Abstract
Obesity-induced inflammation is characterized by recruitment of adipose tissue macrophages that release inflammatory cytokines and chemokines. MIP-1α (macrophage inflammatory protein 1α)/CCL3, a CC chemokine, induces monocyte/macrophage infiltration and thus is implicated in obesity-induced adipose inflammation. Quercetin has been shown to modulate obesity-induced inflammation, but the mechanism of its action remains unclear. Here we demonstrate that quercetin decreases MIP-1α release from adipocytes and macrophages and from cocultured adipocytes/macrophages; it also opposes MIP-1α–induced macrophage infiltration and activation. The inhibitory action of quercetin on the MIP-1α–induced inflammatory responses of macrophages is mediated by downregulation of CCR1/CCR5, and inhibition of activation of JNK, p38 mitogen-activated-protein kinase (MAPK), and IKK as well as IκBα degradation. These findings suggest that quercetin may be a useful agent against obesity-induced adipose tissue inflammation.
Key Words: : adipose tissue, chemokine, inflammation, obesity, quercetin
Introduction
Obesity-induced adipose inflammation contributes to the development of metabolic complications such as insulin resistance and type 2 diabetes mellitus.1–3 Adipose inflammation is characterized by increased infiltration of macrophages into adipose tissue and their activation, leading to enhanced release of proinflammatory cytokines (tumor necrosis factor alpha [TNFα], interlukin-6 [IL-6]), which inhibit insulin signaling in adipose tissue.3–6 In the obese condition, chemokines, which elicit chemotactic activity by binding to their receptors, promote the migration of monocytes/macrophages into inflamed adipose tissue and their activation.7,8 Macrophage inflammatory protein 1α/chemokine (C-C motif ) ligand 3 (MIP-1α/CCL3), which is an endotoxin-inducible C-C family chemokine, is a representative chemokine that plays a pivotal role in the recruitment of monocytes/macrophages.9,10 Recent studies have shown that MIP-1α transcripts and proteins are elevated in the adipose tissue of genetically or diet-induced obese mice,5,11,12 and that circulating levels of MIP-1α are positively correlated with elevated insulin levels in the blood,11,13 this suggests that MIP-1α may be a useful target for modulating obesity-induced adipose inflammation and insulin resistance.
Quercetin (3,3,4,5,7-pentahydroxyflavone), exists mainly in the glycosidic form in which the sugar is conjugated with an aglycone, and is an abundant flavonoid in plants such as apple, tomato, grape, onion, and tea.14,15 It elicits antioxidant and anti-inflammatory activities,16–19 and also protects against metabolic diseases such as fatty liver diseases, insulin resistance, and atherosclerosis, as well as cancer.17,19–22 Since the metabolic diseases are linked to chronic inflammation in adipose tissue, the beneficial effects of quercetin may be related to its anti-inflammatory activity. Indeed, it has been shown to improve obesity-induced inflammatory status and metabolic syndrome in genetically obese Zucker rats.23 However, its effects on adipose inflammation, and the underlying mechanisms, are not yet fully understood.
In this study, we investigated the effect of quercetin on MIP-1α release from adipocytes/macrophages, and on MIP-1α–mediated migration/activation of macrophages. We demonstrated that quercetin suppresses MIP-1α release from adipocytes, macrophages, and cocultured adipocyte/macrophages, and also inhibits its chemotactic action by reducing expression of its receptor and interfering with inflammatory signaling. Quercetin may be a useful agent against obesity-induced adipose tissue inflammation.
Materials And Methods
Materials
Murine RAW 264.7 macrophages were obtained from the Korean Cell Line Bank (KCLB40071). The RPMI 1640 medium and gentamicin were purchased from Gibco BRL. Penicillin–streptomycin and TRIzol were purchased from Invitrogen. Insulin was purchased from Sigma-Aldrich. Quercetin was purchased from Jena Bioscience. Multiwell microchemotaxis chambers were purchased from Neuro Probe, Inc. Diff-Quik was purchased from International Reagent Corp. SYBR premix Ex Taq kit and a Dice Thermal Cycler were purchased from TaKaRa Bio, Inc. An OptEIA™ mouse MIP-1α set was purchased from R&D Systems. p-p38 mitogen-activated protein kinase (MAPK; Thr180/Tyr182), pJNK (c-Jun amino-terminal kinase), total JNK, pIKK, and total IKKβ were obtained from Cell Signaling. pIKKα/β (IκB kinase alpha/beta, Ser180/Ser181) and IκBα were provided by Santa Cruz Biotechnology, Inc.
Animals
Male C57BL/6 mice (8 weeks old; Orient Ltd.) were housed at 22°C and 50% humidity, with a 12-h light–12-h dark cycle. The mice had free access to water and feed. They had a 1 week adaptation period, and were then divided randomly into two groups. One group was given a high-fat diet (HFD, with 60% of calories from fat; (Research Diets), and the other was given a low-fat diet (LFD, 12% of calories from fat; Research Diets) for 10 weeks. The body weight was measured once a week. After 10 weeks, the mice were killed with N2 gas, and their epididymal fat pads were excised for RNA extraction, and frozen in liquid nitrogen. All animal experiments were approved by the Animal Ethics Committee of the University of Ulsan (Ulsan, Republic of Korea) and conformed to the guidelines of the U.S. National Institutes of Health.
Preparation of adipose tissue-conditioned medium
Mesenteric adipose tissue was isolated from obese mice, and all subsequent procedures were performed under a laminar flow hood. The tissue was minced into fragments less than 10 mg in weight and cultured as previously described.8 In brief, 50 mg aliquots of minced adipose tissue fragments were seeded in 10 mL of serum-containing medium in the wells of a 100 mm dish and placed in a humidified incubator at 37°C with 5% CO2. The medium was supplemented with glutamine, 25 mM HEPES, 50 μg/mL gentamicin, and 0.5 μg/mL amphotericin B. The tissue cultures were incubated for 72 h and aliquots of the culture medium were stored at −80°C until use.
Culture of macrophages and 3T3-L1 adipocytes
Murine macrophage RAW 264.7 cells were cultured in the RPMI 1640 medium supplemented with 10% fetal calf serum, 10 mL/L penicillin–streptomycin, and 2 mL/L gentamicin at 37°C and 5% CO2. 3T3-L1 Preadipocytes (ATCC) were cultured in a basal medium consisting of the Dulbecco's modified Eagle's medium (DMEM) supplemented with 200 μM ascorbic acid, 10% newborn calf serum, 10 mg/L penicillin–streptomycin, and 2 mg/L gentamicin at 37°C in a 5% CO2 atmosphere. Differentiation of the 3T3-L1 preadipocytes was induced by adipogenic agents (0.5 mM 3-isobutyl-1-methylxanthine, 0.25 μM dexamethasone, and 10 μg/mL insulin) in the DMEM containing 10% FBS for 2 days after the cells had reached confluence, as described previously. After 40–42 h, the cell culture medium was changed to a maturation medium, consisting of the basal medium with 5 μg/mL insulin. The maturation medium was changed every 2 days for 5 days. Cells (macrophages or adipocytes) were incubated with or without quercetin (2 or 10 μM) and/or obesity-related factors (BSA 50 μM, free fatty acid 500 μM, glucose 25 mM, H2O2 50 μM) in the presence or absence of 0, 2, 10 μM quercetin for 6 h and/or 16 h at 37°C. After treatment, culture supernatants were collected and stored at −20°C.
Coculture of adipocytes and macrophages
Adipocytes and macrophages were cocultured in a contact system as previously described.24 Briefly, RAW 264.7 macrophages (3×105 cells/mL) were plated with serum-starved hypertrophied 3T3-L1 adipocytes, and the cocultures were incubated in the serum-free DMEM for 24 h. RAW 264.7 and 3T3-L1 cells equal in number to those in the coculture that were cultured separately as controls. Quercetin was added to the cocultures at 2 or 10 μM. After 24 h of treatment, culture supernatants were collected and stored at −20°C.
Chemotaxis assay
The migration of macrophages was assessed in a multiwell microchemotaxis chamber.25 Briefly, RAW 264.7 macrophages prepared as above were suspended in the RPMI 1640 medium at 2×106 cells/mL, and 60 μL samples were placed in the upper wells of a 96-well chamber that were separated by an 8 μm polycarbonate filter from the lower wells containing a medium conditioned by mesenteric adipose tissue with or without quercetin and/or MIP-1α. After incubation for 4 h at 37°C, cells that had not migrated were removed by scraping, and those that had migrated across the filter were fixed and stained with Diff-Quik. The cells in four randomly chosen high-power fields (200×) were counted with a light microscope.
Quantitative real-time PCR
RNA was extracted with TRIzol and total RNA (0.5 μg) was reverse-transcribed into cDNA. For real-time reverse transcriptase-PCR assays, the linearity of the amplification of MIP-1α, CCR1, CCR5, 36B4, and β-actin cDNA was established in preliminary experiments. Primers were as follows: mMIP-1α: sense 5′-TGA AAC CAG CAG CCT TTG CTC-3′, antisense 5′-AGG CAT TCA GTT CCA GGT CAG TG-3′; mCCR1: sense 5′-AGT GGG AGT TCA CTC ACC GTA CC-3′, antisense 5′-AGT AAT AGC AAA TAT CAG ACG CAC GG-3′; mCCR5: sense 5′-CGG GAC TGT GAA CAC CTG GA-3′, antisense 5′-TCA AAC TAT GGA AAC AGC CCT CAT C-3′; 36B4: sense 5′-TTC CAG GCT TTG GGC ATC A-3′, antisense 5′-ATG TTC AGC ATG TTC AGC AGT GTG-3′; and β-actin: sense 5′-CAT CCG TAA AGA CCT CTA TGC CAA C-3′, antisense 5′-ATG GAG CCA CCG ATC CAC A-3′. Real-time PCR amplification of the cDNA was performed in duplicate with a SYBR premix Ex Taq kit using a Dice Thermal Cycler. All reactions were performed according to the following schedule: 95°C for 10 sec, and 45 cycles of 95°C for 5 sec and 60°C for 30 sec. The results were analyzed with Real-Time System TP800 software and all values were normalized to the levels of 36B4 or β-actin.
Measurement of MIP-1α concentrations by ELISA
MIP-1α in cultured supernatants was measured by an enzyme-linked immunosorbent assay. The assays were conducted with an OptEIA mouse MIP-1α set. Samples were thawed, diluted appropriately with assay diluent, and assayed. MIP-1α levels were derived from a standard curve constructed with the SOFTmax curve-fitting program (Molecular Devices).
Western blot analysis
Total cellular protein was harvested in a phosphate-buffered saline lysis buffer (10 mM Tris-HCl, 10 mM NaCl, 0.1 mM EDTA, 50 mM NaF, 10 mM Na4P2O7, 1 mM MgCl2, 0.5% deoxycholate, 1% IGEPAL, and protease inhibitor cocktail). Protein (20–50 μg) was subjected to Western blot analysis using polyclonal antibodies to total p-p38 MAPK (Thr180/Tyr182), pJNK (c-Jun amino-terminal kinase), total JNK, pIKK, total IKKβ, pIKKα/β (IκB kinase alpha/beta, Ser180/Ser181), and IκBα. β-actin (Sigma) was used as a loading control.
Statistical analyses
All data are expressed as mean±SEM. Statistical analysis was performed using ANOVA and the Duncan's multiple-range test. Differences were considered to be significant at P<.05.
Results
Levels of MIP-1α mRNA and MIP-1α protein in obese adipose tissue
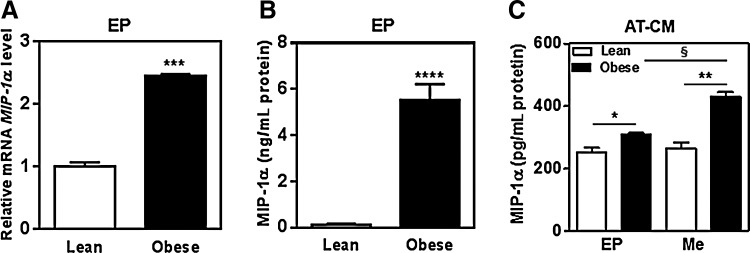
To test whether obesity altered MIP-1α expression in adipose tissue, we compared levels of MIP-1α transcripts and MIP-1α protein in obese and lean adipose tissue. Obese mice fed a HFD (41.38±2.19 g, n=4) had a higher body weight than lean mice fed a LFD (34.79±0.25 g, n=4), and epididymal adipose tissue weight (2.45±0.1 g) in the obese mice was significantly higher than in the lean mice (1.44±0.07 g). As shown in Figure 1A and B, levels of MIP-1α transcripts and MIP-1α protein were significantly higher in the epididymal adipose tissue of the obese mice compared with that of the lean mice. In addition, the amount of MIP-1α protein in the adipose tissue-conditioned medium from the obese mice was significantly higher compared with that from the lean mice (Fig. 1C), and more MIP-1α protein release was detected from mesenteric adipose tissue than from epididymal adipose tissue.
FIG. 1.
Upregulation of MIP-1α in obese adipose tissue. Adipose tissue was isolated from lean mice (n=4) and obese mice (n=4). Epididymal (EP) and mesenteric (Me) adipose tissues of obese and lean mice were incubated for 72 h. (A) MIP-1α mRNA and (B) MIP-1α protein in the epididymal (EP) adipose tissue and (C) MIP-1α protein in the adipose tissue-conditioned medium (AT-CM) were measured by real-time-PCR and ELISA, respectively. MIP-1α levels were normalized to the mRNA levels of the housekeeping gene 36B4, and expressed as ratios. Values are means±SEM. *P<.05, **P<.01, ***P<.005, ****P<.001 vs. control; §P<.05 for mesenteric vs. epididymal adipose tissue.
Obesity-related factors enhance MIP-1α gene expression and MIP-1α protein release
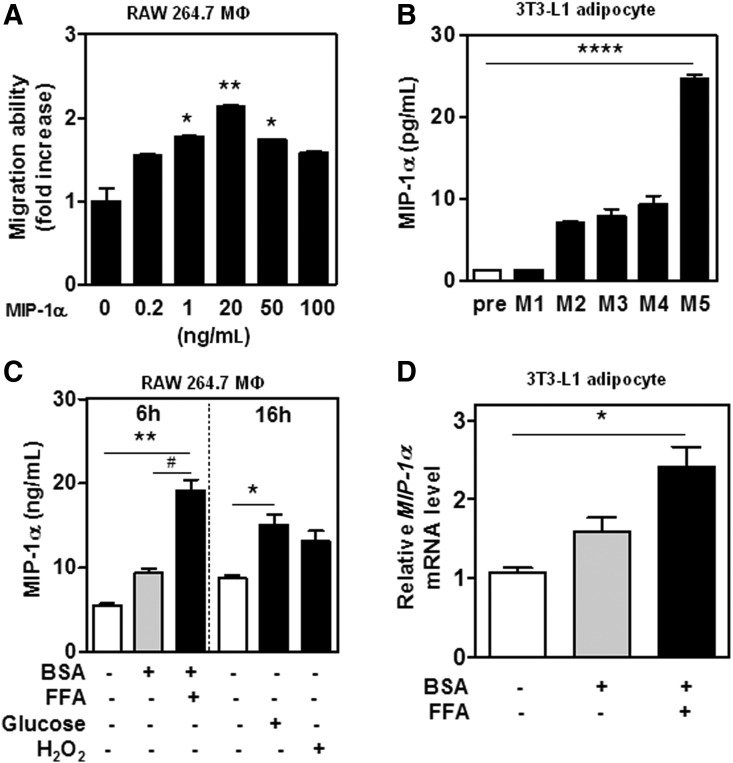
We confirmed that MIP-1α has chemotactic activity for macrophages. The MIP-1α concentration dependence curve of RAW 264.7 macrophage migration was bell-shaped with an optimal response at 20 ng/mL (Fig. 2A), implying that MIP-1α released from adipose tissue should be able to induce macrophage infiltration. In addition, levels of MIP-1α secretion increased during 3T3-L1 adipocyte differentiation and maturation (Fig. 2B). To see whether obesity triggers MIP-1α release from macrophages and adipocytes, we exposed macrophages and adipocytes to obesity-related factors such as free fatty acids (palmitate), glucose, and oxidative stress (hydrogen peroxide). This increased the release of MIP-1α from RAW 264.7 macrophages and also the expression of MIP-1α mRNA in 3T3-L1 adipocytes (Fig. 2C, D).
FIG. 2.
Effects of obesity-related factors on MIP-1α gene expression and release of MIP-1α protein. (A) Macrophage migration in response to MIP-1α. RAW 264.7 macrophages (1×106 cells/mL) were exposed to MIP-1α (0–100 ng/mL) for 4 h. Cells that migrated across the filter were fixed and stained with Diff-Quick and four randomly chosen high-power fields were counted under a light microscope. (B) Release of MIP-1α on preadipocytes (pre) and mature adipocytes (M; number=day). Preadipocytes (5×105 cells/mL) and mature adipocytes are described in Materials and Methods. (C) Release of MIP-1α protein by macrophages treated with obesity-related factors. RAW 264.7 macrophages (MΦ) (1.5×106 cells/mL) were incubated with obesity-related factors (BSA 50 μM, free fatty acid [FFA] 500 μM, glucose 25 mM, or H2O2 50 μM) for 6 or 16 h at 37°C. (D) Expression of MIP-1α mRNA from adipocytes treated with obesity-related factors (FFA). After incubation for 5 days, 3T3-L1 adipocytes were incubated with or without BSA 50 μM and/or FFA 500 μM for 12 h at 37°C. MIP-1α levels were normalized to the mRNA levels of the housekeeping gene 36B4, and expressed as ratios. Values are mean±SEM. *P<.05, **P<.01, ****P<.001 vs. untreated control; #P<.05 vs. negative control. Data presented are representative of three independent experiments.
Effect of quercetin on MIP-1α release from macrophages, adipocytes, and cocultured adipocytes/macrophages
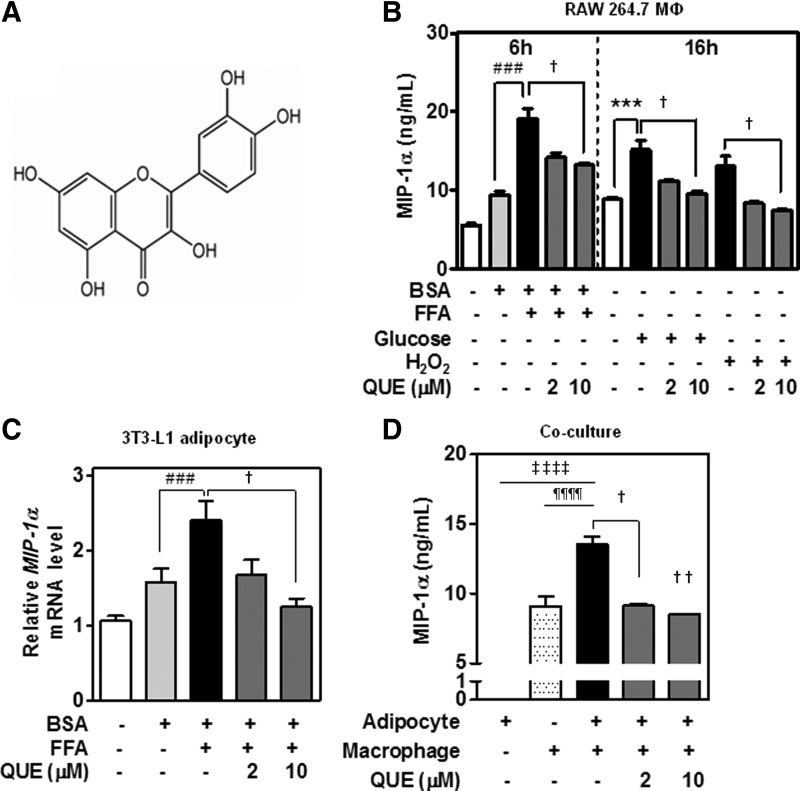
To determine whether quercetin inhibits obesity-induced MIP-1α release from adipose tissue, we measured MIP-1α release from macrophages, adipocytes, and cocultured adipocytes/macrophages treated with obesity-related factors in the presence and absence of quercetin. Quercetin significantly inhibited MIP-1α production by macrophages and reduced MIP-1α transcript levels in adipocytes (Fig. 3B, C). Moreover, it markedly reduced MIP-1α secretion from cocultured adipocytes/macrophages (Fig. 3D).
FIG. 3.
Quercetin decreases MIP-1α release from macrophages, adipocytes, and coculture. (A) Structure of quercetin (3,3′,4′,5,7-pentahydroxyflavone dihydrate). (B–D) Effects of quercetin on release of MIP-1α protein from cells treated with obesity-related factors. (B) RAW 264.7 macrophages (1.5×106 cells/mL) and (C) 3T3-L1 adipocytes were incubated with obesity-related factors (BSA 50 μM, FFA 500 μM, glucose 25 mM, or H2O2 50 μM) together with 0, 2, or 10 μM quercetin (QUE) for 6 h (MΦ) or 16 h (MΦ, adipocyte) at 37°C. (D) 3T3-L1 adipocytes were cocultured with RAW 264.7 macrophages (3×105 cells/mL) together with 0, 2, or 10 μM quercetin, and incubated for 24 h. MIP-1α release was measured by ELISA. MIP-1α levels were normalized to the mRNA levels of the housekeeping gene 36B4, and expressed as ratios. Values are mean±SEM. ***P<.005 vs. untreated control; ###P<.005 vs. negative control; †P<.05, ††P<.01 vs. positive control; ‡‡‡‡P<.001 for adipocytes alone vs. coculture; ¶¶¶¶P<.001 for macrophages alone vs. coculture. Data presented are representative of three independent experiments.
Effect of quercetin on MIP-1α–induced macrophage migration and cytokine release
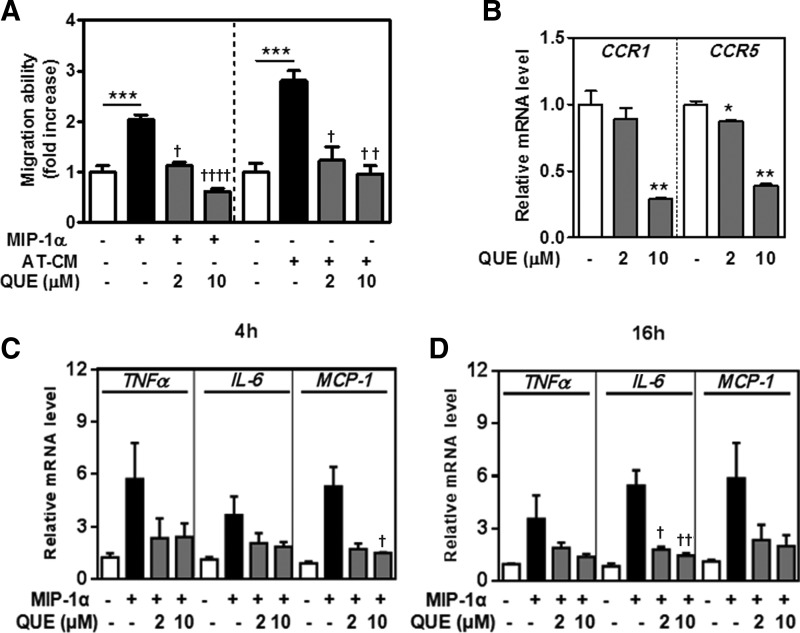
To determine whether quercetin suppresses inflammatory responses to MIP-1α, we prepared an adipose tissue-conditioned medium containing 20 ng/mL of MIP-1α, and treated macrophages with it to induce macrophage infiltration. Quercetin markedly reduced macrophage migration in response to MIP-1α and adipose tissue-conditioned medium (Fig. 4A). Macrophages express CCR1 and CCR5, which are specific receptors for MIP-1α. Therefore, the inflammatory responses of macrophages to MIP-1α are expected to depend on its binding to CCR1 and CCR5. In our experiments, treatment with quercetin decreased macrophage expression of CCR1 and CCR5 in a dose-dependent manner (Fig. 4B), suggesting that the decrease in macrophage migration in response to quercetin was due to decreased numbers of MIP-1α receptors. To examine whether quercetin interfered with MIP-1α–induced macrophage activation leading to the release of inflammatory cytokines, we treated macrophages with MIP-1α in the presence or absence of quercetin. As shown in Figure 4C and D, quercetin tended to reduce TNFα, IL-6, and monocyte chemoattractant protein-1 (MCP-1) mRNA levels at different times.
FIG. 4.
Quercetin decreases MIP-1α–induced macrophage chemotaxis and cytokine release. (A) Effect of quercetin on macrophage migration induced by the adipose tissue-conditioned medium (AT-CM) or MIP-1α. RAW 264.7 macrophages (1.5×106 cells/mL) were pretreated with 0, 2, or 10 μM quercetin for 1 h. They were then placed in the upper wells of 96-well culture chambers separated from the lower wells containing MIP-1α (20 ng/mL) or AT-CM and incubated for 4 h at 37°C. The cells that migrated across the filter were fixed and stained with Diff-Quick, and four randomly chosen high-power fields were counted under a light microscope. (B) Effect of quercetin on CCR1 and CCR5 mRNA in macrophages. RAW 264.7 macrophages were treated with 0, 2, or 10 μM quercetin for 4 h at 37°C. (C, D) Effects of quercetin on expression of cytokine mRNA (TNFα, IL-6, or MCP-1) from macrophages. RAW 264.7 macrophages treated MIP-1α (50 ng/mL) with 0, 2, or 10 μM quercetin for 4 or 6 h. Cytokine (TNFα, IL-6, or MCP-1) mRNA was measured by real-time-PCR and normalized to β-actin. Values are mean±SEM. *P<.05, **P<.01, ***P<.005 vs. untreated control; †P<.05, ††P<.01, ††††P<.001 vs. positive control. Data presented are representative of three independent experiments. IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein 1; TNFα, tumor necrosis factor-alpha.
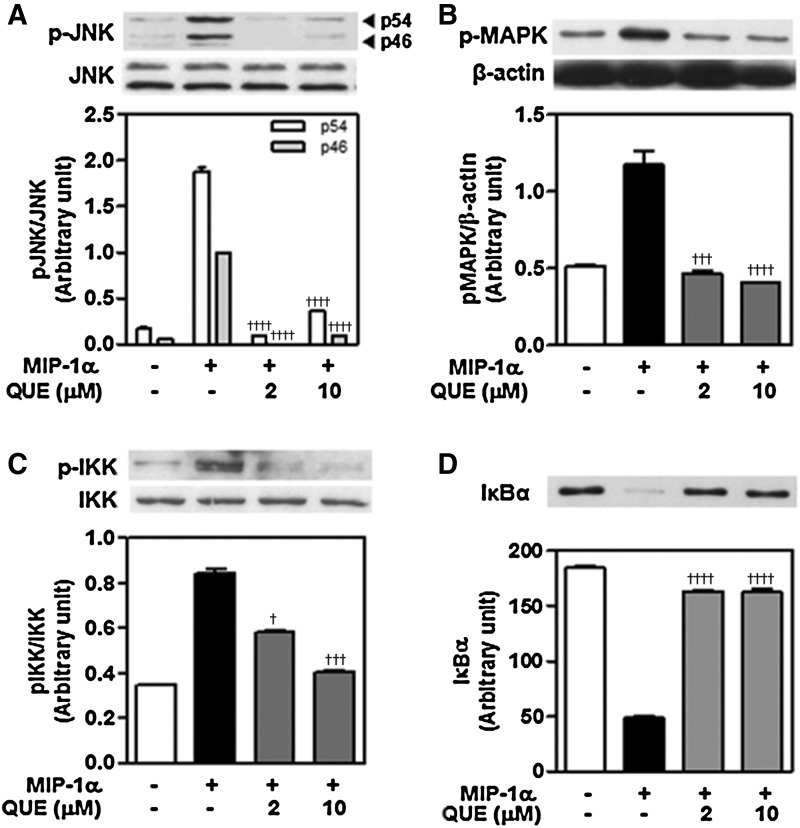
The effect of quercetin on activation of JNK, p38 MAPK, IKK and degradation of IκBα in macrophages induced by MIP-1α
To clarify the underlying mechanism of quercetin action on MIP-1α–induced inflammatory responses such as chemotaxis and cytokine release, we tested whether quercetin modulated MIP-1α–induced inflammatory signaling in macrophages by examining JNK, p38 MAPK, and IKK activation and IκBα degradation. As shown in Figure 5, MIP-1α induced the phosphorylation of JNK, p38 MAPK, and IKK, and IκBα degradation. It inhibited that of JNK (Fig. 5A) and p38 MAPK (Fig. 5B), as well as that of IKK (Fig. 5C), therefore decreasing IκBα degradation (Fig. 5D).
FIG. 5.
Quercetin inhibits the activation of JNK, p38 MAPK, and IKK, and degradation of IκBα in macrophages. Effect of quercetin on the levels of p-JNK (A), p38 MAPK (B), and p-IKK (C), and IκBα degradation (D) induced by MIP-1α- treated macrophages. RAW 264.7 macrophages (1.5×106 cells/mL) were pretreated with quercetin for 30 min and stimulated with MIP-1α 50 ng/mL for 1 h. Cell lysates were fractionated by 11% SDS-PAGE and subjected to Western immunoblot analysis with (p)-JNK, p38 MAPK, (p)-IKK, and IκBα degradation in RAW 264.7 macrophages. †P<.05, †††P<.005, ††††P<.001 vs. positive control. Data presented are representative of three independent experiments. MAPK, mitogen-activated-protein kinase.
Discussion
In this study, we demonstrated that quercetin suppressed MIP-1α release from adipocytes/macrophages, as well as MIP-1α-mediated inflammatory responses in macrophages, by inhibiting the production of its receptors and its signaling activity.
Macrophages infiltrated into adipose tissue, thereby exacerbating the inflammatory responses in adipose tissue by enhancing the production of proinflammatory cytokines/chemokines.1,3,4,6,25 The adipose tissue-derived chemokine, MCP-1/CCL2, stimulates macrophage infiltration into adipose tissue and activate macrophages, and thus are crucial for the amplification of adipose inflammatory responses.5,8 Interestingly, ablation of MCP-1 did not impact the accumulation of macrophages in the adipose tissue of obese mice,26,27 suggesting that other chemokines may be responsible for the infiltration of adipose macrophages. MIP-1α, a CC chemokine secreted by macrophages and activated T-cells, displays strong chemotactic activity for macrophages and is implicated in various inflammatory diseases.9,10 However, its role in obesity-induced inflammation remains unclear. We observed that MIP-1α transcripts and protein were upregulated in the epididymal adipose tissue of obese mice fed an HFD, suggesting that MIP-1α may contribute to the increased infiltration of macrophages into obese adipose tissue. Moreover, we found that mature adipocytes containing large lipid droplets secreted more MIP-1α protein than adipocytes containing smaller lipid droplets, and that obesity-related factors (e.g., FFA and oxidative stress) enhanced MIP-1α release from macrophages. These findings indicate that MIP-1α released from hypertrophic adipocytes and macrophages in obese adipose tissue provokes adipose tissue inflammation. In this context, inhibition of MIP-1α release from adipocytes/macrophages could be useful in protecting against obesity-induced adipose inflammation.
Recent studies have shown that quercetin, a plant aglycone derived from flavonoid glycosides, ameliorates metabolic diseases such as hyperlipidemia, fatty liver diseases, and insulin resistance.21,23 Since adipose inflammation, which is closely associated with the development of the metabolic disease,5 is exacerbated by adipose tissue-derived chemokines, the metabolic improvement brought about by quercetin may be due to its inhibitory action on the release of chemokines such as MIP-1α from adipose tissue. Indeed, we found that quercetin markedly decreased MIP-1α secretion from macrophages and adipocytes treated with obesity-related factors. It also decreased the release of MIP-1α from cocultured macrophages/adipocytes, indicating that it can oppose MIP-1α release from obese adipose tissue. More importantly, it markedly suppressed MIP-1α–induced macrophage infiltration and activation leading to the release of inflammatory cytokines (e.g., TNFα, IL-6, and MCP-1), indicating that it can interfere with amplification of the inflammatory cascade in adipose tissue. Given that the inflammatory mediators exaggerate adipose inflammation and metabolic disturbance, and thus are implicated in obesity-related metabolic complications, including insulin resistance,1,5 the inhibitory action of quercetin may protect against the obesity-related metabolic syndrome. Macrophages express the MIP-1α receptors CCR1 and CCR5 that are specific for MIP-1α, and we found that quercetin downregulated CCR1 and CCR5 gene expression, indicating that the reduced infiltration of macrophages by quercetin may be due to a reduction in MIP-1α receptors.
MIP-1α–mediated signaling is mediated by activation of the PI3K/Akt and MAPK pathways, which lead to macrophage proliferation and migration.28,29 p38 MAPK plays a central role in the regulation of a variety of inflammatory responses such as expression of proinflammatory mediators, leukocyte adhesion, chemotaxis, the oxidative burst, and degranulation.28,29 It has been shown that quercetin inhibits inflammatory responses in LPS-treated U937-derived macrophages and its action is due to inhibiting MAPKs such as ERK and JNK, and transcription factors such as NF-κB and AP-1 that induce inflammatory gene expression.30,31 We found that quercetin inhibited the phosphorylation of JNK and p38 MAPK, as well as of IKK, thus opposing IκB degradation. These findings suggest that it suppresses MIP-1α–induced macrophage infiltration and activation by inhibiting the activation of kinases, following NF-κB inactivation. Further study is needed to clarify the in vivo effects of quercetin in relation to levels of MIP-1α in obese adipose tissue and adipose inflammation.
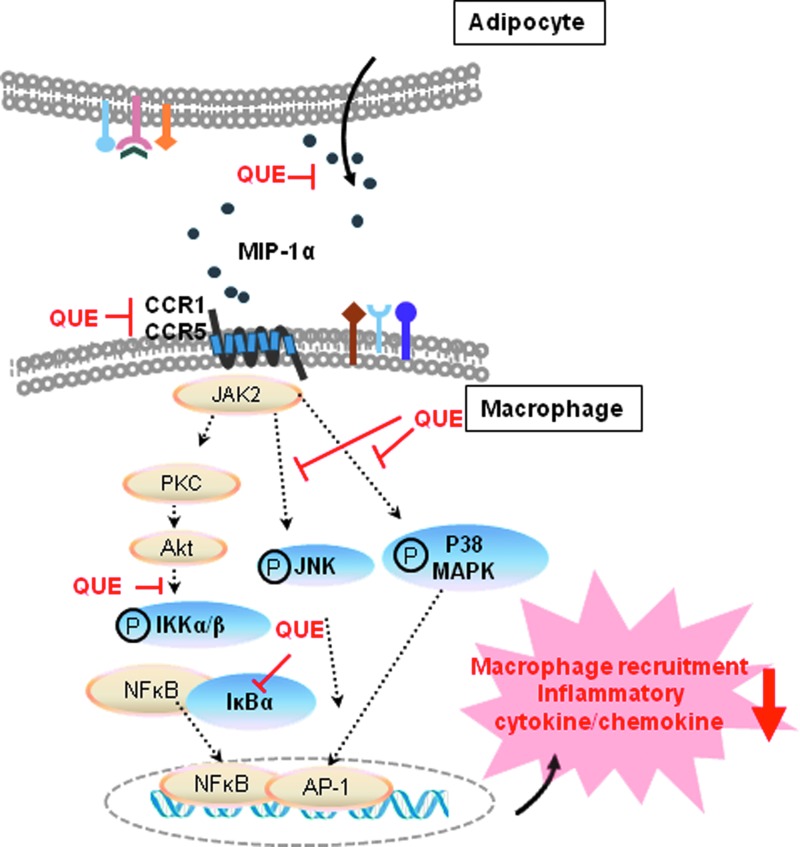
In conclusion, MIP-1α is highly expressed in obese adipose tissue, and obesity-related factors enhance its secretion from adipocytes and macrophages as well as cocultured adipocytes/macrophages. Quercetin suppresses the MIP-1α–mediated migration/activation of macrophages by downregulating CCR1/CCR5 production and inhibiting the activation of inflammatory signaling in macrophages (Fig. 6). Quercetin may be useful for protecting against obesity-induced adipose inflammation and metabolic diseases.
FIG. 6.
Schematic representation of the action of quercetin on MIP-1α–induced adipose inflammatory responses. Quercetin reduces MIP-1α release from adipocytes and/or macrophages; it also inhibits MIP-1α-induced macrophage infiltration and activation by downregulating CCR/CCR5 and by inhibiting activation of JNK, p38 MAPK, and IKK as well as IκBα degradation. Color images available at www.liebertpub.com/jmf
Acknowledgment
This work was supported by the Science Research Center program (Center for Food & Nutritional Genomics Grant (2008-0062618) of the NRF of Korea funded by the MEST.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hotamisligil GS: Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS: Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu R, Kim CS, Kang JH: Inflammatory components of adipose tissue as target for treatment of metabolic syndrome. Forum Nutr 2009;61:95–103 [DOI] [PubMed] [Google Scholar]
- 4.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Barnes GT, Yang Q, et al. : Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang JH, Goto T, Han IS, Kawada T, Kim YM, Yu R: Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity (Silver Spring) 2010;18:780–787 [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD: Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol 2001;175:81–92 [DOI] [PubMed] [Google Scholar]
- 8.Yu R, Kim CS, Kwon BS, Kawada T: Mesenteric adipose tissue-derived monocyte chemoattractant protein-1 plays a crucial role in adipose tissue macrophage migration and activation in obese mice. Obesity (Silver Spring) 2006;14:1353–1362 [DOI] [PubMed] [Google Scholar]
- 9.Maurer M, von Stebut E: Macrophage inflammatory protein-1. Int J Biochem Cell Biol 2004;36:1882–1886 [DOI] [PubMed] [Google Scholar]
- 10.Davatelis G, Tekamp-Olson P, Wolpe SD, et al. : Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J Exp Med 1988;167:1939–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber J, Kiefer FW, Zeyda M, et al. : CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab 2008;93:3215–3221 [DOI] [PubMed] [Google Scholar]
- 12.Jiao P, Chen Q, Shah S, et al. : Obesity-related upregulation of monocyte chemotactic factors in adipocytes: involvement of nuclear factor-kappaB and c-Jun NH2-terminal kinase pathways. Diabetes 2009;58:104–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murdolo G, Kempf K, Hammarstedt A, Herder C, Smith U, Jansson PA: Insulin differentially modulates the peripheral endocannabinoid system in human subcutaneous abdominal adipose tissue from lean and obese individuals. J Endocrinol Invest 2007;30:RC17–RC21 [DOI] [PubMed] [Google Scholar]
- 14.Moskaug JO, Carlsen H, Myhrstad M, Blomhoff R: Molecular imaging of the biological effects of quercetin and quercetin-rich foods. Mech Ageing Dev 2004;125:315–324 [DOI] [PubMed] [Google Scholar]
- 15.Williamson G, Manach C: Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 2005;81:243S–255S [DOI] [PubMed] [Google Scholar]
- 16.Anjaneyulu M, Chopra K: Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol 2004;31:244–248 [DOI] [PubMed] [Google Scholar]
- 17.Overman A, Chuang CC, McIntosh M: Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int J Obes (Lond) 2011;35:1165–1172 [DOI] [PubMed] [Google Scholar]
- 18.Boots AW, Haenen GR, Bast A: Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008;585:325–337 [DOI] [PubMed] [Google Scholar]
- 19.Middleton E, Jr, Kandaswami C, Theoharides TC: The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000;52:673–751 [PubMed] [Google Scholar]
- 20.Plakas SM, Lee TC, Wolke RE: Absence of overt toxicity from feeding the flavonol, quercetin, to rainbow trout (Salmo gairdneri). Food Chem Toxicol 1985;23:1077–1080 [DOI] [PubMed] [Google Scholar]
- 21.Gnoni GV, Paglialonga G, Siculella L: Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. Eur J Clin Invest 2009;39:761–768 [DOI] [PubMed] [Google Scholar]
- 22.Bhaskar S, Kumar KS, Krishnan K, Antony H: Quercetin alleviates hypercholesterolemic diet induced inflammation during progression and regression of atherosclerosis in rabbits. Nutrition 2013;29:219–229 [DOI] [PubMed] [Google Scholar]
- 23.Rivera L, Moron R, Sanchez M, Zarzuelo A, Galisteo M: Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring) 2008;16:2081–2087 [DOI] [PubMed] [Google Scholar]
- 24.Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 25.Kang JH, Kim CS, Han IS, Kawada T, Yu R: Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett 2007;581:4389–4396 [DOI] [PubMed] [Google Scholar]
- 26.Inouye KE, Shi H, Howard JK, et al. : Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 2007;56:2242–2250 [DOI] [PubMed] [Google Scholar]
- 27.Kirk EA, Sagawa ZK, McDonald TO, O'Brien KD, Heinecke JW: Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes 2008;57:1254–1261 [DOI] [PubMed] [Google Scholar]
- 28.Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY: Macrophage inflammatory protein 1-alpha (MIP-1 alpha) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 2003;101:3568–3573 [DOI] [PubMed] [Google Scholar]
- 29.Don MJ, Liao JF, Lin LY, Chiou WF: Cryptotanshinone inhibits chemotactic migration in macrophages through negative regulation of the PI3K signaling pathway. Br J Pharmacol 2007;151:638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter AB, Knudtson KL, Monick MM, Hunninghake GW: The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J Biol Chem 1999;274:30858–30863 [DOI] [PubMed] [Google Scholar]
- 31.Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M: Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J Biol Chem 2000;275:4283–4289 [DOI] [PubMed] [Google Scholar]