Abstract
The bulk of AD research during the last twenty-five years has been Aβ-centric based on a strong faith in the Amyloid Cascade Hypothesis which is not supported by the data on humans. To date, Aβ-based therapeutic clinical trials on sporadic cases of AD have been negative. Although most likely the major reason for the failure is that Aβ is not an effective therapeutic target for sporadic AD, initiation of the treatment at mild to moderate stages of the disease is blamed as too late to be effective. Clinical trials on presymptomatic familial AD cases have been initiated with the logic that Aβ is a trigger of the disease and hence initiation of the Aβ immunotherapies several years before any clinical symptoms would be effective. There is an urgent need to explore targets other than Aβ. There is now increasing interest in inhibiting tau pathology, which does have a far more compelling rationale than Aβ. AD is multifactorial and over 99% of the cases are the sporadic form of the disease. Understanding of the various etiopathogenic mechanisms of sporadic AD and generation of the disease-relevant animal models are required to develop rational therapeutic targets and therapies. Treatment of AD will require both inhibition of neurodegeneration and regeneration of the brain.
Keywords: Aβ, Abnormal hyperphosphorylation of tau, Plaques, Neurofibrillary tangles, Protein Phosphatase-2A, Neuroregeneration, tauopathies
1. Introduction
Alzheimer Disease (AD), which is defined by dementia associated with numerous Aβ plaques and phosphotau neurofibrillary tangles in the brain, especially the hippocampus, is a heterogeneous and a multifactorial disorder [1]. Neither Aβ plaques nor phosphotau neurofibrillary tangles are unique to AD. As many as ~30% of normal aged people have as many Aβ plaques in their brains as in typical cases of AD [2, 3]. Furthermore, in cases of hereditary cerebral hemorrhage with amyloidosis of Dutch origin (HCHWA-D) and sporadic cerebral amyloid angiopathy (SCAA) there is extensive β-amyloidosis in the absence of neurofibrillary tangles [4, 5]. Neurofibrillary tangles of hyperphosphorylated tau is a hallmark of several neurodegenerative diseases called tauopathies which include frontotemporal dementia with Parkinsonism linked to chromosome-17 tau (FTDP-17), Pick disease, cortico-basal degeneration, post-supranuclear palsy, dementia pugilistica/traumatic brain injury/chronic traumatic encephalopathy and Guam Parkinsonism dementia complex. Thus, several different mechanisms are involved in the etiopathogenesis of both plaques and tangles.
In less than 1% of the cases, AD is caused by specific point mutations in amyloid β precursor protein, presenilin-1, or presenilin-2 [6]. All of these three are transmembrane proteins. Mutations in these proteins probably lead to Aβ and tau pathologies by altering the signal transduction, especially involving protein phosphatase-2A (PP2A) and glycogen synthase kinase-3β (GSK-3β) [7]. The remaining over 99% of the AD cases represent the sporadic form of the disease. The exact causes of sporadic AD are not yet established. The presence of one or two APOβ4 alleles increases by ~3.5- and ~10-fold the risk for the disease, respectively, and is generally seen in late onset AD cases [see 8]. Despite the evidence for the multifactorial nature of AD and the involvement of several different mechanisms, because of the immense popularity of the Amyloid Cascade Hypothesis according to which Aβ causes AD, to date most of the therapeutic efforts have been focused on inhibition and removal of Aβ plaques. However, none of these treatments have so far shown any improvement or even reduction in the rate of cognitive impairment. In this article we discuss the likely reasons for the failure of the Aβ-based therapies, and why the focus of the future therapeutic attempts has to be the disease and not just the plaques and tangles. The weaknesses of Aβ as a therapeutic target was also discussed previously [e.g., 9, 10, 11]
2. Plaques and tangles: loss of functions or gain of toxic functions or both
Plaques are extracellular deposits mainly composed of Aβ1-40 and Aβ1-42 which are the metabolites of amyloid precursor protein (APP) generated by its β- and β-secretase cleavage [12-14]. The number of neurofibrillary tangles but not Aβ plaques has been found to correlate with dementia [2, 15, 16]. APP is a transmembrane protein. Its main function is probably synaptic formation and repair [17]. Consistent with its critical role in the maintenance of membrane, APP level is upregulated during neuronal differentiation [18].
APP expression is rapidly upregulated during neural injury, probably to repair the damaged tissue [19]. The APP expression is probably also increased in response to certain genetic, biological, chemical and other environmental insults, all resulting in increased metabolism and production of Aβ. Aβ, though amyloidogenic, is a normal metabolite of APP. Aβ is catabolized by neprilysin and insulin degrading enzyme [20]. An imbalance between the rate of production and clearance of Aβ leads to its deposition as amyloid plaques. APOE and certain other interacting molecules such as heparin sulfates may promote Aβ polymerization in the form of plaques. According to the Amyloid Cascade Hypothesis amyloid β causes neurofibrillary pathology and the disease [21]. The bulk of the studies, however, suggest soluble, especially the oligomeric, Aβ as the main neurotoxic state of the peptide [22]. Thus, it appears that aggregation of Aβ into fibrils could be a neuroprotective response by which the soluble/oligomeric Aβ is packaged by the affected brain into a relatively inert mass. Furthermore, the neurotoxic concentrations of soluble and oligomeric Aβ1-42 in cultured cells are in micromolar whereas its in vivo concentrations seen in the AD brain are in picomolar range.
Despite the evidence for neurotoxicity of Aβ peptide in cultured cells and in vivo in mice and rats reported by several studies [see 23], as many as ~30% of the normal aged humans have as much Aβ plaque load but without corresponding tau pathology in their brains as in typical cases of AD. The brains of cases with hereditary cerebral hemorrhage of the Dutch type show severe Aβ plaque load as congophilic angiopathy but without any tau pathology and dementia [4]. Furthermore, several familial AD presenilin 1 mutations do not result in any increase in Aβ [24]. Thus, the AD-causing APP mutations most likely involve primarily loss of APP function in AD. The mutated APP is unable to maintain synaptogenesis and repair the degenerating synapses; loss of synaptic plasticity precedes any overt Aβ pathology in AD and in transgenic mouse models of AD [25-27].
Tau is the major neuronal microtubule associated protein (MAP). In normal brain tau contains 2–3 moles phosphate per mole of the protein whereas in AD brain it is 3–4-fold hyperphosphorylated [28]. Tau is the major protein subunit of paired helical filaments which make the neurofibrillary tangles [29, 30a]. Tau in neurofibrillary tangles is abnormally hyperphosphorylated [31b]. As much as ~40% of the abnormally hyperphosphorylated tau in AD brain is cytosolic [28, 32].
Normal tau interacts with tubulin and promotes its assembly into microtubules and stabilizes their structure. This biological activity of tau is regulated by its degree of phosphorylation; hyperphosphorylation suppresses its microtubule assembly promoting activity [33]. In AD brain the cytosolic abnormally hyperphosphorylated tau (AD P-tau) instead of interacting with tubulin, binds to normal tau and thereby inhibits the microtubule assembly [28, 34]. Abnormally hyperphosphorylated tau isolated from AD brains sequesters not only normal tau but also the other two neuronal MAPs, MAP1 and MAP2, and disrupts microtubules in vitro [35-37]. While normal tau labels the microtubule network, the AD abnormally hyperphosphorylated tau disrupts it in permeabilized cells in vitro [38]. In vitro dephosphorylation of AD P-tau with protein phosphatase rescues its ability to inhibit microtubule assembly and disrupt the microtubule network [37, 39, 40].
The AD P-tau readily self-assembles into paired helical filaments and its dephosphorylation with protein phosphatase inhibits this aggregation in vitro [40, 41]. While normal tau promotes GTP binding to tubulin and AD P-tau inhibits it, the paired helical filaments have no activity [42]. Unlike AD P-tau, paired helical filaments/neurofibrillary tangles have no effect on microtubule assembly but dephosphorylation of neurofibrillary tangles with protein phosphatases, especially protein phosphatase-2A (PP2A) dissociate the fibrils and the released dephosphorylated protein behaves like normal tau in promoting microtubule assembly [43]. Similarly the in vitro dephosphorylated AD P-tau neither self-assembles nor inhibits but now, instead promotes microtubule assembly [39, 40]. Thus, collectively these findings suggest that in AD the abnormal hyperphosphorylation of tau results in both the loss of normal function and the gain of toxic function.
2.1 Oligomerization and spread of tau pathology
Unlike normal tau, the AD P-tau forms oligomers and as a result sediments at 100,000 to 200,000 × g [28, 34]. The sequestration of normal tau by the AD P-tau is non-saturable and the oligomers so formed lead to their aggregation into filaments [36]. The fine structure and the cytotoxic function of tau oligomers, also called granular tau, has been characterized by Takashima's lab [see 44]. An in vivo confirmation of this seeding of tau pathology was provided by its transmission by intracranial injection of brain extract containing tau filaments from P301S transgenic mice to wild type human tau overexpressing transgenic mice [45, 46]. The nature of tau oligomers, which is probably determined by tau isoform, mutation, hyperphosphorylation and other posttranslational modifications including truncation, characterizes the structure of the tau lesions. Brain homogenates from different human tauopathies used for the in vivo transmission showed the signature tau lesions of the donor brains in the recipient wild type human tau overexpressing transgenic and in non-transgenic mice. Moreover, the tau pathology could be propagated between mouse brains, suggesting a self-propagating behavior of the pathological tau [47]. Expression of P301L mutated human tau in the entorhinal cortex showed the spread of tau pathology in a trans-synaptic manner from entorhinal cortex to limbic and association cortices [48, 49].
All these experimental studies are consistent with the known hierarchical pattern of neurofibrillary pathology in AD [50]. However, in many aged individuals there are numerous neurons with neurofibrillary tangles in the entorhinal cortex and this tau pathology does not spread beyond this region of the brain. Thus, at present it is not clear whether the spread of tau pathology from entorhinal cortex to the limbic region and then to isocortices is spread by simply tau oligomers as shown by Clavaguera et al. [45, 47] in transgenic mice or is mediated by a signal that leads to abnormal hyperphosphorylation of tau which could be transmitted trans-synaptically and/or by the extracellular pathological tau [51, 52]. Alternatively, the human brain could be much more efficient than the rodent brain in dephosphorylating and or degrading the pathological tau oligomers (seeds) and thus, in some individuals the tau pathology may not spread to other regions of the brain causing AD. This could also explain why, unlike in transgenic mice, in AD brain it takes many years for the progression of the tau pathology from entorhinal cortex to the limbic area and isocortices.
3. Etiopathogenesis of neurofibrillary degeneration
3.1 Imbalance between tau protein kinase and phosphatase activities
Tau has 80 Ser/Thr residues which can be phosphorylated and about 50% of them are followed by Pro. Thus, tau is a substrate for several protein kinases which include both proline-directed protein kinases (PDPKs) such as cdk5, GSK-3β, and Dyrk1A, and non-PDPKs such as protein kinase A, calcium, calmodulin activated protein kinase II (CaMKII) and casein-kinase I [53-57]. Thus, more than one combination of protein kinases can produce abnormal hyperphosphorylation of tau [40]. Phosphorylation of tau is mainly regulated by PP2A [58-61]. The activities of several of the tau kinases are regulated by PP2A. Thus, PP2A can regulate the phosphorylation of tau both directly and by inhibiting the activities of several tau protein kinases [62]. PP2A activity is compromised and is probably a cause of the abnormal hyperphosphorylation of tau in AD brain [61, 63, 64].
3.2 Mechanisms involved in familial and sporadic AD
Familial and sporadic AD are caused by different etiological factors and hence involve different upstream pathways. All three proteins, i.e., βAPP, presenilin-1 (PS1) and PS2, certain mutations in which cause AD, are transmembrane proteins. Although only some of these familial AD mutations lead to increase in the generation of Aβ whereas some produce either no significant change or even decrease in Aβ [24], most studies explain the pathology according to the Amyloid Cascade Hypothesis. We postulated that these mutations, probably through alteration in the molecular topology of the plasma and endoplasmic reticulum membranes, dysregulate the signal transduction, affecting downstream PP2A and GSK-3β activities [7]. The decrease in PP2A and increase in GSK-3β cause abnormal hyperphosphorylation of tau on one hand and through phosphorylation of βAPP lead to increase in its amyloidogenic processing on the other hand. In addition to producing tau and Aβ pathologies, the familial AD mutations compromise neuronal plasticity through affecting the expressions and activities of the neurotrophic factors and their receptors [65, 66]. The loss of neuronal plasticity precedes any overt Aβ and tau pathologies, both in AD and in transgenic mouse models of familial AD [26].
Sporadic AD is multifactorial. The exact causes of the disease are not yet established. APOε4 is a risk factor and not a cause of AD. One copy of APOε4 increases the risk by ~3.5 fold and those who inherit two copies of APOε4 have over 10-fold risk of suffering from AD [see 8]. APOε2 appears to nullify the effect of APOε4.
In sporadic AD the PP2A activity in the brain is compromised and is believed to be a cause of both tau and Aβ pathologies [39, 40, 43, 58a, 60, 64, 67, 68b]. PP2A activity can be downregulated by increase in the activities of its two endogenous inhibitors, I1PP2A and I2PP2A[69, 70] or by an increase in the demethylation or phosphotyrosinylation of its catalytic subunit PP2Ac [71-73]. Cerebral ischemia and hypoxia cause acidosis of the tissue that leads to the activation and release of the lysosomal enzyme asparaginyl endopeptidase (AEP) [74, 75]. AEP cleaves I PP2A2 at ASP175 into amino terminal I2NTF and carboxy terminal I2CTF fragments, both of which inhibit PP2A in the neuronal cytoplasm and consequently lead to both tau and Aβ pathologies directly and through activation of tau and APP protein kinases such as GSK-3β [74, 76]. Adeno-associated virus vector-mediated expression of I2NTF and I2CTF in the brain leads to tau and Aβ pathologies and cognitive impairment in rats [77, 78]. In the spinal cord the adeno-associated virus-mediated expression of I2CTF leads to hyperphosphorylation and proliferation of neurofilaments, aggregation and translocation of TDP-43 from the neuronal nucleus to the cytoplasm, increase in ubiquitin expression, loss of motor neurons, and marked motor dysfunction and hind leg paralysis in rats [79]. These findings for the first time provide an explanation and the molecular basis of the involvement of the cerebrovascular changes in AD and ALS and an etiopathogenic relationship between these two major neurodegenerative disorders.
Tau pathology can also result from environmental and endogenous toxins such as β-N-methylamino-L-alanine (BMAA). In Guam Parkinsonism dementia complex (Guam PDC) the PP2A activity is also compromised but due to an increase in the phosphotyrosinylation pTyr307 of PP2Ac (Arif et al. In review). The most probable cause of the increase in pTyr307 PP2Ac is the chronic exposure to BMAA. Brain levels of ~5 mM and ~1 mM have been reported in the postmortem brains of cases with Guam PDC and AD cases from North America, respectively [80, 81]. In primary mouse hippocampal neuronal cultures, metabolically active rat hippocampal slices and in vivo in rat brain, BMAA causes increase in pTyr307 PP2Ac through activating mGluR5 and inhibiting PP2A activity which leads to abnormal hyperphosphorylation of tau and neuronal degeneration (Arif et al. In review). Thus, two independent etiopathogenic mechanisms, one involving ischemia and hypoxia and the other involving an environmental factor and endogenous neurotoxin BMAA, lead downstream to inhibition of PP2A which leads to Alzheimer pathology and neurodegeneration.
3.3 Regulation of tau phosphorylation and aggregation by O-GlcNAcylation
Tau is also highly modified by O-GlcNAcylation, a dynamic posttranslational modification of a protein at Ser/Thr with O-linked β-N-acetylglucosamine (O-GlcNAc) [82, 83]. Five O-GlcNAcylation sites (Thr123, Ser208, Ser238, Ser400, and one site at Ser409, Ser412 or Ser413) of tau protein have been mapped to date [84-86]. O-GlcNAcylation modulates phosphorylation of tau. Inhibition of O-GlcNAcylation leads to hyperphosphorylation of tau both in cultured cells and in vivo in rodents [56, 83]. Consistent to that O-GlcNAcylation can serve as a sensor of intracellular glucose metabolism [87] and reduction of brain glucose metabolism was found to result in decreased O-GlcNAcylation and increased phosphorylation of tau [56, 83, 88]. Importantly, the global O-GlcNAcylation of proteins, especially of tau, is decreased probably as a result of impaired brain glucose metabolism, and the decrease in O-GlcNAcylation correlates to hyperphosphorylation of tau in AD brain [56]. Hyperphosphorylated tau protein purified from AD brains contains approximately five times less O-GlcNAc than normal tau [56]. A deficient glucose metabolism starts to occur before the onset of AD. Thus, it appears that tau pathology and neurodegeneration can be caused by impaired brain glucose metabolism via the down-regulation of tau O-GlcNAcylation in AD [56, 89].
O-GlcNAcylation may also inhibit tau oligomerization. In vitro studies have demonstrated that O-GlcNAcylation of the fourth microtubule-binding repeat of tau inhibits its self-aggregates [90]. O-GlcNAcylation appears to inhibit tau aggregation in vivo as well [91]. A role of O-GlcNAcylation in modulating proteotoxicity was recently reported in C. elegans models of human neurodegenerative diseases [89, 92]. Thus, decreased O-GlcNAcylation may promote tau-mediated neurodegeneration through abnormal hyperphosphorylation and oligomerization of tau.
3.4 Dysregulation of alternative splicing leading to tau pathology
There are six tau isoforms expressed in human central nervous system due to the alternative splicing of exons 2, 3 and 10 from its pre-mRNA. Exon 10 encodes the second microtubule-binding repeat and its alternative splicing generates tau isoforms with 3 or 4 microtubule binding repeats, named 3R-tau or 4R-tau, respectively [93, 94]. Adult human brain expresses approximately equal levels of 3R-tau and 4R-tau [95, 96]. More than half of FTDP-17 tau (FTDP-17 specifically characterized by tau pathology) associated mutations disrupt this balance and cause neurodegeneration [97, 98], suggesting 1:1 ratio of 3R-tau and 4R-tau is required for maintaining normal brain function. Discovery of the mutations that affect the alternative splicing of tau in FTDP-17 tau demonstrates that disruption of 3R-tau/4R-tau balance is sufficient to causes neurodegeneration and dementia. In addition to FTDP tau, alteration of 3R-tau/4R-tau ratio has been seen in other both familial and sporadic human neurodegenerative disorders, such as Pick disease (PiD) (3R-tau>4R-tau), progressive supranuclear palsy (PSP) (4R-tau>3R-tau), corticobasal degeneration (4R-tau>3R-tau), and Down syndrome (3R-tau>4R-tau) [57, 99, 100].
The exon 10 is flanked by unusually large intron 9 (13.6 kb) and intron 10 (3.8 kb) and has two weak splice sites, a weak 5' splice and a weak 3' splice site [101-103]. Alternative splicing of tau exon 10 is regulated by action of trans-acting proteins on cis-elements. Several splicing factors were found to regulate its alternative splicing by acting on different elements in exon 10 and intron 10. It is well known that Ser/Arg rich (SR) proteins, a family of splicing factors, play important roles in the alternative splicing [104]. ASF/SF2 and SC35 promote tau exon 10 inclusion by acting on SC35-like enhancer and poly-purine enhancer at 5’ end of exon 10 [57, 105, 106]. Several other splicing factors were found to work on stem loop of interface region of exon 10 and intron 10 and promote tau exon 10 inclusion [107-111]. The function of splicing factors is tightly regulated by their phosphorylation level. Several kinases have been found to phosphorylate SR proteins and regulate their function [112-117] . Upregulation of Dyrk1A, a tau kinase encoded by a gene located on Down syndrome critical region, suppresses tau exon 10 inclusion, resulting in an increased 3R-tau expression. Therefore, overexpression of Dyrk1A in Down syndrome due to increased gene dosage increases 3R-tau expression, and appears to contribute to earlier onset of tau pathology in this disease [57, 106, 118].
In addition to Dyrk1A, PKA and GSK-3β may also participate in the regulation of tau exon 10 splicing. PKA phosphorylates ASF, 9G8, and SC35 and modulates their function. Opposite to Dyrk1A, activation of PKA or overexpression of PKA catalytic subunits promotes tau exon 10 inclusion. Down-regulation of PKA in AD brain may lead to an increase in 3R-tau expression [119]. GSK-3β is a primary tau kinase and phosphorylates tau at multiple sites [120]. It was reported that GSK-3β interacts with SC35 and phosphorylates SC35-derived peptides. Inhibition of GSK-3β with LiCl promotes neuron to express 4R-tau [121]. Therefore, dysregulated tau exon 10 splicing could be corrected by modulating the function of splicing factors at protein expression or posttranslational level [122, 123].
4. Pathological features other than plaques and tangles in AD brain
A key feature of cerebral aging is the progressive slow loss of axonal and dendritic arborization and eventually loss of many neurons resulting in the shrinkage of the brain. This process of the loss of neuronal plasticity is markedly accelerated in those middle-aged to old-aged individuals who suffer from AD. A normal aged individual is estimated to lose ~0.5% of the brain mass/year as determined by longitudinal structured MRI studies [124]. This rate of loss of brain mass is ~5-fold higher in AD and during 7–10 years of the disease progression an AD patient may lose approximately 200–400 g of brain mass [125]. The neuronal loss is most marked in the hippocampus in AD. The affected brain responds to this loss by activating the dentate gyrus neurogenesis. However, due to the lack of the proper neurotrophic microenvironment in the AD hippocampus, the newborn cells are unable to differentiate into mature functional neurons as detected by the lack of mature MAP2 [126]. Thus. The process of the loss of neuronal/synaptic plasticity continues unstopped and clinically expressed as progressive dementia in AD patients.
5. Therapeutic attempts that failed and therapeutic approaches that look promising
To date most of the treatments tested in human clinical trials were Aβ-based drugs and they were unsuccessful. These therapies included both active and passive immunization to remove Aβ and inhibition of its generation or aggregation [see 11, 127]. At least in the case of active immunization, Aβ plaques were successfully cleared from the brains of AD patients but instead of any decrease in the rate of clinical deterioration, the treated patients showed even worse performance than the placebo-treated controls [128, 129]. Two Phase III clinical trials employing passive Aβ immunotherapy reduced Aβ pathology but failed to show any cognitive benefit [130; Eli Lilly Company Announcement, 2012]. Despite these failures, because of the immense popularity of the Amyloid Cascade Hypothesis, it was concluded that the treatment of mild to moderate AD was probably too late and that treatment of the prodromal stage of the disease was probably required. Based on this reasoning, two clinical trials, one on a large cohort of familial AD caused by a presenilin-1 mutation(s) in Colombia, South America and another in the U.S. and Europe on familial AD cases (the DiAN study) have been initiated. Moreover, a passive Aβ immunotherapy clinical trial, the Salnuzumab study which failed in mild to moderate patients, now has been initiated in only early-mild to mild cases. By 2016 we expect to learn the outcomes of these Aβ immunotherapies on prodromal to very early stages of AD.
We have to also consider the possibility that Aβ is not a useful drug target. The Amyloid Cascade Hypothesis which posits that Aβ causes AD by inducing neurofibrillary pathology and leads to neurodegeneration and dementia is deeply flawed (Fig. 1). There are at least as many as 30% of the normal aged people who have as much Aβ load in the form of plaques except lacking the dystrophic neurites with tau pathology in their brains as in typical cases of AD [see 131]. Both HCHWA-D, and SCAA are characterized by extensive Aβ deposits in the absence of neurofibrillary pathology [4, 5]. Conversely, all tauopathies except AD and Down syndrome are characterized by tau pathology in the absence of Aβ pathology and show dementia; cases of progressive supranuclear palsy with tau pathology localized in the brain stem show motor dysfunction. In contrast, the density of Aβ plaques does not correlate with dementia [2].
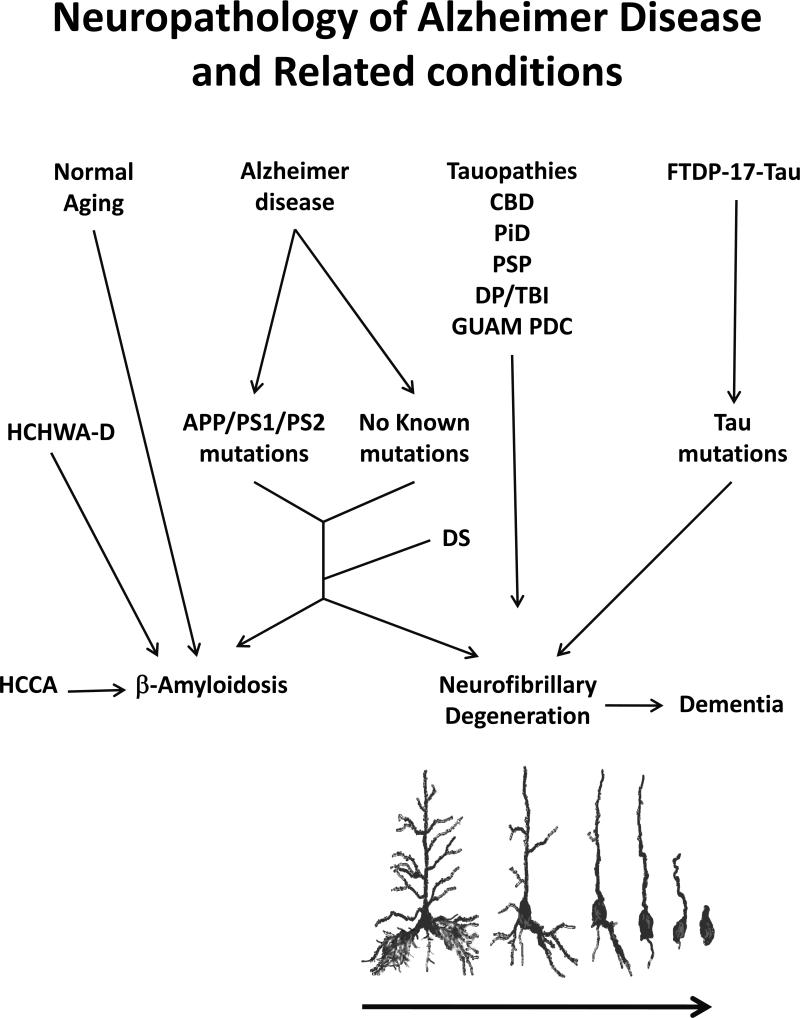
Figure 1. Neuropathology of Alzheimer disease (AD) and related conditions.
Both AD and adults with Down syndrome (DS) are neuropathologically characterized by β-amyloidosis and phosphotau neurofibrillary degeneration. While familial AD is caused by certain mutations in βAPP, presenilin 1 (PS1) and PS2 proteins, the exact causes of sporadic AD, which accounts for over 99% of the cases, are not yet established. Besides normal aged cases, around 30% of whom have as much Aβ plaque load in their brains as in a typical case of AD, extensive β-amyloidosis in the absence of neurofibrillary pathology is a hallmark of hereditary cerebral hemorrhage with amyloidosis of Dutch origin (HCHWA-D) and sporadic cerebral congophilic angiopathy (SCCA). Conversely, several tauopathies such as corticobasal degeneration (CBD), Pick disease (PiD), progressive supranuclear palsy (PSP), dementia pugilistica/traumatic brain injury (DP/TBI) and Guam Parkinsonism dementia complex (Guam PDC) are characterized by phosphotau neurofibrillary pathology in the absence of Aβ plaque. Moreover, several intronic and extronic mutations in tau gene in frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17 tau) cause phosphotau neurofibrillary pathology. Tau pathology in the neocortex in tauopathies is associated with dementia. Neurofibrillary degeneration is a slow chronic progressive process which is seen as retrograde degeneration and takes place over a period of several months to years.
Despite these human brain data that are completely inconsistent with the Amyloid Cascade Hypothesis, several labs keep interpreting their data from transgenic mouse models to fit the hypothesis. For instance, crossing mutated APP overexpression transgenic mice with tau knockout mice which attenuated cognitive deficit was interpreted solely on the Amyloid Cascade Hypothesis line that Aβ-induced neurotoxicity and thus the disease required tau [132]. The very same data could also be due to the fact that mutated APP results in an increase in GSK-3 activity probably due to attenuation of the PI3-AKT-GSK-3 signaling pathway which leads to both tau pathology and Aβ deposits, and that it is the tau hyperphosphorylation and not the Aβ pathology which causes cognitive impairment in the mutated βAPP transgenic mice [133-135].
Probably there are several different etiopathogenic mechanisms of the formation of Aβ deposits. APP is a rapid stress response protein, and age-associated oxidative stress and other factors involving the accumulated effect of environmental toxins probably leads to an imbalance between the production and the clearance of Aβ. In familial AD, because of mutations in the transmembrane proteins, APP, presenilin (PS)-1 and PS-2, and in sporadic AD, probably because of dysregulation of neurotrophic and other factors such as ischemia and hypoxia, the signal transduction is altered. The resulting downstream imbalance between protein kinase and protein phosphatase activities on one hand leads to abnormal hyperphosphorylation of tau, leading to neurofibrillary degeneration, and on the other hand to an increase in the amyloidogenic processing of APP such as due to its phosphorylation by GSK-3 at Thr668 and increase in β-secretase activity [136-139].
A potentially more serious aspect of Aβ pathology which has received relatively little attention in the AD field is the congophilic angiopathy. In the cerebral blood vessels the deposition of Aβ as plaques can cause hypoperfusion of the brain and lead to hypoxia and ischemia of the brain. Ischemic changes in the brain can lead to the release and activation of asparaginyl endopeptidase from the neuronal lysosomes to the cytoplasm [74, 75]. The asparaginyl endopeptidase in the neuronal cytoplasm causes the cleavage and the translocation of the inhibitor-2 of protein phosphatase-2A and consequently the abnormal hyperphosphorylation of tau [74].
The failure of Aβ-based clinical trials for therapy of AD has now shifted attention to other drug targets, especially tau. To date two Phase II clinical trials for tau-based therapies have been reported. One trial employed methylene blue as an inhibitor of tau aggregation (Remblar Tau Rx, UK and Singapore). For reasons unknown, the low dose Remblar (60 mg) showed some beneficial effect but the higher dose (100 mg) was non-effective and no trial results have been reported in the literature. At present this compound in a new formulation is in Phase III clinical trial for AD. A clinical trial employing a small molecule inhibitor of GSK-3β in Phase II clinical trials of both progressive supranuclear palsy and AD were negative. This failure is suspected to be due to the low dose of the drug which, because of its toxicity in liver and kidney, could not be tested at a dose that can significantly inhibit GSK-3β activity; full posthoc data are awaited.
Like Aβ immunotherapy, active immunization with tau phosphopeptides has been reported to successfully remove tau aggregates and improve neurobehavior in various mutated tau overexpression transgenic mice [140-142]. Active immunization with normal full-length human tau was found to produce AD-like pathology and encephalomyelitis in C57/BL6 mice [143]. Passive immunotherapy for tau has also been shown to successfully reduce tau pathology and improve/rescue neurobehavioral deficits in tau overexpression transgenic mice [144, 145]. Axon Neuroscience, Vienna, Austria, is currently conducting a Phase I clinical trial for the development of an active tau immunization-based vaccine. Although the initial report on tau immunotherapy reported the take up of the IgG in the affected neurons [140], several studies observed the presence of tau in the extracellular space [146] and the spread of tau pathology through this pool of the protein [45, 48, 49, 51, 52, 147, 148]. If the extracellular tau is the culprit, then the tau immunotherapy should be effective.
Inhibition of abnormal hyperphosphorylation of tau remains one of the most promising therapeutic approaches to AD and related tauopathies. PP2A is the major regulator of tau phosphorylation [58-61]. In the case of tau protein kinases more than one combination of non-PDPKs and PDPKs can abnormally hyperphosphorylate tau [40]. Thus, a rescue of PP2A activity which is compromised in AD brain [63, 64, 149] is one of the most attractive drug targets. A cause of decrease in PP2A activity is the cleavage and translocation of its inhibitor I2PP2A [69]. Direct inhibition of I2PP2A or asparaginyl endopeptidase that causes its cleavage and translocation are attractive drug targets. Reduction of pTyr307 PP2Ac by antagonizing mGluR5 or inhibiting Src activity are targets for Guam PD, AD and ALS cases involving inhibition of PP2A due to increase in pTyr307 PP2Ac. Reduction of tau hyperphosphorylation by inducing increase in its O-GlcNAcylation is another promising strategy; increase in O-GlcNAcylation by inhibiting O-GlcNAcylase, the enzyme that hydrolyzes and removes the O-GlcNAc from proteins is currently in early clinical trials [91]. Rescue of dysregulated exon 10 splicing by modulation of the splicing factors at protein expression or posttranslational modification level is another therapeutic approach. The use of microtubule stabilizing drugs to rescue microtubule network disruption is in Phase II clinical trial by Bristol Myer Squibb (The Epithelon drug trial).
The treatment of AD patients along with inhibition of neurodegeneration will also require neural regeneration. After all, the AD brain suffers from unsuccessful neurogenesis and a very marked loss of neuronal/synaptic plasticity and these deficits even precede overt Aβ and tau pathologies. One of the most promising approaches for neural regeneration is the development of neurotrophic compounds that can provide the biochemical microenvironment conducive to successful neurogenesis and rescue of neuronal plasticity. Peptidergic neurotrophic compounds based on ciliary neurotrophic factor (CNTF) and brain derived neurotrophic factor (BDNF) are among the most promising drug candidates [150-152]. A CNTF peptidergic compound was found to successfully rescue the dentate gyrus neurogenesis and rescue neuronal/synaptic plasticity in aged mice [151, 153, 154], in a 3xTg-AD transgenic mouse model of AD [26], in an AAV1-I2NTF-CTF rat model of sporadic AD [77], and in Ts65Dn trisomic Down syndrome mouse model [155]. In all these studies the chronic treatment with CNTF peptidergic compounds showed a significant improvement in cognitive performance and no side effects were found. Similarly, the development of compounds that can modulate BDNF [150] and direct administration of BDNF showed neuroprotective effects and improvement in cognitive performance in several transgenic mouse models and in non-human primates [156]. Thus, a combination of drugs that can inhibit neurodegeneration of the AD type and drugs that can stimulate neural regeneration of the affected brain has to be the future direction to intervene and treat AD and related neurodegenerative conditions.
Acknowledgements
We thank Dr. Ezzat El-Akkad for the preparation of Figure 1 and Ms. Janet Murphy for secretarial assistance. Studies reviewed in this article from our labs were supported in part by the New York State Office of People with Developmental Disabilities, NIH grant AG019158, FIRCA Award TW008744, Zenith Award ZEN-12-241433 from Alzheimer's Association, Chicago, IL, and grant #20121203 from the Alzheimer's Drug Discovery Foundation, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iqbal K, Flory M, Khatoon S, Soininen H, Pirttila T, Lehtovirta M, et al. Subgroups of Alzheimer's disease based on cerebrospinal fluid molecular markers. Ann Neurol. 2005;58:748–57. doi: 10.1002/ana.20639. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–42. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 3.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, et al. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–89. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 4.van Duinen SG, Castano EM, Prelli F, Bots GT, Luyendijk W, Frangione B. Hereditary cerebral hemorrhage with amyloidosis in patients of Dutch origin is related to Alzheimer disease. Proc Natl Acad Sci USA. 1987;84:5991–4. doi: 10.1073/pnas.84.16.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coria F, Castano EM, Frangione B. Brain amyloid in normal aging and cerebral amyloid angiopathy is antigenically related to Alzheimer's disease beta-protein. Am J Pathol. 1987;129:422–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65:664–70. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal K, Grundke-Iqbal I. Metabolic/signal transduction hypothesis of Alzheimer's disease and other tauopathies. Acta Neuropathol (Berl) 2005;109:25–31. doi: 10.1007/s00401-004-0951-y. [DOI] [PubMed] [Google Scholar]
- 8.Roses AD. On the discovery of the genetic association of Apolipoprotein E genotypes and common late-onset Alzheimer disease. J Alzheimers Dis. 2006;9:361–6. doi: 10.3233/jad-2006-9s340. [DOI] [PubMed] [Google Scholar]
- 9.Neve RL, Robakis NK. Alzheimer's disease: a re-examination of the amyloid hypothesis. TrendsNeurosci. 1998;21:15–9. doi: 10.1016/s0166-2236(97)01168-5. [DOI] [PubMed] [Google Scholar]
- 10.Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH. Amyloid-independent mechanisms in Alzheimer's disease pathogenesis. J Neurosci. 2010;30:14946–54. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacobini E, Gold G. Alzheimer disease therapy-moving from amyloid-beta to tau. Nat Rev Neurol. 2013 doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 12.Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 13.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–6. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 15.Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B. Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol (Berl) 1987;74:209–25. doi: 10.1007/BF00688184. [DOI] [PubMed] [Google Scholar]
- 16.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 17.Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–21. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts GW, Gentleman SM, Lynch A, Graham DI. beta A4 amyloid protein deposition in brain after head trauma. Lancet. 1991;338:1422–3. doi: 10.1016/0140-6736(91)92724-g. [DOI] [PubMed] [Google Scholar]
- 20.Miners JS, Barua N, Kehoe PG, Gill S, Love S. Abeta-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol. 2011;70:944–59. doi: 10.1097/NEN.0b013e3182345e46. [DOI] [PubMed] [Google Scholar]
- 21.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 22.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shioi J, Georgakopoulos A, Mehta P, Kouchi Z, Litterst CM, Baki L, et al. FAD mutants unable to increase neurotoxic Abeta 42 suggest that mutation effects on neurodegeneration may be independent of effects on Abeta. J Neurochem. 2007;101:674–81. doi: 10.1111/j.1471-4159.2006.04391.x. [DOI] [PubMed] [Google Scholar]
- 25.Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:167–79. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard J, Wanka L, Tung YC, Cardenas-Aguayo Mdel C, LaFerla FM, Iqbal K, et al. Pharmacologic reversal of neurogenic and neuroplastic abnormalities and cognitive impairments without affecting Abeta and tau pathologies in 3xTg-AD mice. Acta Neuropathol. 2010;120:605–21. doi: 10.1007/s00401-010-0734-6. [DOI] [PubMed] [Google Scholar]
- 27.Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987;78:151–64. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 28.Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993;268:24374–84. [PubMed] [Google Scholar]
- 29.Iqbal K, Wisniewski HM, Shelanski ML, Brostoff S, Liwnicz BL, Terry RD. Protein changes in senile dementia. Brain Res. 1974;77:337–43. doi: 10.1016/0006-8993(74)90798-7. [DOI] [PubMed] [Google Scholar]
- 30.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–9. [PubMed] [Google Scholar]
- 31.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iqbal K, Grundke-Iqbal I, Wisniewski HM. Neuronal cytoskeleton in aging and dementia. Prog Brain Res. 1986;70:279–88. doi: 10.1016/s0079-6123(08)64310-1. [DOI] [PubMed] [Google Scholar]
- 33.Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259:5301–5. [PubMed] [Google Scholar]
- 34.Iqbal K, Grundke-Iqbal I, Zaidi T, Merz PA, Wen GY, Shaikh SS, et al. Defective brain microtubule assembly in Alzheimer's disease. Lancet. 1986;2:421–6. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- 35.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso AD, Grundke-Iqbal I, Iqbal K. Alzheimer's disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–7. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 37.Alonso AD, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–6. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Chohan MO, Grundke-Iqbal I, Iqbal K. Disruption of microtubule network by Alzheimer abnormally hyperphosphorylated tau. Acta Neuropathol (Berl) 2007;113:501–11. doi: 10.1007/s00401-007-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang JZ, Grundke-Iqbal I, Iqbal K. Restoration of biological activity of Alzheimer abnormally phosphorylated tau by dephosphorylation with protein phosphatase-2A, -2B and -1. Brain Res Mol Brain Res. 1996;38:200–8. doi: 10.1016/0169-328x(95)00316-k. [DOI] [PubMed] [Google Scholar]
- 40.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–8. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khatoon S, Grundke-Iqbal I, Iqbal K. Guanosine triphosphate binding to beta-subunit of tubulin in Alzheimer's disease brain: role of microtubule-associated protein tau. J Neurochem. 1995;64:777–87. doi: 10.1046/j.1471-4159.1995.64020777.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang JZ, Gong CX, Zaidi T, Grundke-Iqbal I, Iqbal K. Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J Biol Chem. 1995;270:4854–60. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 44.Maeda S, Sahara N, Saito Y, Murayama M, Yoshiike Y, Kim H, et al. Granular tau oligomers as intermediates of tau filaments. Biochemistry. 2007;46:3856–61. doi: 10.1021/bi061359o. [DOI] [PubMed] [Google Scholar]
- 45.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nature Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clavaguera F, Grueninger F, Tolnay M. Intercellular transfer of tau aggregates and spreading of tau pathology: Implications for therapeutic strategies. Neuropharmacology. 2014;76(Pt A):9–15. doi: 10.1016/j.neuropharm.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 47.Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA. 2013;110:9535–40. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, et al. Trans-synaptic spread of tau pathology in vivo. PloS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–97. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 51.Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–10. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu JW, Herman M, Liu L, Simoes S, Acker CM, Figueroa H, et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem. 2013;288:1856–70. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau protein by casein kinase-1 converts it to an abnormal Alzheimer-like state. J Neurochem. 1995;64:1420–3. doi: 10.1046/j.1471-4159.1995.64031420.x. [DOI] [PubMed] [Google Scholar]
- 54.Singh TJ, Haque N, Grundke-Iqbal I, Iqbal K. Rapid Alzheimer-like phosphorylation of tau by the synergistic actions of non-proline-dependent protein kinases and GSK-3. FEBS Lett. 1995;358:267–72. doi: 10.1016/0014-5793(94)01445-7. [DOI] [PubMed] [Google Scholar]
- 55.Singh TJ, Zaidi T, Grundke-Iqbal I, Iqbal K. Modulation of GSK-3-catalyzed phosphorylation of microtubule-associated protein tau by non-proline-dependent protein kinases. FEBS Lett. 1995;358:4–8. doi: 10.1016/0014-5793(94)01383-c. [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Shi J, Tanimukai H, Gu J, Gu J, Grundke-Iqbal I, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer's disease. Brain. 2009;132:1820–32. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi J, Zhang T, Zhou C, Chohan MO, Gu X, Wegiel J, et al. Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome. J Biol Chem. 2008;283:28660–9. doi: 10.1074/jbc.M802645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennecib M, Gong C, Wegiel J, Lee MH, Grundke-Iqbal I, Iqbal K. Inhibition of protein phosphatases and regulation of tau phosphorylation in rat brain. Alzheimer's Reports. 2000;3:295–304. [Google Scholar]
- 59.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Inhibition of PP-2A upregulates CaMKII in rat forebrain and induces hyperphosphorylation of tau at Ser 262/356. FEBS Lett. 2001;490:15–22. doi: 10.1016/s0014-5793(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 60.Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem. 2000;275:5535–44. doi: 10.1074/jbc.275.8.5535. [DOI] [PubMed] [Google Scholar]
- 61.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–50. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 62.Iqbal K, Alonso A, Chen S, Chohan MO, El-Akkad E, Gong CX, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–8. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 64.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–7. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 65.Castellani RJ, Honda K, Zhu X, Cash AD, Nunomura A, Perry G, et al. Contribution of redox-active iron and copper to oxidative damage in Alzheimer disease. Ageing Res Rev. 2004;3:319–26. doi: 10.1016/j.arr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Priller C, Dewachter I, Vassallo N, Paluch S, Pace C, Kretzschmar HA, et al. Mutant presenilin 1 alters synaptic transmission in cultured hippocampal neurons. J Biol Chem. 2007;282:1119–27. doi: 10.1074/jbc.M605066200. [DOI] [PubMed] [Google Scholar]
- 67.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Alzheimer's disease abnormally phosphorylated tau is dephosphorylated by protein phosphatase-2B (calcineurin). J Neurochem. 1994;62:803–6. doi: 10.1046/j.1471-4159.1994.62020803.x. [DOI] [PubMed] [Google Scholar]
- 68.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000;485:87–93. doi: 10.1016/s0014-5793(00)02203-1. [DOI] [PubMed] [Google Scholar]
- 69.Tanimukai H, Grundke-Iqbal I, Iqbal K. Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer's disease. Am J Pathol. 2005;166:1761–71. doi: 10.1016/S0002-9440(10)62486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–96. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–4. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 72.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, et al. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 73.Zhou XW, Gustafsson JA, Tanila H, Bjorkdahl C, Liu R, Winblad B, et al. Tau hyperphosphorylation correlates with reduced methylation of protein phosphatase 2A. Neurobiol Dis. 2008;31:386–94. doi: 10.1016/j.nbd.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 74.Basurto-Islas G, Grundke-Iqbal I, Tung YC, Liu F, Iqbal K. Activation of Asparaginyl Endopeptidase Leads to Tau Hyperphosphorylation in Alzheimer's Disease. J Biol Chem. 2013;288:17495–507. doi: 10.1074/jbc.M112.446070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z, Jang SW, Liu X, Cheng D, Peng J, Yepes M, et al. Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol Cell. 2008;29:665–78. doi: 10.1016/j.molcel.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnaud L, Chen S, Liu F, Li B, Khatoon S, Grundke-Iqbal I, et al. Mechanism of inhibition of PP2A activity and abnormal hyperphosphorylation of tau by I(2)(PP2A)/SET. FEBS Lett. 2011;585:2653–9. doi: 10.1016/j.febslet.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bolognin S, Blanchard J, Wang X, Basurto-Islas G, Tung YC, Kohlbrenner E, et al. An experimental rat model of sporadic Alzheimer's disease and rescue of cognitive impairment with a neurotrophic peptide. Acta Neuropathol. 2012;123:133–51. doi: 10.1007/s00401-011-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X, Blanchard J, Kohlbrenner E, Clement N, Linden RM, Radu A, et al. The carboxy-terminal fragment of inhibitor-2 of protein phosphatase-2A induces Alzheimer disease pathology and cognitive impairment. FASEB J. 2010;24:4420–32. doi: 10.1096/fj.10-158477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Blanchard J, Grundke-Iqbal I, Wegiel J, Deng HX, Siddique T, et al. Alzheimer disease and amyotrophic lateral sclerosis: an etiopathogenic connection. Acta Neuropathol. 2013 doi: 10.1007/s00401-013-1175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murch SJ, Cox PA, Banack SA, Steele JC, Sacks OW. Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol Scand. 2004;110:267–9. doi: 10.1111/j.1600-0404.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 81.Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, et al. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer's disease. Acta Neurol Scand. 2009;120:216–25. doi: 10.1111/j.1600-0404.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 82.Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996;271:28741–4. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- 83.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:10804–9. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Udeshi ND, O'Malley M, Shabanowitz J, Hunt DF, Hart GW. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics : MCP. 2010;9:153–60. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuzwa SA, Yadav AK, Skorobogatko Y, Clark T, Vosseller K, Vocadlo DJ. Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids. 2011;40:857–68. doi: 10.1007/s00726-010-0705-1. [DOI] [PubMed] [Google Scholar]
- 86.Smet-Nocca C, Broncel M, Wieruszeski JM, Tokarski C, Hanoulle X, Leroy A, et al. Identification of O-GlcNAc sites within peptides of the Tau protein and their impact on phosphorylation. Mol Biosyst. 2011;7:1420–9. doi: 10.1039/c0mb00337a. [DOI] [PubMed] [Google Scholar]
- 87.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 88.Li X, Lu F, Wang JZ, Gong CX. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur J Neurosci. 2006;23:2078–86. doi: 10.1111/j.1460-9568.2006.04735.x. [DOI] [PubMed] [Google Scholar]
- 89.Gong CX, Liu F, Iqbal K. O-GlcNAc cycling modulates neurodegeneration. Proc Natl Acad Sci USA. 2012;109:17319–20. doi: 10.1073/pnas.1215395109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu CH, Si T, Wu WH, Hu J, Du JT, Zhao YF, et al. O-GlcNAcylation modulates the self-aggregation ability of the fourth microtubule-binding repeat of tau. Biochem Biophys Res Commun. 2008;375:59–62. doi: 10.1016/j.bbrc.2008.07.101. [DOI] [PubMed] [Google Scholar]
- 91.Yuzwa SA, Shan X, Macauley MS, Clark T, Skorobogatko Y, Vosseller K, et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nature Chem Biol. 2012;8:393–9. doi: 10.1038/nchembio.797. [DOI] [PubMed] [Google Scholar]
- 92.Wang P, Lazarus BD, Forsythe ME, Love DC, Krause MW, Hanover JA. O-GlcNAc cycling mutants modulate proteotoxicity in Caenorhabditis elegans models of human neurodegenerative diseases. Proc Natl Acad Sci USA. 2012;109:17669–74. doi: 10.1073/pnas.1205748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. Embo J. 1989;8:393–9. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–33. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- 95.Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. Embo J. 1990;9:4225–30. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kosik KS, Orecchio LD, Bakalis S, Neve RL. Developmentally regulated expression of specific tau sequences. Neuron. 1989;2:1389–97. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- 97.D'Souza I, Schellenberg GD. Regulation of tau isoform expression and dementia. Biochim Biophys Acta. 2005;1739:104–15. doi: 10.1016/j.bbadis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Liu F, Gong CX. Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener. 2008;3:8. doi: 10.1186/1750-1326-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshida M. Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology. 2006;26:457–70. doi: 10.1111/j.1440-1789.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 100.Hogg M, Grujic ZM, Baker M, Demirci S, Guillozet AL, Sweet AP, et al. The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol. 2003;106:323–36. doi: 10.1007/s00401-003-0734-x. [DOI] [PubMed] [Google Scholar]
- 101.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 102.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–41. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.D'Souza I, Schellenberg GD. Determinants of 4-repeat tau expression. Coordination between enhancing and inhibitory splicing sequences for exon 10 inclusion. J Biol Chem. 2000;275:17700–9. doi: 10.1074/jbc.M909470199. [DOI] [PubMed] [Google Scholar]
- 104.Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta. 2005;1739:91–103. doi: 10.1016/j.bbadis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 105.D'Souza I, Schellenberg GD. Arginine/serine-rich protein interaction domain-dependent modulation of a tau exon 10 splicing enhancer: altered interactions and mechanisms for functionally antagonistic FTDP-17 mutations Delta280K AND N279K. J Biol Chem. 2006;281:2460–9. doi: 10.1074/jbc.M505809200. [DOI] [PubMed] [Google Scholar]
- 106.Qian W, Liang H, Shi J, Jin N, Grundke-Iqbal I, Iqbal K, et al. Regulation of the alternative splicing of tau exon 10 by SC35 and Dyrk1A. Nucleic Acids Res. 2011;39:6161–71. doi: 10.1093/nar/gkr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Gao L, Tse SW, Andreadis A. Heterogeneous nuclear ribonucleoprotein E3 modestly activates splicing of tau exon 10 via its proximal downstream intron, a hotspot for frontotemporal dementia mutations. Gene. 2010;451:23–31. doi: 10.1016/j.gene.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y, Wang J, Gao L, Stamm S, Andreadis A. An SRp75/hnRNPG complex interacting with hnRNPE2 regulates the 5' splice site of tau exon 10, whose misregulation causes frontotemporal dementia. Gene. 2011;485:130–8. doi: 10.1016/j.gene.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao L, Wang J, Wang Y, Andreadis A. SR protein 9G8 modulates splicing of tau exon 10 via its proximal downstream intron, a clustering region for frontotemporal dementia mutations. Mol Cell Neurosci. 2007;34:48–58. doi: 10.1016/j.mcn.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ray P, Kar A, Fushimi K, Havlioglu N, Chen X, Wu JY. PSF suppresses tau exon 10 inclusion by interacting with a stem-loop structure downstream of exon 10. J Mol Neurosci. 2011;45:453–66. doi: 10.1007/s12031-011-9634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kar A, Havlioglu N, Tarn WY, Wu JY. RBM4 interacts with an intronic element and stimulates tau exon 10 inclusion. J Biol Chem. 2006;281:24479–88. doi: 10.1074/jbc.M603971200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gui JF, Lane WS, Fu XD. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 1994;369:678–82. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- 113.Wang HY, Lin W, Dyck JA, Yeakley JM, Songyang Z, Cantley LC, et al. SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J Cell Biol. 1998;140:737–50. doi: 10.1083/jcb.140.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Colwill K, Pawson T, Andrews B, Prasad J, Manley JL, Bell JC, et al. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. Embo J. 1996;15:265–75. [PMC free article] [PubMed] [Google Scholar]
- 115.Rossi F, Labourier E, Forne T, Divita G, Derancourt J, Riou JF, et al. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature. 1996;381:80–2. doi: 10.1038/381080a0. [DOI] [PubMed] [Google Scholar]
- 116.Kvissel AK, Orstavik S, Eikvar S, Brede G, Jahnsen T, Collas P, et al. Involvement of the catalytic subunit of protein kinase A and of HA95 in pre-mRNA splicing. Exp Cell Res. 2007;313:2795–809. doi: 10.1016/j.yexcr.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 117.Patel NA, Kaneko S, Apostolatos HS, Bae SS, Watson JE, Davidowitz K, et al. Molecular and genetic studies imply Akt-mediated signaling promotes protein kinase CbetaII alternative splicing via phosphorylation of serine/arginine-rich splicing factor SRp40. J Biol Chem. 2005;280:14302–9. doi: 10.1074/jbc.M411485200. [DOI] [PubMed] [Google Scholar]
- 118.Yin X, Jin N, Gu J, Shi J, Zhou J, Gong CX, et al. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A) modulates serine/arginine-rich protein 55 (SRp55)-promoted Tau exon 10 inclusion. J Biol Chem. 2012;287:30497–506. doi: 10.1074/jbc.M112.355412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi J, Qian W, Yin X, Iqbal K, Grundke-Iqbal I, Gu X, et al. Cyclic AMP-dependent protein kinase regulates the alternative splicing of tax exon 10L A mechanism involved in tau pathology of Alzheimer disease. J Biol Chem. 2011;286:14639–48. doi: 10.1074/jbc.M110.204453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, et al. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. JNeurochem. 2003;87:1333–44. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- 121.Hernandez F, Perez M, Lucas JJ, Mata AM, Bhat R, Avila J. Glycogen synthase kinase-3 plays a crucial role in tau exon 10 splicing and intranuclear distribution of SC35. Implications for Alzheimer's disease. J Biol Chem. 2004;279:3801–6. doi: 10.1074/jbc.M311512200. [DOI] [PubMed] [Google Scholar]
- 122.Rodriguez-Martin T, Anthony K, Garcia-Blanco MA, Mansfield SG, Anderton BH, Gallo JM. Correction of tau mis-splicing caused by FTDP-17 MAPT mutations by spliceosome-mediated RNA trans-splicing. Hum Mol Genet. 2009;18:3266–73. doi: 10.1093/hmg/ddp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Avale ME, Rodriguez-Martin T, Gallo JM. Trans-splicing correction of tau isoform imbalance in a mouse model of tau mis-splicing. Hum Mol Genet. 2013;22:2603–11. doi: 10.1093/hmg/ddt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fox NC, Freeborough PA, Rossor MN. Visualisation and quantification of rates of atrophy in Alzheimer's disease. Lancet. 1996;348:94–7. doi: 10.1016/s0140-6736(96)05228-2. [DOI] [PubMed] [Google Scholar]
- 125.Chan D, Fox NC, Jenkins R, Scahill RI, Crum WR, Rossor MN. Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology. 2001;57:1756–63. doi: 10.1212/wnl.57.10.1756. [DOI] [PubMed] [Google Scholar]
- 126.Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, et al. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008;67:78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iqbal K, Grundke-Iqbal I. Alzheimer's disease, a multifactorial disorder seeking multitherapies. Alzheimers & Dementia. 2010;6:420–4. doi: 10.1016/j.jalz.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–62. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 129.Boche D, Donald J, Love S, Harris S, Neal JW, Holmes C, et al. Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Abeta42 immunisation in Alzheimer's disease. Acta Neuropathol. 2010;120:13–20. doi: 10.1007/s00401-010-0705-y. [DOI] [PubMed] [Google Scholar]
- 130.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–72. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 131.Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 133.DaRocha-Souto B, Coma M, Perez-Nievas BG, Scotton TC, Siao M, Sanchez-Ferrer P, et al. Activation of glycogen synthase kinase-3 beta mediates beta-amyloid induced neuritic damage in Alzheimer's disease. Neurobiol Dis. 2012;45:425–37. doi: 10.1016/j.nbd.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–9. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou F, Gong K, Song B, Ma T, van Laar T, Gong Y, et al. The APP intracellular domain (AICD) inhibits Wnt signalling and promotes neurite outgrowth. Biochim Biophys Acta. 2012;1823:1233–41. doi: 10.1016/j.bbamcr.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 136.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB J. 2004;18:902–4. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 137.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–9. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 138.Rezai-Zadeh K, Douglas Shytle R, Bai Y, Tian J, Hou H, Mori T, et al. Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer's disease beta-amyloid production. J Cell Mol Med. 2009;13:574–88. doi: 10.1111/j.1582-4934.2008.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma SL, Pastorino L, Zhou XZ, Lu KP. Prolyl isomerase Pin1 promotes amyloid precursor protein (APP) turnover by inhibiting glycogen synthase kinase-3beta (GSK3beta) activity: novel mechanism for Pin1 to protect against Alzheimer disease. J Biol Chem. 2012;287:6969–73. doi: 10.1074/jbc.C111.298596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–29. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bi M, Ittner A, Ke YD, Gotz J, Ittner LM. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PloS One. 2011;6:e26860. doi: 10.1371/journal.pone.0026860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Troquier L, Caillierez R, Burnouf S, Fernandez-Gomez FJ, Grosjean ME, Zommer N, et al. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012;9:397–405. doi: 10.2174/156720512800492503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rosenmann H, Grigoriadis N, Karussis D, Boimel M, Touloumi O, Ovadia H, et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol. 2006;63:1459–67. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- 144.Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286:34457–67. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–14. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–7. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Clavaguera F, Lavenir I, Falcon B, Frank S, Goedert M, Tolnay M. “Prion-like” templated misfolding in tauopathies. Brain Pathol. 2013;23:342–9. doi: 10.1111/bpa.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Soto C. In vivo spreading of tau pathology. Neuron. 2012;73:621–3. doi: 10.1016/j.neuron.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 149.Gong CX, Grundke-Iqbal I, Damuni Z, Iqbal K. Dephosphorylation of microtubule-associated protein tau by protein phosphatase-1 and -2C and its implication in Alzheimer disease. FEBS Lett. 1994;341:94–8. doi: 10.1016/0014-5793(94)80247-5. [DOI] [PubMed] [Google Scholar]
- 150.Cardenas-Aguayo Mdel C, Kazim SF, Grundke-Iqbal I, Iqbal K. Neurogenic and neurotrophic effects of BDNF peptides in mouse hippocampal primary neuronal cell cultures. PloS One. 2013;8:e53596. doi: 10.1371/journal.pone.0053596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chohan MO, Li B, Blanchard J, Tung YC, Heaney AT, Rabe A, et al. Enhancement of dentate gyrus neurogenesis, dendritic and synaptic plasticity and memory by a neurotrophic peptide. Neurobiol Aging. 2011;32:1420–34. doi: 10.1016/j.neurobiolaging.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 152.Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. The J Clin Invest. 2010;120:1774–85. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blanchard J, Chohan MO, Li B, Liu F, Iqbal K, Grundke-Iqbal I. Beneficial Effect of a CNTF Tetrapeptide on Adult Hippocampal Neurogenesis, Neuronal Plasticity, and Spatial Memory in Mice. J Alzheimers Dis. 2010;21:1185–95. doi: 10.3233/JAD-2010-1000069. [DOI] [PubMed] [Google Scholar]
- 154.Li B, Wanka L, Blanchard J, Liu F, Chohan MO, Iqbal K, et al. Neurotrophic peptides incorporating adamantane improve learning and memory, promote neurogenesis and synaptic plasticity in mice. FEBS Lett. 2010;584:3359–65. doi: 10.1016/j.febslet.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 155.Blanchard J, Bolognin S, Chohan MO, Rabe A, Iqbal K, Grundke-Iqbal I. Rescue of synaptic failure and alleviation of learning and memory impairments in a trisomic mouse model of down syndrome. J Neuropathol Exp Neurol. 2011;70:1070–9. doi: 10.1097/NEN.0b013e318236e9ad. [DOI] [PubMed] [Google Scholar]
- 156.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–7. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]