Abstract
The urothelium is a stratified epithelium that prevents exchange of water and toxic substances between the urinary tract and blood. It is composed of Keratin-5-expressing-basal-cells (K5-BCs), intermediate cells and superficial cells specialized for synthesis and transport of uroplakins that assemble into the apical barrier. K5-BCs are considered to be a progenitor cell type in the urothelium and other stratified epithelia. Fate mapping studies however, reveal that P-cells, a transient population, are urothelial progenitors in the embryo, intermediate cells are superficial cell progenitors in the adult regenerating urothelium, and K5-BCs are a distinct lineage. Our studies indicate that retinoids, potent regulators of ES cells and other progenitors, are also required in P-cells and intermediate cells for their specification. These observations have important implications for tissue engineering and repair, and ultimately, may lead to treatments that prevent loss of the urothelial barrier, a major cause of voiding dysfunction and bladder pain syndrome.
INTRODUCTION
The urothelium is a stratified epithelium derived from endoderm (Wells and Melton, 1999) that extends from the renal pelvis to the proximal urethra that serves as a crucial barrier between the blood and urine. The mature urothelium consists of a layer of Keratin-5 expressing basal cells (K5-BCs), intermediate cells (I-cells) and a luminal layer of superficial cells (S-cells). S-cells are a terminally differentiated and are specialized for synthesis and transport of uroplakins (Upks), a family of molecules that assemble into apical crystalline plaque that is water proof and damage resistant [reviewed in: (Khandelwal et al., 2009; Wu et al., 2009)]. Damage to the urothelial barrier can compromise bladder function, lead to inflammation, and expose sub-urothelial nerve fiber receptors to urinary toxins, a possible mechanism behind chronic bladder pain or interstitial cystitis (Wyndaele and De Wachter, 2003). Thus, identification of urothelial progenitors and the signaling pathways that regulate them will be important for designing strategies for tissue augmentation and regeneration.
The urothelium is distinguishable in the mouse embryo on E11.5 when the bladder begins to form at the anterior aspect of the urogenital sinus. It is thought to assemble in a linear sequence, beginning with K5-BC progenitors that produce I-cells and S-cells that populate upper layers (Shin et al., 2011). The adult urothelium is quiescent but can rapidly regenerate in response to acute damage such as urinary tract infection or exposure to drugs and toxins [reviewed in: (Khandelwal et al., 2009)]. The injury response begins with desquamation of the damaged urothelium, followed by a massive wave of proliferation that reconstitutes the urothelial barrier within 72h, observations that suggest the existence of a progenitor population. Fate mapping studies using a TM-inducible ShhCreERT2;mTmG to indelibly label Shh+ cells support the existence of a population of Shh-expressing progenitors in the adult which are proposed to be K5-BCs (Shin et al., 2011). It remains unclear, however, whether these progenitors are also important for generating the urothelium during embryonic development.
Retinoic acid (RA) is a potent signaling molecule that regulates self-renewal and pluripotency and specification in ES cells and other progenitors, by inducing chromatin modifications in regulatory regions of RA-responsive genes (Kashyap et al., 2011). Retinoids are important in adults for vision and fertility, maintaining a wide variety of specialized epithelia (Wolbach and Howe, 1925), and are critical regulators of organogenesis. RA is synthesized from retinol, an inactive precursor that is taken up by cells and converted to retinoic acid in a two step process by retinol dehydrogenase-10 (Rdh10) and retinaldehyde dehydrogenase-2 (Raldh2), enzymes that are selectively expressed in cells where active RA signaling is required (Duester, 2008; Niederreither and Dolle, 2008). Once available, RA regulates transcription by binding to and activating RA receptors (Rars), a family of eight transcription factors that are widely expressed in adults and embryos. Rars control transcription by binding to RA response elements in promoter regions of target genes in association with Rxrs, a second family of nuclear receptors. In the absence of RA, Rar/Rxr heterodimers are frozen in an inactive conformation, however RA binding to the Rar/Rxr heterodimer induces a conformational change, converting the inactive complex to a transcriptionally active state (Samarut and Rochette-Egly, 2012)
The observations that RA regulates the adult steady state urothelium (Liang et al., 2005), together with recent studies showing that RA can induce ES cells to differentiate into urothelial cells (Mauney et al., 2010) suggest that retinoids may be important regulators of urothelial differentiation in vivo. To address this, we examined the requirement for RA-signaling in urothelial cells by expressing a dominant inhibitory form of Retinoic acid receptor alpha (Rara), RaraT403 in urothelial progenitors. RaraT403 lacks the ligand dependent activation domain that is critical for recruiting histone modifiers (Kashyap et al., 2011) and is thus a potent inhibitor of endogenous RA signaling in vivo and in vitro (Blumberg et al., 1997; Damm et al., 1993). RaraDN has been inserted into the Rosa26 locus (Soriano, 1999) after a floxed STOP sequence to generate [Gt(ROSA)26Sor)] mice (hereafter called RaraDN mice). We showed previously that Cre-dependent expression of RaraDN generates a collection of defects that are virtually identical to those observed in RA-deficiency and in mutants lacking components of the RA-signaling pathway (Table S1) that increase the severity of phenotypes in a dose dependent manner (Chia et al., 2011; Rosselot et al., 2010). Importantly, defects induced by expression of RaraDN appear to be specific for Rar-signaling, since we have not observed abnormalities that could be linked to inhibition of transcription via other nuclear receptor family members (Table S1).
The Shh-expressing population in the adult urothelium contains progenitors that have long term regenerative capacity and which have been proposed to be K5-BCs (Shin et al., 2011). We show here that the Shh-expressing population in embryos contains K5-BCs as well as two additional cell types: P-cells, which are present in the embryonic urothelium but not in the adult, and I-cells, which are present in the embryonic and adult urothelium. Lineage studies using a Krt5CreERT2 line to indelibly label K5-BCs and their daughters indicate that that K5-BCs are unlikely to be progenitors in the embryo or in adults. On the other hand, we find that P-cells, a transient urothelial cell type, are progenitors in the embryo and I-cells are progenitors in the adult regenerating urothelium, and we show that retinoids are required both in P-cells and I-cells for their specification. These observations could have important implications for tissue engineering and repair and may lead to treatments for patients with voiding dysfunctions and/or painful bladder syndrome that are associated with loss of the urothelial barrier function.
RESULTS
Shh-expressing cells are progenitors in the embryonic urothelium
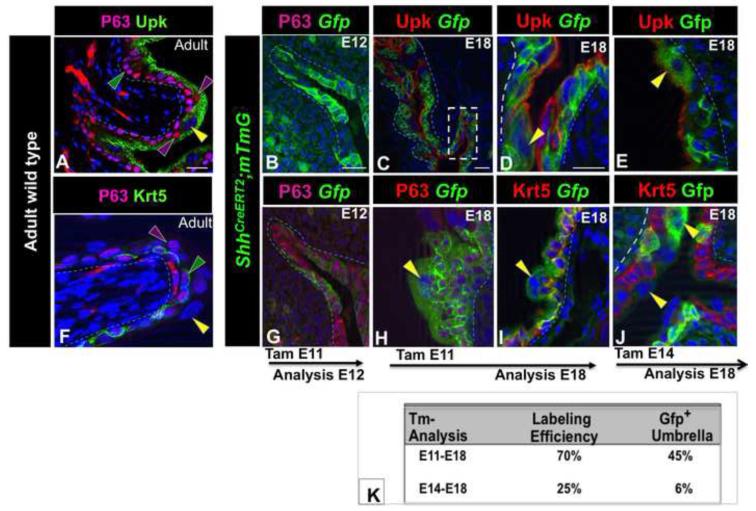
The mature urothelium is composed of a layer of basal cells that are positive for Krt5 and P63 (K5-BCs), 1-2 layers of intermediate cells (I-cells) that express Upk and P63 but not Krt5 and a luminal layer of superficial cells (S-cells) that express Upk, but not Krt5 or P63 [(Fig. 1A,F); in this figure and in subsequent figures, yellow arrowheads designate S-cells, purple arrowheads designate I-cells and green arrowheads designate K5-BCs]. Recent fate mapping studies using ShhCreERT2+/−;mTmG mice to indelibly label Shh+ cells and their daughters, support the existence of a population of Shh-expressing progenitors with long term regenerative potential (Shin et al., 2011). Based on the co-localization of Shh and Krt5, a marker of K5-BCs, it was proposed that the urothelial progenitor is a K5-BC. An interesting question, however, is whether this progenitor population also participates in de novo urothelial formation in the embryo.
Figure 1. Shh-expressing cells are progenitors in the developing urothelium.
A. A section from an adult urothelium stained with Uroplakin (Upk; green) and P63 (pink). B. A section from an E12 ShhCreERT2;mTmG embryo treated with TM at E11. C. Upk-expression (red) in an E18 ShhCreERT2;mTmG embryo exposed to TM on E11. D. Higher magnification of C. E. Upk expression (red) in a section from an E18 ShhCreERT2;mTmG embryo exposed to TM on E14. F. A section from an adult urothelium stained with Krt5 (green) and P63 (pink). G. P63 expression in the urothelium from an E12 ShhCreERT2;mTmG embryo treated with TM on E11. H. P63-expression (pink) in an E18 ShhCreERT2;mTmG embryo exposed to TM on E11. I. Krt5-expression (red) in an E18 ShhCreERT2;mTmG embryo exposed to TM on E11. J. Krt5-expression (red) in an E18 ShhCreERT2;mTmG embryo exposed to TM on E14. K. A table showing the labeling efficiency after TM treatment at E11 vs. E14, and the percentage of S-cells in E18 ShhCreERT2;mTmG embryos expressing the Gfp lineage tag 7 days and 4 days after TM treatment, respectively. Yellow arrowheads: S-cells, Green arrowheads, K5-BCs, purple arrowheads; I-cells. mTmG Gfp-positive cells are green. Magnifications: A,B,C,G,F 20X ; D,E, H,I,J 40X . All scale bars are 50μm. (See Also Figure S1).
ShhCreERT2+/− mice which harbor a TM inducible form of Cre (Harfe et al., 2004), were crossed with Gt(ROSA)26SortdTomato,−EGFP [(Rosa26-membrane-Tomato/membrane-Gfp reporter mice); hereafter called mTmG mice]. In TM treated ShhCreERT2+/−;mTmG mice, membrane bound Gfp is expressed in cells that undergo Cre-mediated recombination and membrane bound Tomato is expressed constitutively from the Rosa26 promoter in cells where recombination has not taken place (Muzumdar et al., 2007). We used this reporter line in fate mapping experiments to evaluate the potential of the Shh+ population in the developing urothelium. We first examined the specificity of Cre-dependent recombination by comparing the distribution of Shh mRNA with the Gfp-lineage tag in ShhCreERT2;mTmG embryos. In situ hybridization analysis indicates that Shh mRNA is expressed in virtually all urothelial cells between E10 and E13, becoming restricted to the basal and intermediate layers by E14, when S-cells begin to form in the upper layer (Fig. S1A-C). To evaluate the initial distribution of Cre-recombination, we analyzed the urothelium in TM treated ShhCreERT2;mTmG embryos after a short chase. TM was administered at E11, when the urothelium begins to form, and at E14, when the urothelium is stratified, and embryos were analyzed 24h later. In E12 ShhCreERT2;mTmG embryos exposed to TM at E11, Gfp-labeling was throughout the developing urothelium in a pattern that overlaps well with endogenous Shh mRNA (Fig. 1B,G; Fig. S1; compare A and D). In E15 ShhCreERT2;mTmG embryos exposed to TM on E14, Gfp-labeling was present in the intermediate and basal layers where Shh-mRNA is expressed, but was undetectable in the superficial cell layer, where Shh mRNA is down-regulated (Fig. S1B,E-I). Together these findings indicate that Cre-dependent recombination in ShhCreERT2;mTmG embryos is restricted to Shh-expressing cells.
To evaluate whether Shh+ cells can generate superficial cell daughters, ShhCreERT2;mTmG embryos were exposed to TM on E11 or E14, and analyzed at E18 when the urothelium is stratified and mature S-cells occupy the luminal layer. In E18 embryos exposed to TM at E11, Gfp-expression was present in 70% of urothelial cells including the superficial cell compartment where labeling was in 45% of the population (Fig. 1C,D,H,I,K). In E18 embryos exposed to TM at E14, 25% of urothelial cells were Gfp labeled including 6% of the superficial cell population (Fig. 1E,J,K). Since Cre-mediated recombination peaks between 6 and 48h after TM exposure (Hayashi and McMahon, 2002), these observations suggest that Shh-expressing progenitors are present in the urothelium between E11.5 and E16.
The urothelium stratifies in a unique manner
According to the current thinking, the urothelium stratifies in a similar fashion as does the skin, beginning with K5-BC progenitors that produce mature cell types that progressively populate the upper layers. A surprising observation however, is that Krt5, an early marker of K5-BCs, is barely detectable prior to E15, a stage when S-cells and I-cells have already formed (Fig. S2A-C,G-I). We therefore examined the composition of the Shh+ population to determine the ontogeny of different cell types and to identify potential progenitor populations. Shh is secreted and Upk, the definitive marker of both I-cells and S-cells, is expressed on the apical surface making it difficult to distinguish individual positive cells (e.g. Fig. S2G-L). We therefore used ShhGfp/Cre and ShhnLacZ reporter mice (Harfe et al., 2004; Lewis et al., 2004) to define cell types present in the Shh+ population and we generated Up2-Cfp reporter mice that express Cfp driven by Up2 regulator sequences to evaluate the distribution of Upk-expressing cells (Fig. S2M,N).
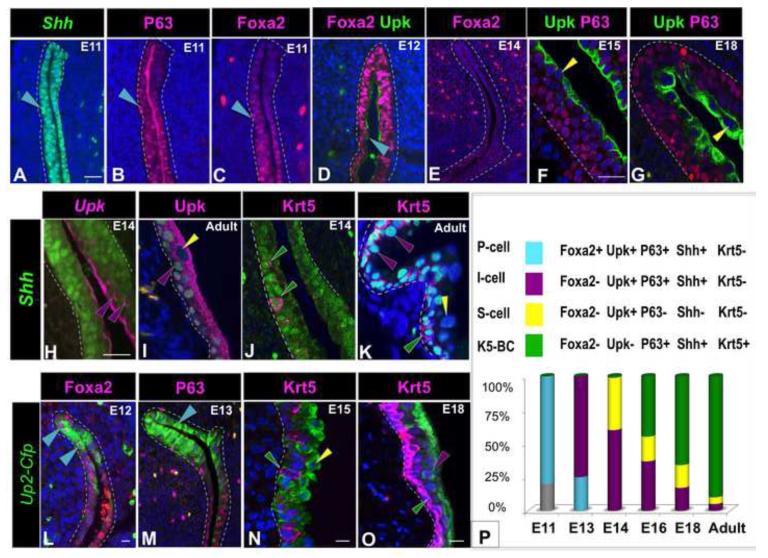
Marker analysis of ShhGfp/Cre and Shh-nlacZ mice revealed at that the Shh-expressing population in the embryonic urothelium contains 4 cell types: an undifferentiated endodermal population (Foxa2+ Upk− P63+ Shh+ Krt5−), P-cells (Foxa2+ Upk+ P63+ Shh+ Krt5−): which are a transient cell type abundant between E11 and E13 but undetectable at later stages (Fig. 2A-E); I-cells (Foxa2− Upk+ P63+ Shh+ Krt5−): which are abundant in the basal and intermediate layers at E14, and in adults reside in the intermediate layer where they comprise 5% of the urothelial population (Fig. 2,H,I,K,O); and K5-BCs (Foxa2− Upk− P63+ Shh+ Krt5+): which are first detected between E14 and E15 and by E18 are the majority of cells in the urothelium (Fig. 2J,K,N,O). S-cells, which are negative for Shh-expression (Foxa2− Upk+ P63− Shh− Krt5−), are first detectable in an immature mononucleated form at E14 (Fig. 2F) and by E18 are multinucleated, resembling their mature counterparts (Fig. 2G). Analysis of the distribution of UP2-Cfp activity confirmed these observations: Up2-Cfp+ P-cells co-expressing Foxa2 were detected between E11 and E13, while Up2-Cfp expression was restricted to intermediate and S-cells at later stages (Fig. 2L-M and not shown). At birth, 90% of cells in the urothelium are K5-BCs, which occupy the basal layer, 5% are I-cells and 5% are S-cells (Fig. 2P).
Figure 2. The Shh-population contains multiple cell types.
A. A section from an E11 ShhGfp/Cre embryo immunostained for expression of Gfp (green nuclear staining). B. A serial section from the same embryo as in (A) stained for expression of P63 (pink). C. A serial section from the same embryo as in (A) stained for expression of Foxa2. D. A section from an E12 embryo showing P-cells expressing Foxa2 (pink) and Upk (green) expression. E. A section from an E14 embryo stained with Foxa2 antibody (pink) which is undetectable. F. A section from an E14 embryo stained for expression of Upk (green) and P63 (pink). G. A section from the urothelium of an E18 embryo stained for expression of P63 (pink) and Upk (green). H. A section from an E14 ShhGfp/Cre embryo (Gfp is green nuclear staining) stained for expression of Upk (pink). I. A section from an adult ShhnlacZ embryo (nlacZ is green nuclear staining) stained with Upk antibody (pink). J. A section from an E14 ShhGfp/Cre embryo (Gfp is green nuclear staining) stained for expression of Krt5 (pink). K. A section from an adult ShhnlacZ embryo (nlacZ is green nuclear staining) stained for expression Krt5 (pink). L. A section from an E12 Up2-Cfp embryo (Cfp detected with anti-Gfp antibody is shown in green) stained for expression of Foxa2 (pink). M. A section from a E13 Up2-Cfp embryo stained for expression of P63 (pink). Cfp detected with anti-Gfp antibody is shown in green. N. A section from an E15 Up2-Cfp embryo stained for expression of Krt5 (Cfp detected with anti-Gfp antibody is shown in green). O. A section from an E18 Up2-Cfp embryo stained with Krt5 antibody (pink). Cfp detected with anti-Gfp antibody is shown in green. P. A schematic showing the color code for different urothelial cell types and the relative proportions in the embryonic and adult urothelium. In this and subsequent figures: S-cells are marked with yellow arrowheads, I-cells with purple arrowheads, K5-BCs with green arrowheads, and P-cells, with blue-green arrowheads. Magnifications: A-D; L,M 20X; E 10X; F,G 40X; H-K 40X ;N ,O x40. Scale bars: 50μm. (See also Figure S2).
Fate mapping indicates that the K5-BCs are not urothelial progenitors in the embryonic or adult urothelium
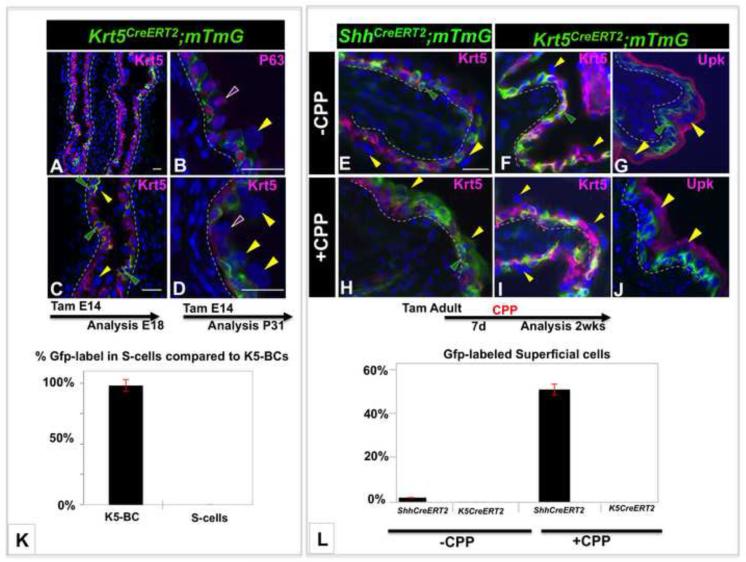
The observations that (i) K5-BCs are undetectable in the urothelium between E11 and E14 when progenitor potential is high, (ii) K5-BCs form after S-cells, I-cells and P-cells, suggests that K5-BCs are unlikely to be urothelial progenitors during development. To directly address this question we performed fate-mapping studies using Cre-lox recombination to indelibly label K5-BCs and their daughters. Tg(KRT5CreERT2) mice (hereafter referred to as K5CreERT2 mice) express a transgene containing Krt5 regulatory sequences fused to the TM-inducible Cre/ERT2 cassette that drives Cre-dependent recombination in Krt5 expressing cells, including epidermis (Indra et al., 1999). Based on the distribution of Krt5 expression, K5-BCs appear between E14 and E15. We therefore exposed Krt5CreERT2;mTmG embryos to TM on E14 and analyzed the distribution of Gfp-positive cells after 4 days, which in experiments with the ShhCreERT2;mTmG line as a lineage marker, was sufficient time to label 6% of the superficial cell population (Fig. 1E,J,K). In E18 Krt5CreERT2;mTmG embryos exposed to TM on E14, Cre-dependent recombination occurred in about 22% of urothelial cells, and labeling was confined almost exclusively to the K5-BC population (Fig. 3A,C). Similar findings were obtained in experiments where TM was administered at E14 in utero and embryos were analyzed after either one month or three months; lineage-tagged cells were almost exclusively K5-BCs with an occasional Gfp labeled cell in the intermediate layer (Fig. 3B,D,K and not shown). These results suggest that K5-BCs are not urothelial progenitors during development.
Figure 3. K5-BCs are unlikely to be urothelial progenitors.
A-D. Lineage studies in the embryonic urothelium using the Krt5CreERT2;mTmG line to follow the fate of K5-BCs. A. section from a Krt5CreERT2;mTmG E18 embryo exposed to TM at E14 stained for expression of Krt5 (pink). Cells expressing the Gfp-lineage marker detected with Gfp antibody are green. B. A section from a Krt5CreERT2;mTmG embryo exposed to TM at E14 and analyzed 1 month later stained for expression of P63 (pink). Cells expressing the Gfp-lineage marker detected with Gfp antibody are green C. A higher magnification of the section in (A). D. A section from a Krt5CreERT2;mTmG embryo exposed to TM at E14 and analyzed after 1 month stained for expression of Krt5 (pink). Cells expressing the Gfp-lineage marker detected with Gfp antibody are green. E. A section from a TM treated adult ShhCreERT2;mTmG mouse that did not receive CPP, stained for expression of Krt5 (pink). Cells expressing the Gfp-lineage marker detected with Gfp antibody are green. F-G. Sections from adult TM treated Krt5CreERT2;mTmG mice that did not receive CPP, stained for expression of Krt5 (pink in F) and Upk (pink in G). H. A section from a TM treated adult ShhCreERT2;mTmG mouse analyzed 2 weeks after CPP administration stained for expression of Krt5 (pink). I-J. Sections from a TM treated adult Krt5CreERT2;mTmG mouse stained for expression of Krt5 (pink in I) or Upk (pink in J) 2 weeks after CPP treatment. Cells expressing the Gfp-lineage marker detected with Gfp antibody are green K. A graph showing the distribution of lineage tagged cells in the K5-BC and superficial compartments in Krt5CreERT2;mTmG mice exposed to TM on 14, in utero and analyzed at E18 or P31. L A graph showing a comparison of lineage tracing studies in Krt5CreERT2;mTmG and ShhCreERT2;mTmG mice with and without CPP treatment. S-cells are marked with yellow arrowheads, I-cells with purple arrowheads, K5-BCs with green arrowheads. For quantification, a minimum of three independent experiments were performed, and the average ± SEM was plotted. Magnifications: A 20X ; C 40X; B,D 40X, E-J 30X. Scale bars: 50μm. (See also Figure S3).
K5-BCs have been proposed to be progenitors with long-term regenerative capacity in the adult urothelium based on fate mapping experiments using the ShhCreERT2;mTmG line (Shin et al., 2011) which in adults, drives Cre-dependent recombination in I-cells as well as in K5-BCs (Fig. 2J,K; Fig. S1F-I). To directly determine whether K5-BC have regenerative potential in the adult urothelium, we performed parallel fate mapping experiments using the Krt5Cre/ERT2;mTmG and ShhCreERT2;mTmG as lineage markers for K5-BCs and Shh-expressing cells respectively, after treatment with cyclophosphamide (CPP), which induces a rapid cycle of injury and repair (Farsund and Dahl, 1978). To assess the kinetics of regeneration in our experimental setting, CPP-treated and untreated controls were injected with EdU (5-ethynyl-2′-deoxyuridine) to label cells in S-phase, and proliferation was measured at 24h, 48h and 72h. Consistent with the low rate of turnover in the adult urothelium, few proliferating cells were present in controls that had not been treated with CPP (Fig. S3A), however in CPP-treated animals, proliferation increased after 24h, peaking at 48h when 33% of cells in the urothelium were Edu+ (Fig. S3B,C). To evaluate the kinetics of urothelial regeneration during this 3day period, we stained CPP treated adults and controls for expression of Upk, which labels both intermediate and S-cells. Analysis 24h and 48h after CPP treatment revealed a decreased thickness of the urothelium and down-regulation of Upk expression compared to untreated controls, indicating that extensive exfoliation had taken place (Fig. S3D-F). By 72h after CPP-treatment, the thickness of the urothelium and expression levels of Upk-distribution were similar to controls, indicating that the urothelium was reconstituted (Fig. S3D,G).
We next performed damage and regeneration experiments with the ShhCreERT2;mTmG and Krt5CreERT2;mTmG lines. Adult mice were given 3 doses of TM over a one-week period to activate Cre-dependent recombination and expression of the Gfp-lineage tag. CPP was administered 1 week after the last TM injection to induce a round of damage and repair. Analysis was performed 2 weeks after CPP exposure when regeneration is complete and the urothelium has returned to a quiescent state. Analysis of ShhCreERT2;mTmG mice that did not receive CPP revealed a small number of lineage-tag expressing cells (Fig. 3E), however in CPP-treated ShhCreERT2;mTmG mice, Gfp-expression was present in 70% of the cells in the urothelium, including 50% of the superficial cell population (Fig. 3H,L). These findings demonstrate that Shh-expressing cells can generate superficial cell daughters after CPP-induced injury. In parallel experiments with K5CreERT2;mTmG mice as a lineage marker, 60% of the urothelium was Gfp-positive after Tm-induction, indicating that recombination was robust, however expression of the lineage tag was almost entirely restricted to the K5-BC population in both CPP treated and untreated adults (Fig. 3F,G,I,J,L). The observation that K5-BCs do not generate detectable numbers of S-cells during regeneration suggests that I-cells rather than K5-BCs must be the superficial progenitor.
P-cells are a newly discovered progenitor population in the developing urothelium
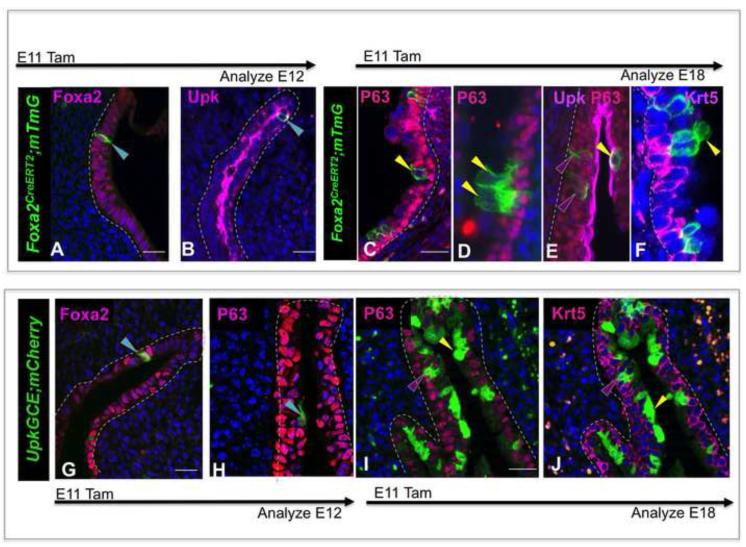
Our studies suggest that P-cells (Foxa2+ P63+ Shh+ Upk+ Krt5−) are a transient population present in the embryonic urothelium between E11 and E13; a period when fate mapping indicates that progenitor potential is high (Fig. 1). To evaluate whether P-cells can produce other urothelial cell types, we used a TM-inducible Foxa2CreERT line (Frank et al., 2007) in fate mapping experiments. We first examined the specificity of Cre-dependent recombination in the Foxa2CreERT;mTmG line by exposing embryos to TM at E11 and analyzing the distribution of the Gfp lineage marker after a short, 24h, chase. Gfp expression was seen in a small number of P-cells that co-express Foxa2, P63 and Upk, while Gfp labeled cells were undetectable in the urothelium of embryos that were not exposed to TM (Fig. 4A,B and not shown), indicating that Cre-dependent recombination is TM-dependent and is initially confined to P-cells. Analysis of TM pulsed embryos after a longer, 7day chase, revealed expression of Gfp in 10-14% of cells in the intermediate and superficial populations (Fig. 4C-F). Although almost 90% of urothelial cells are K5-BCs at this stage, expression of the Gfp-lineage tag was rare or undetectable in the K5-BC population (Fig. 4C-F), suggesting that K5-BCs arise from a distinct progenitor cell type.
Figure 4. P-cells are a transient progenitor population in the embryonic urothelium.
A-F. Lineage tracing with the Foxa2CreERT2;mTmG line. A. Expression of the Gfp lineage tag (detected with antibody, green) in P-cells stained for expression of Foxa2 cells (pink) in an E12 embryo, 24h after TM exposure on E11. B. Expression of the Gfp lineage tag (detected with antibody, green) in P-cells expressing Upk (pink) in an E12 embryo 24h after TM exposure on E11. C. A section from an E18 Foxa2CreERT2;mTmG embryo exposed to TM on E11 stained for expression of P63 (pink). D. A section from an E18 Foxa2CreERT2;mTmG embryo exposed to Tam at E11 stained for expression of Upk (pink) and P63 (red). E A section from an E18 Foxa2CreERT2;mTmG embryo exposed to Tam on E11stained for expression of Krt5 (pink). F. A section from an E18 Foxa2CreERT2;mTmG embryo exposed to Tam at E11, stained with Krt5 (pink) showing a cluster of lineage-marked cells (detected with Gfp antibody, green). G. Expression of the mCherry lineage tag (green, detected with an antibody directed against Rfp) in P cells in an E12 Upk3aGCE;mCherry embryo exposed to Tamoxifen on E11 and stained for expression of Foxa2 (pink). H. Expression of the mCherry lineage tag (green, detected with an antibody directed against Rfp) in P cells in an E12 Upk3aGCE;mCherry embryo exposed to Tamoxifen on E11 and stained for expression of P63 (pink). I. Expression of the mCherry lineage tag (green, detected with an antibody directed against Rfp) in intermediate and superficial cells in an E18 Upk3aGCE;mCherry embryo exposed to Tamoxifen on E11 and stained for expression of P63 (pink). J. Expression of the mCherry lineage tag (green, detected with an antibody directed against Rfp) in intermediate and superficial cells in an E18 Upk3aGCE;mCherry embryo exposed to Tamoxifen on E11 and stained for expression of Krt5 (pink). Magnifications: A,B, G 20X ; H 40X;C,D 20X;E,20X and F,2X. Scale bars: 50μm.
In parallel experiments, we traced the fate of P-cells using the Upk3aGCE;mCherry line (www.gudmap.org), in which Tm-inducible mCherry expression is detected by antibody staining (mCherry is shown in green in Fig. 4 and in subsequent figures). Analysis of E12 Upk3aCCE;mCherry embryos 24h after Tm exposure revealed mCherry labeling in a small number of P-cells co-expressing Foxa2, Upk and P63 (Fig. 4G,H), indicating that recombination at this stage is restricted to the P-cell compartment. Analysis after a 7-day chase period revealed expression of the mCherry lineage tag in about 33% of the superficial cell population and 10% of the intermediate cell population (Fig. 4I,J), but again, we did not observe expression in K5-BCs. These studies suggest that P-cells are intermediate cell and superficial cell progenitors. However, since intermediate and superficial cells form within 24h of one another (E13, and E14, respectively), it is unclear from our studies whether superficial cells are direct descendants of P-cells or whether they are derived from intermediate cells, which are P-cell daughters. Together, these studies suggest that P-cells are intermediate cell and superficial cell progenitors. However, since intermediate and S-cells form within 24h of one another (E13, and E14, respectively), it is unclear from our studies whether S-cells are direct descendants of P-cells or whether they are derived from I-cells, which are P-cell daughters.
I-cells are likely to be superficial cell progenitors in the regenerating adult urothelium
Fate mapping studies indicate that the Shh-expressing population contains urothelial progenitors in adults (Shin et al., 2011). We find that Shh is localized in K5-BCs, which fate mapping studies suggest are unlikely to be urothelial progenitors (Fig. 3), and in I-cells which have not been assessed for progenitor potential. We therefore used the Upk3aGCE;mCherry line in fate mapping studies to determine whether I-cells can generate superficial cells in the adult regenerating urothelium. Upk3aGCE;mCherry adults were treated first with TM, then 1 week later with CPP to induce a round of regeneration, and analysis was performed 2 weeks later to evaluatethe distribution of mCherry expressing cells.
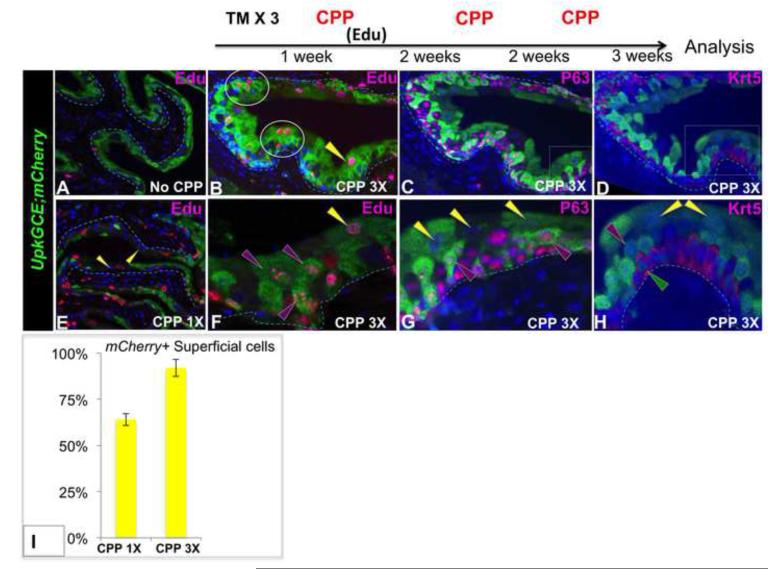
Analysis of TM induced Upk3aGCE;mCherry control mice that had not received CPP, revealed mCherry-labeling in I-cells and S-cells, however few were Edu+ (Fig. 5A), consistent with the low rate of proliferation in the adult steady state urothelium (Jost, 1989). However, analysis of Upk3aGCE;mCherry adults after CPP treatment revealed Edu labeling in 45% of I-cells and in 67% of S-cells (Fig. 5E). CPP induces death and desquamation of the superficial layer (Fig. S3), thus the presence of Edu+ lineage tagged S-cells strongly suggests that they are intermediate cell daughters. To evaluate whether I-cells can self-renew and produce superficial cell daughters after serial CPP damage and regeneration, Upk3aGCE;mCherry mice were Upk3aGCE;mCherry adults were given TM 3 times over a one week period to activate Cre-dependent recombination, then CPP was administered 1 week, 3 weeks and 5 weeks after the last dose of TM. Edu was given 48h after the first CPP dose to label cells in S-phase. Analysis after 3 rounds of injury and repair revealed an increase in the percentage of lineage-labeled S-cells [(92% compared to 67% after 1 dose of CPP (Fig. 5B,C,D,F,G,H,I)]. The observation that the numbers of lineage marked S-cells increase after serial damage and regeneration suggests that I-cells are superficial cell progenitors, and that they can self-renew. Since the Edu concentration is reduced by half each time a cell divides, the presence of S-cells expressing high levels of Edu suggests that they are either derived directly from intermediate cell progenitors that divide slowly, or are derived from a transit amplifying intermediary cell type.
Figure 5. I-cells are a superficial progenitor population in adults.
A. A section showing the urothelium of a Upk3aGCE;mCherry adult that did not receive CPP mCherry detected with an antibody directed against Rfp is shown in green and Edu-expressing cells are pink. B-H Sections from an Upk3aGCE;mCherry adult after 3 rounds of CPP-induced damage and repair. (B): Stained with Edu and mCherry (C) Stained with P63 and mCherry (D) Stained with Krt5 and mCherry. E. A section of an Upk3aGCE;mCherry adult after 1 round of CPP-induced damage and repair, showing the distribution of mCherry expression (green) and Edu (pink). F,G,H, Higher magnification of B,C,D, respectively. For quantification, a minimum of three independent experiments were performed, and the average ± SEM was plotted. Magnifications: A,E 20X; B,C,D 20X and 2x zoomed; F,G,H 40X and 2X zoomed. Scale bars 50uM.
Retinoid signaling is selectively expressed in P-cells during development, and is up regulated in the regenerating urothelium
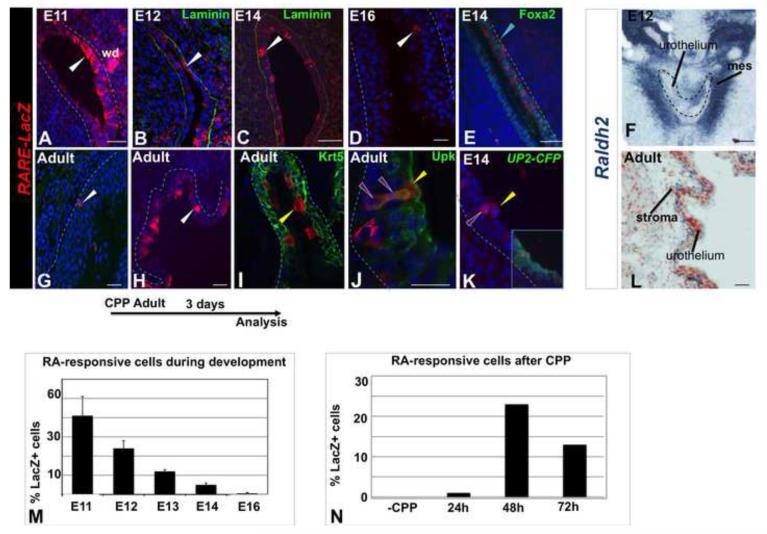
Retinoic acid (RA) can induce embryonic stem cells to differentiate into urothelial cells in culture (Mauney et al., 2010), suggesting that retinoids may be important for controlling urothelial specification in vivo. To begin to address this, we analyzed wild type embryos to assess the distribution of RA-responsive cells using the RARE-LacZ reporter line. RARE-LacZ mice harbor a transgene containing LacZ fused to an RA-response element which is expressed in cells where RA is available and RA-receptor signaling is active (Rossant et al., 1991). Analysis of RARE-LacZ expression during development revealed that LacZ-positive cells were most abundant between E11 and E14 and decreased to low levels at later stages (Fig. 6A-D). Analysis at of the distribution of RARE-LacZ activity at E12 revealed expression in P-cells (Fig. 6E), and by E14, LacZ expression was localized predominantly in intermediate and S-cells (Fig. 6K). These studies suggest that the RA-responsive cells are most abundant in the embryonic urothelium between E11 and E14, when urothelial progenitors are also present (Fig.1).
Figure 6. RA-signaling is selectively up-regulated in the embryonic urothelium and in the adult urothelium during regeneration.
A. A section showing the urothelium in an E11 RARE-lacZ reporter embryo showing LacZ expression (red) detected with antibody staining. B. A section showing the distribution of RA-responsive cells in the urothelium of an E12 RARE-lacZ reporter (red). C. A section from an E14 RARE-lacZ reporter embryo stained for lacZ expression (red). D. A section from an E12 RARE-lacZ reporter embryo stained for lacZ expression (red) and Foxa2 (green). E. A section from an E14 Up2-Cfp;RARE-lacZ reporter embryo stained for lacZ expression (red) and Up2-Cfp (green). F. In situ hybridization showing expression of Raldh2 in sub-urothelial mesenchyme in an E12 embryo. G. A section from the urothelium of adult RARE-lacZ reporter mouse stained for lacZ expression (red). H. G. A section from the urothelium of adult RARE-lacZ reporter mouse 48h after administration of CPP stained for lacZ expression (red) and Krt5 (green). I. A section from the urothelium of adult RARE-lacZ reporter mouse 48h after administration of CPP, stained for lacZ expression (red) and Upk (green). K. Same section as in (E) showing only the red channel. L. In situ hybridization showing expression of Raldh2 in sub-urothelial stroma in a wild type adult mouse. For quantification, a minimum of three independent experiments were performed, and the average ± SEM was plotted.Magnifications: A –C,F 20X; D,E. Scale bars: 50μm
RA-deficiency in mammals results in squamous metaplasia in the adult urothelium (Liang et al., 2005; Wolbach and Howe, 1925), indicating that retinoids are normally required for maintenance of the adult steady state urothelium. Analysis of the adult RARE-lacZ mice revealed low numbers of RA-responsive cells (Fig. 6G), suggesting that low levels of RA-signaling are adequate to maintain superficial cell renewal in the steady state urothelium, which has a very slow rate of turnover. To evaluate whether RA-signaling increases in response to injury, RARE;LacZ mice were treated with CPP then analyzed during the first 3 days post-treatment, to determine the numbers and distribution of LacZ-expressing cells. This analysis revealed a dramatic increase in the LacZ+ population, which followed similar kinetics as proliferation (Fig. 6G-H, Fig. S3C). Marker analysis during this 72h period revealed that the majority of LacZ+ cells were intermediate and S-cells (Fig. 6I,J), suggesting that RA-signaling may be important in in these populations for regeneration after damage. These findings suggest that low levels of RA-signaling maintain steady state urothelial cell renewal, while high levels of RA-signaling may be important for renewal of the superficial layer after injury.
RA-receptors are only active when bound to RA, which is synthesized from retinol (vitamin A) in a temporally and spatially restricted manner by RA-synthesizing enzymes. To identify the source of RA that regulates urothelial formation and regeneration, we performed in situ hybridization analysis to assess the distribution of Aldh1a2 (hereafter-called Raldh2), an enzyme required for RA-synthesis (Niederreither et al., 1999). These experiments reveal that Raldh2 expression in the bladder is restricted to the mesenchyme just below the urothelium where expression was highest between E12 and E13 (Fig. 6F). In adults, Raldh2 expression persisted in in the sub-urothelial stroma (Fig. 6L), a domain important for regulating urothelial maintenance and regeneration via Wnt, Bmp and Shh signaling (Mysorekar et al., 2009; Shin et al., 2011). These observations suggest that RA synthesized in the stromal compartment may be important for regulating RA-receptor signaling in the embryonic and adult urothelium.
RA signaling is required for urothelial specification
While the above experiments demonstrated that RA signaling is active in P-cells at a stage in embryonic development when progenitor potential is high, it was still unclear what role, if any, RA signaling has in urothelial specification. To directly address this question, we used Cre-Lox recombination to express RaraDN, a dominant inhibitory RA-receptor, in the Shh-positive population in the embryo, which our studies indicate contains urothelial progenitors (Fig. 1). To do this, we used the ShhCre/+ line (Harfe et al., 2004) which drives Cre-dependent recombination in more than 90% of cells in the developing urothelium in combination with the Rosa26 mTmG reporter [(Muzumdar et al., 2007) ; Fig. S1J)]. RaraDN is a truncated form of Rara that has been inserted in the Rosa26 locus downstream of a floxed stop cassette where it is activated by Cre-mediated recombination. Our previous studies demonstrated that expression of the RaraDN mutant receptor blocks transcription by endogenous RA-receptors and importantly, we found that defects in embryos expressing 2 copies of the RaraDN allele are more severe than those in embryos expressing one allele (Chia et al., 2011; Rosselot et al., 2010), indicating that RaraDN inhibits RA-signaling in a dose-dependent manner.
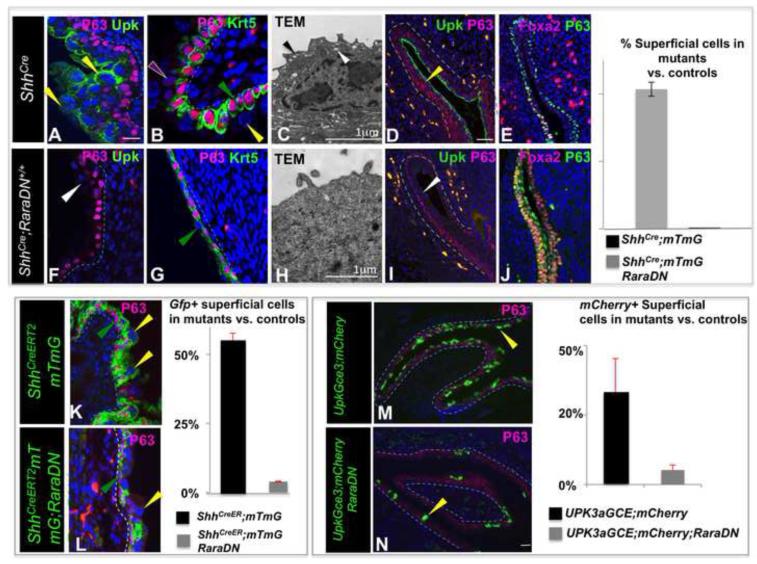
To evaluate whether RaraDN is an efficient suppressor of RA-signaling in the urothelium, we generated ShhCre/+; RaraDN+/+ mutants expressing two copies of the RaraDN, and we examined whether expression of the RaraDN led to a reduction in expression of RA-responsive genes. Analysis of E18 ShhCre/+ controls and ShhCre; RaraDN+/+ mutants revealed that Ret and Rarb2, two genes whose expression is RA-dependent, were down regulated in mutants compared to controls (Fig. S4A,B,D,E). These findings suggested that the RaraDN was efficiently inhibiting RA-signaling. For further confirmation, we compared the distribution of RA-responsive cells in mutants and controls using the RARE-lacZ reporter line. Analysis of ShhCre;RARE-LacZ controls at E11, revealed large numbers of LacZ+ cells (Fig. S4C), however in ShhCre; RaraDN;RARE-LacZ mutants, the number of LacZ-expressing cells was greatly reduced (Fig. S4C,F). The observation that Rarb and Ret are down-regulated in ShhCre; RaraDN mutants, together with the reduction in the numbers of RA-responsive cells in the mutants, suggests that RaraDN driven by ShhCre efficiently inhibits RA-signaling in urothelial cells.
In E18 ShhCre/+ control embryos, the urothelium is fully stratified epithelium containing K5-BCs, one to two layers of I-cells and a layer of mature, multinucleated S-cells (Fig. 7A). However, in 5/7 ShhCre; RaraDN+/+ mutants examined, there was only a single layer of P63-expressing cells, few if any morphologically distinguishable S-cells and Upk a marker of intermediate and S-cells, was down-regulated (Fig. 7F). On the other hand, K5-BCs expressing Krt5 and P63 lined the basal layer in mutants, suggesting that formation of this population is retinoid-independent (Fig. 7B,G). TEM analysis of the urothelium in controls revealed the prominent apical plaque and fusiform vesicles that are unique features of S-cells (Fig. 7C, black and white arrowheads, mark apical plaque and vesicles, respectively) were absent from S-cells in mutants, which instead displayed microvilli (Fig. 7H, black arrowhead), structures not found on the surface of wild type S-cells. These studies suggest that RA-signaling is normally required for formation of intermediate and S-cells.
Figure 7. Retinoids are required for urothelial formation.
A. P63 (pink) and Upk (green) staining in an E18 control ShhCre/+ embryo. B. Krt5 (green) and P63 (pink) staining in the urothelium of an E18 control ShhCre/+ embryo. C. Transmission electron microscopy showing the apical surface of an ShhCre/+ embryo. D. A section from an E14 ShhCre/+ control embryo stained with Upk (green) and P63 (pink). E. A section from an E14 ShhCre/+ embryo stained with Foxa2 (pink) and P63 (green). F. P63 (pink) and Upk (green) staining in a section from an E18 ShhCre/+; RaraDN mutant embryo. G. Krt5(green) and P63 (pink) staining in the urothelium of an E18 ShhCre/+; RaraDN mutant embryo. H. Transmission electron microscopy showing the apical surface of an E18 ShhCre/+; RaraDN mutant urothelial cell. I. A section from an E14 ShhCre/+;RaraDN mutant embryo stained with Upk (green) and P63 (pink). J. A section from an E14 ShhCre/+;RaraDN mutant embryo stained with Foxa2 (pink) and P63 (green). K. P63 (pink) staining in an E18 control ShhCreERT2;mTmG embryo exposed to TM on E11 (the Gfp lineage-tag is green). L. P63 (pink) staining in an E18 ShhCreERT2;mTmG;RaraDN mutant embryo exposed to TM on E11 (the Gfp lineage-tag is green). M. P63 (pink) staining in an E18 Upk3aGCE;mCherry control embryo exposed to TM on E11 (mCherry is shown in green). N. P63 (pink) staining in an E18 Upk3aGCE;mCherry;RaraDN mutant embryo exposed to TM on E11 (mCherry is shown in green). For quantification, a minimum of three independent experiments were performed, and the average ± SEM was plotted. Magnifications: A,B,D,E,F,G,I,J 20X; C,H 31,000X; KL, 40X; M,N 20X. Scale bars: 50μm. (See also S4).
We next investigated which cell types normally mediate RA-signaling. To begin to examine the temporal requirement for RA-signaling, we used the TM inducible ShhCreERT2;mTmG line to express RaraDN at E11, when our studies indicate that P-cell progenitors are abundant. ShhCreERT2;mTmG controls and ShhCreERT2;mTmG; RaraDN mutants were exposed to TM at E11 and analyzed at E18. This analysis revealed a moderate reduction in overall numbers of intermediate and S-cells compared to the controls, and a dramatic reduction in the numbers of Gfp-labeled S-cells (Fig. 7K,L), supporting the suggestion that RA-signaling is normally required in urothelial progenitors for formation of S-cells. Since RaraDN inhibits RA-signaling in a dose-dependent manner (Blumberg et al., 1997; Damm et al., 1993; Rajaii et al., 2008), the reduced severity of the urothelial phenotype in ShhCreERT2;mTmG; RaraDN mutants compared to the constitutive ShhCre; RaraDN+/+ line is likely to be due to expression of one vs. two copies of DN, respectively.
Impaired superficial cell formation at E18 could indicate a role for retinoids for survival of urothelial progenitors or could indicate a role for RA in specification of urothelial progenitors. We did not detect intermediate or S-cells in ShhCre+/−; RaraDN+/+ mutants at any stage examined (Fig. 7D,I), suggesting that these cell types failed to form. Consistent with this, TUNEL analysis of mutants did not reveal increased apoptosis in the urothelium compared to controls (data not shown). P-cells, the first urothelial cell type, are transiently present in the urothelium between E11 and E13 (Fig. 4). They are distinguishable from endoderm by expression of Upk, and from other urothelial cell types by expression of Foxa2, which is down-regulated after E13 (Fig. 2). Immunostaining of E14 ShhCre+/− controls revealed undetectable expression of Foxa2, as expected, however E14 ShhCre+/−; RaraDN+/+ mutants contained large numbers of Foxa2 expressing cells which were also positive for Shh and P63, but lack expression of Upk expression or Krt18, Krt20 and other urothelial markers (Fig. 7J and not shown). The persistence of this population expressing endodermal markers in ShhCre+/−; RaraDN+/+ mutants and absence of intermediate and S-cells, suggests that retinoids may normally be important in endoderm for specification of P-cells.
To directly examine the requirement for RA-signaling in P-cells, we first attempted to use the Foxa2CreERT2;mTmG line to express a single copy of RaraDN, which in ShhCreERT2;mTmG mice resulted in reduction of the number of lineage tagged S-cells. We were, however, unable to obtain Foxa2CreERT2;mTmG; RaraDN mutants, most likely due to embryonic lethality, since Foxa2CreERT2 labels cells in the heart and vasculature which are also regulated by RA-signaling (Li et al., 2012). We therefore used the Upk3aGCE line to express the RaraDN, which our studies indicate selectively labels P-cells after a TM pulse at E11 (Fig. 4G,H). E11 Upk3aGCE;mCherry controls and Upk3aGCE;mCherry;RaraDN mutants were exposed to TM and analyzed at E18 to determine the distribution of lineage tagged intermediate and S-cells. Analysis of controls revealed abundant mCherry labeled superficial and I-cells as expected (Fig. 7M), however the number of mCherry-labeled cells in mutants was greatly reduced (Fig. 7N). This phenotype was virtually identical to that obtained in with ShhCreERT2;mTmG;DN mutants, which also express one allele of RaraDN (compare Fig. 7K and 7L). Together these results suggest that retinoids control urothelial formation by regulating P-cell specification.
RA signaling is required for urothelial regeneration
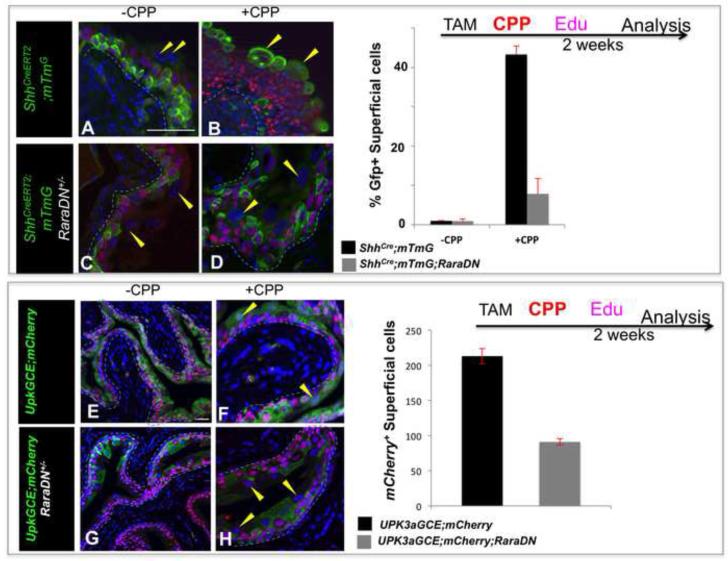
We found that RA-signaling is selectively up regulated in the urothelium following CPP-induced injury, suggesting that retinoids may control regeneration (Fig. 6). To address this further, we first used the ShhCreERT2 line, which drives expression in urothelial progenitors, to express the RaraDN. ShhCreERT2; RaraDN;mTmG mutants and ShhCreERT2;mTmG controls were treated with TM, and CPP was administered after 1 week to induce damage and repair. Controls and mutants were analyzed after 2 weeks to evaluate the distribution of lineage tagged-cells in the CPP-treated urothelium. In animals that had not received CPP, Gfp-labeled cells were predominantly in the basal and intermediate layers and a small number of lineage-tagged S-cells were detectable (Fig. 8A,C). Analysis of ShhCreERT2;mTmG controls after CPP treatment revealed expression of the Gfp-lineage tag in 40% of the superficial cell population indicating that these were daughters of Shh+ progenitors (Fig. 8B). In ShhCreERT2;RaraDN;mTmG mutants however, there were 10-fold fewer Gfp-labeled S-cells compared to controls, (Fig. 8D). These results indicate that, RA signaling is important in an Shh-expressing cell type for urothelial regeneration.
Figure 8. Retinoids are required for urothelial regeneration.
A. P63 expression in a control ShhCreERT2;mTmG adult that has not received CPP. B. P63 expression (pink) in a ShhCreERT2;mTmG adult analyzed 2 weeks after CPP treatment. C. P63 expression in a mutant ShhCreERT2;mTmG; RaraDN adult that has not received CPP. D. P63 (pink) expression in a CPP-treated ShhCreERT2;mTmG;RaraDN mutant adult analyzed 2 weeks after CPP treatment. E. P63 expression (pink) in a control Upk3aGCE;mCherry adult that has not received CPP (mCherry is shown in green). F. P63 (pink) expression in a CPP-treated Upk3aGCE;mCherry adult analyzed 2 weeks after CPP treatment. mCherry is shown in green. G. P63 (pink) expression in a Upk3aGCE;mCherry;RaraDN mutant adult that did not receive CPP. mCherry is shown in green. H. P63 (pink) expression in a Upk3aGCE;mCherry RaraDN mutant 2 weeks after CPP treatment. mCherry is shown in green. For quantification a minimum of three independent experiments were performed, and the average ± SEM was plotted. Magnifications: A-H 20X. Scale bars 50μm.
To evaluate the requirement for RA-signaling in I-cells, which our studies suggest are superficial cell progenitors, we performed CPP-induced injury using Upk3aGCE;mCherry as a lineage marker. Upk3aGCE;mCherry mice were treated with TM then 1 week later with CPP. Edu was administered 48h after CPP treatment to label proliferating cells. Analysis of Upk3aGCE;mCherry control mice 2 weeks after CPP treatment revealed extensive mCherry labeling in the intermediate and superficial cell populations (Fig. 8E,F). In Upk3aGCE;mCherry; RaraDN mutants however, the overall numbers of S-cells were reduced by about 40% compared to controls and the proportion of mCherry-labeled S-cells was also reduced by nearly 50% (Fig. 8G,H), suggesting that RA-signaling is normally required in I-cells for regeneration of the adult urothelium. As expected, we did not observe defects in regeneration in Krt5CreERT2;RaraDN mutants (data not shown).
Previous studies suggest that K5-BCs, which are progenitors in skin and other stratified epithelia, are also progenitors in the adult urothelium (Shin et al., 2011). We show by fate mapping however, that K5-BCs are unlikely to be progenitors either in the embryo or adult regenerating urothelium. Our studies suggest that formation and regeneration of the urothelium depends on distinct progenitor populations: P-cells a transient cell type abundant during early stages of urothelial development, and I-cells that serve as progenitors in the adult regenerating urothelium. We show that retinoids, potent signaling molecules that regulate specification and self-renewal of ES cells and other progenitor cell types, are required in P-cells for their specification during development, and in I-cells for regeneration in response to injury. The identification of novel urothelial progenitors and the observation that urothelial formation and regeneration depends on retinoid signaling in these progenitors could have important implications for tissue engineering and repair. Ultimately, these findings may lead to treatments that prevent loss of the urothelial barrier associated with chronic injury, a major cause of voiding dysfunction and bladder pain syndrome in humans.
DISCUSSION
Recent studies indicate that the adult urothelium contains a population of Shh-expressing cells that have long term regenerative potential and these cells have been proposed to be K5-BCs (Kurzrock et al., 2008; Shin et al., 2011; Thangappan and Kurzrock, 2009). Our fate mapping studies, however, suggest that K5-BCs rarely if ever produce intermediate or S-cells and that the intermediate/superficial cell compartment arises from a separate lineage. We show that P-cells, are transient progenitors in the embryonic urothelium, and we show that I-cells are superficial cell progenitors in the regenerating adult urothelium (see model). Retinoids are potent transcriptional regulators that can induce embryonic stem cells to form urothelial cells in vitro (Mauney et al., 2010). Our studies demonstrate that impaired RA-signaling leads to loss of the intermediate and superficial cell populations during development due to failure in P-cell specification, and, and we find that RA-signaling is also important in I-cells in adults, for regeneration after injury. That K5-BCs are unlikely progenitors in the embryo or adult challenges the current thinking, and raises the possibility that other specialized epithelia may develop from novel progenitor populations.
K5-BC cells and the intermediate/superficial populations originate from different lineages
Lineage studies using Krt5CreERT2;mTmG to permanently label K5-BCs and their daughters indicate that K5-BCs may self-renew, but rarely if ever generate other urothelial cell types. On the other hand, we find that neither P-cells nor I-cells can generate K5-BC daughters in lineage studies, suggesting that the intermediate/superficial cell compartment and K5-BCs are derived from independent lineages. Recent fate mapping studies using a constitutive P63Cre line [DeltaNp63(+/Cre);ROSA26(EYFP)] to label P63-expressing cells and their daughters, suggest that K5-BCs and the intermediate/superficial cell populations arise from a common progenitor (Pignon et al., 2013). However, since the Cre line is constitutive, it will likely label P63-expressing endodermal population that generates epithelia lining the digestive, respiratory and genital and urinary tract epithelia. Krt5-expressing cells are present in the urethra at early stages of bladder urogenital sinus development, but are only detected in the bladder after I-cells and S-cells form. Whether urothelial K5-BCs derive from these urethral Krt5-expressing cells is an interesting possibility.
RA-dependent transcription regulates multiple steps of urothelial development and regeneration
The endoderm is patterned along the rostro-caudal axis to generate a number of organs, including the thyroid, thymus, lung, stomach, intestine, pancreas, and the bladder, and retinoids have been shown to be important in endoderm for establishing this regional patterning and cell type specification (Bayha et al., 2009). An example of the multiple functions of RA-signaling in organ formation is the pancreas, where RA acts at the stage of specification (Martin et al., 2005; Molotkov et al., 2005) and at later stages, is required for formation of insulin-producing beta cells (Dalgin et al., 2011; Stafford and Prince, 2002). In addition, RA can also induce stem cells in culture to differentiate into pancreatic cell types culture (Shim et al., 2007). Retinoids may act in a similar manner in the urothelium. Retinoids induce ES cells to differentiate into urothelial cell types (Mauney et al., 2010) and our studies suggest RA signaling controls specification of P-cell progenitors in the embryonic urothelium and I-cell progenitors during regeneration. It would not be surprising if RA is also an important regulator of I- cells in the steady state adult urothelium, a question that we will address in future studies.
Retinoids control pluripotency and specification of progenitors and stem cell populations (Soprano et al., 2007; Wang et al., 2011; Wichterle and Peljto, 2008). Recent studies suggest that this RA regulates the state change from pluripotency/self-renewal to differentiation via an epigenetic mechanism in which RA-binding to Rar/Rxr complexes in regulatory regions of target genes relieves polycomb repression by inducing a conformational change in the RA-receptors [reviewed in: (Gudas and Wagner, 2011)]. This RA-induced conformational change is mediated by the ligand dependent activating domain (AF2) which is deleted in the RaraDN mutant receptor, hence it would not be surprising if RaraDN expression in urothelial progenitors inhibited their ability to undergo a state change. It will be interesting to determine whether RA signaling acts by positively regulating sets of target genes in urothelial progenitors or by relieving repression. The identification of novel urothelial progenitors whose specification is regulated by retinoids, could have important implications for tissue engineering and repair, and ultimately, may lead to treatments that prevent loss of the urothelial barrier, a major cause of voiding dysfunction and bladder pain syndrome in humans.
EXPERIMENTAL PROCEDURES
Mice
Animals were housed in the animal facility of Irving Cancer Research Center, Columbia University; all animal works were approved by IACUC protocol. Littermates were used for all experiments in which wild type and mutant embryos were compared and n=3 animals were analyzed unless otherwise specified.
Chemical injury
For chemical injury, cyclophosphamide (CPP, Sigma Cat#: C7397) was dissolved in PBS (15 mg/ml) and given to mice at a dose of 150 mg /kg by IP injection.
Proliferation
Mice were injected IP with EdU at dose of 0.1 mg/20g. Proliferating cells were detected on frozen sagittal bladder sections according to the manufacturers protocol (Click-iT® EdU cell proliferation assay kit, Invitrogen Cat#: C-10419).
Histology, immunohistochemistry, and non-radioactive in situ hybridization
Tissues were fixed o/n with 4% PFA. Cryosections were 7μm and 14μm for immunostaining and in situ hybridization, respectively. In situ hybridization analysis with digoxigenin-labeled riboprobes was essentially as described elsewhere (Mendelsohn et al., 1999).
Supplementary Material
Highlights.
The urothelium regenerates, but can be damaged by chronic exposure to toxins or UTI
We identify progenitors critical for urothelial formation and regeneration
Development and repair utilize two separate populations of progenitors
RA is critical in urothelial progenitors for specification and regeneration
ACKNOWLEDGEMENTS
We thank Fanghua Li and Jean-Marc Garnier for technical assistance, Indira Mysorekar, Doris Herzlinger Frank Costantini and Lori Sussel for discussions and critical reading of the manuscript, Xue-Ru Wu for the Upk2 promoter construct, Jeff Whitsett for the Foxa2 antibody. This work was supported by grants from NIDDK and the TJ Martell foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- Bayha E, Jorgensen MC, Serup P, Grapin-Botton A. Retinoic acid signalingorganizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE. 2009;4:e5845. doi: 10.1371/journal.pone.0005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Bolado J, Jr., Moreno TA, Kintner C, Evans RM, Papalopulu N. An essential role for retinoid signaling in anteroposterior neural patterning. Development. 1997;124:373–379. doi: 10.1242/dev.124.2.373. [DOI] [PubMed] [Google Scholar]
- Chia I, Grote D, Marcotte M, Batourina E, Mendelsohn C, Bouchard M. Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development. 2011;138:2089–2097. doi: 10.1242/dev.056838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgin G, Ward AB, Hao LT, Beattie CE, Nechiporuk A, Prince VE. Zebrafish mnx1 controls cell fate choice in the developing endocrine pancreas. Development. 2011;138:4597–4608. doi: 10.1242/dev.067736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K, Heyman RA, Umesono K, Evans RM. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:2989–2993. doi: 10.1073/pnas.90.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsund T, Dahl E. Cell kinetics of mouse urinary bladder epithelium. III. A histologic and ultrastructural study of bladder epithelium during regeneration after a single dose of cyclophosphamide, with special reference to the mechanism by which polyploid cells are formed. Virchows Archiv B: Cell pathology. 1978;26:215–223. [PubMed] [Google Scholar]
- Frank DU, Elliott SA, Park EJ, Hammond J, Saijoh Y, Moon AM. System for inducible expression of cre-recombinase from the Foxa2 locus in endoderm, notochord, and floor plate. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:1085–1092. doi: 10.1002/dvdy.21093. [DOI] [PubMed] [Google Scholar]
- Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. Journal of cellular physiology. 2011;226:322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen- inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost SP. Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57:27–36. doi: 10.1007/BF02899062. [DOI] [PubMed] [Google Scholar]
- Kashyap V, Gudas LJ, Brenet F, Funk P, Viale A, Scandura JM. Epigenomic reorganization of the clustered Hox genes in embryonic stem cells induced by retinoic acid. J Biol Chem. 2011;286:3250–3260. doi: 10.1074/jbc.M110.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal P, Abraham SN, Apodaca G. Cell Biology and Physiology of the Uroepithelium. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelial stem cells. Am J Physiol Renal Physiol. 2008;294:F1415–1421. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Li P, Pashmforoush M, Sucov HM. Mesodermal retinoic acid signaling regulates endothelial cell coalescence in caudal pharyngeal arch artery vasculogenesis. Dev Biol. 2012;361:116–124. doi: 10.1016/j.ydbio.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FX, Bosland MC, Huang H, Romih R, Baptiste S, Deng FM, Wu XR, Shapiro E, Sun TT. Cellular basis of urothelial squamous metaplasia: roles of lineage heterogeneity and cell replacement. J Cell Biol. 2005;171:835–844. doi: 10.1083/jcb.200505035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dolle P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Ramachandran A, Yu RN, Daley GQ, Adam RM, Estrada CR. All-trans retinoic acid directs urothelial specification of murine embryonic stem cells via GATA4/6 signaling mechanisms. PloS one. 2010;5:e11513. doi: 10.1371/journal.pone.0011513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126:1139–1148. doi: 10.1242/dev.126.6.1139. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post- implantation development [see comments] Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci U S A. 2013;110:8105–8110. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaii F, Bitzer ZT, Xu Q, Sockanathan S. Expression of the dominant negative retinoid receptor, RAR403, alters telencephalic progenitor proliferation, survival, and cell fate specification. Dev Biol. 2008;316:371–382. doi: 10.1016/j.ydbio.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, et al. Non-cell-autonomous retinoid signaling is crucial for renal development. Development. 2010;137:283–292. doi: 10.1242/dev.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarut E, Rochette-Egly C. Nuclear retinoic acid receptors: conductors of the retinoic acid symphony during development. Mol Cell Endocrinol. 2012;348:348–360. doi: 10.1016/j.mce.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R, Kim JH. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano DR, Teets BW, Soprano KJ. Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitamins and hormones. 2007;75:69–95. doi: 10.1016/S0083-6729(06)75003-8. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Thangappan R, Kurzrock EA. Three clonal types of urothelium with different capacities for replication. Cell Prolif. 2009;42:770–779. doi: 10.1111/j.1365-2184.2009.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang J, Liu H, Lu D, Chen X, Zenonos Z, Campos LS, Rad R, Guo G, Zhang S, et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18283–18288. doi: 10.1073/pnas.1100893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Vertebrate endoderm development. Annual review of cell and developmental biology. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Peljto M. Differentiation of mouse embryonic stem cells to spinal motor neurons. Current protocols in stem cell biology Chapter. 2008;1 doi: 10.1002/9780470151808.sc01h01s5. Unit 1H 1 1-1H 1 9. [DOI] [PubMed] [Google Scholar]
- Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009;75:1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndaele JJ, De Wachter S. The basics behind bladder pain: a review of data on lower urinary tract sensations. Int J Urol. 2003;10(Suppl):S49–55. doi: 10.1046/j.1442-2042.10.s1.11.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.