higher risk of ATDH because of enzyme induction. Patients with ongoing alcohol abuse and concomitant use of other hepatotoxic drugs also have an increased risk of hepatotoxicity. In the USPHS surveillance study[87], alcohol consumption appeared to more than double the rate of probable isoniazid hepatitis, with daily consumption increasing the rate more than four times. It is highly likely that this subgroup of patients may have additional risk for hepatotoxicity as compared to other patient groups with cirrhosis, and warrants close monitoring.
Genetic polymorphisms in drug-metabolizing enzymes affect enzyme activity. This may lead to differences in treatment response or drug toxicity, for example, due to an increased formation of reactive metabolites. Data on genetic risk factors for ATDH are still limited. Human genetic studies have shown that cytochrome P450 2E1 (CYP2E1) is involved in ATDH. Huang et al[71] demonstrated that slow acetylators for isoniazid have a more than twofold risk of developing ATDH compared with fast acetylators. Deficiency of glutathione S-transferase (GST) activity, because of homozygous null mutations at GSTM1 and GSTT1 loci, may modulate susceptibility to drug- and xenobiotic-induced hepatotoxicity. Polymorphisms at GSTM1, GSTT1 and CYP2E1 loci have been linked to various forms of liver injury[88].
Prevention of ATDH
There are few effective treatments available for ATDH. This emphasizes the importance of early detection of hepatotoxicity and prompt withdrawal of the offending drug. Polypharmacy should be avoided to prevent inadvertent use of other potentially hepatotoxic drugs. Close clinical and biochemical monitoring are strictly needed for early detection of this potentially reversible liver injury.
Genetic profiling of patients for polymorphisms associated with increased risk of hepatotoxicity will be helpful but is currently not available in the clinical setting. NAT2 genotype could be used to divide patients into low and high isoniazid dose groups. N-Acetyl cysteine (NAC) has been shown in one study to prevent antitubercular-therapy-induced hepatotoxicity[89]. In that RCT, 60 new TB patients aged ≥ 60 years were randomized into two groups. In Group I (n = 32), the drug regimen included daily doses of isoniazid, rifampicin, pyrazinamide, and ethambutol. Patients in Group II (n = 28) were treated with the same regimen and NAC. The mean values of aspartate aminotransferase and alanine aminotransferase were significantly higher in group I than in group II (with NAC) after 1 and 2 wk of treatment[89]. This study proved that NAC protects against antitubercular-drug-induced hepatotoxicity. More studies are needed on the potentially protective effect of such compounds in humans and possible interactions with antitubercular drugs. A hepatoprotective effect of silymarin on ATDH has been shown in rats[90]. A study in patients with cirrhosis is warranted to demonstrate that NAC efficacy may strengthen the already depleted armor in the fight against TB.
The herbal formulation of Curcuma longa and Tinospora cordifolia prevented hepatotoxicity significantly and improved the disease outcome as well as patient compliance, without any toxicity or side effects in a randomized study[91]. Caution must be exercised before using any indigenous drug formulation due to unknown drug interactions and side effects. Ultimately, a strategy that incorporates new analytical approaches - addressing both the immune response and pharmacogenetic vulnerability - can be envisioned.
MDR TB
Many studies for risk factors for drug-resistant TB have found that the presence of hepatic cirrhosis is a risk factor for the development of drug-resistant TB[92]. A study for risk factors for drug-resistant TB found that the prevalence of drug-resistant TB was 46% among patients with cirrhosis, although the number of patients with cirrhosis was only 11[93]. Drug resistance may occur from reduced immune response and the inability to use the most potent drugs in many patients due to the risk of hepatotoxicity.
IFN-induced reactivation of TB - a special scenario
The standard of care for patients with chronic hepatitis C is pegylated IFN-α (Peg-IFN) and ribavirin. IFN treatment induces immunomodulation[94]. Theoretically, IFN-induced immunomodulation should increase TB occurrence as well as other bacterial infections but there is a paucity of reported cases. There have been few case reports of patients developing reactivation of TB as a consequence of IFN therapy, but overall, there is a paucity of data about development of TB in patients after IFN treatment[95-98]. Recent unpublished data from India have shown 10 cases of IFN-induced reactivation of TB. There are many cases of TB occurring after completion of treatment[99]. There could well be under-reporting of cases, leading to lower incidence of TB seen with IFN administration, and such under-reporting is normally high in developing countries. Hence, there is a need for close surveillance of TB in patients receiving IFN for hepatitis C.
New drugs
There is an urgent need for development of new drugs with high efficacy and low hepatotoxicity to reduce the incidence of ATDH. A new drug, bedaquiline, has recently been approved for the treatment of MDR TB[100]. Bedaquiline is a member of the diarylquinoline class of drugs and has a unique mechanism of action, targeting ATP synthase of M. tuberculosis. ATP synthase is used by the bacterium for generation of its energy supply. Bedaquiline is active against both M. tuberculosis and drug-resistant bacteria that cause MDR TB. Laboratory tests and clinical trials have shown it to have strong bactericidal and sterilizing properties[100]. More data on the safety of this drug are required. Moxifloxacin has been shown to be the most efficacious fluoroquinolone in vitro. Many studies with this drug in various combinations are ongoing[101]. Many drugs are in various stages of development, namely DprE inhibitors, indazoles, mycobacterial gyrase inhibitors, pyrazinamide analogs, nitroimidazoles and RNA polymerase inhibitors[102,103].
CONCLUSION
Patients with cirrhosis are predisposed to TB, especially extrapulmonary TB. Diagnosis of TB in patients with cirrhosis is challenging, due to hampered immune response and reduced sensitivity of the available diagnostic tests. Successful completion of antitubercular drug therapy remains a challenge in patients with cirrhosis due to reduced hepatic reserve and higher incidence of hepatotoxicity. Close monitoring and early detection are the mainstay to prevent drug-induced liver injury. Successful reintroduction of antitubercular drugs is possible and should be done in stable patients. Liver transplantation is possible in patient’s not recovering but post-transplantation antitubercular therapy is difficult with ongoing immunosuppression. Ongoing research for potent nonhepatotoxic antitubercular drugs should be expedited.
FUTURE DIRECTIONS
RCTs are needed to decide the optimal regimen of antitubercular therapy in cirrhosis, depending on Child’s score. Better diagnostic methods are needed for detection of latent TB, especially in patients with hepatitis C, prior to starting IFN regimens and as part of pre-transplant evaluation. The efficacy of hepatoprotective agents to reduce drug-induced liver injury when given in combination with antitubercular therapy needs to be studied.
Footnotes
P- Reviewers: Costantini S, Paramesh AS S- Editor: Qi Y L- Editor: Kerr C E- Editor: Ma S
References
- 1.Gutierrez MC, Brisse S, Brosch R, Fabre M, Omaïs B, Marmiesse M, Supply P, Vincent V. Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:e5. doi: 10.1371/journal.ppat.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global tuberculosis report 2012. Geneva, World Health Organization. 2012. Retrieved April 3. 2013. Available from: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 3.TB Statistics for India. TB Facts (2012). Retrieved April 3. 2013. Available from: http://www.tbfacts: tb-statistics-india.html. [Google Scholar]
- 4.World Health Organization, et al. Global tuberculosis control: surveillance, planning and financing. WHO/HTM/ TB/2009.411. Retrieved April 3, 2013. WHO, Geneva: Switzerland; 2009. [Google Scholar]
- 5.Harada H, Murai S, Kojima H, Tokita H, Kamitsukasa H, Yagura M. [Diagnosis and treatment of pulmonary tuberculosis complicated with chronic liver disease] Nihon Rinsho. 1998;56:3212–3216. [PubMed] [Google Scholar]
- 6.Tiribelli C, Croce LS, Polo S, Sodde M, Stanta G. Incidence of hepatocellular carcinoma in Italy: what could we learn from autoptic studies? Ital J Gastroenterol. 1991;23:448–451. [PubMed] [Google Scholar]
- 7.Cho YJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Clinical characteristics of tuberculosis in patients with liver cirrhosis. Respirology. 2007;12:401–405. doi: 10.1111/j.1440-1843.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 8.Thulstrup AM, Mølle I, Svendsen N, Sørensen HT. Incidence and prognosis of tuberculosis in patients with cirrhosis of the liver. A Danish nationwide population based study. Epidemiol Infect. 2000;124:221–225. doi: 10.1017/s0950268899003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saigal S, Nandeesh HP, Agarwal SR, Misra A, Jain SK, Sarin SK. High prevalence and profile of tuberculosis in chronic liver disease patients. Gastroenterology. 1998;114:A38. [Google Scholar]
- 10.Baijal R, Praveenkumar HR, Amarapurkar DN, Nagaraj K, Jain M. Prevalence of tuberculosis in patients with cirrhosis of liver in western India. Trop Doct. 2010;40:163–164. doi: 10.1258/td.2010.090463. [DOI] [PubMed] [Google Scholar]
- 11.Mehta JB, Dutt A, Harvill L, Mathews KM. Epidemiology of extrapulmonary tuberculosis. A comparative analysis with pre-AIDS era. Chest. 1991;99:1134–1138. doi: 10.1378/chest.99.5.1134. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez OY, Adams G, Teeter LD, Bui TT, Musser JM, Graviss EA. Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int J Tuberc Lung Dis. 2003;7:1178–1185. [PubMed] [Google Scholar]
- 13.Iglesias M, Macías MA, Correro F, Soria MJ. [Miliary tuberculosis and cirrhosis] Rev Clin Esp. 1994;194:68–69. [PubMed] [Google Scholar]
- 14.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Shawcross DL, Wright GA, Stadlbauer V, Hodges SJ, Davies NA, Wheeler-Jones C, Pitsillides AA, Jalan R. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology. 2008;48:1202–1212. doi: 10.1002/hep.22474. [DOI] [PubMed] [Google Scholar]
- 16.Laso FJ, Lapeña P, Madruga JI, San Miguel JF, Orfao A, Iglesias MC, Alvarez-Mon M. Alterations in tumor necrosis factor-alpha, interferon-gamma, and interleukin-6 production by natural killer cell-enriched peripheral blood mononuclear cells in chronic alcoholism: relationship with liver disease and ethanol intake. Alcohol Clin Exp Res. 1997;21:1226–1231. [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Fricke I, Mitchell D, Mittelstädt J, Lehan N, Heine H, Goldmann T, Böhle A, Brandau S. Mycobacteria induce IFN-gamma production in human dendritic cells via triggering of TLR2. J Immunol. 2006;176:5173–5182. doi: 10.4049/jimmunol.176.9.5173. [DOI] [PubMed] [Google Scholar]
- 19.López M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol. 2003;170:2409–2416. doi: 10.4049/jimmunol.170.5.2409. [DOI] [PubMed] [Google Scholar]
- 20.Chen YC, Hsiao CC, Chen CJ, Chin CH, Liu SF, Wu CC, Eng HL, Chao TY, Tsen CC, Wang YH, et al. Toll-like receptor 2 gene polymorphisms, pulmonary tuberculosis, and natural killer cell counts. BMC Med Genet. 2010;11:17. doi: 10.1186/1471-2350-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pimentel-Nunes P, Roncon-Albuquerque R, Gonçalves N, Fernandes-Cerqueira C, Cardoso H, Bastos RP, Marques M, Marques C, Alexandre Sarmento J, Costa-Santos C, et al. Attenuation of toll-like receptor 2-mediated innate immune response in patients with alcoholic chronic liver disease. Liver Int. 2010;30:1003–1011. doi: 10.1111/j.1478-3231.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 22.Dhiman RK. Tuberculous peritonitis: towards a positive diagnosis. Dig Liver Dis. 2004;36:175–177. doi: 10.1016/j.dld.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Schirren CA, Jung MC, Zachoval R, Diepolder H, Hoffmann R, Riethmüller G, Pape GR. Analysis of T cell activation pathways in patients with liver cirrhosis, impaired delayed hypersensitivity and other T cell-dependent functions. Clin Exp Immunol. 1997;108:144–150. doi: 10.1046/j.1365-2249.1997.d01-985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirisi M, Vitulli D, Falleti E, Fabris C, Soardo G, Del Forno M, Bardus P, Gonano F, Bartoli E. Increased soluble ICAM-1 concentration and impaired delayed-type hypersensitivity skin tests in patients with chronic liver disease. J Clin Pathol. 1997;50:50–53. doi: 10.1136/jcp.50.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Çelikbilek M, Selçuk H, Yilmaz U. The effect of hepatotropic virus (HBV-HCV) infections on tuberculin skin test in patients with cirrhosis. Turk J Gastroenterol. 2012;23:234–238. doi: 10.4318/tjg.2012.0341. [DOI] [PubMed] [Google Scholar]
- 26.Mazurek GH, Weis SE, Moonan PK, Daley CL, Bernardo J, Lardizabal AA, Reves RR, Toney SR, Daniels LJ, LoBue PA. Prospective comparison of the tuberculin skin test and 2 whole-blood interferon-gamma release assays in persons with suspected tuberculosis. Clin Infect Dis. 2007;45:837–845. doi: 10.1086/521107. [DOI] [PubMed] [Google Scholar]
- 27.Sharma SK, Mohanan S, Sharma A. Relevance of latent TB infection in areas of high TB prevalence. Chest. 2012;142:761–773. doi: 10.1378/chest.12-0142. [DOI] [PubMed] [Google Scholar]
- 28.Greco S, Girardi E, Navarra A, Saltini C. Current evidence on diagnostic accuracy of commercially based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis. Thorax. 2006;61:783–790. doi: 10.1136/thx.2005.054908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greco S, Rulli M, Girardi E, Piersimoni C, Saltini C. Diagnostic accuracy of in-house PCR for pulmonary tuberculosis in smear-positive patients: meta-analysis and metaregression. J Clin Microbiol. 2009;47:569–576. doi: 10.1128/JCM.02051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steingart KR, Dendukuri N, Henry M, Schiller I, Nahid P, Hopewell PC, Ramsay A, Pai M, Laal S. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol. 2009;16:260–276. doi: 10.1128/CVI.00355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim NJ, Choo EJ, Kwak YG, Lee SO, Choi SH, Woo JH, Kim YS. Tuberculous peritonitis in cirrhotic patients: comparison of spontaneous bacterial peritonitis caused by Escherichia coli with tuberculous peritonitis. Scand J Infect Dis. 2009;41:852–856. doi: 10.3109/00365540903214264. [DOI] [PubMed] [Google Scholar]
- 33.Hillebrand DJ, Runyon BA, Yasmineh WG, Rynders GP. Ascitic fluid adenosine deaminase insensitivity in detecting tuberculous peritonitis in the United States. Hepatology. 1996;24:1408–1412. doi: 10.1002/hep.510240617. [DOI] [PubMed] [Google Scholar]
- 34.Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 35.Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, Arrese M, Soza A, Viviani P, Letelier LM. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705–710. doi: 10.1097/00004836-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Grönhagen-Riska C, Hellstrom PE, Fröseth B. Predisposing factors in hepatitis induced by isoniazid-rifampin treatment of tuberculosis. Am Rev Respir Dis. 1978;118:461–466. doi: 10.1164/arrd.1978.118.3.461. [DOI] [PubMed] [Google Scholar]
- 37.Senousy BE, Belal SI, Draganov PV. Hepatotoxic effects of therapies for tuberculosis. Nat Rev Gastroenterol Hepatol. 2010;7:543–556. doi: 10.1038/nrgastro.2010.134. [DOI] [PubMed] [Google Scholar]
- 38.Park WB, Kim W, Lee KL, Yim JJ, Kim M, Jung YJ, Kim NJ, Kim DH, Kim YJ, Yoon JH, et al. Antituberculosis drug-induced liver injury in chronic hepatitis and cirrhosis. J Infect. 2010;61:323–329. doi: 10.1016/j.jinf.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Vilchèze C, Jacobs WR. The mechanism of isoniazid killing: clarity through the scope of genetics. Annu Rev Microbiol. 2007;61:35–50. doi: 10.1146/annurev.micro.61.111606.122346. [DOI] [PubMed] [Google Scholar]
- 40.Ellard GA, Gammon PT. Pharmacokinetics of isoniazid metabolism in man. J Pharmacokinet Biopharm. 1976;4:83–113. doi: 10.1007/BF01086149. [DOI] [PubMed] [Google Scholar]
- 41.Nelson SD, Mitchell JR, Timbrell JA, Snodgrass WR, Corcoran GB. Isoniazid and iproniazid: activation of metabolites to toxic intermediates in man and rat. Science. 1976;193:901–903. doi: 10.1126/science.7838. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell JR, Zimmerman HJ, Ishak KG, Thorgeirsson UP, Timbrell JA, Snodgrass WR, Nelson SD. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med. 1976;84:181–192. doi: 10.7326/0003-4819-84-2-181. [DOI] [PubMed] [Google Scholar]
- 43.Gent WL, Seifart HI, Parkin DP, Donald PR, Lamprecht JH. Factors in hydrazine formation from isoniazid by paediatric and adult tuberculosis patients. Eur J Clin Pharmacol. 1992;43:131–136. doi: 10.1007/BF01740658. [DOI] [PubMed] [Google Scholar]
- 44.Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128:116–123. doi: 10.1378/chest.128.1.116. [DOI] [PubMed] [Google Scholar]
- 45.American Thoracic Society. CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1–77. [PubMed] [Google Scholar]
- 46.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 47.Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell. 2001;104:901–912. doi: 10.1016/s0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 48.Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–1477. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 49.Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123:1649–1658. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- 50.Ijaz K, Jereb JA, Lambert LA, Bower WA, Spradling PR, McElroy PD, Iademarco MF, Navin TR, Castro KG. Severe or fatal liver injury in 50 patients in the United States taking rifampin and pyrazinamide for latent tuberculosis infection. Clin Infect Dis. 2006;42:346–355. doi: 10.1086/499244. [DOI] [PubMed] [Google Scholar]
- 51.Girling DJ. The hepatic toxicity of antituberculosis regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle. 1978;59:13–32. doi: 10.1016/0041-3879(77)90022-8. [DOI] [PubMed] [Google Scholar]
- 52.Arbex MA, Varella Mde C, Siqueira HR, Mello FA. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations. Part 1: first-line drugs. J Bras Pneumol. 2010;36:626–640. doi: 10.1590/s1806-37132010000500016. [DOI] [PubMed] [Google Scholar]
- 53.Shibata K, Fukuwatari T, Sugimoto E. Effects of dietary pyrazinamide, an antituberculosis agent, on the metabolism of tryptophan to niacin and of tryptophan to serotonin in rats. Biosci Biotechnol Biochem. 2001;65:1339–1346. doi: 10.1271/bbb.65.1339. [DOI] [PubMed] [Google Scholar]
- 54.From the Centers for Disease Control and Prevention. Fatal and severe hepatitis associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection--New York and Georgia, 2000. JAMA. 2001;285:2572–2573. [PubMed] [Google Scholar]
- 55.Belanger AE, Besra GS, Ford ME, Mikusová K, Belisle JT, Brennan PJ, Inamine JM. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci USA. 1996;93:11919–11924. doi: 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010;375:2100–2109. doi: 10.1016/S0140-6736(10)60359-9. [DOI] [PubMed] [Google Scholar]
- 57.Lalloo UG, Ambaram A. New antituberculous drugs in development. Curr HIV/AIDS Rep. 2010;7:143–151. doi: 10.1007/s11904-010-0054-4. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. WHO: Geneva; 2010. p. (WHO/HTM/TB/2009.420). Available from: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf. [PubMed] [Google Scholar]
- 59.Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465–471. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- 60.Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 61.Døssing M, Wilcke JT, Askgaard DS, Nybo B. Liver injury during antituberculosis treatment: an 11-year study. Tuber Lung Dis. 1996;77:335–340. doi: 10.1016/s0962-8479(96)90098-2. [DOI] [PubMed] [Google Scholar]
- 62.Ormerod LP, Horsfield N. Frequency and type of reactions to antituberculosis drugs: observations in routine treatment. Tuber Lung Dis. 1996;77:37–42. doi: 10.1016/s0962-8479(96)90073-8. [DOI] [PubMed] [Google Scholar]
- 63.Tost JR, Vidal R, Caylà J, Díaz-Cabanela D, Jiménez A, Broquetas JM. Severe hepatotoxicity due to anti-tuberculosis drugs in Spain. Int J Tuberc Lung Dis. 2005;9:534–540. [PubMed] [Google Scholar]
- 64.van Hest R, Baars H, Kik S, van Gerven P, Trompenaars MC, Kalisvaart N, Keizer S, Borgdorff M, Mensen M, Cobelens F. Hepatotoxicity of rifampin-pyrazinamide and isoniazid preventive therapy and tuberculosis treatment. Clin Infect Dis. 2004;39:488–496. doi: 10.1086/422645. [DOI] [PubMed] [Google Scholar]
- 65.Teleman MD, Chee CB, Earnest A, Wang YT. Hepatotoxicity of tuberculosis chemotherapy under general programme conditions in Singapore. Int J Tuberc Lung Dis. 2002;6:699–705. [PubMed] [Google Scholar]
- 66.Fernández-Villar A, Sopeña B, Fernández-Villar J, Vázquez-Gallardo R, Ulloa F, Leiro V, Mosteiro M, Piñeiro L. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004;8:1499–1505. [PubMed] [Google Scholar]
- 67.Pukenyte E, Lescure FX, Rey D, Rabaud C, Hoen B, Chavanet P, Laiskonis AP, Schmit JL, May T, Mouton Y, et al. Incidence of and risk factors for severe liver toxicity in HIV-infected patients on anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2007;11:78–84. [PubMed] [Google Scholar]
- 68.Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996;9:2026–2030. doi: 10.1183/09031936.96.09102026. [DOI] [PubMed] [Google Scholar]
- 69.Saigal S, Agarwal SR, Nandeesh HP, Sarin SK. Safety of an ofloxacin-based antitubercular regimen for the treatment of tuberculosis in patients with underlying chronic liver disease: a preliminary report. J Gastroenterol Hepatol. 2001;16:1028–1032. doi: 10.1046/j.1440-1746.2001.02570.x. [DOI] [PubMed] [Google Scholar]
- 70.Breen RA, Miller RF, Gorsuch T, Smith CJ, Schwenk A, Holmes W, Ballinger J, Swaden L, Johnson MA, Cropley I, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax. 2006;61:791–794. doi: 10.1136/thx.2006.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, Chang FY, Lee SD. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology. 2003;37:924–930. doi: 10.1053/jhep.2003.50144. [DOI] [PubMed] [Google Scholar]
- 72.Sharma SK, Balamurugan A, Saha PK, Pandey RM, Mehra NK. Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during antituberculosis treatment. Am J Respir Crit Care Med. 2002;166:916–919. doi: 10.1164/rccm.2108091. [DOI] [PubMed] [Google Scholar]
- 73.Ungo JR, Jones D, Ashkin D, Hollender ES, Bernstein D, Albanese AP, Pitchenik AE. Antituberculosis drug-induced hepatotoxicity. The role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med. 1998;157:1871–1876. doi: 10.1164/ajrccm.157.6.9711039. [DOI] [PubMed] [Google Scholar]
- 74.Sharifzadeh M, Rasoulinejad M, Valipour F, Nouraie M, Vaziri S. Evaluation of patient-related factors associated with causality, preventability, predictability and severity of hepatotoxicity during antituberculosis [correction of antituberclosis] treatment. Pharmacol Res. 2005;51:353–358. doi: 10.1016/j.phrs.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 75.Pande JN, Singh SP, Khilnani GC, Khilnani S, Tandon RK. Risk factors for hepatotoxicity from antituberculosis drugs: a case-control study. Thorax. 1996;51:132–136. doi: 10.1136/thx.51.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhardwaj V, Agrawal M, Suri T, Sural S, Kashyap R, Dhal A. Evaluation of adequacy of short-course chemotherapy for extraspinal osteoarticular tuberculosis using 99mTc ciprofloxacin scan. Int Orthop. 2011;35:1869–1874. doi: 10.1007/s00264-010-1162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Black M, Mitchell JR, Zimmerman HJ, Ishak KG, Epler GR. Isoniazid-associated hepatitis in 114 patients. Gastroenterology. 1975;69:289–302. [PubMed] [Google Scholar]
- 78.Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 79.Lew W, Pai M, Oxlade O, Martin D, Menzies D. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med. 2008;149:123–134. doi: 10.7326/0003-4819-149-2-200807150-00008. [DOI] [PubMed] [Google Scholar]
- 80.Schenker S, Martin RR, Hoyumpa AM. Antecedent liver disease and drug toxicity. J Hepatol. 1999;31:1098–1105. doi: 10.1016/S0168-8278(99)80325-0. [DOI] [PubMed] [Google Scholar]
- 81.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 82.Chemotherapy and management of tuberculosis in the United Kingdom: recommendations of the Joint Tuberculosis Committee of the British Thoracic Society. Thorax. 1990;45:403–408. doi: 10.1136/thx.45.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Migliori GB, Raviglione MC, Schaberg T, Davies PD, Zellweger JP, Grzemska M, Mihaescu T, Clancy L, Casali L. Tuberculosis management in Europe. Task Force of the European Respiratory Society (ERS), the World Health Organisation (WHO) and the International Union against Tuberculosis and Lung Disease (IUATLD) Europe Region. Eur Respir J. 1999;14:978–992. doi: 10.1183/09031936.99.14497899. [DOI] [PubMed] [Google Scholar]
- 84.Ichai P, Saliba F, Antoun F, Azoulay D, Sebagh M, Antonini TM, Escaut L, Delvart V, Castaing D, Samuel D. Acute liver failure due to antitubercular therapy: Strategy for antitubercular treatment before and after liver transplantation. Liver Transpl. 2010;16:1136–1146. doi: 10.1002/lt.22125. [DOI] [PubMed] [Google Scholar]
- 85.Lefeuvre S, Rebaudet S, Billaud EM, Wyplosz B. Management of rifamycins-everolimus drug-drug interactions in a liver-transplant patient with pulmonary tuberculosis. Transpl Int. 2012;25:e120–e123. doi: 10.1111/j.1432-2277.2012.01561.x. [DOI] [PubMed] [Google Scholar]
- 86.Lee BH, Koh WJ, Choi MS, Suh GY, Chung MP, Kim H, Kwon OJ. Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy. Chest. 2005;127:1304–1311. doi: 10.1378/chest.127.4.1304. [DOI] [PubMed] [Google Scholar]
- 87.Kopanoff DE, Snider DE, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991–1001. doi: 10.1164/arrd.1978.117.6.991. [DOI] [PubMed] [Google Scholar]
- 88.Roy B, Chowdhury A, Kundu S, Santra A, Dey B, Chakraborty M, Majumder PP. Increased risk of antituberculosis drug-induced hepatotoxicity in individuals with glutathione S-transferase M1 ‘null’ mutation. J Gastroenterol Hepatol. 2001;16:1033–1037. doi: 10.1046/j.1440-1746.2001.02585.x. [DOI] [PubMed] [Google Scholar]
- 89.Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, Velayati AA. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur J Gastroenterol Hepatol. 2010;22:1235–1238. doi: 10.1097/MEG.0b013e32833aa11b. [DOI] [PubMed] [Google Scholar]
- 90.Tasduq SA, Peerzada K, Koul S, Bhat R, Johri RK. Biochemical manifestations of anti-tuberculosis drugs induced hepatotoxicity and the effect of silymarin. Hepatol Res. 2005;31:132–135. doi: 10.1016/j.hepres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Adhvaryu MR, Reddy N, Vakharia BC. Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach. World J Gastroenterol. 2008;14:4753–4762. doi: 10.3748/wjg.14.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishioka SA. Cirrhosis as a risk factor to drug-resistant tuberculosis. Eur Respir J. 1996;9:2188–2189. doi: 10.1183/09031936.96.09102188. [DOI] [PubMed] [Google Scholar]
- 93.Arévalo M, Solera J, Cebrian D, Bartolomé J, Robles P. Risk factors associated with drug-resistant Mycobacterium tuberculosis in Castilla-la-Mancha (Spain) Eur Respir J. 1996;9:274–278. doi: 10.1183/09031936.96.09020274. [DOI] [PubMed] [Google Scholar]
- 94.Itoh Y, Okanoue T, Enjyo F, Morimoto M, Takeuchi T, Kagawa K, Kashima K. Elevated interleukin-6 and gamma-globulin during interferon therapy of hepatitis B. Am J Gastroenterol. 1992;87:1485–1487. [PubMed] [Google Scholar]
- 95.Sabbatani S, Manfredi R, Marinacci G, Pavoni M, Cristoni L, Chiodo F. Reactivation of severe, acute pulmonary tuberculosis during treatment with pegylated interferon-alpha and ribavirin for chronic HCV hepatitis. Scand J Infect Dis. 2006;38:205–208. doi: 10.1080/00365540500263268. [DOI] [PubMed] [Google Scholar]
- 96.Belkahla N, Kchir H, Maamouri N, Ouerghi H, Hariz FB, Chouaib S, Chaabouni H, Mami NB. [Reactivation of tuberculosis during dual therapy with pegylated interferon and ribavirin for chronic hepatitis C] Rev Med Interne. 2010;31:e1–e3. doi: 10.1016/j.revmed.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 97.Farah R, Awad J. The association of interferon with the development of pulmonary tuberculosis. Int J Clin Pharmacol Ther. 2007;45:598–600. doi: 10.5414/cpp45598. [DOI] [PubMed] [Google Scholar]
- 98.Babudieri S, Soddu A, Murino M, Molicotti P, Muredda AA, Madeddu G, Fois AG, Zanetti S, Pirina P, Mura MS. Tuberculosis screening before anti-hepatitis C virus therapy in prisons. Emerg Infect Dis. 2012;18:689–691. doi: 10.3201/eid1804.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar N, Shah V, Kumar R, Sarin SK. Development of tuberculosis in patients on interferon treatment for HCV related CLD. J Hepatology. 2013;58:S345–S346. [Google Scholar]
- 100.Deoghare S. Bedaquiline: a new drug approved for treatment of multidrug-resistant tuberculosis. Indian J Pharmacol. 2013;45:536–537. doi: 10.4103/0253-7613.117765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seung KJ, Becerra MC, Atwood SS, Alcántara F, Bonilla CA, Mitnick CD. Salvage therapy for multidrug-resistant tuberculosis. Clin Microbiol Infect. 2013:Epub ahead of print. doi: 10.1111/1469-0691.12335. [DOI] [PubMed] [Google Scholar]
- 102.Dooley KE, Nuermberger EL, Diacon AH. Pipeline of drugs for related diseases: tuberculosis. Curr Opin HIV AIDS. 2013;8:579–585. doi: 10.1097/COH.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Available from: http://www.tballiance:downloads/Pipeline/TBA-Pipeline-Q3-2013.pdf.


