Abstract
Dural ateriovenous fistula (DAVF) at the craniocervical junction is rare. We report a patient presenting with brainstem dysfunction as an uncommon onset. Brainstem lesion was suggested by magnetic resonance image study. Angiogram revealed a DAVF at a high cervical segment supplied by the meningeal branch of the right vertebral artery, with ascending and descending venous drainage. Complete obliteration of the fistula was achieved via transarterial Onyx embolization. Clinical cure was achieved in the follow-up period; meanwhile, imaging abnormalities of this case disappeared. Accordingly, we hypothesize that a brainstem lesion of this case was caused by craniocervical DAVF, which induced venous hypertension. Thus, venous drainage patterns should be paid attention to because they are important for diagnosis and theraputic strategy.
Keywords: Dural arteriovenous fistula, Brainstem dysfunction, Diagnosis, Venous congestion
INTRODUCTION
Spinal dural ateriovenous fistula (SDAVF) is the lesion within dural leaflets due to direct arterial-to-venous shunt. Dural ateriovenous fistula (DAVF) at the craniocervical junction is rare, as an uncommon type of SDAVF, which has been documented in sevral case reports2,3,4,8). Cases usually present with subarachnoid hemorrhage (SAH) or myelopathy. We report a patient who presented brainstem dysfunction due to DAVF at the craniocervical junction that was cured by transarterial Onyx embolization. It is a very rare case when symptoms are caused by abnomal venous drainage. On the other hand, Onyx embolization for DAVF in this area has rarely been described. Drainage patterns should be analyzed because they are important for diagnosis and theraputic strategy. However, there are few reports regarding drainage patterns for this rare complication.
CASE REPORT
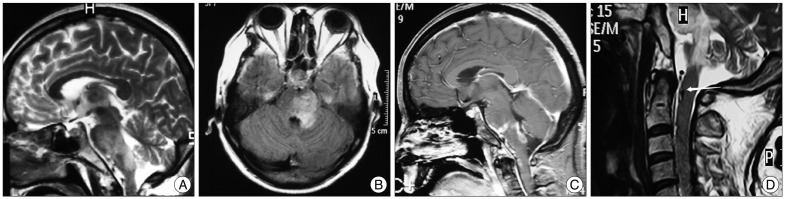
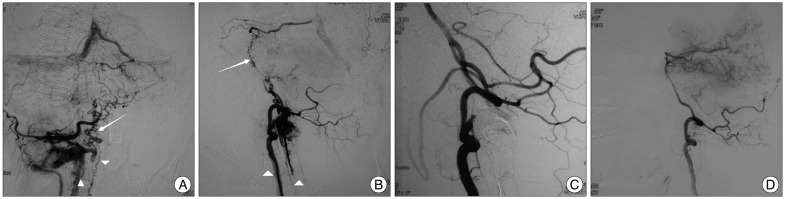
A 46-year-old female patient presented with a history of one month's duration of vertigo, gait, nausea, vomiting and dysphagia, which developed progressively. Head computered tomography scan revealed a low density lesion at the brainstem. She was referred for a brain MRI study with a preliminary diagnosis of brainstem infarction in a local hospital. T2-weight MRI and FLAIR image showed high signal intensity swelling from pons to medulla oblongata (Fig. 1A, B). Low signal intensity and partial enhancement in the same territory were detected on T1-weight and contrast enhanced imagings (Fig. 1C). The patient was transfered to our hospital. Neurologic examination revealed attenuation of left-side gag reflex and right-side nasolabial groove. Tongue extension moved to the right and muscular strength of left limbs was IV in this case. Left-side finger-to-nose test was postive. Spinal MRI disclosed abnormal flow void at ventral surface from craniocervical junction to cervical cord, suggesting engorged vessels (Fig. 1D). Cerebral angiography performed five days after admission revealed DAVF with a meningeal branch originated from the radicular artery of the right C2 segment of VA as a feeding vessel, draining via abnormally hypertrophic pontomesencephalic veins ascending into basal vein retrogradely and descending into the anterior spinal vein, anterior internal vertebral venous plexus and vertebral artery venous plexus (Fig. 2A, B). Arterial feeder originated from external carotid artery (ECA) was not disclosed from bilateral ECA angiography. A diagnosis of DAVF at the craniocervical junction with venous congestion of brainstem was made.
Fig. 1.
Sagittal T2-weight MRI (A) and FLAIR image (B) demonstrating abnormalities from pons to medulla oblongata. C : Sagittal contrast enhanced imaging demonstrating abnormal enhancing lesions located at pontobulbar junction, dorsal oblongata and craniocervical junction. D : Sagittal cervical T2-weight MRI demonstrating abnormal flow void (arrow) at ventral surface from craniocervical junction to cervical cord.
Fig. 2.
A and B : Right VA angiogram, posterior-anterior and lateral view, demonstrating DAVF with a meningeal branch originated from radicular artery of the right C2 segment of VA as a feeding vessel, draining via abnormally hypertrophic pontomesencephalic veins (arrows) ascending from basal vein retrogradely into straight sinus and descending into the anterior spinal vein, anterior internal vertebral venous plexus and vertebral artery venous plexus (arrowheads). C : Right VA angiogram immediately after embolization, lateral view, demonstrating complete obliteration of the fistula. D : Right VA angiogram six months after embolization, lateral view, demonstrating complete obliteration of the fistula and disappearance of abnormal venous drainage. VA : vertebral artery, DAVF : dural ateriovenous fistula.
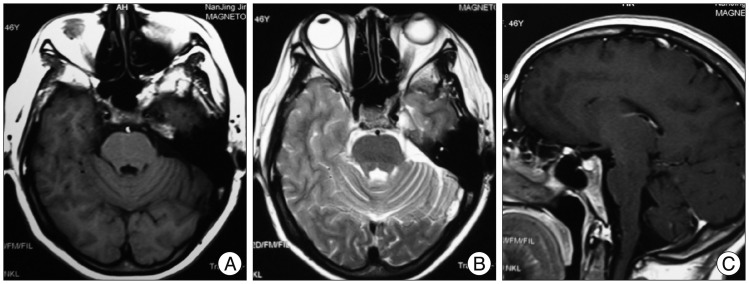
The patient underwent a transarterial endovascular embolization with Onyx-18 (ev3, Irvine, CA, USA). All procedures were performed under general anesthesia. A six-French sheath was introduced in the right-side femoral artery. Intravenous heparin was given before the guiding catheter was advanced to keep activated clotting time from 200 to 300 seconds. A six-French Envoy guiding catheter (Cordis Endovascular, Miami Lakes, FL, USA) and a Marathon microcatheter (ev3, Irvine, CA, USA) were used. Marathon catheter was advanced to the feeding artery. Onyx-18 was injected using real-time digital subtraction fluoroscopic mapping after the microcatheter lumen was flushed with 0.3 mL dimethyl sulfoxide (DMSO, ev3, Irvine, CA, USA). The injection was terminated when the angiogram confirmed complete obliteration of the fistula (Fig. 2C). Low-molecular-weight heparin (LMWH) injection, aspirin and clopidogrel were administrated after endovascular treatment. LMWH 4100 U, twice daily, was administrated for three days. Meanwhile, aspirin (100 mg) and clopidogrel (75 mg), once a day, were administrated for one month. There were not any therapy-related complications. Previous neurological deficits improved gradually after embolization. This patient has been followed-up for six months and recovered completely without any neurological deficits. Cerebral angiography performed at six months after embolization showed complete obliteration of the fistula and disappearance of abnormal venous drainage (Fig. 2D). MRI did not demostrate any abnormalities in the brainstem (Fig. 3).
Fig. 3.
Follow-up six-month brain MRI. Axial T1-weight imaging (A), axial T2-weight imaging (B), and contrast enhanced T1-weight imaging (C) demonstrating disappeared brainstem abnormal signals.
DISCUSSION
SDAVF is a common type of spinal vascular malformation in adult that usually affects the lumbar or lower thoracic spine. This is uncommon at the craniocervical junction. Review of the literature revealed that DAVFs occuring at the craniocervical junction had been called DAVFs at the foramen magnum and often present with progressive myelopathy or SAH2,3,4,8). Only one case in this anatomical area has been reported to involve brainstem dysfunction10). Brainstem dysfunction as an initial symptom has been documented in several cases of CCFs or intracranial DAVFs1,6,9,12,15,17,18). Congestion caused by venous hypertension was also considered as a probable pathological mechanism.
In here we describe a case presenting with brainstem dysfunction at onset. Symptoms developed progressively for one month. This onset was different to brainstem infarction whose progression is acute or subacute. An MRI study detected abnormal flow void, suggesting engorged vessels. A cerebral angiogram confirmed a DAVF at the craniocervical junction that was supplied by the meningeal branch originated from the radicular artery of the right VA with ascending and descending venous drainage. DAVF ought to be considered for these dysfunctions to avoid catastrophic outcomes such as brainstem hemorrhage or necrosis which might result from erroneous diagnosis followed by incorrect therapy8,11,15).
Brainstem venous congetion may be caused by elevated venous pressure secondary to arterial pressure via fistula. In previously reported cases of SDAVFs, three major anomalous venous drainage patterns were recognized : ascending, descending and undetected8,13). Ascending venous drainage into intracranial sinus was considered to account for SAH caused by venous hypertension. Moreover, a descending venous drainage was considered for myelopathy. Ascending along with descending venous drainage was detected in the present case, resulting in dysfunction due to brainstem congestion. The previous two cases of cervical DAVFs and one of lumbar spinal DAVF that presented brainstem dysfunctions demonstrated a similar ascending venous drainage (Table 1)10,19,20). The pathological mechanism is thought to involve blood flow of the venous drainage. Venous system of the brainstem is complicated because of the anastomosis between each other. Venous flow drains into the transverse sinus, the petrosal sinus and the straight sinus under normal physiological conditions14). In a retrograde ascending venous drainage system of the cervical DAVF if the venous flow increases precipitously, an elevated hemodynamic stress, varix formation or even hemorrhage may occur5,8). However, this patient and the other three cases reported with ascending drainage presented braistem dysfunction rather than hemorrhage (Table 1). We found that retrograde flow in each case was slow and the draining volume was not very high. Under this condition, normal brainstem venous drainage would be stagnant, resulting in congestion and edema, similarly to myelopathy caused by venous hypertension as it is seen in some cases of SDAVFs7). Therefore, the aim of treatment is to obliterate abnormal shunt and venous drainage as well as relieving venous congestion.
Table 1.
Summary of case reports of brainstem venous congestion due to SDAVF
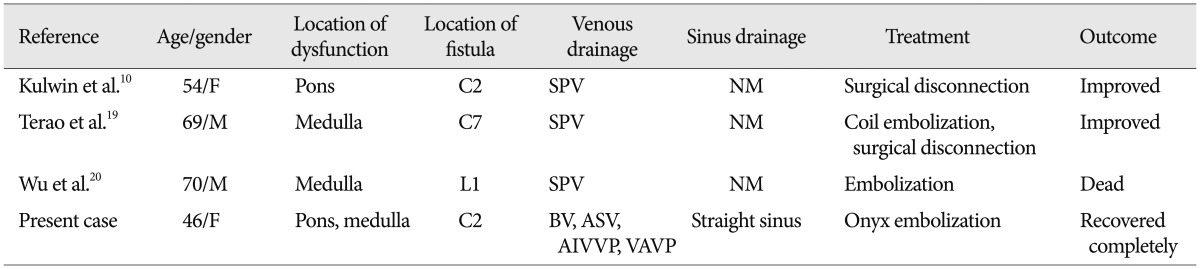
F : female, M : male, SPV : spinal perimedullary vein, BV : basal vein, ASV : anterior spinal vein, AIVVP : anterior internal vertebral venous plexus, VAVP : vertebral artery venous plexus, NM : not mentioned, SDAVF : spinal dural ateriovenous fistula
Onyx, as a novel liquid embolic agent, has being an alternative option in the treatment of cerebral and spinal vascular malformations16). Surgical approach would be difficult and at high risk when the fistula and draining veins are located at the ventral side of the foramen magnum. This patient was treated with endovascular treatment using transarterial Onyx embolization, which is safer and more controllable than former endovascular theraputic approaches. Onyx-18 was injected with a "hold-reinjection" technique to avoid glue migration to the proximal trunk of the feeding artery under real-time digital subtraction fluoroscopic mapping. Complete obliteration of the fistula was achieved; meanwhile, anomalous venous drainage disapeared. Anticoagulant and antiplatelet therapy were performed to prevent thrombosis caused by blood flow stasis in the arterialized drainage vein due to closure of a fistula. This patient recovered completely without any neurological deficits as determined during the follow-up period. Brainstem anomalous signals of T1-weight, T2-weight and contrast-enhanced T1-weight imaging had disapeared by the latest MRI study, which verified the lesion congestion rather than infarction.
CONCLUSION
Retrograde ascending venous drainage of the DAVF at the craniocervical junction, when venous flow is not high, may induce brainstem venous congestion. Congestion could be reversible if DAVF is considered as a differential diagnosis for brainstem dysfunction of undetermined origin and appropriate treatment was performed promptly to avoid poor outcomes. Moreover, onyx embolization is an alternative option for the treatment of DAVF at the craniocervical junction.
Acknowledgements
We thank Juan Pablo de Rivero Vaccari, Ph.D. for his revision about English grammar and vocabulary.
References
- 1.Bussière M, Lownie SP, Pelz DM, Nicolle D. Direct carotid-cavernous fistula causing brainstem venous congestion. J Neuroophthalmol. 2009;29:21–25. doi: 10.1097/WNO.0b013e31818e3db9. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Wang Q, Tian Y, Gu Y, Xu B, Leng B, et al. Dural arteriovenous fistulae at the craniocervical junction : the relation between clinical symptom and pattern of venous drainage. Acta Neurochir Suppl. 2011;110(Pt 2):99–104. doi: 10.1007/978-3-7091-0356-2_18. [DOI] [PubMed] [Google Scholar]
- 3.Fassett DR, Rammos SK, Patel P, Parikh H, Couldwell WT. Intracranial subarachnoid hemorrhage resulting from cervical spine dural arteriovenous fistulas : literature review and case presentation. Neurosurg Focus. 2009;26:E4. doi: 10.3171/FOC.2009.26.1.E4. [DOI] [PubMed] [Google Scholar]
- 4.Guo LM, Zhou HY, Xu JW, Wang GS, Tian X, Wang Y, et al. Dural arteriovenous fistula at the foramen magnum presenting with subarachnoid hemorrhage : case reports and literature review. Eur J Neurol. 2010;17:684–691. doi: 10.1111/j.1468-1331.2009.02895.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamada J, Yano S, Kai Y, Koga K, Morioka M, Ishimaru Y, et al. Histopathological study of venous aneurysms in patients with dural arteriovenous fistulas. J Neurosurg. 2000;92:1023–1027. doi: 10.3171/jns.2000.92.6.1023. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki M, Murakami K, Tomita T, Numagami Y, Nishijima M. Cavernous sinus dural arteriovenous fistula complicated by pontine venous congestion. A case report. Surg Neurol. 2006;65:516–518. doi: 10.1016/j.surneu.2005.06.044. discussion 519. [DOI] [PubMed] [Google Scholar]
- 7.Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas : a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129(Pt 12):3150–3164. doi: 10.1093/brain/awl220. [DOI] [PubMed] [Google Scholar]
- 8.Kai Y, Hamada J, Morioka M, Yano S, Mizuno T, Kuratsu J. Arteriovenous fistulas at the cervicomedullary junction presenting with subarachnoid hemorrhage : six case reports with special reference to the angiographic pattern of venous drainage. AJNR Am J Neuroradiol. 2005;26:1949–1954. [PMC free article] [PubMed] [Google Scholar]
- 9.Kai Y, Hamada JI, Morioka M, Yano S, Ushio Y. Brain stem venous congestion due to dural arteriovenous fistulas of the cavernous sinus. Acta Neurochir (Wien) 2004;146:1107–1111. doi: 10.1007/s00701-004-0315-3. discussion 1111-1112. [DOI] [PubMed] [Google Scholar]
- 10.Kulwin C, Bohnstedt BN, Scott JA, Cohen-Gadol A. Dural arteriovenous fistulas presenting with brainstem dysfunction : diagnosis and surgical treatment. Neurosurg Focus. 2012;32:E10. doi: 10.3171/2012.2.FOCUS1217. [DOI] [PubMed] [Google Scholar]
- 11.Lin CH, Lo YK, Lin YT, Li JY, Lai PH, Gau YY. Central vasomotor failure in a patient with medulla arteriovenous fistula. Acta Neurol Taiwan. 2006;15:192–196. [PubMed] [Google Scholar]
- 12.Murata H, Kubota T, Murai M, Kanno H, Fujii S, Yamamoto I. Brainstem congestion caused by direct carotid-cavernous fistula--case report. Neurol Med Chir (Tokyo) 2003;43:255–258. doi: 10.2176/nmc.43.255. [DOI] [PubMed] [Google Scholar]
- 13.Park KW, Park SI, Im SB, Kim BT. Spinal dural arteriovenous fistula with supply from the lateral sacral artery-case report and review of literature- J Korean Neurosurg Soc. 2009;45:115–117. doi: 10.3340/jkns.2009.45.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhoton AL., Jr The posterior fossa veins. Neurosurgery. 2000;47(3 Suppl):S69–S92. doi: 10.1097/00006123-200009001-00012. [DOI] [PubMed] [Google Scholar]
- 15.Shintani S, Tsuruoka S, Shiigai T. Carotid-cavernous fistula with brainstem congestion mimicking tumor on MRI. Neurology. 2000;55:1929–1931. doi: 10.1212/wnl.55.12.1929. [DOI] [PubMed] [Google Scholar]
- 16.Stiefel MF, Albuquerque FC, Park MS, Dashti SR, McDougall CG. Endovascular treatment of intracranial dural arteriovenous fistulae using Onyx : a case series. Neurosurgery. 2009;65(6 Suppl):132–139. doi: 10.1227/01.NEU.0000345949.41138.01. discussion 139-140. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi S, Tomura N, Watarai J, Mizoi K, Manabe H. Dural arteriovenous fistula of the cavernous sinus with venous congestion of the brain stem : report of two cases. AJNR Am J Neuroradiol. 1999;20:886–888. [PMC free article] [PubMed] [Google Scholar]
- 18.Teng MM, Chang T, Pan DH, Chang CN, Huang CI, Guo WY, et al. Brainstem edema : an unusual complication of carotid cavernous fistula. AJNR Am J Neuroradiol. 1991;12:139–142. [PMC free article] [PubMed] [Google Scholar]
- 19.Terao T, Taniguchi M, Ide K, Shinozaki M, Takahashi H. Cervical dural arteriovenous fistula presenting with brainstem dysfunction : case report and review. Spine (Phila Pa 1976) 2006;31:E722–E727. doi: 10.1097/01.brs.0000232701.53604.4e. [DOI] [PubMed] [Google Scholar]
- 20.Wu SL, Tsui HW, Chuang YC, Kuo HC, Chen CJ. Lumbar spinal dural arteriovenous fistula presenting with brainstem symptomatology : a case report. Acta Radiol. 2006;47:730–732. doi: 10.1080/02841850600767732. [DOI] [PubMed] [Google Scholar]