Abstract
Aster yomena is used in traditional remedies to treat cough, asthma and insect bites; however, its therapeutic mechanism is not completely understood. To elucidate the anti-asthmatic effect of A. yomena, we investigated the anti-asthmatic characteristics of an alcohol extract of A. yomena in an ovalbumin (OVA)-induced murine asthma model. In this study, we showed that A. yomena extract inhibited the overall pathophysiological features of asthma by suppressing Th2 responses and enzymes associated with the production of inflammatory mediators. This suppression resulted in decreased Th2 type cytokines and eosinophils in the bronchoalveolar lavage fluid and OVA-specific IgE in serum. Additionally, A. yomena extract significantly decreased airway hyperresponsiveness and abrogated the histopathological changes in the lungs, which reached normal levels in the OVA-challenged mice treated with A. yomena extract. These findings suggest that A. yomena could be a promising natural agent for treating bronchial asthma in humans.
Key Words: : airway hyperresponsiveness, Aster yomena, OVA-induced asthma
Introduction
Allergic asthma is defined as a chronic respiratory disease characterized by airway inflammation, persistent airway hyperresponsiveness (AHR), and reversible airway obstruction.1,2 Allergic airway inflammation is thought be driven by an imbalance of Th1/Th2 cytokine production, leading to the production of IgE and recruitment of eosinophils in the airways.3 Based on a growing understanding of the pathogenesis of allergic asthma, various management strategies, including inhaled corticosteroids and β2 agonists, are focused primary on decreasing inflammation and relaxing bronchoconstriction.4 However, these therapies often result in undesirable systemic side effects, especially when used in large doses over an extended period of time.5 Thus, researchers continue to seek and evaluate additional therapeutics with fewer side effects, preferably of natural origin, for the treatment of allergic asthma.6,7
Aster yomena, a Korean edible vegetable, is a perennial herb found in Korea, China, Japan, and Siberia, that is used as a type of folk medicine to treat cough, asthma, and insect bites. Whereas A. yomena has been shown to have antioxidant activity,8,9 there are little experimental data describing its anti-asthmatic effects. To further characterize the anti-asthmatic effect of A. yomena, we used an ovalbumin (OVA)–induced murine asthma model to investigate the anti-inflammatory and anti-asthmatic characteristic effects of A. yomena. In this study, we showed that A. yomena clearly inhibited overall pathophysiological features of asthma by suppressing Th2 responses.
Materials and Methods
Animals
Six-week-old female Balb/c mice were purchased from Joongang Laboratory Animal Co. (Seoul, Republic of Korea) and maintained under specific pathogen-free conditions in the animal facility at Seoul National University College of Medicine. All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Institute (IACUC) of Laboratory Animal Resources at Seoul National University.
Preparation of plant material
A. yomena was obtained from the Plant Extract Bank (Daejeon, Republic of Korea). Dried A. yomena was milled into powder and extracted with 70% ethanol by stirring for 24 h at room temperature (RT). The extract was filtered with a 0.45 μm filter, and the filtrates were lyophilized with a freeze dryer. The dry residue was reconstituted with phosphate-buffered saline (PBS) to the desired final concentration.
Gas chromatograph and mass spectrometry analysis
An Agilent 6890/5975 inert gas chromatograph and mass spectrometer (GC-MS) system (Agilent Technologies, Palo Alto, CA, USA) was used to analyze the fingerprint of A. yomena extract. The extracts were separated by a 6890-N GC on a DB-5MS capillary column (60 m length×250 μm internal diameter, 1.4 μm film thickness) with helium as the carrier gas with pressure-controlled flow set at 1 mL/min. The injection port was set at 250°C, the oven was set on a gradient as follows: 50–150°C at 10°C/min, 150–200°C at 7°C/min, and 200–250°C at 5°C/min. The samples were injected in split mode as 10:1 and were submitted to electrospray ionization and detected by a 5975 MS (mass scan range, 29–800 amu). The mass spectrum for each peak was compared with the compounds in the library (Agilent Data Analysis software; Agilent Technologies) of known spectral data for compound identification.
Sensitization and challenge
Mice were divided into the following groups: (1) sham sensitization (hereinafter referred to as “controls”) plus challenge with PBS, (2) sensitization with sterile lipopolysaccharide (LPS)-free ovalbumin (OVA; Sigma-Aldrich, St. Louis, MO, USA) by intraperitoneal injection (i.p.) plus challenge with OVA (intranasal [i.n.]) and PBS by oral administration (p.o.), and (3) sensitization with OVA plus challenge with OVA and Aster yomena extract (1g/kg) p.o. Briefly, mice were actively sensitized with 75 μg OVA (i.p.) emulsified in 2 mg aluminum hydroxide (Sigma-Aldrich) in 200 μL PBS on days 0 and 7. Starting on day 14, mice were challenged with 50 μg OVA (i.n.) in 20 μL PBS on days 14–16 and 21–23. Mice were treated with A. yomena extract (1g/kg p.o.) via a stainless steel needle in 200 μL PBS 1 h before each of the OVA challenges on days 12–16 and 19–23. One day after the last challenge, the methacholine bronchial provocation test (MBPT) was used to assess airway function, and then mice were sacrificed to determine the pathophysiological features of asthma.
AHR measurement
Whole-body plethysmography (Buxco, Troy, NY, USA) was used to measure the AHR to increasing doses of nebulized methacholine (Mch, Sigma-Aldrich) administered by an ultrasonic nebulizer (NE-U12, Omron, Japan) as described previously.10 The quantified alterations were expressed as enhanced pause (Penh) as a main indicator of airway obstruction. Penh is directly correlated with airway resistance in the animal. Aerosolized Mch was nebulized through the inlet of the main chamber for 2 min, and the response to each dose was subsequently measured for 3 min. The average Penh for 3 min was used to compare the results among experimental groups.
Bronchoalveolar lavage (BAL) analysis
BAL fluid (BALF) inflammatory cells were obtained as described previously.10 Briefly, the tracheas of anesthetized mice were exposed and cut just below the larynx. A polyurethane flexible tube was placed 6 mm into the trachea, and the lung was flushed twice with the same bolus of sterile PBS. BAL was performed using 1 mL of saline, with a BALF return average of 80%. BALF samples were centrifuged at 1000 g at 4°C for 10 min. The cell pellets were resuspended in PBS and centrifuged onto microscope slides at 800 g for 10 min using cytospin. Slides were air-dried, fixed for 10 min in a synthetic mountant (Histomount™, Ted Pella, Redding, CA, USA) and stained with Diff-Quik (Sysmex, Kobe, Japan). A minimum of 100 cells/slide were examined under a light microscope to obtain a differential leukocyte count, and were classified as macrophages, lymphocytes, neutrophils, or eosinophils.10
Measurement of cytokine levels in BALF by enzyme-linked immunosorbent assay and flow cytometry
Interleukin (IL)-4, IL-13, and IL-17 levels in the BALF supernatant were quantified by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. To assess intracellular cytokine production, BAL cells were stimulated with 50 ng/mL phorbol-12-myristate-13-acetate (PMA) and incubated with 1 μg/mL ionomycin in the presence of Golgiplug™ (BD Biosciences, San Diego, CA, USA) for 4 h at 37°C. Cells were fixed, permeabilized and stained with anti-mouse CD4, IL-13 Abs or isotype controls. Samples were collected using BD LSRII® flow cytometer (BD Biosciences) and collected data were analyzed using the FlowJo® ver. 9.6 software (Tree Star, Ashland, OR, USA).
Determination of serum OVA-specific IgE
The presence of serum OVA-specific IgE was determined by ELISA. Briefly, each well of a microtiter plate was coated with 100 μL OVA (50 μg/mL) and incubated at 4°C overnight. After blocking with 3% skim milk, the wells were incubated at room temperature for 2 h with 50 μL of diluted mouse serum for IgE. After washing, 100 μL diluted biotin-labeled goat anti-mouse IgE Abs were added to the wells and incubated for 1 h at room temperature. The wells were incubated with diluted streptavidin-peroxidase for 1 h, followed by incubation with 100 μL 3,3′,5,5′–tetramethylbenzidine for 10 min at room temperature. The absorbance at 450 nm was then read using an automated microplate reader.
Histopathological study
Lungs were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 3-μm sections. Lung sections were stained with hematoxylin and eosin (H&E) for histopathological analysis. Image acquisition and processing were performed using a Leica microscope and the Leica Application Suite (Leica Microsystems, Buffalo Grove, IL, USA).
Reverse transcription polymerase chain reaction assays
Total RNA was isolated from lung tissues with a TRIzol® Plus RNA Purification Kit (Life Technologies, Grand Island, NY, USA), and cDNA was synthesized using a Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science, Indianapolis, IN, USA). Conventional PCR products were generated with the Hotstar Taq Master Mix Kit (QIAGEN, Valencia, CA, USA) using specific primers.
Statistical analysis
All data are expressed as means±SEM. Data were compared using the unpaired two-tailed Student's t-test using GraphPad Prism ver. 5.01 (GraphPad Software, La Jolla, CA). P<.05 were considered to indicate statistical significance.
Results and Discussion
A. yomena ethanol extract suppresses airway inflammation in a murine asthma model
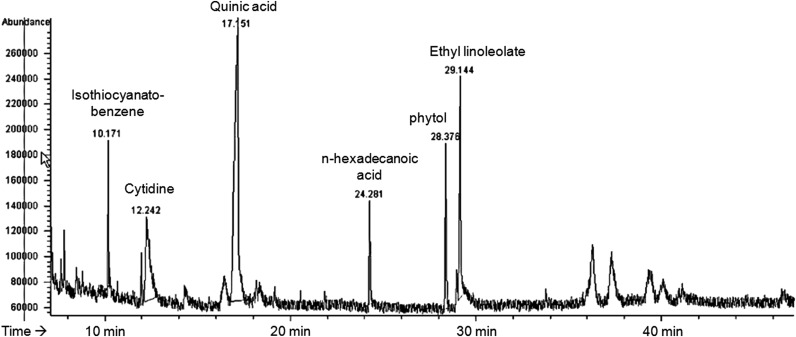
A. yomena ethanol extract was prepared for GC analyses using GC-MS. Peaks within chromatograms were identified by comparison to the retention times of standards and by parallel GC-MS analysis. The fingerprint of A. yomena ethanol extract showed a chromatographic profile with peaks with retention times of 10–30 min (Fig. 1). GC-MS analysis of A. yomena ethanol extract revealed the existence of the isothiocyanato-benzene, cytidine, quinic acid, n-hexadecanoic acid, phytol, and ethyl linoleolate (Fig. 1).
FIG. 1.
Fingerprint of an ethanol extract of Aster yomena. GC-MS analysis of A. yomena showed the existence of the isothiocyanato-benzene, cytidine, quinic acid, n-hexadecanoic acid, phytol, and ethyl linoleolate compared with known spectral data. GC-MS, gas chromatography/mass spectrometry.
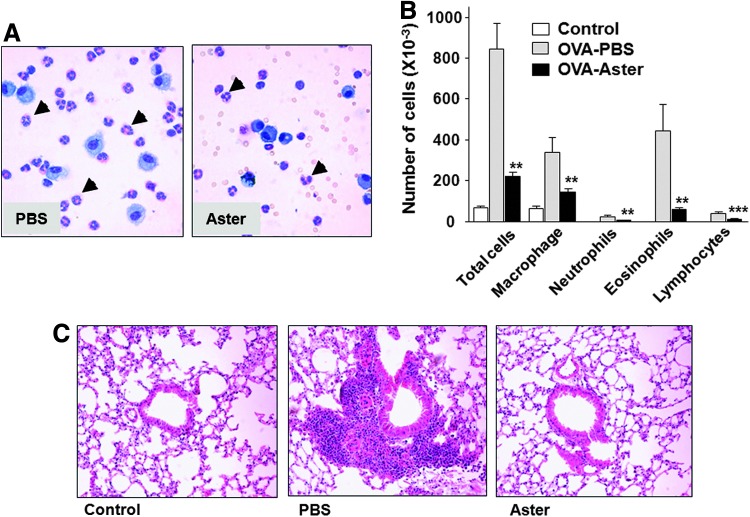
Then, we evaluated the effect of A. yomena on eosinophilia in BALF in the murine asthma model by measuring the total numbers and types of cells present. The OVA-induced murine model is a well-described allergic asthma model that reproduces the airway eosinophilia, pulmonary inflammation, Th2-biased immunological responses and elevated IgE levels found during asthma attacks.11 In OVA-challenged mice administered PBS, numbers of inflammatory cells, especially macrophages and eosinophils, were considerably increased compared to control mice (Fig. 2A, B), indicating that our asthma model was well established. In contrast, the number of total cells, eosinophils and other inflammatory cells in BALF were markedly decreased in A. yomena extract–treated mice compared with PBS-treated mice (Fig. 2A, B). Moreover, histological analysis revealed an anti-asthmatic effect of A. yomena as demonstrated by pulmonary infiltration of leukocytes in the peribronchiole and mucous production. Administration of A. yomena extract led to reduced accumulation of inflammatory cells in the peribronchiole compared with those in PBS-treated OVA-challenged mice (Fig. 2C), indicating that A. yomena extract inhibits airway inflammation in allergic asthma.
FIG. 2.
Decreased numbers of cells in bronchoalveolar lavage (BAL) fluid and histopathological changes in ovalbumin (OVA)-challenged mice treated with an ethanol extract of A. yomena. (A) Representative photographs of a differential cell count by Diff-Quik staining. Bronchoaveolar lavage fluid (BALF) cells from phosphate-buffered saline–treated (PBS) or A. yomena extract–treated (Aster) OVA-challenged mice were centrifuged onto microscope slides and stained with Diff-Quik. Leukocytes were classified as macrophages, lymphocytes, neutrophils, or eosinophils (arrow head). Magnification, ×400. (B) Differences between OVA-challenged mice treated with PBS (gray) and those treated with A. yomena extract (black) were evaluated by an unpaired, two-tailed Student's t-test (**P<.01, ***P<.001). Data are expressed as means±SEM (n=5–8). (C) Lung histology was examined in control mice and PBS-treated or A. yomena extract–treated OVA-challenged mice by hematoxylin and eosin (H&E) staining of paraffin-embedded sections. Magnification, ×200. Color images available online at www.liebertpub.com/jmf
A. yomena extract reduces AHR in the murine asthma model
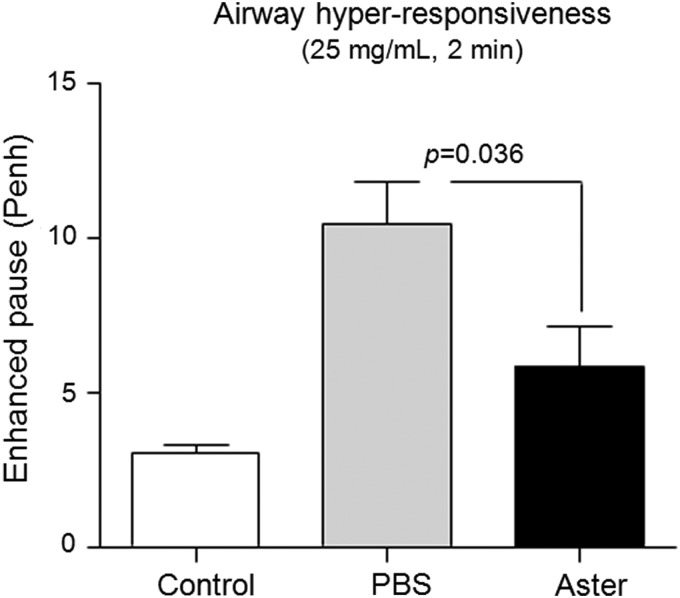
To investigate whether administration of A. yomena extract affected AHR, we performed whole-body plethysmography by measuring the Penh response to nebulized Mch. AHR was significantly lower in A. yomena extract–treated mice compared to PBS-treated mice (Fig. 3). These data were consistent with the histopathology of mouse lungs treated with A. yomena extract.
FIG. 3.
Analysis of airway hyperresponsiveness (AHR) to methacholine in mice exposed to OVA or control (open shapes). One day after the last OVA challenge, airway function was evaluated by treating with 25 mg/mL nebulized methacholine. The average enhanced pause (Penh) for 3 min was used to compare the results from PBS-treated (gray) and A. yomena extract–treated (black) OVA-challenged groups using the unpaired two-tailed Student's t-test. Data are expressed as means±SEM (n=5–8).
A. yomena extract suppresses Th2 responses in the murine asthma model
The immunological responses involved in asthma are characterized by proliferation and activation of Th2 cells, setting off an allergic cascade that includes IgE synthesis, chemokine production, airway eosinophilia, smooth muscle hyperplasia, mucus production, and AHR.3 In particular, Th2-type cytokines may stimulate mucus overproduction and hypersecretion in the lung.12 In addition to Th2-type cytokines, Th17 cells enhance Th2 cell-mediated eosinophilic as well as neutrophilic airway inflammation in the murine asthma model.13
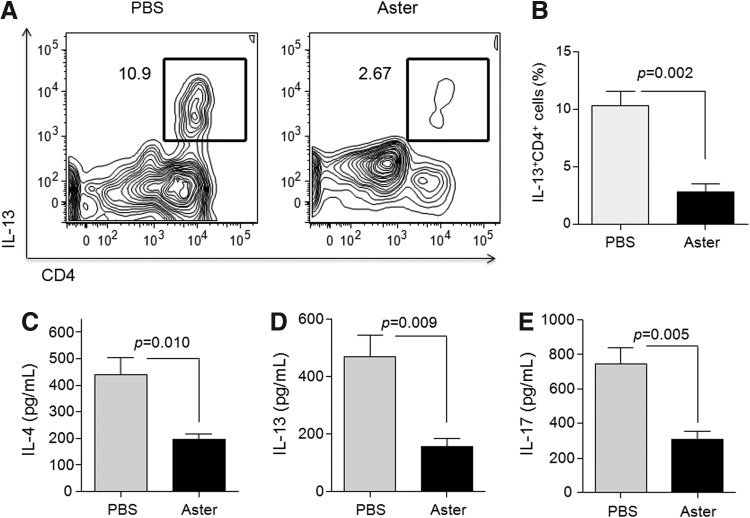
We measured the levels of Th2 cytokines and IL-17 accompanying asthma in BALF to determine the mechanisms underlying A. yomena extract–mediated inhibition of airway inflammation. The frequency of IL-13-producing CD4+ T cells in BAL was significantly decreased in A. yomena extract–treated mice compared to PBS-treated mice (Fig. 4A, B). Moreover, administration of A. yomena extract significantly suppressed the increased levels of IL-4, IL-13, and IL-17 in the BALF of OVA-challenged mice, compared to those in PBS-treated mice (Fig. 4C, D).
FIG. 4.
Effect of A. yomena extract on cytokine production by BAL cells. (A) Representative dot plot of the expression of intracellular interleukin (IL)-13 in CD4+ T cells. (B) The frequencies of IL-13-producing CD4+ T cells in PBS-treated versus A. yomena extract–treated mice are shown. Data are expressed as means±SEM (n=4). (C-E) IL-4, IL-13, and IL-17 levels in the BAL fluid (BALF) were measured by enzyme-linked immunosorbent assay (ELISA). Data are expressed as means±SEM (n=3–5). P values were calculated using an unpaired two-tailed Student's t-test.
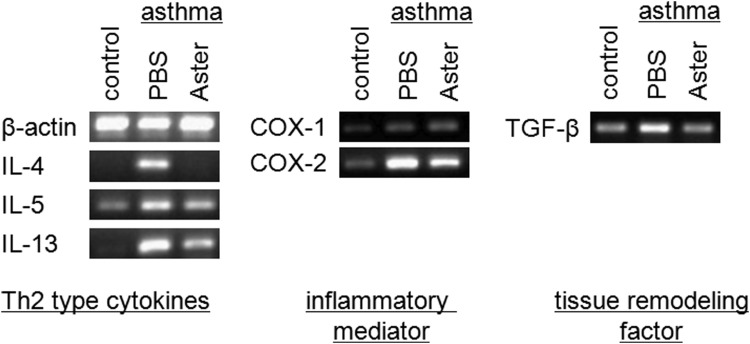
We further investigated the mechanism underlying the reduced pulmonary inflammation in A. yomena extract–treated OVA-challenged mice by determining the expression of several genes in the lung: Th2-type cytokines (e.g., IL-4, IL-5, and IL-13), tissue remodeling factor (e.g., TGF-β), and enzymes (e.g., COX-1 and COX-2). Interestingly, induction of TGF-β expression was found in the BALF from OVA-induced mice,14 suggesting a potential mechanism for airway remodeling by the deposition of extracellular matrix proteins such as collagen. The COX-1 isoform is expressed constitutively, whereas the COX-2 isoform is an inducible agonist that plays a critical role in the production of proinflammatory lipid mediators such as 5-lipoxygenase, arachidonic acid products and leukotrienes. Patients with allergic inflammation and asthma showed elevated expression of COX-2.15 As a consequence of IgE-mediated mast cell degranulation after bronchial antigen challenge in allergic asthmatics, increased levels of leukotrienes are found in the BALF of asthmatic patients.16 In this study, the expression levels of Th2-type cytokines, COX-2 and TGF-β were markedly decreased in the lungs of A. yomena extract–treated OVA-challenged mice compared to those treated with PBS (Fig. 5), suggesting that A. yomena extract may ameliorate the pathophysiological features of asthma in the lung by suppressing the inflammatory mediators and factors involved in tissue remodeling.
FIG. 5.
Effect of A. yomena extract on gene expression in lung tissues from OVA-induced asthmatic mice. Gene expression in lung tissues was assayed by reverse-transcription polymerase chain reaction (RT-PCR). Representative RT-PCR products for IL-4, IL-5, IL-13, COX-1, COX-2, TGF-β, and β-actin (internal control) are shown.
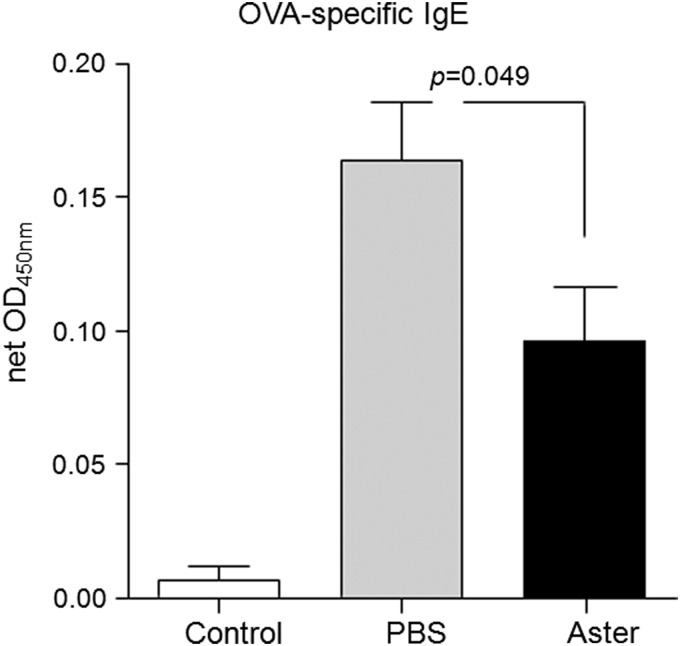
The production of specific or total IgE is strongly associated with asthma, indicating that IgE is a crucial factor in the systemic asthmatic response.17 In this study, we assayed serum IgE levels in OVA-challenged mice by ELISA to investigate the systemic effect of A. yomena extract. IgE induced by Th2-type cytokines (primarily IL-4 and IL-5) in an allergic response stimulate eosinophilopoiesis, regulate eosinophil functions, and promote the growth of mucosal-type mast cells. As expected, serum IgE levels were significantly decreased in A. yomena extract–treated OVA-challenged mice compared with those treated with PBS (Fig. 6).
FIG. 6.
Lower serum IgE levels in OVA-induced mice treated with A. yomena extract. Serum IgE levels were measured by ELISA. Results are expressed as net optical density (OD) by subtracting background OD. Data are expressed as means±SEM (n=5–8). P values were calculated using an unpaired two-tailed Student's t-test.
Conclusion
Our data demonstrate that the ethanol extract of the herb A. yomena suppressed pulmonary inflammation and AHR, key characteristics of asthma, in the OVA-induced asthma model by suppressing Th2 responses. We are currently determining the exact composition of A. yomena extract to determine the factors involved in this inhibition. Our findings raise the clinically important possibility that A. yomena extract could be an effective natural anti-asthmatic agent in humans.
Acknowledgments
This work was supported in part by the Cooperative Research Program for Agriculture Science & Technology Development (PJ007492 to H.-R.K.), the Rural Development Administration, Republic of Korea, a grant from the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs (A103001 to H.-R.K.) and a National Research Foundation of Korea Grant funded by the Korean Government (2011-0006498 to H.-R.K.) as well as the Seoul National University Hospital Research Fund (2012-1280 to H.-R.K.).
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: www.textcheck.com/certificate/rYKcxF.
Author Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Wills-Karp M: Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 1999;17:255–281 [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF, Jr: Asthma. N Engl J Med 2001;344:350–362 [DOI] [PubMed] [Google Scholar]
- 3.Renauld JC: New insights into the role of cytokines in asthma. J Clin Pathol 2001;54:577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corry DB: Emerging immune targets for the therapy of allergic asthma. Nat Rev Drug Discov 2002;1:55–64 [DOI] [PubMed] [Google Scholar]
- 5.Nelson HS: Is there a problem with inhaled long-acting beta-adrenergic agonists? J Allergy Clin Immunol 2006;117:3–16; quiz 17. [DOI] [PubMed] [Google Scholar]
- 6.Li XM: Treatment of asthma and food allergy with herbal interventions from traditional chinese medicine. Mt Sinai J Med 2011;78:697–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Natarajan S, Bae H, Jung SK, Cruikshank W, Remick DG: Herbal medicine treatment reduces inflammation in a murine model of cockroach allergen-induced asthma. Ann Allergy Asthma Immunol 2011;107:154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng TB, Liu F, Lu Y, Cheng CH, Wang Z: Antioxidant activity of compounds from the medicinal herb Aster tataricus. Comp Biochem Physiol C Toxicol Pharmacol 2003;136:109–115 [DOI] [PubMed] [Google Scholar]
- 9.Oh YC, Cho WK, Jeong YH, et al. : A novel herbal medicine KIOM-MA exerts an anti-inflammatory effect in LPS-stimulated RAW 264.7 macrophage cells. Evid Based Complement Alternat Med 2012;2012:462383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon SG, Lee CG, Oh MH, et al. : Recombinant basic fibroblast growth factor inhibits the airway hyperresponsiveness, mucus production, and lung inflammation induced by an allergen challenge. J Allergy Clin Immunol. 2007;119:831–837 [DOI] [PubMed] [Google Scholar]
- 11.Kumar RK, Herbert C, Foster PS: The “classical” ovalbumin challenge model of asthma in mice. Curr Drug Targets 2008;9:485–494 [DOI] [PubMed] [Google Scholar]
- 12.Hauber HP, Foley SC, Hamid Q: Mucin overproduction in chronic inflammatory lung disease. Can Respir J 2006;13:327–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakashin H, Hirose K, Maezawa Y, et al. : IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 2008;178:1023–1032 [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Zhang G, Qin X, et al. : Inhibition of allergen-induced airway remodeling by neonatal bacillus Calmette-Guerin vaccination is associated with interferon-gamma-producing T cells but not regulatory T cells in mice. Ann Allergy Asthma Immunol 2011;107:163–170 [DOI] [PubMed] [Google Scholar]
- 15.Pang L: COX-2 expression in asthmatic airways: the story so far. Thorax 2001;56:335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzel SE, Westcott JY, Larsen GL: Bronchoalveolar lavage fluid mediator levels 5 minutes after allergen challenge in atopic subjects with asthma: relationship to the development of late asthmatic responses. J Allergy Clin Immunol 1991;87:540–548 [DOI] [PubMed] [Google Scholar]
- 17.Platts-Mills TA: The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med 2001;164(8 Pt 2):S1–S5 [DOI] [PubMed] [Google Scholar]