Abstract
AIM: To evaluate the technical feasibility of a modified tapered metal tip and low profile introducer for one-step endoscopic ultrasound (EUS)-guided biliary drainage (EUS-BD) in a new experimental biliary dilatation porcine model.
METHODS: A novel dedicated device for one-step EUS-guided biliary drainage system (DEUS) introducer has size 3F tapered catheter with size 4F metal tip for simple puncture of the intestinal wall and liver parenchyma without graded dilation. A self-expandable metal stent, consisting of both uncovered and nitinol-covered portions, was preloaded into DEUS introducer. After establishment of a biliary dilatation model using endoscopic hemoclips or band ligation with argon plasma coagulation in 9 mini-pigs, EUS-BD using a DEUS was performed following 19-G needle puncture without the use of fistula dilation devices.
RESULTS: One-step EUS-BD was technically successful in seven pigs [7/9 (77.8%) as intention to treat] without the aid of devices for fistula dilation from the high body of stomach or far distal esophagus to the intrahepatic (n = 2) or common hepatic (n = 5) duct. Primary technical failure occurred in two cases that did not show adequate biliary dilatation. In seven pigs with a successful bile duct dilatation, the technical success rate was 100% (7/7 as per protocol). Median procedure time from confirmation of the dilated bile duct to successful placement of a metallic stent was 10 min (IQR; 8.9-18.1). There were no immediate procedure-related complications.
CONCLUSION: Modified tapered metal tip and low profile introducer may be technically feasible for one-step EUS-BD in experimental porcine model.
Keywords: Endoscopic ultrasound, Biliary drainage, Biliary dilation, Feasibility, Complications
Core tip: Endoscopic ultrasound (EUS)-guided biliary drainage (EUS-BD) has been restricted due to limited data and dedicated devices to perform safely without multi-step processes. We report a pilot study of a modified tapered metal tip and low profile introducer for one-step EUS-BD in a new experimental biliary dilatation porcine model. EUS-guided hepaticoenterostomy using a novel dedicated device for one-step EUS-BD system (DEUS) in an experimental bile duct dilatation porcine model might be technically feasible and effective. One-step stent insertion and deployment precluded the need for additional fistula dilation devices for graded dilation or needle cautery incision, without involving complex procedures or DEUS-related complications.
INTRODUCTION
Percutaneous transhepatic biliary drainage (PTBD) is most predominant salvage procedure used to access the biliary tract after failed endoscopic retrograde cholangiopancreatography (ERCP). However, PTBD-related adverse event rates of 9%-33% and mortality rates of 2%-15% have been reported[1-4]. Furthermore, in terms of the quality of life, long-term placement of external catheters is very uncomfortable for the patient.
Endoscopic ultrasound-guided biliary drainage (EUS-BD) has been introduced as an effective alternative to PTBD after failed ERCP. The potential benefits of EUS-BD include internal drainage, thereby avoiding long term external drainage in cases where external PTBD drainage catheters cannot be internalized. EUS-guided hepaticogastrostomy (EUS-HG), which is a specialized form of EUS-BD that allows biliary drainage from the intrahepatic bile duct to the stomach, can also be used as an additional and effective treatment alternative after failed ERCP. However, EUS-HG with transluminal stenting has inherent procedural complexity, as it requires fistula dilation prior to stenting, resulting in prolonged procedure time, loss of the guidewire, and several adverse events, including stent migration or bile peritonitis[5-10]. Clinical practice in this area has been restricted due to limited data, and specialized devices to perform this procedure safely are needed.
At present, dedicated endoscopic devices to EUS-BD/HG with transluminal stenting are limited. Therefore, we developed a novel device dedicated to one-step EUS-guided biliary drainage system (DEUS), which has a modified tapered metal tip and low profile introducer including partially covered self-expandable metal stent for one-step transluminal stenting after EUS-guided needle puncture without graded dilation or use of cautery incision. To tailor treatment to individual patients clinical training with this stent should be simple without serious complications. Furthermore, there is no proven biliary dilatation animal model suitable for evaluation of the efficacy of this specialized stent. This pilot animal study was performed to evaluate the technical feasibility and clinical outcomes of DEUS in an experimental biliary dilatation porcine model.
MATERIALS AND METHODS
This pilot proof-of-concept study was performed in accordance with the rules of the Institutional Animal Care Committee and the National Center of Efficacy Evaluation for the Development of Health Products Targeting Digestive Disorders. This study was approved by the Committee on Animal Research at Inha University and the Animal Protection Committee of the Korean government.
In vivo study using a porcine animal model
The in vivo experiments were performed using nine domesticated female mini-pigs with a mean weight of 40 kg (range 38-42 kg). Before endoscopic procedures, the animals were fasted for 48 h. On the day of the procedure, animals were sedated by intramuscular injection of tiletamine/zolazepam (Zoletil; 5 mg/kg) and xylazine (Narcoxyl; 2 mg/kg). Anesthesia was administered by intravenous injection (0.1 mg/kg) followed by continuous infusion (4-6 mg/h) of vecuronium. After tracheal intubation, the animals were ventilated with a 1:1 mixture of 1.0%-2.0% inspired isoflurane (Ifran®, Hana Pharm Co Ltd) and oxygen (5-10 mL/kg per minute). The animals were placed recumbently on their left sides on a fluoroscopy table. Vital signs were continuously monitored during the procedure. Prophylactic antibiotics were administered 3 d before and after EUS-guided intervention. The animals were maintained on their usual diet for 7 d following successful transluminal stent insertion. All animals were euthanized by potassium chloride overdose 7 d post-stent insertion.
Preparation of the experimental biliary dilatation porcine model
To date, no suitable biliary dilatation model or adequate dilatation training in animal studies has been available. Adequate biliary dilatation is essential for successful EUS-HE in porcine models. In preliminary to EUS-HE, we established a biliary dilatation model using endoclip closure or endoscopic band ligation (EBL) of the major papilla followed by argon plasma coagulation (APC System, power setting 40 W, gas flow 2 L/min; ERBE Elektromedizin GmbH, Tubingen, Germany) in the ampullary orifice (Figure 1). First, we made biliary dilatation model using endoclip closure with APC. After 2 wk, we evaluated the efficacy of this first biliary dilatation model by EUS. Depending on the intra-/extra-hepatic bile duct dilatation, we attempted EUS-HE or switched to a second dilatation model using repeated clipping or EBL with APC (Figure 2). We evaluated duct dilatation by EUS at the same time intervals and attempted EUS-HE. This experimental animal model was created by three endoscopists (Park DH, Lee TH, Choi JH).
Figure 1.
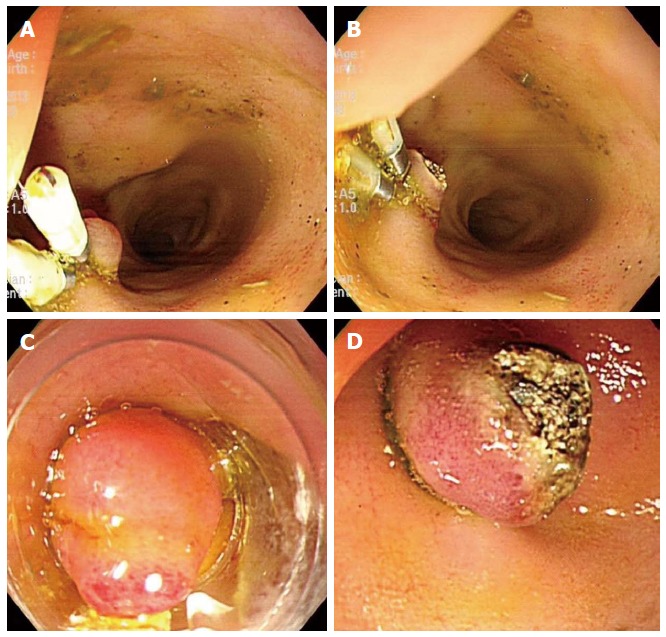
Experimental biliary dilatation model using endoclips and endoscopic band ligation with argon plasma coagulation. A, B: Argon plasma coagulation (APC) was performed on the orifice of ampulla on the duodenal bulb following multiple rounds of hemoclipping on the ampulla of Vater; C, D: In an alternate model, endoscopic band ligation was performed on the ampulla of Vater, again followed by APC in the same manner.
Figure 2.
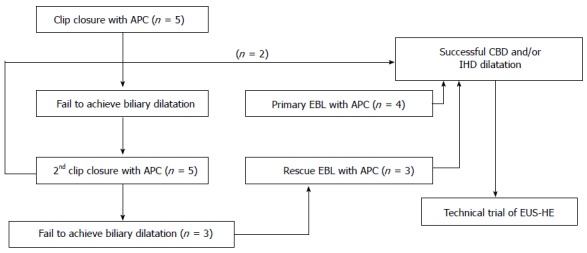
Flow of experimental endoscopic biliary dilatation using clip closure and/or endoscopic band ligation with argon plasma coagulation in a porcine model. APC: Argon plasma coagulation; EBL: Endoscopic band ligation; IHD: Intrahepatic bile duct; CBD: Common bile duct; EUS-HE: Endoscopic ultrasound-guided hepaticoenterostomy.
A novel dedicated DEUS
The DEUS introducer (Standard Sci Tech Inc., Seoul, South Korea) has 3F catheter with 4F tapered pentagonal metal tip for simple puncture of the intestinal wall and liver parenchyma without the need of graded dilation devices (Figure 3). The tapered metal tip and catheter may reduce wall resistance and enhance pushability during one-step tract puncture via a guidewire. The outer sheath of the delivery catheter is size 7F, which provides good pushability and adequate resistance. The concept of this 3F distal/4F metal tip device was inspired by a 4F tip balloon catheter with stainless steel stylet (Hurricane Balloon, Boston Scientific, Natick, Mass) that we found to have high primary fistula puncture and dilation performance during EUS-HG on our institutional experiences. A self-expandable metal stent, consisting of both uncovered and nitinol-covered portions, was preloaded into the catheter. The uncovered proximal end of the stent (8 mm in diameter and 15 mm in length), which is funnel shaped to prevent small-branched bile duct obstruction and distal migration, was placed into the bile duct. The body and distal portions of the stent were covered with a silicone membrane (6 mm in diameter and 35 or 45 mm in length) for the prevention of bile leaks, and the distal end was equipped with four flaps for the prevention of inward stent migration (Figure 3).
Figure 3.
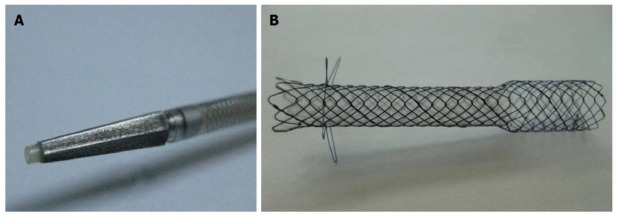
Gross finding of dedicated device for one-step endoscopic ultrasound-guided biliary drainage system. A: The 7F delivery sheath and 3F catheter with 4F tapered pentagonal metal tip; B: Gross finding of the metallic stent. The uncovered proximal end of the stent (8 mm in diameter and 15 mm in length), the body and distal portions of the covered stent with a silicone membrane (6 mm in diameter and 35 or 45 mm in length), and the distal end with four flaps.
One-step EUS-HE technique
EUS-HE was performed using a GF-UCT 240 linear-array echoendoscope together with the EU-ME-1 Ultrasound System (Olympus Medical Systems, Tokyo, Japan) by two experienced endoscopists (Park DH, Lee TH). We performed EUS-HE using the porcine biliary dilatation model after confirming the route and dilatation of the biliary duct via EUS-guided contrast injection into the intrahepatic bile duct (IHD) or common bile duct (CBD) with the endoscope positioned in the stomach high body or the duodenal bulb. If the IHD was dilatated > 5 mm, then it was punctured directly via the liver parenchyma. However, in cases of inadequate IHD dilatation (< 5 mm), despite adequate CBD dilatation (> 7 mm), the puncture was made near the hilum through the liver parenchyma. Since the porcine ampulla of Vater is anatomically much closer to the duodenal bulb, choledochoduodenostomy is technically restricted; consequently, we initially planned to perform a hepaticoenterostomy; hepatic gastrostomy on the high body of stomach or hepaticoesophagostomy on the far distal esophagus. We punctured the bile duct using a 19-G fine aspiration needle (EZ Shot2TM, Olympus Medical Systems, Tokyo, Japan) followed by bile aspiration and radiopaque contrast injection to delineate the CBD and IHD under fluoroscopy. Next, we inserted a 0.025-inch guidewire (VisiGlide, Olympus Medical Systems, Tokyo, Japan) through the EUS-guided 19-G needle and coiled it into the bile duct lumen for transluminal stent placement. EUS-HE was performed according to the direction that the guidewire was inserted into the CBD/IHD. Without using a graded dilation catheter, bougie, or needle-knife cautery, the DEUS was directly inserted and the metallic stent then sequentially deployed over the guidewire (Figure 4). Technical success was defined as metal stent placement along the intestine to the bile duct without the use of fistula dilation methods, such as a catheter, bougie, balloon dilator or needle-knife, and by the flow of contrast medium and/or bile through the stent. Early adverse events were defined as any procedure or stent-related complications that occurred within 48 h.
Figure 4.
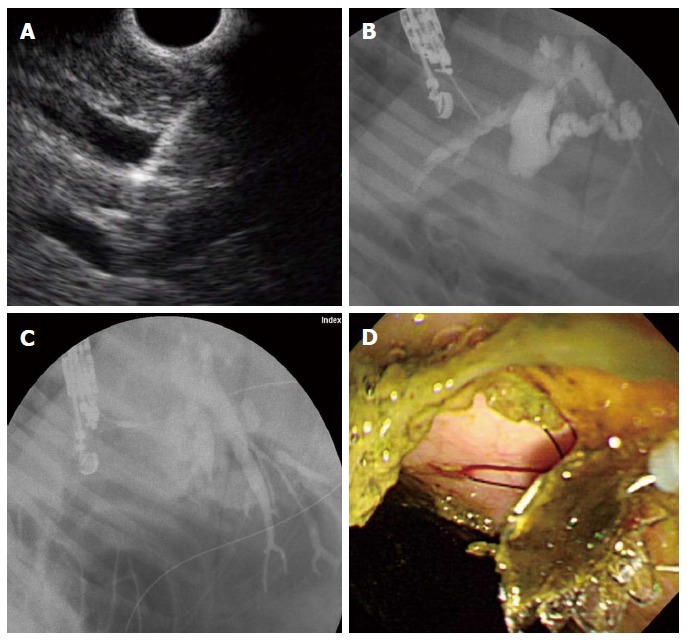
Endoscopic ultrasound-guided hepaticoenterostomy. A: Linear endoscopic ultrasound (EUS) image shows a 19-G needle puncture targeting the intrahepatic bile duct (IHD; B: Fluoroscopic image reveals marked IHD and common bile duct dilatation with ampullary obstruction; C: After EUS-guided 19-G needle puncture of the IHD, the device for one-step endoscopic ultrasound-guided biliary drainage system was inserted and the stent released under the guidance of EUS and fluoroscopy; D: Following successful deployment of the stent, the bile passage was seen in the high body of stomach.
Pathologic examination
The liver and intestine from esophagus to duodenum were excised for gross examination. Following examination for bile leakage, perforation, or stent dislocation, the tissue specimens near the stent location were fixed for 72 h in formalin and then sectioned. Sectioned tissues were stained with hematoxylin-eosin and Wheatley’s trichrome. The severity of inflammation, fibrosis, mucosal sloughing, or hyperplasia were evaluated in each section. All pathologic examinations were performed by a single experienced gastrointestinal pathologist (Cho HD).
RESULTS
Technical feasibility of DEUS
One-step EUS-HE was successful in seven porcine subjects [7/9 (77.8%) as intention to treat], all of which underwent transluminal stenting following EUS-guided 19G needle puncture without graded fistula dilation. The route of EUS-HE was from the high body of stomach (n = 4) or far distal esophagus (n = 3) to the IHD (n = 2) or CHD (n = 5). The median procedure time from the time of confirmation of dilated IHD and/or CHD to that of successful stent placement was 10 minutes (IQR; 8.9-18.1). The stent used was either 5 cm (n = 2) or 6 cm (n = 4) in length. In one case both 5- and 6-cm stents were used due to underestimation of the tract length (Table 1). Six of seven pigs underwent successful EBL plus APC (n = 4, primary band ligation with APC; n = 3, band ligation after failure of second clipping with APC). In the two cases of primary clipping with APC, only one had successful stent placement following the repeated puncture and insertion trial. Primary technical failure occurred in two cases due to inadequate dilatation of the CBD or IHD as compared with the successfully dilated cases (7.3 mm vs 11.7 mm in CBD, P = 0.013; 1.8 mm vs 4.3 mm in IHD, P = 0.037). In the seven pigs with adequate bile duct dilatation, the technical success of DEUS was 100% (7/7 as per protocol).
Table 1.
Outcomes of a novel dedicated device for one-step EUS-guided biliary drainage system and associated complications
| CBD diameter, mm | IHD dilatation, mm | Route | Stent length, cm | Technical success | Procedure time, min | Adverse events | Mortality |
| 6.2 | 1.6 | - | - | Failure | 20 | - | - |
| 13.2 | 5.2 | S to IHD | 6 | Success | 10 | - | - |
| 8.4 | 2.0 | - | - | Failure | 31 | - | - |
| 12.5 | 6.2 | S to CHD | 5, 6 | Success | 8.4 | - | - |
| 13.6 | 5.8 | E to IHD | 5 | Success | 16.2 | Bile leakage1 | Expire, day 3 |
| 11 | 3.3 | E to CHD | 5 | Success | 8 | - | - |
| 9.2 | 3.5 | S to CHD | 6 | Success | 9.4 | - | - |
| 12.8 | 3.8 | E to CHD | 6 | Success | 13.2 | - | - |
| 10 | 2.8 | S to CHD | 6 | Success | 10 | - | - |
Related to multiple needle puncture, no obvious bile leakage on persistent areas on necropsy. CBD: Common bile duct; CHD: Common hepatic duct; IHD: Intrahepatic bile duct; S: The high body of stomach; E: Far distal esophagus.
Procedure-related complications and pathology
There were no immediate post-procedure or stent-related complications. One pig died 2 d after the procedure from bile leakage, according to autopsy. This subject underwent numerous needle punctures (5 attempts) and contrast injections prior to stent insertion. Necropsy findings in the other pigs showed no signs of bile peritonitis, major vessel injuries, or adjacent organ damage. The gross pathology specimens showed a well-demarcated lesion from the distal esophagus to the IHD through the surrounding liver parenchyma. Microscopic examination revealed mild inflammation and necrotic tissues around the metallic stent location but without complications (Figure 5).
Figure 5.
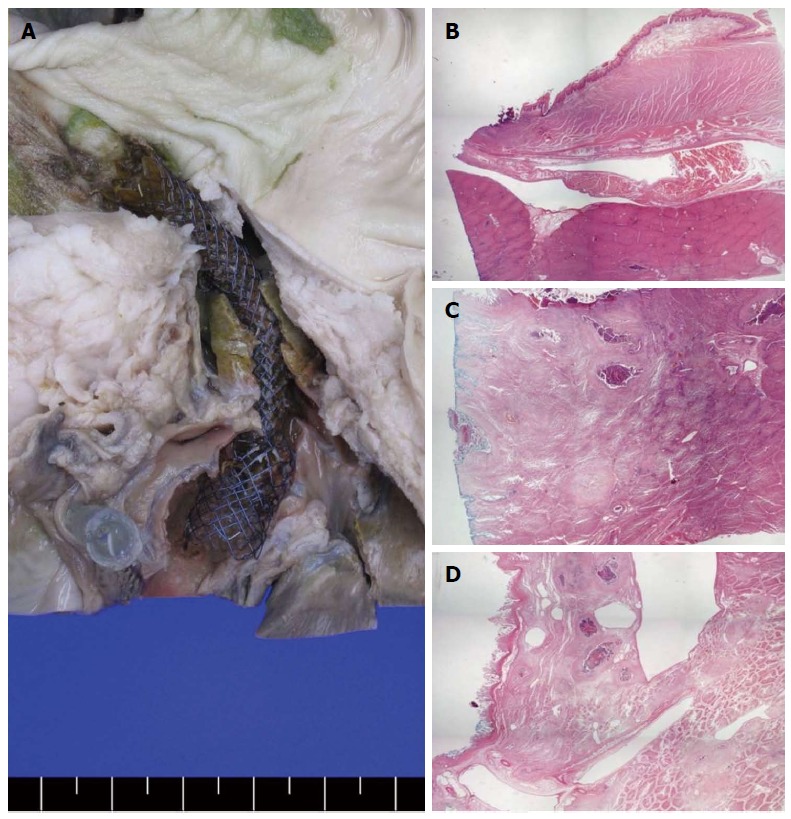
Gross and microscopic findings in the autopsy specimens. A: Gross hepaticoenterostomy specimens showed that stent insertion along the far distal esophagus to the intrahepatic bile duct (IHD) via the liver parenchyma did not cause complications, such as stent dislocation or migration; B-D: Microscopic findings showed the distal esophagus (B), liver parenchyma (C), and IHD (D) sections adjacent to the inserted metallic stent. Surrounding mild inflammation and necrotic tissue were seen but without any other complications, such as abscess or perforation.
DISCUSSION
EUS-BD with transluminal stenting has an inherent procedural complexity, especially in the case of graded dilation prior to stent insertion. Repeated dilation can be performed with sequential use of 4F ERCP catheters followed by 6F and 7F bougie dilators. Alternatively, needle-knives or cystotome cautery incision followed by stent placement can be used[5-17]. However, the drawback of a needle knife is the higher probability of adverse events as compared with graded dilation[6,7]. In addition, these multi-step procedures may be time consuming and increase the risk of procedure-related complications, such as bile leak, pneumoperitoneum, or bleeding. At present, no dedicated endoscopic devices for EUS-BD with transluminal stenting are available, except for a pseudocyst or gallbladder drainage[18].
To overcome these problems, we explored a modified delivery system involving deployment of a self-expandable metal stent for one-step EUS-guided HE. First, the DEUS delivery catheter has a 3F catheter tip and tapered pentagonal 4F metal tip for simple puncture, precluding the need for dilation devices, such as bougies, catheters, needle-knives, or balloon dilations. Furthermore, the stent introducer is also composed of a 7F introducer sheath, which may have reduced wall resistance and good pushability compared with conventional 8F, or 8.5F stent introducers for EUS-HE. Metallic stent in DEUS is the first device targeted by EUS-HE. DEUS exhibited high technical feasibility in the biliary dilatation model without use of any dilation processes despite the small number of cases. In the seven successful stent placement cases, puncture of the high body of stomach or far distal esophagus was relatively easy and potentially reduces procedure time, since DEUS does not require sequential dilation. Second, the design of the newly developed stent comprises an uncovered proximal segment to minimize obstruction of the bile duct side branches, and the remaining longer portion is covered with silicone to prevent bile leakage; it also has multiple flaps on the distal portion to prevent inward migration. No stent migration was evident in any of the successful cases. According to pathologic evaluation, hepaticoenterostomy caused well-demarcated tract maturation without bile leakage and peri-fistula abscess formation according to the short-term follow-up. This suggests that EUS-HE with metal stenting may cause minimal tissue damage around the fistula.
Regarding complications, there were no immediate DEUS-related complications during the procedure. In the success groups of the initial trial, stent insertion on the first attempt, deployment, and bile drainage were all successful. The one mortality case underwent multiple rounds of clipping for duct dilatation, and we attempted several IHD and CBD punctures to estimate duct dilatation and obstruction. Although successful placement of the stent was achieved, the repeated needle punctures (5 attempts) and contrast injections required may have induced delayed bile leakage or microperforations. At autopsy, large amounts of bile were detected in the peritoneum, but neither migration nor inappropriate positioning of the stent was detected. In addition, no obvious bile leakage was noted on necropsy. Relatively short duration of and inadequate bile duct dilatation may cause technical difficulties and even mortality. CBD and IHD dilatations in the two failed cases were less than those in the successful cases. If adequate dilatation is achieved during long-term periods, needle puncture and stent insertion may be more technically feasible.
In addition, there was no available bile duct dilatation porcine model for EUS-BD. In our experimental model, we found that EBL plus APC showed sufficient IHD and CBD dilatation compared with multiple rounds of endoclipping plus APC. In the EBL model, we were able to insert DEUS directly after 2 wk in four of the cases, and after 1 wk in the other three cases, which had initially failed the second round of endoclipping. As a training model for porcine biliary dilatation, EBL with APC may be effective for EUS-guided biliary intervention. However further evaluation of the use of APC and the time period necessary for ideal biliary dilatation should be performed, especially for IHD dilatation, which was not as great as that of the CBD during the observational period.
The limitations of this study included the small sample size and a non-comparative design, as we did not make comparisons with other current metal or plastic stents. Second, our result regarding procedure time may be exaggerated in this porcine model. The procedure time was relatively short in this study. Despite of the advance of devices, it may be due to the relatively thin gastric and esophageal wall in pigs. Third, we autopsied all pigs after short-term follow-up. Even though the live pigs were healthy until the time of autopsy, long-term follow up is necessary to evaluate stent patency and complications. Fourth, bile duct dilatation or obstruction by clipping/EBL plus APC was estimated by serial EUS prior to endoscopy. Less invasive methods and effective dilatation models with respect to technique and timing should be developed for more advanced training.
In conclusion, this modified tapered metal tip and low profile 7F introducer may be technically feasible for EUS-HE in an experimental bile duct dilatation porcine model. One-step EUS-HE using DEUS did not require additional fistula dilation devices for graded dilation or needle knife cautery or involve complex procedures. However, this in vivo study was limited by the small number of subjects. Based on this pilot study results, human clinical trials may be warranted to confirm technical feasibility and safety.
COMMENTS
Background
Endoscopic ultrasound (EUS)-guided biliary drainage (EUS-BD) may be an effective alternative to percutaneous transhepatic biliary drainage after failed endoscopic retrograde cholangiopancreatography (ERCP). However, its clinical practice has been restricted due to limited data and devices to perform the procedure safely.
Research frontiers
Although EUS-BD may be a useful alternative, there have been few studies proving the feasibility and safety of dedicated devices to EUS-BD. In this study, the authors demonstrated the technical feasibility of a novel dedicated device for one-step EUS-BD in a new experimental biliary dilatation porcine model.
Innovations and breakthroughs
EUS-BD with transluminal stenting has inherent procedural complexity, such as sequential dilation of the fistula after needle puncture prior to stenting, resulting in a prolonged procedure time, loss of the guidewire, or several adverse events. This is the first pilot study to report that a novel dedicated device for one-step EUS-BD in an experimental bile duct dilatation porcine model was technically feasible and relatively safe.
Applications
By experimental animal study, EUS-BD using this device may represent a future strategy for therapeutic intervention in the treatment of patients with malignant biliary obstruction and/or who failed ERCP.
Terminology
One-step EUS-guided hepaticoenterostomy (EUS-HE): One-step EUS-HE is the placement of biliary stents without the aid of devices for fistula dilation from the high body of stomach or far distal esophagus to the intrahepatic or common hepatic duct.
Peer review
The authors examined the feasibility of a modified delivery system for one-step EUS-BD. It is an in vivo pilot study in a biliary dilatation porcine model and, despite the small number of cases, the device developed by the authors could be an alternative to the usual technique, less complicated and offering reduction of the procedure time.
Footnotes
Supported by the Soonchunhyang University Research Fund, a research grant (2013-7202) from the Asan Institute for Life Sciences, Asan Medical Center and the National Center of Efficacy Evaluation for the Development of Health Products Targeting Digestive Disorders
P- Reviewers: Leitman IM, Velayos B S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Beissert M, Wittenberg G, Sandstede J, Beer M, Tschammler A, Burghardt W, Jahns R, Hahn D. Metallic stents and plastic endoprostheses in percutaneous treatment of biliary obstruction. Z Gastroenterol. 2002;40:503–510. doi: 10.1055/s-2002-32806. [DOI] [PubMed] [Google Scholar]
- 2.Doctor N, Dick R, Rai R, Dafnios N, Salamat A, Whiteway H, Dooley J, Davidson BR. Results of percutaneous plastic stents for malignant distal biliary obstruction following failed endoscopic stent insertion and comparison with current literature on expandable metallic stents. Eur J Gastroenterol Hepatol. 1999;11:775–780. doi: 10.1097/00042737-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Kühn JP, Busemann A, Lerch MM, Heidecke CD, Hosten N, Puls R. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts compared with patients with dilated intrahepatic bile ducts. AJR Am J Roentgenol. 2010;195:851–857. doi: 10.2214/AJR.09.3461. [DOI] [PubMed] [Google Scholar]
- 4.Weber A, Gaa J, Rosca B, Born P, Neu B, Schmid RM, Prinz C. Complications of percutaneous transhepatic biliary drainage in patients with dilated and nondilated intrahepatic bile ducts. Eur J Radiol. 2009;72:412–417. doi: 10.1016/j.ejrad.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Park do H. Endoscopic ultrasonography-guided hepaticogastrostomy. Gastrointest Endosc Clin N Am. 2012;22:271–280, ix. doi: 10.1016/j.giec.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–1284. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 7.Park do H, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video) Gastrointest Endosc. 2013;78:91–101. doi: 10.1016/j.gie.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Park do H, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168–2174. doi: 10.1038/ajg.2009.254. [DOI] [PubMed] [Google Scholar]
- 9.Park do H, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos) Gastrointest Endosc. 2010;71:413–419. doi: 10.1016/j.gie.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Eum J, Park do H, Ryu CH, Kim HJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with a fully covered metal stent as a novel route for natural orifice transluminal endoscopic biliary interventions: a pilot study (with videos) Gastrointest Endosc. 2010;72:1279–1284. doi: 10.1016/j.gie.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Fujita N, Horaguchi J, Noda Y, Kobayashi G. Current issues regarding endosonography-guided biliary drainage for biliary obstruction. Dig Endosc. 2010;22 Suppl 1:S132–S136. doi: 10.1111/j.1443-1661.2010.00971.x. [DOI] [PubMed] [Google Scholar]
- 12.Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52–59. doi: 10.1016/j.gie.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 13.Ramírez-Luna MA, Téllez-Ávila FI, Giovannini M, Valdovinos-Andraca F, Guerrero-Hernández I, Herrera-Esquivel J. Endoscopic ultrasound-guided biliodigestive drainage is a good alternative in patients with unresectable cancer. Endoscopy. 2011;43:826–830. doi: 10.1055/s-0030-1256406. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen-Tang T, Binmoeller KF, Sanchez-Yague A, Shah JN. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232–236. doi: 10.1055/s-0029-1243858. [DOI] [PubMed] [Google Scholar]
- 15.Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y, et al. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239–1245. doi: 10.1038/ajg.2011.84. [DOI] [PubMed] [Google Scholar]
- 16.Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Moriyasu F, et al. Endoscopic ultrasonography-guided biliary drainage. J Hepatobiliary Pancreat Sci. 2010;17:611–616. doi: 10.1007/s00534-009-0196-1. [DOI] [PubMed] [Google Scholar]
- 17.Prachayakul V, Aswakul P. A novel technique for endoscopic ultrasound-guided biliary drainage. World J Gastroenterol. 2013;19:4758–4763. doi: 10.3748/wjg.v19.i29.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–876. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]


