Abstract
We sought to determine if single-dose external beam radiation therapy (EBRT) could modulate the expression signature of T-cell costimulatory and coinhibitory molecules in human prostate cancer (PCa) cell lines in vitro. We investigated the functional impact of irradiated PCa cells with a modulated costimulatory profile on responder T-cell activity. We used three PCa cell lines (DU145, PC3, and LNCaP) and two epithelial cell lines from noncancerous prostate and lung tissue. After 72 hours of EBRT, surface expression of four immunostimulatory molecules (CD70, CD275/ICOSL, CD134L/OX40L, and CD137L/41BBL) and two immunosuppressive markers (CTLA-4/CD152 and PD-L1/CD274) were evaluated by flow cytometry. We evaluated the impact of several radiation doses and the longevity of modulated expression. We examined the functional impact of radiation-induced modulation of cancer cells by cytotoxic T cells (CTL) cytotoxicity and ELISPOT assay for interferon-gamma (IFN-γ) production. Last, we evaluated whether IFN-γ-induced PD-L1 expression could be reversed by EBRT. After 10 Gy EBRT, expression of OX40L and 41BBL increased in all three PCa cell lines; expression of CD70 and ICOSL increased in PC3 cells. Conversely, a decrease in PD-L1 expression in DU145 and PC3 cells was detectable up to 144 hours after EBRT. No PD-L1 was detected in LNCaP. Epithelial cells from normal prostate were not modulated by radiation. CTL cytolytic activity and IFN-γ production were enhanced by interaction with irradiated PCa cells. Finally, EBRT failed to prevent IFN-γ-induced upregulation of PD-L1. We demonstrate that a single dose of EBRT increased surface expression of costimulatory molecules and decreased the expression of coinhibitory molecules in human PCa cell lines. Changes in irradiated tumor cells led to functional enhancement of T-cell activity, despite EBRT failing to reduce IFN-γ-induced expression of PD-L1. These data suggest that combining radiotherapy with T-cell stimulating immunotherapy may be an attractive strategy for cancer treatment.
Key words: : immunomodulator, immunotherapy, irradiation, preclinical study, prostate cancer
Introduction
Traditionally, radiation therapy (RT) for prostate cancer (PCa) has been employed as a tumoricidal modality, with cancer-cell death as the intended goal. Recently, a new role for RT has emerged based on its ability to stimulate antitumor immune responses. In preclinical models, RT has been shown to alter tumor-cell phenotype,1 increase the availability of released tumor-associated antigens for uptake by circulating dendritic cells,2 and increase production of inflammatory cytokines,3 all of which can contribute to tumor-specific immune responses. Clinical studies have provided further evidence that RT enhances antitumor immune responses, particularly when combined with cancer immunotherapy (CIT) strategies.4–8 CIT is becoming an essential component of the cancer treatment arsenal and was recently selected by Science as their Breakthrough of the Year for 2013. The main types of CIT being used to treat cancer fall into one of three categories, namely monoclonal antibodies (mAbs), nonspecific immunotherapies, and cancer vaccines. Over the past 15 years, the U.S. Food and Drug Administration (FDA) has approved approximately a dozen mAbs to treat certain cancers.9 Furthermore, interleukin-2, a nonspecific immunotherapy, was the first true immunotherapy agent to be used as a single agent to treat renal cell carcinoma.10–12 Interestingly, the first FDA-approved cancer vaccine was PROVENGE® for treatment of PCa.13
The objective of this study was to explore the hypothesis that exposure of PCa cells to single-dose external beam radiation therapy (EBRT) could enhance antitumor CD8+ cytotoxic T cells (CTL) activity through increased expression of costimulatory molecules and decreased expression of coinhibitory molecules. We chose two members of the tumor necrosis factor superfamily (TNFSF) of receptors that have been reported to robustly enhance CTL activity, namely OX40 ligand (OX40L/TNFSF4/CD134L/CD252) and 41BB ligand (41BBL/TNFSF9/CD137L).14 Activating signals to T cells through the cognate receptors OX40 and 41BB improves effector CTL survival, proliferation, and activity. Additionally, we examined two other T-cell activating signals, CD70 and ICOSL. The CD27-CD70 signaling promotes optimal T-cell activation of antigen-naïve T cells,15,16 while the ICOSL-ICOS interaction can efficiently stimulate proliferation, cytokine production, and effector T-cell generation.17
In addition to costimulatory molecules, T cells express several inhibitory proteins, such as CTLA-4 and PD-1.18 CTLA-4 and PD-1 deliver inhibitory signals to T cells upon ligation by CD80/86 or PD-L1, respectively.14 Blockade of these inhibitory receptors can augment T-cell function.18 In 2011 FDA approval of a CTLA-4 blocking antibody (Yervoy®/ipilimumab) for melanoma marked a major milestone for CIT, and PD-1 blocking antibodies are in clinical development.19
Thus, we sought to determine the effects of EBRT on the expression of immunostimulatory (OX40L, 41BBL, ICOSL, and CD70) and immunosuppressive (CTLA-4 and PD-L1) proteins on the surface of three human PCa cell lines and two normal epithelial cell lines in vitro. We then investigated whether irradiation of PCa cells led to enhanced T-cell activity and increased production of interferon-gamma (IFN-γ). Prior studies have reported that while the initial increase in IFN-γ production activates an immune response, it may also exert negative feedback by stimulating the expression of PD-L1.20,21 Therefore, we also tested whether EBRT could reverse this feedback loop and further provide support for the use of EBRT as an adjuvant to immunotherapy for PCa.
Materials and Methods
Cell lines
Androgen-resistant human PCa cell lines (PC3 and DU145) and an androgen-sensitive PCa cell line (LNCaP) were purchased from American Type Culture Collection. Normal prostate epithelial cells (PrECs) were purchased from Lonza. The murine total prostate-specific antigen (TPSA) cell line was created by transfection of PSA expression plasmid in the TRAMPC-1 murine prostate adenocarcinoma cell line, as previously described.22,23
Tumor irradiation
PCa cell lines were irradiated at 80%–85% confluence in 15 mL of media in a T75 flask. Cells were treated with a single fraction of 10 Gy, except for the dose-escalation experiment, where irradiation was executed in 5-Gy increments to 15 Gy in one administration. A Cs-137 source (Gammacell-1000; AECL/Nordion) at a dose rate of 0.70 Gy/min was used for all treatments.
Flow cytometric analysis
Tumor cell surface staining was performed using primary labeled antibodies matched with the appropriate isotype controls. Six immune markers were examined: four immunostimulatory (CD70-FITC, CD275/ICOS-L-PE, CD134-L/OX40-L-PE, and CD137-L/41BB-L-PE) and two immunosuppressive (CTLA-4/CD152-PE and PD-L1/CD274-PE). Flow cytometry was performed 72 hours after irradiation unless otherwise specified. Antibodies were purchased from BioLegend or BD Biosciences. PCa cell lines were treated with 10 ng recombinant human IFN-γ (R&D Systems) for 24 hours, then analyzed by flow cytometry for PD-L1. Stained cells were acquired with the Becton Dickinson D×P10 FACSCalibur, using FlowJo analysis software (BD PharMingen). Results in percent-positive cells represent the average of 3 experiments. Isotype control staining was <5% for all samples analyzed. Cell viability was >85% in all studies. Dead cells were excluded from the analysis based on scatter profile.
Functional studies
Human
The HLA-A2-restricted, CEA-specific, CD8+ cytotoxic T-cell line (designated CEA CTL) that recognizes the CEA peptide epitope YLSGANLNL (CAP-1)24 was maintained, propagated, and utilized, as previously described.25
Murine
The mouse IFN-γ ELISPOT kit (ALP) (Mabtech) was followed per protocol and performed as previously described, with slight modifications.20 Briefly, plates (Millipore) were coated overnight with anti-mouse IFN-γ mAb (clone: AN18, 15 mg/mL) and washed six times. Splenocytes were harvested from C57BL/6 mice inoculated with a PSA-based Listeria monocytogenes vaccine, which has been previously described.23
Statistical analysis
Statistical analysis was derived from a Student's t-test using a two-tailed distribution and calculated at 95% confidence. p-Values<0.05 were considered statistically significant. Significant differences in the distribution of flow cytometry analysis data were determined by the Kolmogorov–Smirnov test using CellQuest software (BD Biosciences).
Results
Single-dose EBRT modulates the molecular signature of T-cell costimulatory and coinhibitory molecules on human PCa cells
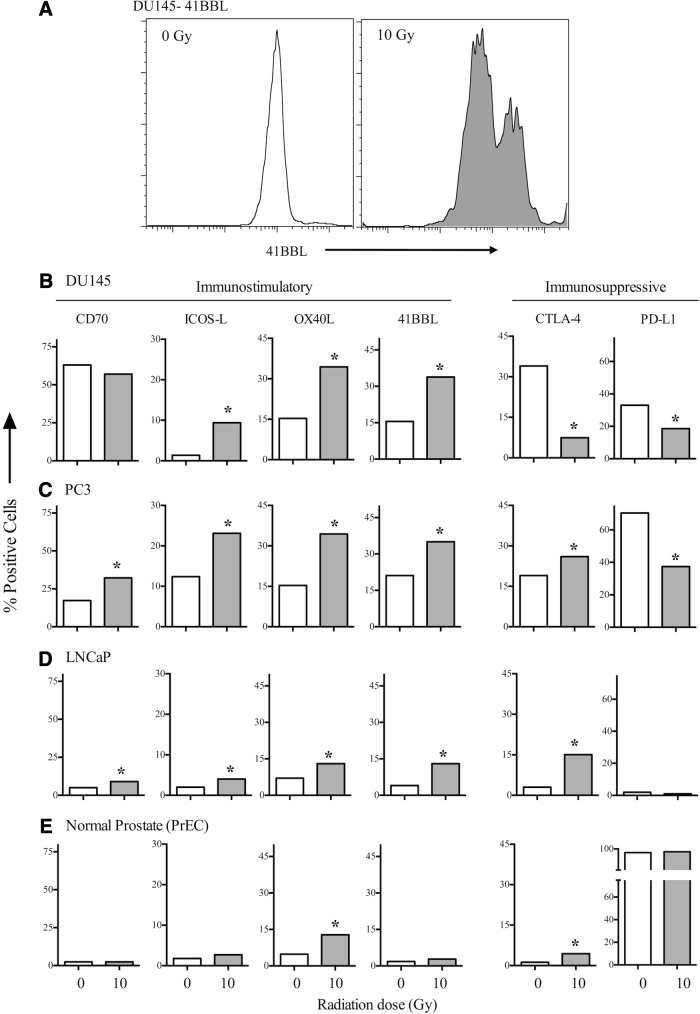
To determine whether EBRT had surface marker modulation capabilities, we began by exposing the PCa and PrEC lines to 10 Gy in a single administration. As seen in Figure 1B, a single fraction of 10 Gy induced a 2.2-fold increase in 41BBL (15.5% in untreated cells vs. 33.8% in treated cells) in DU145 cells. Additionally, there was a fivefold increase in ICOS-L and a ninefold increase in OX40L (0 Gy: 15.4% vs. 10 Gy: 34.4%; p<0.05). CD70 expression showed relatively no change in treated cells (57%) vs. untreated cells (63%). Conversely, expression of the immunosuppressive molecules CTLA-4 and PDL-1 decreased fivefold and twofold, respectively (p<0.05). Treating PC3 (Fig. 1C) and LNCaP (Fig. 1D) cells with a single fraction of 10 Gy also resulted in significant increases in the expression of immunostimulatory molecules and a significant decrease in the expression of the immunosuppressive molecule PD-L1, but not CTLA-4. To determine whether the radiation-induced modulation of costimulatory molecules was restricted to tumor cells, we examined PrECs. Interestingly, these cells' very high basal expression of PDL-1 did not change after RT (Fig. 1E). In addition, the low expression of costimulatory molecules did not significantly increase after EBRT, with the exception of OX40L, which was detected in 4.8% of untreated cells and in 12.8% of treated cells. Overall, the total level of OX40L was much lower in irradiated normal PrECs (<15%) than in irradiated DU145 and PC3 cells (>30%).
FIG. 1.
Irradiated PCa cells modulate the balance of positive and negative costimulatory molecules. (A) DU145 PCa cells were irradiated with 10 Gy or left untreated (0 Gy). Cells were analyzed after 72 hours for surface expression of 41BBL. (B) DU145 cells were irradiated with 10 Gy or left untreated (0 Gy). Numbers indicate percent-positive cells, and each molecule is graphed on an independent scale. (C) PC3 cells, (D) LNCaP cells, and (E) PrECs. *Statistical significance relative to untreated cells. PCa, prostate cancer; PrECs, prostate epithelial cells.
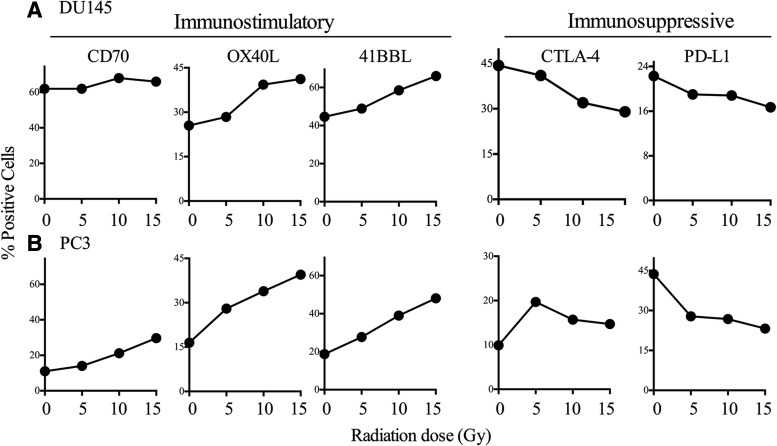
Radiation-induced changes in expression of costimulatory and coinhibitory molecules in PCa cells are dose-dependent
To determine the effect of dose on the modulation of costimulatory molecule expression, we treated DU145 and PC3 cell lines with a single fraction of 0, 5, 10, or 15 Gy EBRT. We then observed increased expression of OX40L and 41BBL and decreased expression of CTLA-4 and PD-L1 at all dose levels (Fig. 2A, B). In DU145 cells (Fig. 2A), OX40L expression increased from 25.5% to 28.4% after 5 Gy, with the largest increase seen after treatment with 10 and 15 Gy (39.3% and 41.2%, respectively). Similarly, 41BBL expression increased in a dose-dependent manner from 42% in untreated cells to 58.5% and 66% (p=0.02) after treatment with 10 and 15 Gy, respectively. Expression levels of both CTLA-4 and PD-L1 decreased as the dose increased. As seen with single-dose treatment (Fig. 1), expression of CD70 was not modulated by EBRT doses above 10 Gy (Fig. 2A). In PC3 cells (Fig. 2B), OX40L expression increased after EBRT doses as low as 5 Gy and doubled after 10 Gy (33.9%) and 15 Gy (39.5%) compared with no treatment (16.5%). Similarly, expression of 41BBL increased from 18.7% to 27.7% after 5 Gy and more than doubled after 10 Gy (39%) and 15 Gy (48.1%) (p=0.04). Unlike DU145 cells, in PC3 cells, CD70 expression increased with each 5-Gy increase in radiation: from 11.2% in untreated cells to 14%, 21.2% (p=0.02), and 29.7% (p=0.009). On the other hand, expression of PD-L1 decreased in PC3 cells from 43.7% in untreated cells to 27.8%, 26.8%, and 23.2% after 5, 10, and 15 Gy, respectively. A similar decrease in PD-L1 and CTLA-4 expression was seen in DU145 cells (Fig. 2A). There was a slight (statistically insignificant) increase in CTLA-4 expression in PC3 cells after 5 Gy (19.7%) compared with 0 Gy (9.9%) (p=0.07). Modulation of CTLA-4 did not appear to be dose-dependent, and expression levels declined at 10 Gy (15.7%) and 15 Gy (14.7%) compared with 5 Gy.
FIG. 2.
Irradiated PCa cells modulate the balance of positive and negative costimulatory molecules in a dose-dependent manner. (A) DU145 cells and (B) PC3 cells. Cells were analyzed 72 hours after EBRT. Numbers indicate percent-positive cells. EBRT, external beam radiation therapy.
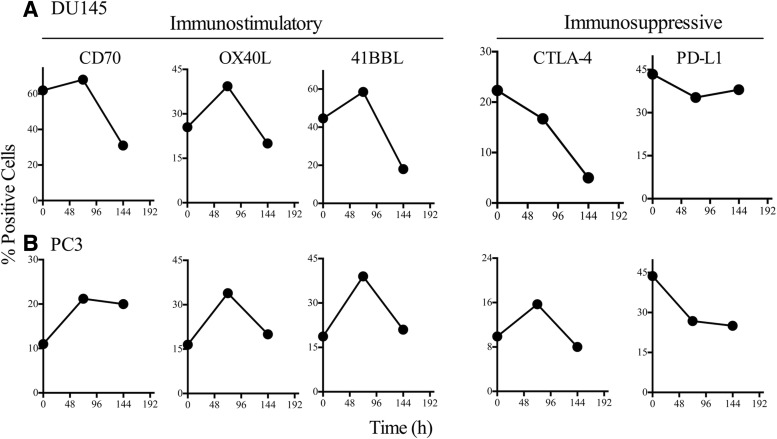
Reduced expression of coinhibitory molecule PD-L1 is sustained in RT-treated tumor cells
Next, we investigated whether radiation-induced modulation of costimulatory molecules was sustainable over time. We irradiated DU145 and PC3 cells with a single fraction of 10 Gy and performed flow cytometry either 72 hours (3 days) or 144 hours (6 days) post-treatment. In DU145 cells, expression of CD70, OX40L, and 41BBL increased 72 hours post-treatment compared with untreated cells, but decreased 144 hours post-treatment compared with untreated cells (Fig. 3A). In PC3 cells, expression of CD70, OX40L, and 41BBL decreased 144 hours post-treatment, but not below baseline levels observed in untreated cells (Fig. 3B). CTLA-4 expression slightly declined at both 72 and 144 hours post-treatment in DU145 cells. In PC3 cells, levels of CTLA-4 slightly increased 72 hours post-treatment (0 Gy 9.9% vs. 10 Gy 15.7%), but returned to baseline (8%) after 144 hours. In contrast, PD-L1 expression declined in both tumor cell lines and remained stable 144 hours post-treatment. Overall, modulation of positive costimulatory molecules was temporary and most robust 72 hours post-treatment, while modulation of the coinhibitory molecule PD-L1 was sustained.
FIG. 3.
PCa cells maintain a modulated costimulatory molecule profile for ≥72 hours after radiation exposure. (A) DU145 cells and (B) PC3 cells were irradiated with 10 Gy or left untreated (0 Gy). Cells were analyzed 0, 72, or 144 hours after irradiation. Numbers indicate percent-positive cells.
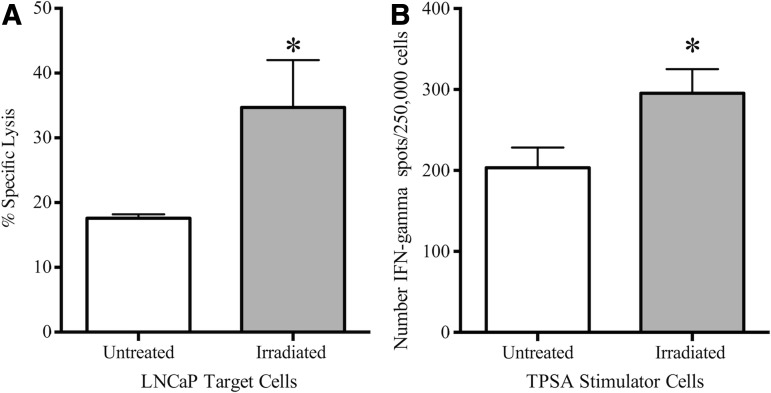
EBRT-induced modulation of tumor cells results in productive interactions with T cells
We next examined the functional consequences for CTL activity of phenotypic modulation by EBRT. First, we used irradiated LNCaP cells as targets for cytolysis by CEA-specific CD8+ CTLs (Fig. 4A). We detected 18% cytolysis of untreated LNCaP cells by CEA-specific T cells. After treatment of these cells with 10 Gy of radiation, CTL killing significantly increased (35%; p≤0.001 vs. no treatment). Second, splenocytes from mice vaccinated with a PSA-based Listeria vaccine were incubated with either unirradiated TPSA cells or cells treated with a single 10-Gy dose of radiation (Fig. 4B). Incubation with irradiated cells increased IFN-γ production 1.45-fold (p=0.05) over responder splenocytes stimulated with untreated TPSA cells, as detected by ELISPOT.
FIG. 4.
Functional consequences of modulating the costimulatory profile of PCa cells via radiation. (A) LNCaP cells were irradiated with 10 Gy (gray bar) or left untreated (0 Gy, open bar). After 72 hours, cells were used as targets in an 18-hour CTL lysis assay. (B) Splenocytes from mice vaccinated with a Listeria-based PSA vaccine were incubated with either untreated TPSA cells (open bar) or TPSA cells irradiated with 10 Gy (gray bar). Graph shows IFN-γ-producing cells/250,000 plated cells as detected by ELISPOT. *Statistical significance relative to untreated cells. CTL, cytotoxic T cells; IFN-γ, interferon-gamma; TPSA, total prostate-specific antigen.
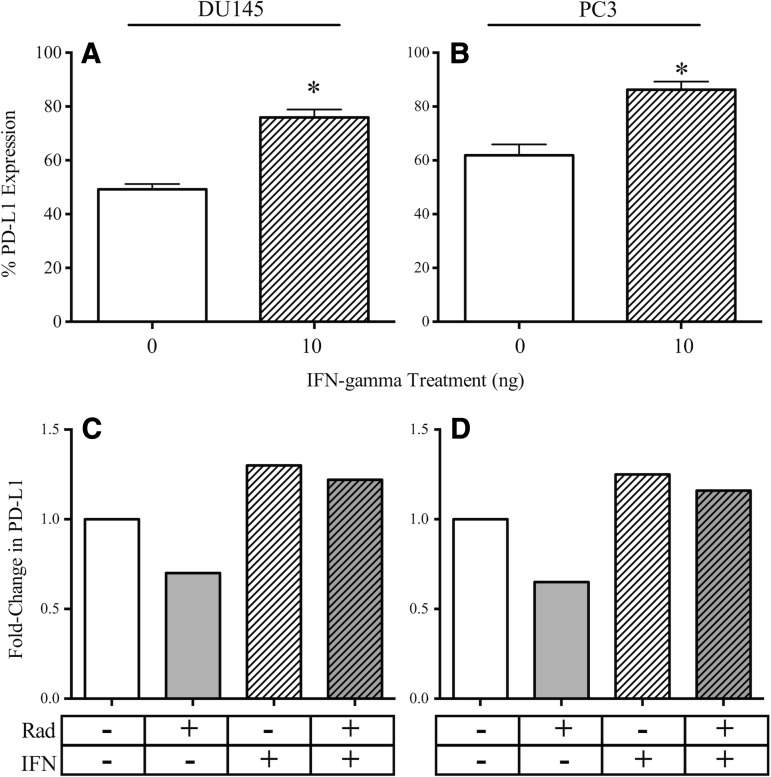
Expression of PD-L1 in DU145 and PC3 cells increases with IFN-γ treatment, and this increase is not abrogated by EBRT
IFN-γ production was enhanced in T cells after interaction with PCa tumor cells whose costimulatory molecules had been modulated by radiation. Several reports have linked IFN-γ production from T cells with increased PD-L1 expression on tumor cells.26–28 Thus, we investigated whether a potentially negative feedback loop exists in PCa cells. We treated DU145 and PC3 cells with recombinant human IFN-γ for 24 hours, harvested the cells, and evaluated PDL-1 expression by flow cytometry. PD-L1 expression on DU145 cells increased significantly, from 49.2% (untreated cells) to 75.9% (IFN-γ-treated cells) (p=0.0006) (Fig. 5A). Similarly, PD-L1 expression on PC3 cells increased from 61.9% (untreated cells) to 86.3% (IFN-γ-treated cells) (p=0.03) (Fig. 5B). We hypothesized that the PD-L1 increase in response to IFN-γ would be inhibited in irradiated cells. Consistent with our previous results, radiation mediated a decrease in PD-L1. However, as depicted in Figure 5C and D, exposure of irradiated DU145 and PC3 cells to IFN-γ demonstrated an increase of PD-L1 expression similar to that noted on unirradiated cells.
FIG. 5.
PD-L1 upregulation in PCa cells after treatment with recombinant human IFN-γ is not abrogated by radiation. (A) DU145 cells and (B) PC3 cells were treated with 10 ng IFN-γ (hatched bar) or left untreated (open bar). Surface PDL-1 was analyzed after 72 hours. Numbers indicate percent-positive cells. *Statistical significance relative to untreated cells. (C) DU145 cells and (D) PC3 cells were irradiated (10 Gy, gray bar), treated with 10 ng IFN-γ (hatched bar) or first irradiated, then treated with IFN-γ after 24 hours (hatched gray bar); untreated (open bar). Surface PDL-1 was analyzed after 72 hours. Numbers indicate fold change normalized to untreated cells.
Discussion
In this study, we examined the response of three PCa cell lines to a single fraction of radiation. After 10 Gy, EBRT increased surface expression of the immunostimulatory markers OX40L and 41BBL in all three cell lines. Expression of CD70 also increased after RT in PC3 cells, but not in the DU145 or LNCaP cell lines (Fig. 1). Conversely, expression of PD-L1, a transmembrane protein that transmits inhibitory signals to CTLs29,30 decreased in all three cell lines after EBRT. Importantly, irradiation did not alter the immunosuppressive profile of normal PrECs, whose high baseline expression of PD-L1 was maintained after irradiation (Fig. 1E). EBRT did not significantly modulate the slight expression of costimulatory molecules in PrECs, a finding also seen in normal human bronchial epithelial cells that likely helps to diminish autoimmune responses.29
CTLA-4 expression, which is expressed on helper T cells and suppresses the immune response, was uniquely modulated by EBRT among the three tumor cells lines: a decrease in DU145 cells, an increase in LNCaP cells, and a small, insignificant increase in PC3 cells (Fig. 1).
To our knowledge, this is the first study to explore the impact of radiation-induced modulation of costimulatory and coinhibitory molecules in PCa cells and normal epithelial cells. Our results suggest that RT may be employed to specifically modulate gene expression within PCa cells.
Previous preclinical studies established that antitumor T-cell activation was profoundly dependent on the relative timing of RT and immunotherapy.31 In our study, OX40L and 41BBL were upregulated 72 hours post-RT, but these increases were not detectable 144 hours post-RT (Fig. 3). In contrast, PD-L1 reduction was sustained. These data suggest a potential therapeutic window for the optimal timing of combination treatment with RT and CIT. Moreover, the RT-induced changes observed were dose-dependent (Fig. 2). While PCa continues to be most commonly treated with fractionated RT delivered in 1.8–2 Gy per fraction,6,7 our data suggest a potential immunologic benefit of treatment with higher doses per fraction. Consistent with our data of immunostimulation after treatment with 15 Gy, preclinical murine studies also showed that following a dose of 15 Gy in a single fraction increased the number of tumor-reactive T cells. However, this was accompanied by a parallel increase in regulatory T cells (Tregs), which are known to suppress tumor-specific immunity.32 These findings, though, may not be the case in human tumor cells, as we saw an increase in OX40L, which have been shown to reduce Tregs.33 Taken together, these data suggest that an optimal fractionation scheme may exist to promote immunostimulatory activity, above which the balance may shift toward immunosuppression.
To demonstrate that EBRT can not only alter the balance of immune-relevant signals, but also elicit a more robust immune response from T cells, we tested the sensitivity of irradiated target cells to CTL-mediated killing. Cytolysis of LNCaP tumor cells significantly increased after the tumor cells had been exposed to 10 Gy (Fig. 4A). We incubated splenocytes from mice vaccinated with a Listeria-based PSA vaccine with a murine tumor-cell line expressing PSA. When treated with 10 Gy of radiation, the TPSA cells showed a significant increase in IFN-γ production compared with incubation with untreated cells (Fig. 4B). These data mirror results reported in other human tumor-cell types, including colorectal and head and neck carcinoma cells, where RT enhanced cytolysis by CTLs.1,34
Here, combining immune cells with irradiated targets increased levels of IFN-γ. It has been reported that IFN-γ increases expression of PD-L1 on tumor cells,20,21 which diminishes antitumor immune responses. Confirming these reports, our study showed a significant increase in surface expression of PD-L1 in both DU145 and PC3 cells after treatment with IFN-γ (Fig. 5). However, we also demonstrated that a single fraction of EBRT reduced PD-L1 expression from baseline levels in both cell lines (Figs. 1–3, 5). It was interesting to note that EBRT did not abrogate IFN-γ-induced PD-L1 upregulation, suggesting that an alternate pathway may be involved.
Modulation of costimulatory molecules (such as OX40L and 41BBL) and coinhibitory molecules (such as PD-L1) appears to be particularly important for maintaining effective immune responses against self-antigens presented by tumor cells.35–37 Here, we report that costimulatory molecules are increased and coinhibitory signaling proteins are decreased in PCa cells in response to single-dose EBRT, which led to enhanced CTL-mediated killing and increased production of IFN-γ (Fig. 6). Such mechanisms could be effective even in the absence of immunogenic cell death38,39 and would be useful against radioresistant cancer cells. A thorough understanding of the molecular mechanism(s) that result in radiotherapy's ability to enhance immune attack will help us capitalize on these biological changes, enabling us to design CIT approaches in combination with RT.
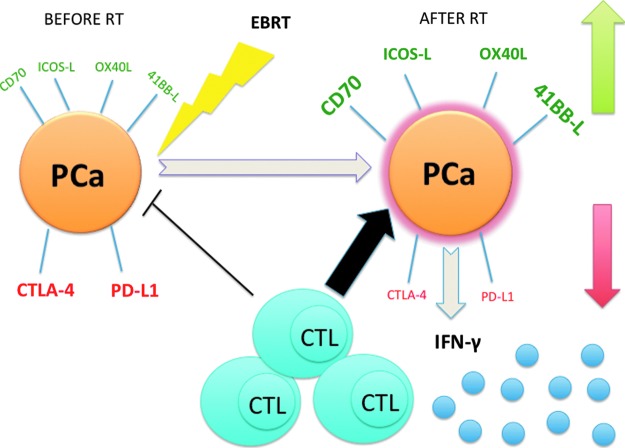
FIG. 6.
EBRT increases expression of cell surface immunostimulatory proteins and decreases expression of cell surface immunoinhibitory proteins leading to activation of CTLs and IFN-γ production. Untreated PCa cells display low levels of stimulatory proteins (green) and high levels of inhibitory proteins (red). After treatment with EBRT, PCa cells increased their expression of stimulatory proteins (green) and decreased their expression of inhibitory proteins (red), thereby, preferentially activating CTL-mediated cell killing and production of IFN-γ.
Acknowledgments
We thank Bonnie L. Casey and Debra Weingarten for editorial assistance in the preparation of this article. This research was supported by NIH Grant No. R01 EB009040 and the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Disclosure Statement
The authors have no financial, commercial, or other conflicts of interest to declare.
References
- 1.Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 2004;64:7985. [DOI] [PubMed] [Google Scholar]
- 2.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 2007;204:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005;174:7516. [DOI] [PubMed] [Google Scholar]
- 4.Nesslinger NJ, Sahota RA, Stone B, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res 2007;13:1493. [DOI] [PubMed] [Google Scholar]
- 5.Schaue D, Comin-Anduix B, Ribas A, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res 2008;14:4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res 2008;14:5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 2005;11:3353. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman HL, Divgi CR. Optimizing prostate cancer treatment by combining local radiation therapy with systemic vaccination. Clin Cancer Res 2005;11(19 Pt 1):6757. [DOI] [PubMed] [Google Scholar]
- 9.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012;12:278. [DOI] [PubMed] [Google Scholar]
- 10.George S, Pili R, Carducci MA, et al. Role of immunotherapy for renal cell cancer in 2011. J Natl Compr Canc Netw 2011;9:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akaza H, Tsukamoto T, Fujioka T, et al. Combined immunotherapy with low-dose IL-2 plus IFN-alpha for metastatic renal cell carcinoma: Survival benefit for selected patients with lung metastasis and serum sodium level. Jpn J Clin Oncol 2011;41:1023. [DOI] [PubMed] [Google Scholar]
- 12.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006;6:836. [DOI] [PubMed] [Google Scholar]
- 13.May KF, Jr., Gulley JL, Drake CG, et al. Prostate cancer immunotherapy. Clin Cancer Res 2011;17:5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol 2004;16:321. [DOI] [PubMed] [Google Scholar]
- 15.Keller AM, Schildknecht A, Xiao Y, et al. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity 2008;29:934. [DOI] [PubMed] [Google Scholar]
- 16.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev 2009;229:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kober J, Leitner J, Klauser C, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol 2008;38:2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baitsch L, Legat A, Barba L, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One 2012;7:e30852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Feng Y, Lu L, et al. Interferon-gamma-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology 2012;217:385. [DOI] [PubMed] [Google Scholar]
- 21.Schoop R, Wahl P, Le Hir M, et al. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant 2004;19:2713. [DOI] [PubMed] [Google Scholar]
- 22.Hannan R, Zhang H, Wallecha A, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer Immunol Immunother 2012;61:2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahabi V, Reyes-Reyes M, Wallecha A, et al. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother 2008;57:1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang KY, Zaremba S, Nieroda CA, et al. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst 1995;87:982. [DOI] [PubMed] [Google Scholar]
- 25.Tsang KY, Zhu M, Nieroda CA, et al. Phenotypic stability of a cytotoxic T-cell line directed against an immunodominant epitope of human carcinoembryonic antigen. Clin Cancer Res 1997;3(12 Pt 1):2439. [PubMed] [Google Scholar]
- 26.Eppihimer MJ, Gunn J, Freeman GJ, et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 2002;9:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.English K, Barry FP, Field-Corbett CP, et al. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett 2007;110:91. [DOI] [PubMed] [Google Scholar]
- 28.Rowe JH, Ertelt JM, Way SS. Innate IFN-gamma is essential for programmed death ligand-1-mediated T cell stimulation following Listeria monocytogenes infection. J Immunol 2012;189:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris TJ, Hipkiss EL, Borzillary S, et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate 2008;68:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burocchi A, Pittoni P, Gorzanelli A, et al. Intratumor OX40 stimulation inhibits IRF1 expression and IL-10 production by Treg cells while enhancing CD40L expression by effector memory T cells. Eur J Immunol 2011;41:3615. [DOI] [PubMed] [Google Scholar]
- 34.Gelbard A, Garnett CT, Abrams SI, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res 2006;12:1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilcox RA, Tamada K, Strome SE, et al. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol 2002;169:4230. [DOI] [PubMed] [Google Scholar]
- 36.Cannons JL, Lau P, Ghumman B, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol 2001;167:1313. [DOI] [PubMed] [Google Scholar]
- 37.Croft M, So T, Duan W, et al. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 2009;229:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: Immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012;11:215. [DOI] [PubMed] [Google Scholar]
- 39.Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51. [DOI] [PubMed] [Google Scholar]