Abstract
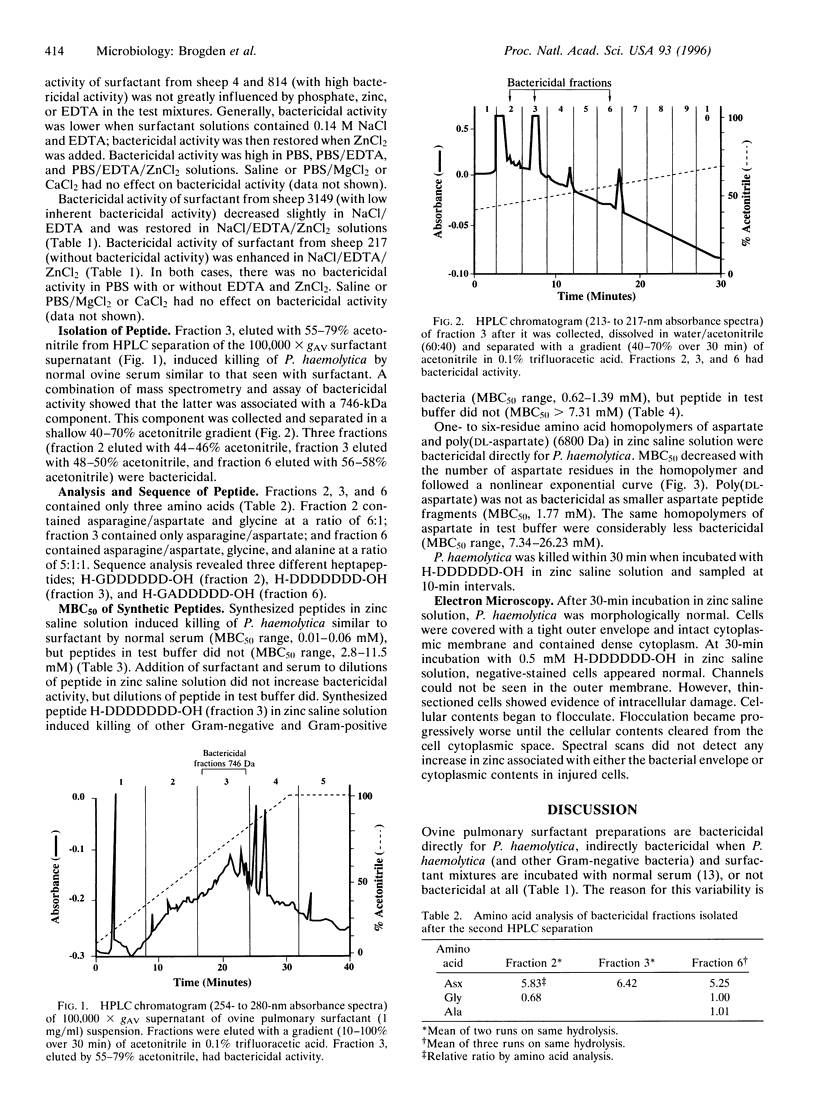
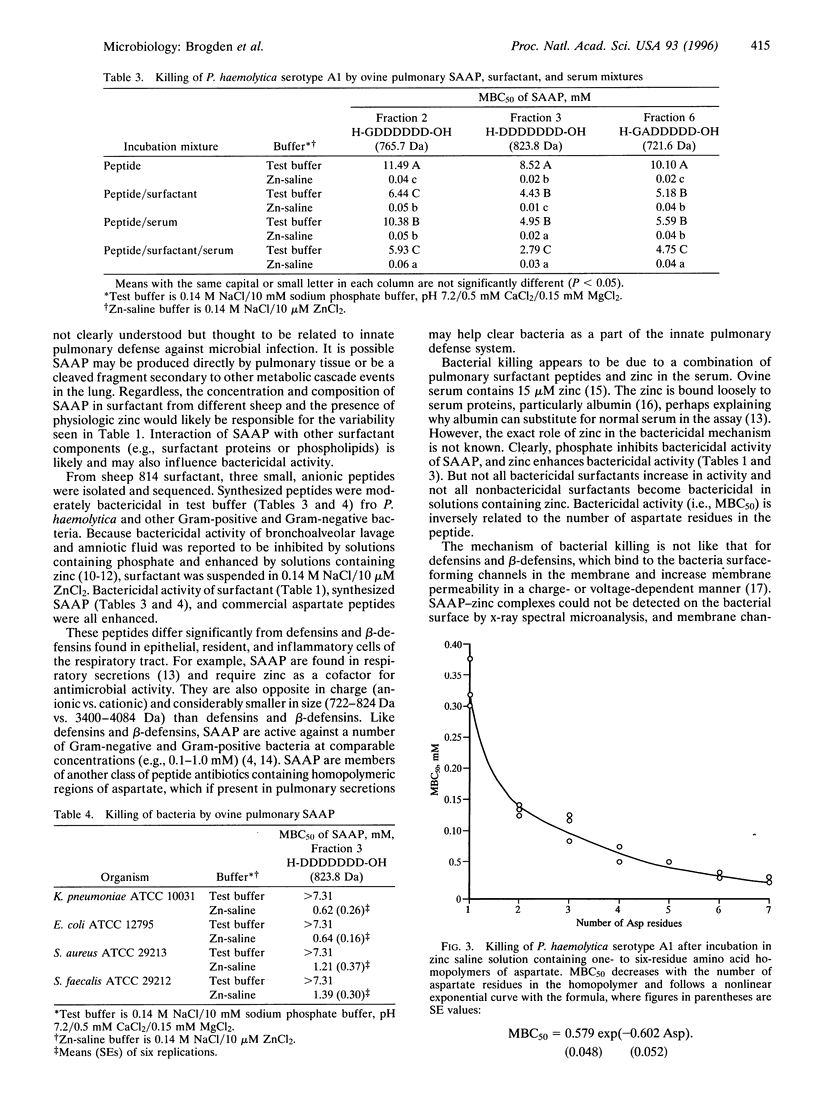
Ovine pulmonary surfactant is bactericidal for Pasteurella haemolytica when surfactant and bacteria mixtures are incubated with normal ovine serum. To isolate this component, surfactant (1 mg/ml) was centrifuged at 100,000 x gav, and the supernatant was fractionated by HPLC. Fractions were eluted with acetonitrile (10-100%)/0.1% trifluoracetic acid and tested for bactericidal activity. Amino acid and sequence analysis of three bactericidal fractions showed that fraction 2 contained H-GDDDDDD-OH, fraction 3 contained H-DDDDDDD-OH, and fraction 6 contained H-GADDDDD-OH. Peptides in 0.14 M NaCl/10 microM ZnCl2 (zinc saline solution) induced killing of P. haemolytica and other bacteria comparable to defensins and beta-defensins [minimal bactericidal concentration (MBC)50 range, 0.01-0.06 mM] but not in 0.14 M NaCl/10 mM sodium phosphate buffer, pH 7.2/0.5 mM CaCl2/0.15 mM MgCl2 (MBC50 range, 2.8-11.5 mM). Bactericidal activity resided in the core aspartate hexapeptide homopolymeric region, and MBC50 values of aspartate dipeptide-to-heptapeptide homopolymers were inversely proportional to the number of aspartate residues in the peptide. P. haemolytica incubated with H-DDDDDD-OH in zinc saline solution was killed within 30 min. Ultrastructurally, cells contained flocculated intracellular constituents. In contrast to cationic defensins and beta-defensins, surfactant-associated anionic peptides are smaller in size, opposite in charge, and are bactericidal in zinc saline solution. They are members of another class of peptide antibiotics containing aspartate, which when present in pulmonary secretions may help clear bacteria as a part of the innate pulmonary defense system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman H. G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Brogden K. A. Ovine pulmonary surfactant induces killing of Pasteurella haemolytica, Escherichia coli, and Klebsiella pneumoniae by normal serum. Infect Immun. 1992 Dec;60(12):5182–5189. doi: 10.1128/iai.60.12.5182-5189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle A. J., Uchida D., Ackerman S. J., Mitzner W., Irvin C. G. Role of cationic proteins in the airway. Hyperresponsiveness due to airway inflammation. Am J Respir Crit Care Med. 1994 Nov;150(5 Pt 2):S63–S71. doi: 10.1164/ajrccm/150.5_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- Diamond G., Jones D. E., Bevins C. L. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G., Zasloff M., Eck H., Brasseur M., Maloy W. L., Bevins C. L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Boose D., LaForce F. M. Isolation of an antibacterial peptide from human lung lavage fluid. J Infect Dis. 1985 Jun;151(6):1123–1129. doi: 10.1093/infdis/151.6.1123. [DOI] [PubMed] [Google Scholar]
- Funkhouser J. D., Tangada S. D., Peterson R. D. Ectopeptidases of alveolar epithelium: candidates for roles in alveolar regulatory mechanisms. Am J Physiol. 1991 Jun;260(6 Pt 1):L381–L385. doi: 10.1152/ajplung.1991.260.6.L381. [DOI] [PubMed] [Google Scholar]
- Ganz T., Lehrer R. I. Defensins. Curr Opin Immunol. 1994 Aug;6(4):584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Defensins. Eur J Haematol. 1990 Jan;44(1):1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Shiffer K. Structures and properties of the surfactant-associated proteins. Annu Rev Physiol. 1991;53:375–394. doi: 10.1146/annurev.ph.53.030191.002111. [DOI] [PubMed] [Google Scholar]
- LaForce F. M., Boose D. S. Effect of zinc and phosphate on an antibacterial peptide isolated from lung lavage. Infect Immun. 1984 Sep;45(3):692–696. doi: 10.1128/iai.45.3.692-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Selsted M. E., Szklarek D., Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983 Oct;42(1):10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D., Rayner J., Couto M., Ganz T. Cationic defensins arise from charge-neutralized propeptides: a mechanism for avoiding leukocyte autocytotoxicity? J Leukoc Biol. 1992 Jun;51(6):634–639. doi: 10.1002/jlb.51.6.634. [DOI] [PubMed] [Google Scholar]
- Prasad A. S., Oberleas D. Binding of zinc to amino acids and serum proteins in vitro. J Lab Clin Med. 1970 Sep;76(3):416–425. [PubMed] [Google Scholar]
- Schlievert P., Johnson W., Galask R. P. Isolation of a low-molecular-weight antibacterial system from human amniotic fluid. Infect Immun. 1976 Nov;14(5):1156–1166. doi: 10.1128/iai.14.5.1156-1166.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Lehrer R. I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983 Dec 10;258(23):14485–14489. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S. Purification, primary structure, and antimicrobial activities of a guinea pig neutrophil defensin. Infect Immun. 1987 Sep;55(9):2281–2286. doi: 10.1128/iai.55.9.2281-2286.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Golde L. M., Batenburg J. J., Robertson B. The pulmonary surfactant system: biochemical aspects and functional significance. Physiol Rev. 1988 Apr;68(2):374–455. doi: 10.1152/physrev.1988.68.2.374. [DOI] [PubMed] [Google Scholar]
- Wilde C. G., Griffith J. E., Marra M. N., Snable J. L., Scott R. W. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J Biol Chem. 1989 Jul 5;264(19):11200–11203. [PubMed] [Google Scholar]



